Hybrid Quinolinyl Phosphonates as Heterocyclic Carboxylate Isosteres: Synthesis and Biological Evaluation against Topoisomerase 1B (TOP1B)
Abstract
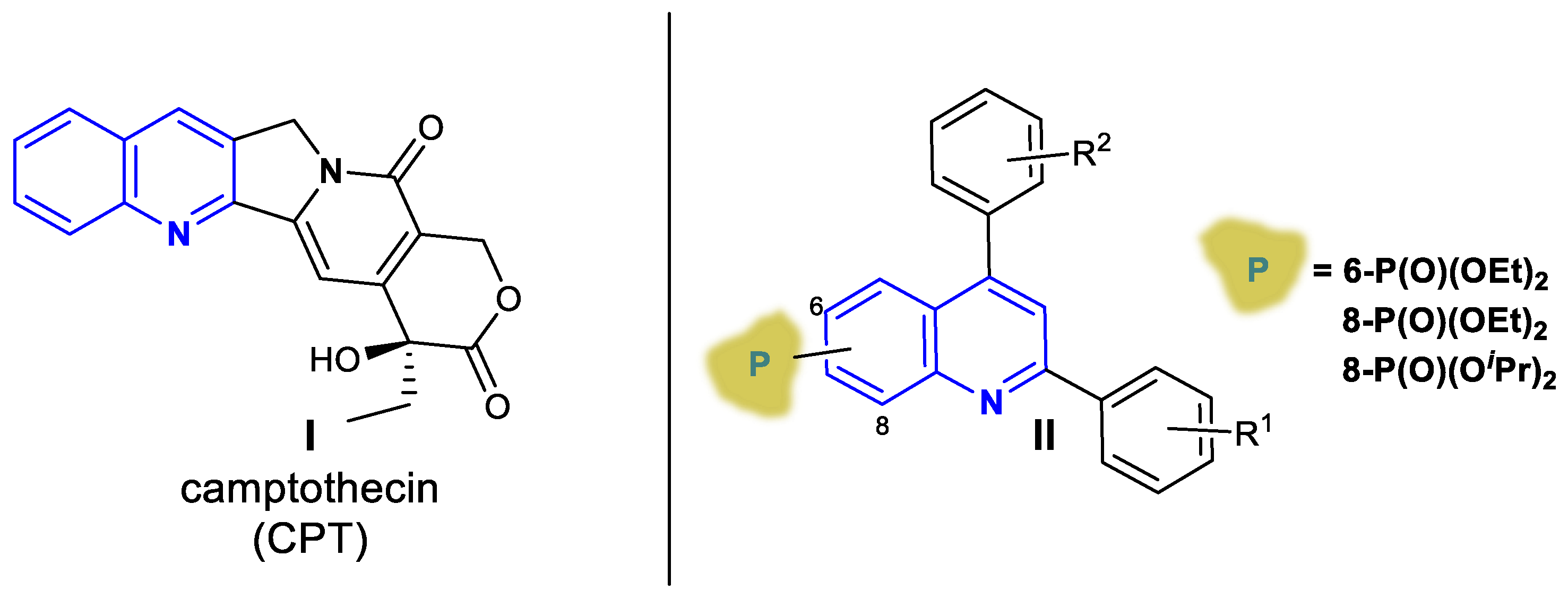
1. Introduction
2. Results and Discussion
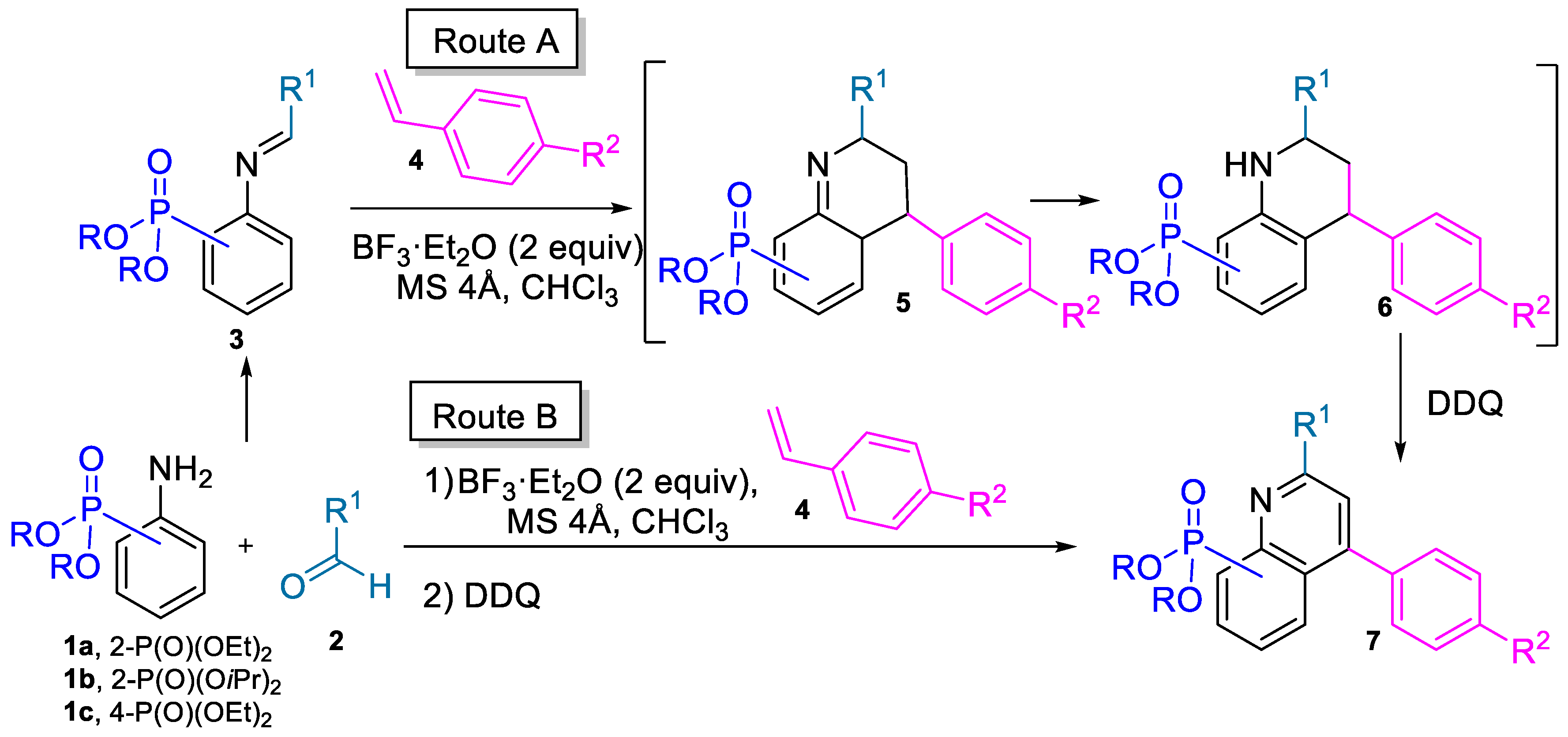
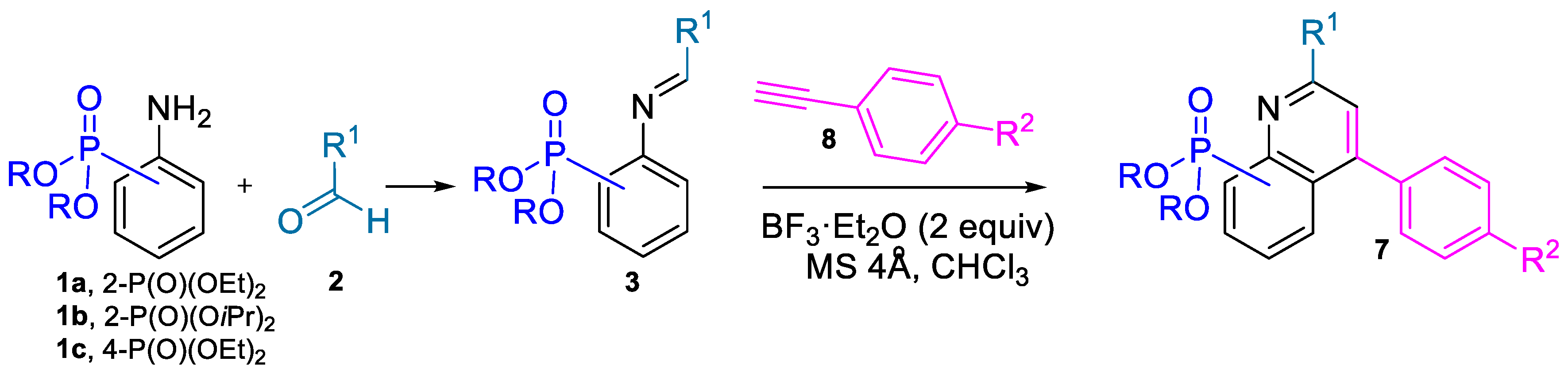
2.1. Chemistry
2.2. In Vitro Cytotoxicity
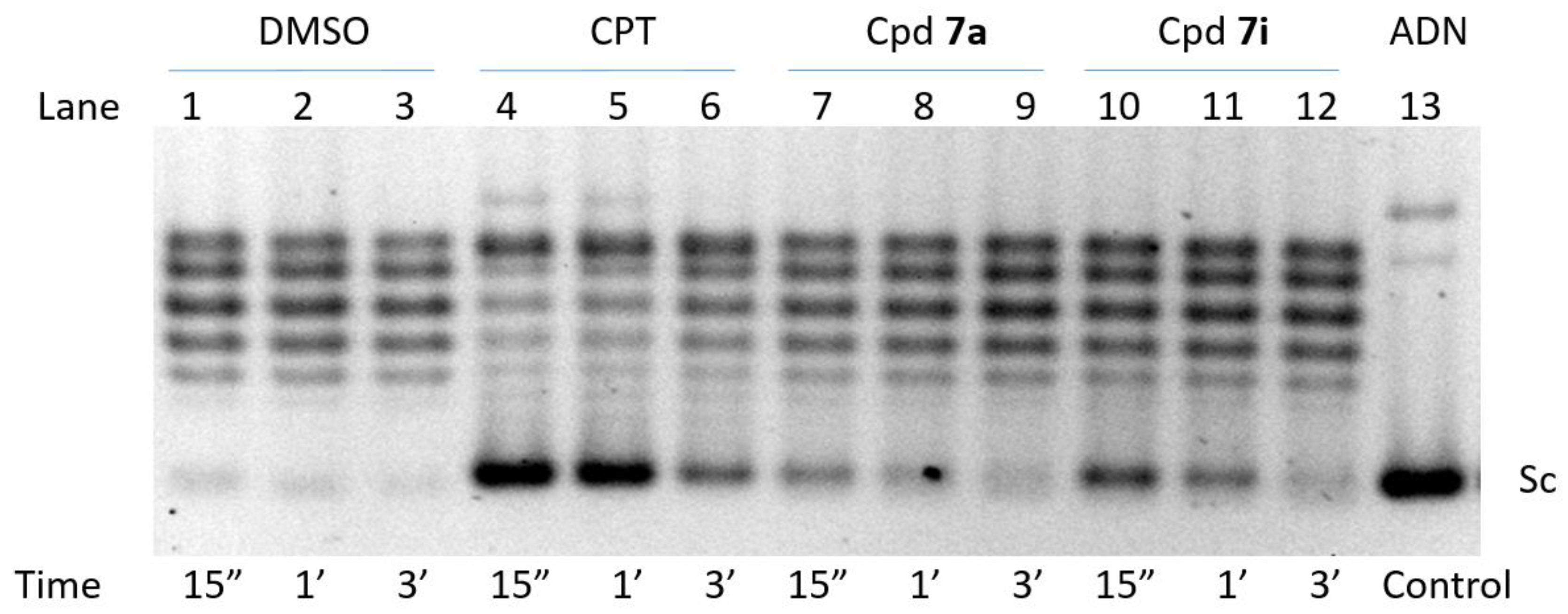
2.3. Inhibition of Human TOP1B (hTOP1B)
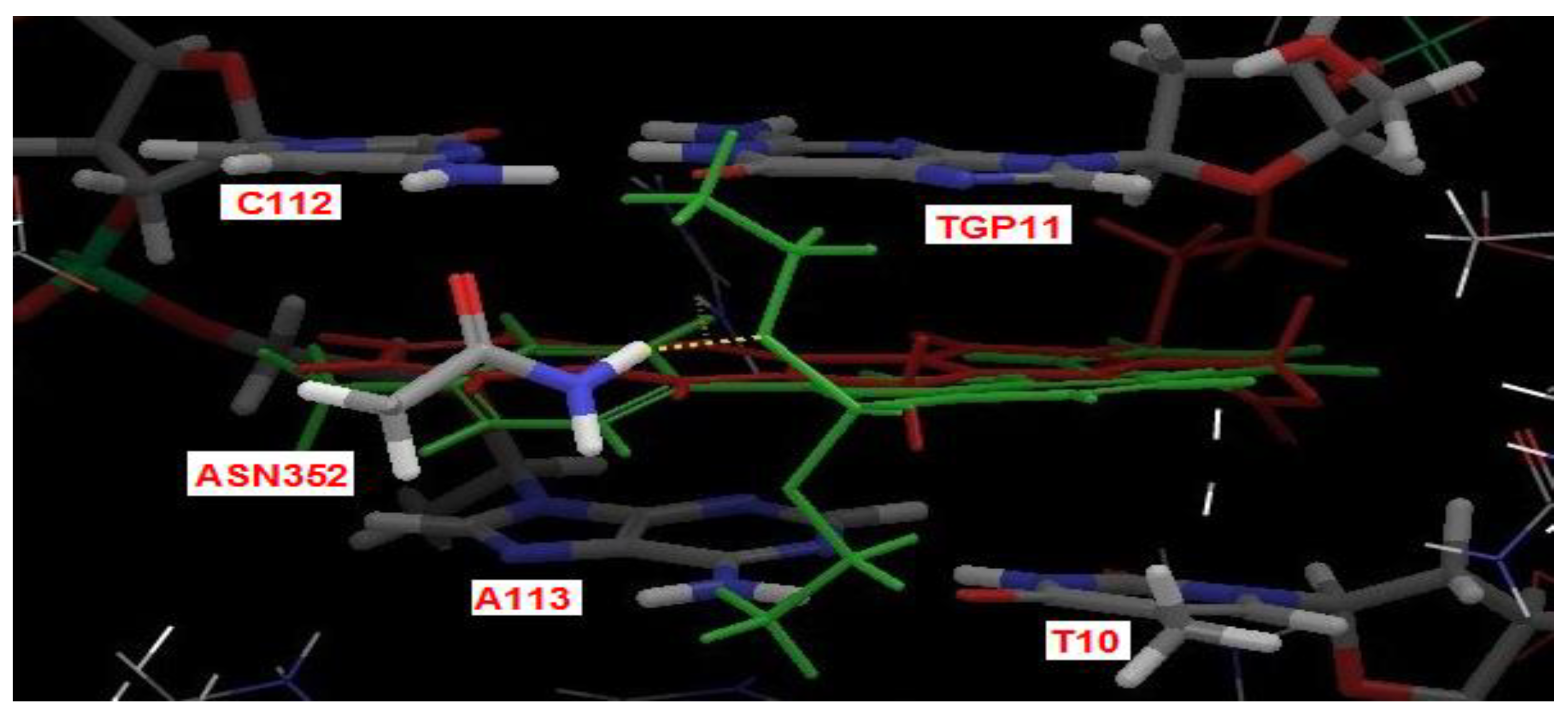
2.4. Docking Study
2.5. Antileishmanial Activity of New Quinolinylphosphonate Derivatives
2.6. Inhibition of Leishmanial TOP1 (LTOP1B)
3. Materials and Methods
3.1. Chemistry
3.1.1. General Methods
3.1.2. Compounds Purity Analysis
3.1.3. Synthesis of Aminophenyl Phosphonates 1
3.1.4. Synthesis of Quinolinyl Phosphonates
3.2. Biology
3.2.1. Materials
3.2.2. Expression and Purification of Human Topoisomerase 1B
3.2.3. Cytotoxicity Assays
3.2.4. hTOP1B DNA Relaxation Assays
3.2.5. Docking Study
3.2.6. In Vitro L. infantum Promastigotes Assays
3.2.7. Ex Vivo Murine Splenic Explant Cultures
3.2.8. Selectivity Index (SI) Determination
3.2.9. Purification of Leishmanial Topoisomerase 1B
3.2.10. LTOP1B Relaxation Activity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMB | Amphotericin B |
| CCK8 | Cell counting kit |
| CPT | Camptothecin |
| DDQ | Dichloro-5,6-dicyanobenzoquinone |
| MCR | Multicomponent reaction |
| SDS | Sodium dodecyl sulfate; SI, selectivity index |
| TOP1B | DNA topoisomerase 1B |
| TLC | Thin layer chromatography |
References
- International Agency for Research on Cancer, GLOBOCAN 2018. Available online: http://gco.iarc.fr/today/home (accessed on 13 November 2020).
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef]
- Reguera, R.M.; Elmahallawy, E.K.; García-Estrada, C.; Carbajo-Andrés, R.; Balaña-Fouce, R. DNA Topoisomerases of Leishmania Parasites; Druggable Targets for Drug Discovery. Curr. Med. Chem. 2019, 26, 5900–5923. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, N.; Miyagawa, K. Targeting DNA damage response in cancer therapy. Cancer Sci. 2014, 105, 370–388. [Google Scholar] [CrossRef]
- Villa, H.; Otero Marcos, A.R.; Reguera, R.M.; Balaña-Fouce, R.; García-Estrada, C.; Pérez-Pertejo, Y.; Tekwani, B.L.; Myler, P.J.; Stuart, K.D.; Bjornsti, M.A.; et al. A novel active DNA topoisomerase I in Leishmania donovani. J. Biol. Chem. 2003, 278, 3521–3526. [Google Scholar] [CrossRef]
- Balaña-Fouce, R.; Alvarez-Velilla, R.; Fernández-Prada, C.; García-Estrada, C.; Reguera, R.M. Trypanosomatids topoisomerase re-visited. New structural findings and role in drug discovery. Int. J. Parasitol. Drugs Drug Resist. 2014, 24, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Champoux, J.J. DNA Topoisomerases: Structure, Function, and Mechanism. Annu. Rev. Biochem. 2001, 70, 369–413. [Google Scholar] [CrossRef] [PubMed]
- Selas, A.; Martin-Encinas, E.; Fuertes, M.; Masdeu, C.; Rubiales, G.; Palacios, F.; Alonso, C. A patent review of topoisomerase I inhibitors (2016-present). Expert Opin. Ther. Pat. 2021, 31, 473–508. [Google Scholar] [CrossRef] [PubMed]
- Alonso, C.; González, M.; Palacios, F.; Rubiales, G. Study of the hetero-[4+2]-cycloaddition reaction of aldimines and alkynes. Synthesis of 1,5-naphthyridine and isoindolone derivatives. J. Org. Chem. 2017, 82, 6379–6387. [Google Scholar] [CrossRef]
- Alonso, C.; Fuertes, M.; González, M.; Rubiales, G.; Palacios, F. Synthesis and biological evaluation of indeno[1,5]naphthyridines as topoisomerase I (TopI) inhibitors with antiproliferative activity. Eur. J. Med. Chem. 2016, 115, 179–190. [Google Scholar] [CrossRef]
- Alonso, C.; Fuertes, M.; González, M.; Rubiales, G.; Rodríguez-Gascón, A.; Rubiales, G.; Palacios, F. Synthesis and biological evaluation of 1,5-naphthyridines as topoisomerase I inhibitors. A new family of antiproliferative agents. Curr. Top. Med. Chem. 2014, 14, 2722–2728. [Google Scholar] [CrossRef]
- Tejeria, A.; Perez-Pertejo, Y.; Reguera, R.M.; Balana-Fouce, R.; Alonso, C.; Gonzalez, M.; Rubiales, G.; Palacios, F. Substituted 1,5-naphthyridine derivatives as novel antileishmanial agents. Synthesis and biological evaluation. Eur. J. Med. Chem. 2018, 152, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Tejeria, A.; Perez-Pertejo, Y.; Reguera, R.M.; Balana-Fouce, R.; Alonso, C.; Fuertes, M.; Gonzalez, M.; Rubiales, G.; Palacios, F. Antileishmanial effect of new indeno-1,5-naphthyridines, selective inhibitors of Leishmania infantum type IB DNA topoisomerase. Eur. J. Med. Chem. 2016, 124, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Akkachairin, B.; Rodphon, W.; Reamtong, O.; Mungthin, M.; Tummatorn, J.; Thongsornkleeb, C.; Ruchirawat, S. Synthesis of neocryptolepines and Carbocycle-Fused Quinolines and Evaluation of Their Anticancer and Antiplasmodial Activities. Bioorg. Chem. 2020, 98, 103732. [Google Scholar] [CrossRef] [PubMed]
- Ahadi, H.; Emami, S. Modification of 7-piperazinylquinolone antibacterials to promising anticancer lead compounds: Synthesis and in vitro studies. Eur. J. Med. Chem. 2020, 187, 111970. [Google Scholar] [CrossRef] [PubMed]
- Musiol, R. An overview of quinoline as a privileged scaffold in cancer drug discovery. Expert Opin. Drug Discov. 2017, 12, 583–597. [Google Scholar] [CrossRef] [PubMed]
- Afzal, O.; Kumar, S.; Haider, M.R.; Ali, M.R.; Kumar, R.; Jaggi, M.; Bawa, S. A review on anticancer potential of bioactive heterocycle quinolone. Eur. J. Med. Chem. 2015, 97, 871–910. [Google Scholar] [CrossRef]
- Maslanka, M.; Mucha, A. Recent developments in peptidyl diaryl phoshonates as inhibitors and activity-based probes for serine proteases. Pharmaceuticals 2019, 12, 86. [Google Scholar] [CrossRef] [PubMed]
- Patani, G.A.; LaVoie, E.J. Bioisosterism: A Rational Approach in Drug Design. Chem. Rev. 1996, 96, 3147–3176. [Google Scholar] [CrossRef]
- Rye, C.S.; Baell, J.B. Phosphate isosteres in medicinal chemistry. Curr. Med. Chem. 2005, 12, 3127–3141. [Google Scholar] [CrossRef]
- Berube, G. An overview of molecular hybrids in drug Discovery. Expert Opin. Drug Discov. 2016, 11, 281–305. [Google Scholar] [CrossRef] [PubMed]
- Fortin, S.; Berube, G. Advances in the development of hybrid anticancer drugs. Expert Opin. Drug Discov. 2013, 8, 1029–1047. [Google Scholar] [CrossRef] [PubMed]
- Alonso, C.; Fuertes, M.; Martin-Encinas, E.; Selas, A.; Rubiales, G.; Tesauro, C.; Knudssen, B.R.; Palacios, F. Novel topoisomerase I inhibitors. Syntheses and biological evaluation of phosphorus substituted quinoline derivates with antiproliferative activity. Eur. J. Med. Chem. 2018, 149, 225–237. [Google Scholar] [CrossRef]
- Tejería, A.; Pérez-Pertejo, Y.; Reguera, R.M.; Carbajo-Andrés, R.; Balaña-Fouce, R.; Alonso, C.; Martin-Encinas, E.; Selas, A.; Rubiales, G.; Palacios, F. Antileishmanial activity of new hybrid tetrahydroquinoline and quinoline derivatives with phosphorus substituents. Eur. J. Med. Chem. 2019, 162, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Ghashghaei, O.; Masdeu, C.; Alonso, C.; Palacios, F.; Lavilla, R. Recent advances of the Povarov reaction in medicinal chemistry. Drug Discov. Today 2018, 29, 71–79. [Google Scholar] [CrossRef]
- Cadogan, J.I.G.; Sears, D.J.; Smith, D.M. The reactivity of organophosphorus compounds. Part XXV. Displacement of activated aromatic nitro-groups by tervalent phosphorus reagents. J. Chem. Soc. 1969, 1314–1318. [Google Scholar] [CrossRef]
- Staker, B.L.; Feese, M.D.; Cushman, M.; Pommier, Y.; Zembower, D.; Stewart, L.; Burgin, A.B. Structures of three classes of anticancer agents bound to the human topoisomerase I-DNA covalent complex. J. Med. Chem. 2005, 48, 2336–2345. [Google Scholar] [CrossRef] [PubMed]
- Pommier, Y.; Marchand, C. Interfacial inhibitors: Targeting macromolecular complexes. Nat. Rev. Drug Discov. 2012, 11, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.M.; Rajan, R.; Mondragon, A. Structural studies of type I topoisomerases. Nucleic Acids Res. 2009, 37, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Marchand, C.; Antony, S.; Kohn, K.W.; Cushman, M.; Ioanoviciu, A.; Staker, B.L.; Burgin, A.B.; Stewart, L.; Pommier, Y. A novel norindeniosoquinoline structure reveals a common interfacial inhibitor paradigm for ternary trapping of the topoisomerase I-DNA covalent complex. Mol. Cancer Ther. 2006, 5, 287–295. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Koster, D.A.; Paile, K.; Bot, E.S.M.; Bjornsti, M.A.; Dekker, N.H. Antitumour drugs impede DNA uncoiling by topoisomerase I. Nature 2007, 448, 213–217. [Google Scholar] [CrossRef]
- Lauria, A.; Ippolito, M.; Almerico, A.M. Molecular docking approach on the Topoisomerase I inhibitors series included in the NCI anti-cancer agents mechanism database. J. Mol. Model. 2007, 13, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Lisby, M.; Krogh, B.O.; Boege, F.; Westergaard, O.; Knudsen, B.R. Camptothecins inhibit the utilization of hydrogen peroxide in the ligation step of topoisomerase I catalysis. Biochemistry 1998, 37, 10815–10827. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, B.R.; Straub, T.; Boege, F. Separation and functional analysis of eukaryotic DNA topoisomerases by chromatography and electrophoresis. J. Chromatogr. B Biomed. Appl. 1996, 684, 307–321. [Google Scholar] [CrossRef]
- Schrödinger Release 2015-1: Maestro, version 10.1; Schrödinger, L.L.C.: New York, NY, USA, 2015.
- Schrödinger Release 2015-1: Glide, version 6.9; Schrödinger, L.L.C.: New York, NY, USA, 2015.
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef]
- Schrödinger Release 2015-1: Protein Preparation Wizard; epik 3.1; Schrödinger, L.L.C.: New York, NY, USA, 2015.
- Calvo-Álvarez, E.; Stamatakis, K.; Punzón, C.; Álvarez-Velilla, R.; Tejería, A.; Escudero-Martínez, J.M.; Pérez-Pertejo, Y.; Fresno, M.; Balaña-Fouce, R.; Reguera, R.M. Infrared fluorescent imaging as a potent tool for in vitro, ex vivo and in vivo models of visceral leishmaniasis. PLoS Negl. Trop. Dis. 2015, 9, e0003666. [Google Scholar] [CrossRef]
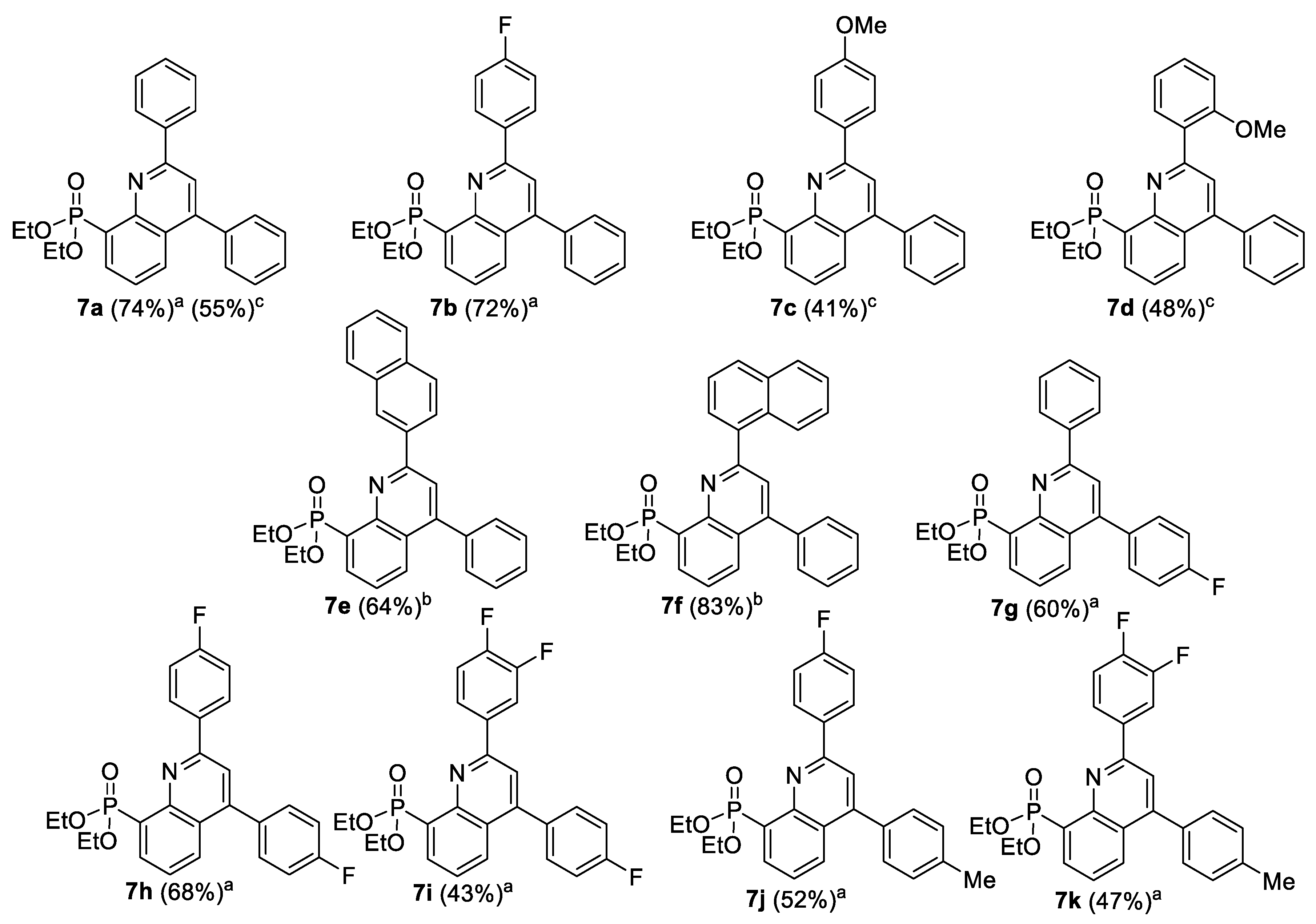
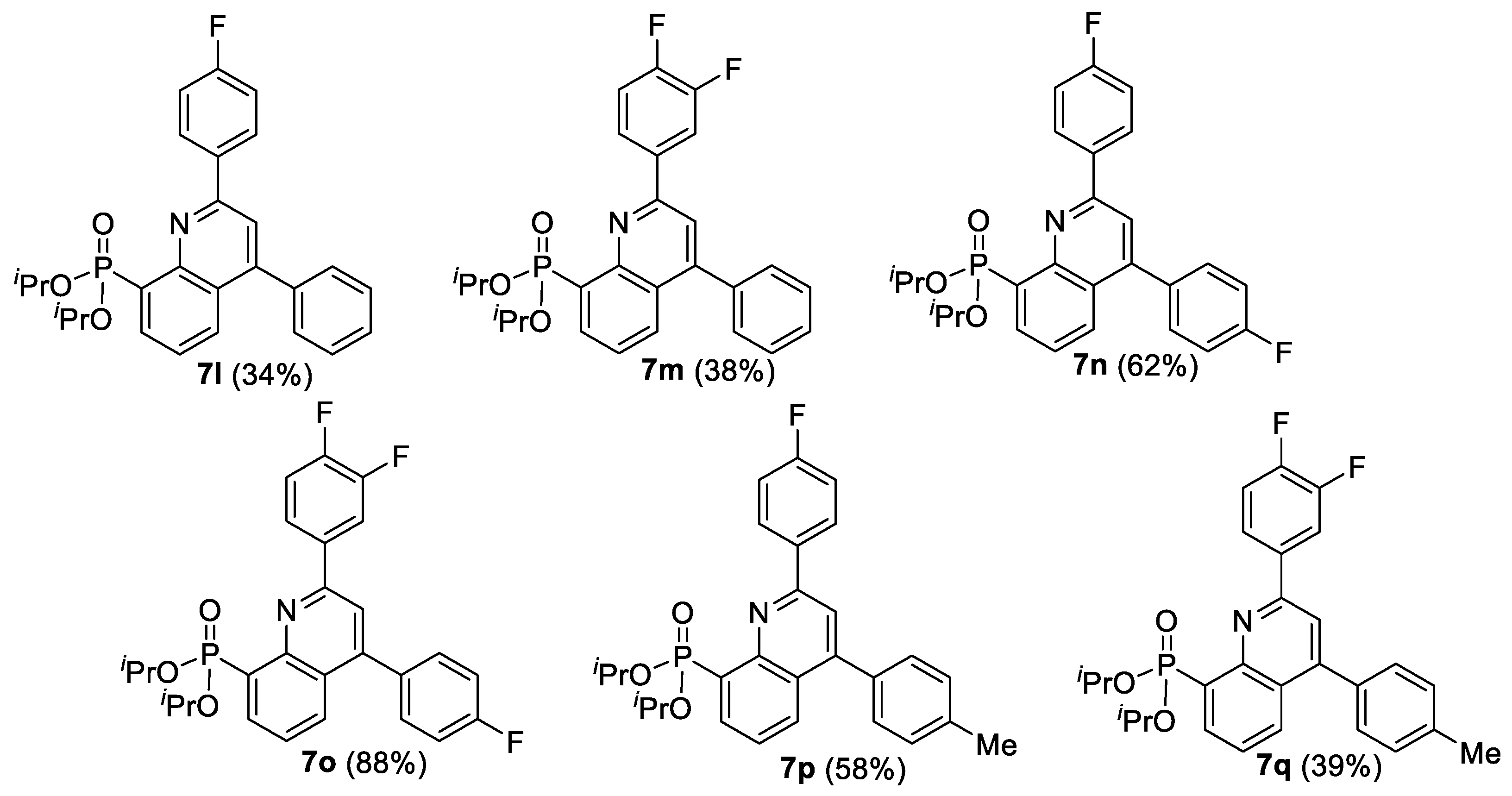
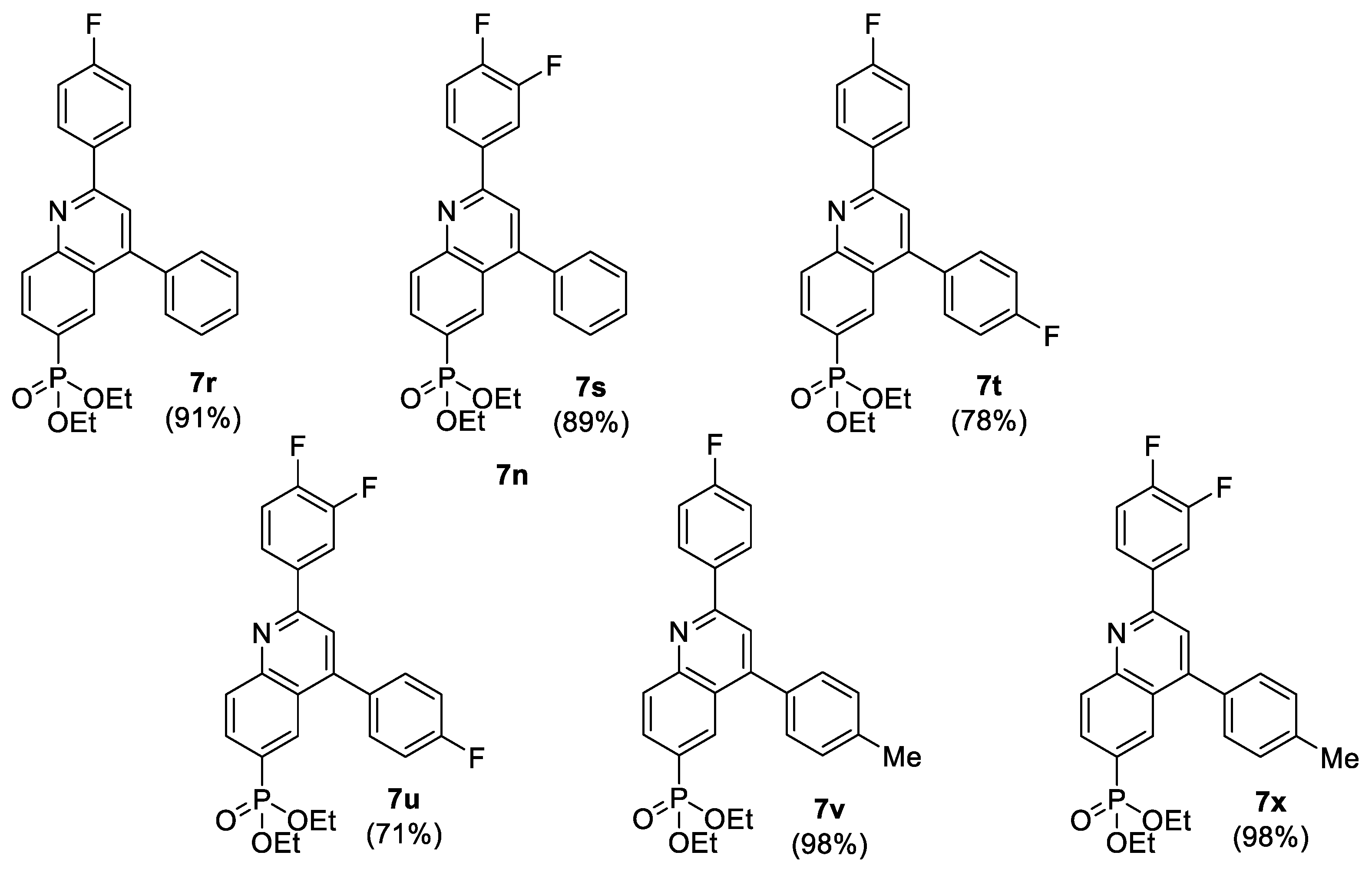
| Entry | Comp. | PO(OR)2 | R1 | R2 | Cytotoxicity IC50 (µM) a | |||
|---|---|---|---|---|---|---|---|---|
| Lung A549 | Ovarian SKOV3 | Kidney HEK293 | MRC-5 | |||||
| 1 | CPT | (1.0 ± 0.06)·10−3 | (5.5 ± 0.01)·10−3 | - | (1.7 ± 0.96)·10−5 | |||
| 2 | 7a | 8-(PO(OEt)2) | C6H5 | H | 2.26 ± 0.59 | 9.30 ± 0.76 | 27.29 ± 2.22 | >50 |
| 3 | 7b | 8-(PO(OEt)2) | 4-F-C6H4 | H | 2.16 ± 0.29 | 5.29 ± 1.42 | 43.04 ± 4.65 | >50 |
| 4 | 7c | 8-(PO(OEt)2) | 4-OMe-C6H4 | H | 12.59 ± 1.30 | 25.04 ± 3.60 | 38.29 ± 8.90 | >50 |
| 5 | 7d | 8-(PO(OEt)2) | 2-OMe-C6H4 | H | 12.26 ± 4.10 | 19.38 ± 1.60 | >50 | >50 |
| 6 | 7e | 8-(PO(OEt)2) | 2-naphthyl | H | 3.18 ± 0.27 | 1.33 ± 0.76 | 48.14 ± 6.39 | >50 |
| 7 | 7f | 8-(PO(OEt)2) | 1-naphthyl | H | 2.66 ± 0.16 | 4.54 ± 0.52 | 35.67 ± 3.13 | >50 |
| 8 | 7g | 8-(PO(OEt)2) | C6H5 | F | 2.27 ± 0.32 | 9.29 ± 1.79 | 24.43 ± 3.46 | >50 |
| 9 | 7h | 8-(PO(OEt)2) | 4-F-C6H4 | F | 3.07 ± 0.22 | 9.79 ± 0.59 | 15.66 ± 1.74 | >50 |
| 10 | 7i | 8-(PO(OEt)2) | 3,4-F2-C6H3 | F | 4.64 ± 0.30 | 37.59 ± 9.10 | >50 | >50 |
| 11 | 7j | 8-(PO(OEt)2) | 4-F-C6H4 | Me | 0.83 ± 0.01 | 10.24 ± 0.38 | >50 | >50 |
| 12 | 7k | 8-(PO(OEt)2) | 3,4-F2-C6H3 | Me | 2.72 ± 0.37 | 10.59 ± 1.13 | 22.26 ± 1.36 | >50 |
| 13 | 7l | 8-(PO(OiPr)2) | 4-F-C6H4 | H | 5.01 ± 2.70 | 18.73 ± 1.98 | 10.12 ± 1.26 | >50 |
| 14 | 7m | 8-(PO(OiPr)2) | 3,4-F2-C6H3 | H | 18.39 ± 1.4 | 12.13 ± 1.7 | >50 | >50 |
| 15 | 7n | 8-(PO(OiPr)2) | 4-F-C6H4 | F | 10.55 ± 1.73 | 19.82 ± 3.83 | 15.01 ± 0.99 | >50 |
| 16 | 7o | 8-(PO(OiPr)2) | 3,4-F2-C6H3 | F | 6.79 ± 3.87 | 22.12 ± 4.47 | 21.04 ± 4.05 | >50 |
| 17 | 7p | 8-(PO(OiPr)2) | 4-F-C6H4 | Me | 6.84 ± 1.74 | 12.13 ± 1.68 | 14.38 ± 1.69 | >50 |
| 18 | 7q | 8-(PO(OiPr)2) | 3,4-F2-C6H3 | Me | 8.24 ± 2.42 | 4.67 ± 1.50 | 12.32 ± 0.71 | >50 |
| 19 | 7r | 6-(PO(OEt)2) | 4-F-C6H4 | H | 2.80 ± 1.24 | 3.03 ± 1.24 | 36.86 ± 8.06 | >50 |
| 20 | 7s | 6-(PO(OEt)2) | 3,4-F2-C6H3 | H | 6.19 ± 0.67 | >50 | 19.18 ± 1.13 | >50 |
| 21 | 7t | 6-(PO(OEt)2) | 4-F-C6H4 | F | 2.85 ± 1.26 | >50 | >50 | >50 |
| 22 | 7u | 6-(PO(OEt)2) | 3,4-F2-C6H3 | F | 4.33 ± 1.11 | >50 | >50 | >50 |
| 23 | 7v | 6-(PO(OEt)2) | 4-F-C6H4 | Me | 0.84 ± 0.23 | 41.61 ± 10.01 | >50 | >50 |
| 24 | 7x | 6-(PO(OEt)2) | 3,4-F2-C6H3 | Me | 3.05 ± 0.86 | >50 | >50 | >50 |
| Entry | Comp. | PO(OR)2 | R1 | R2 | % Inhibition a | ||
|---|---|---|---|---|---|---|---|
| 15 s | 1 min | 3 min | |||||
| 1 | CPT | 80 | 76 | 3 | |||
| 2 | 7a | 8-(PO(OEt)2) | C6H5 | H | 20 | 28 | 1 |
| 3 | 7b | 8-(PO(OEt)2) | 4-F-C6H4 | H | 54 | 61 | 21 |
| 4 | 7c | 8-(PO(OEt)2) | 4-OMe-C6H4 | H | 11 | 9 | 4 |
| 5 | 7d | 8-(PO(OEt)2) | 2-OMe-C6H4 | H | 22 | 16 | 3 |
| 6 | 7e | 8-(PO(OEt)2) | 2-naphthyl | H | 17 | 36 | 4 |
| 7 | 7f | 8-(PO(OEt)2) | 1-naphthyl | H | 27 | 28 | 8 |
| 8 | 7g | 8-(PO(OEt)2) | C6H5 | F | 28 | 36 | 3 |
| 9 | 7h | 8-(PO(OEt)2) | 4-F-C6H4 | F | 27 | 19 | 4 |
| 10 | 7i | 8-(PO(OEt)2) | 3,4-F2-C6H3 | F | 47 | 37 | 2 |
| 11 | 7j | 8-(PO(OEt)2) | 4-F-C6H4 | Me | 65 | 67 | 3 |
| 12 | 7k | 8-(PO(OEt)2) | 3,4-F2-C6H3 | Me | 63 | 95 | 5 |
| 13 | 7l | 8-(PO(OiPr)2) | 4-F-C6H4 | H | 18 | 13 | 2 |
| 14 | 7m | 8-(PO(OiPr)2) | 3,4-F2-C6H3 | H | 26 | 32 | 3 |
| 15 | 7n | 8-(PO(OiPr)2) | 4-F-C6H4 | F | 59 | 57 | 7 |
| 16 | 7o | 8-(PO(OiPr)2) | 3,4-F2-C6H3 | F | 29 | 27 | 3 |
| 17 | 7p | 8-(PO(OiPr)2) | 4-F-C6H4 | Me | 32 | 46 | 4 |
| 18 | 7q | 8-(PO(OiPr)2) | 3,4-F2-C6H3 | Me | 51 | 35 | 6 |
| 19 | 7r | 6-(PO(OEt)2) | 4-F-C6H4 | H | 9 | 15 | 3 |
| 20 | 7s | 6-(PO(OEt)2) | 3,4-F2-C6H3 | H | 9 | 13 | 2 |
| 21 | 7t | 6-(PO(OEt)2) | 4-F-C6H4 | F | 21 | 18 | 5 |
| 22 | 7u | 6-(PO(OEt)2) | 3,4-F2-C6H3 | F | 27 | 21 | 14 |
| 23 | 7v | 6-(PO(OEt)2) | 4-F-C6H4 | Me | 22 | 30 | 12 |
| 24 | 7x | 6-(PO(OEt)2) | 3,4-F2-C6H3 | Me | 18 | 25 | 5 |
| Entry | Comp. | PO(OR)2 | R1 | R2 | EC50 L. Infantum | IC50 Splenocites | SI | LTOP1B Inhibition | |
|---|---|---|---|---|---|---|---|---|---|
| Promastigotes | Amastigotes | ||||||||
| 1 | 7a | 8-(PO(OEt)2) | C6H5 | H | 0.91 ± 0.04 | 4.03 ± 0.30 | 3.61 ± 0.45 | 0.9 | 58.87 |
| 2 | 7b | 8-(PO(OEt)2) | 4-F-C6H4 | H | 7.63 ± 0.76 | 14.56 ± 1.30 | 12.24 ± 1.77 | 0.8 | 24.43 |
| 3 | 7c | 8-(PO(OEt)2) | 4-OMe-C6H4 | H | 9.46 ± 1.67 | 8.23 ± 1.70 | 15.90 ± 1.65 | 1.9 | 45.45 |
| 4 | 7d | 8-(PO(OEt)2) | 2-OMe-C6H4 | H | 11.37 ± 0.62 | 19.46 ± 2.38 | 30.09 ± 6.05 | 1.5 | 46.41 |
| 5 | 7e | 8-(PO(OEt)2) | 2-naphthyl | H | 20.38 ± 1.57 | 20.44 ± 5.62 | 51.62 ± 1.52 | 2.5 | 18.36 |
| 6 | 7f | 8-(PO(OEt)2) | 1-naphthyl | H | 20.55 ± 2.19 | 19.66 ± 2.39 | 12.22 ± 1.49 | 0.6 | 7.5 |
| 7 | 7g | 8-(PO(OEt)2) | C6H5 | F | 16.05 ± 1.94 | 11.89 ± 5.17 | 33.86 ± 7.98 | 2.8 | 16.49 |
| 8 | 7h | 8-(PO(OEt)2) | 4-F-C6H4 | F | 8.43 ± 0.92 | 24.17 ± 2.37 | 20.26 ± 7.49 | 0.8 | 22.44 |
| 9 | 7i | 8-(PO(OEt)2) | 3,4-F2-C6H3 | F | 6.35 ± 0.16 | 13.43 ± 5.53 | 20.64 ± 3.11 | 1.5 | 54.01 |
| 10 | 7j | 8-(PO(OEt)2) | 4-F-C6H4 | Me | 7.29 ± 0.94 | 9.80 ± 0.48 | 14.54 ± 1.56 | 1.5 | 41.21 |
| 11 | 7k | 8-(PO(OEt)2) | 3,4-F2-C6H3 | Me | 2.59 ± 0.48 | 26.23 ± 3.65 | 24.07 ± 7.79 | 0.9 | 77.02 |
| 12 | 7l | 8-(PO(OiPr)2) | 4-F-C6H4 | H | 9.57 ± 0.4 | 19.47 ± 2.23 | 22.1 ± 4.7 | 1.1 | 3.55 |
| 13 | 7m | 8-(PO(OiPr)2) | 3,4-F2-C6H3 | H | 11.18 ± 0.74 | 10.20 ± 1.34 | 19.79 ± 3.76 | 1.9 | 5.41 |
| 14 | 7n | 8-(PO(OiPr)2) | 4-F-C6H4 | F | 4.86 ± 0.5 | 5.52 ± 1.11 | 15.29 ± 1.72 | 2.8 | 43.31 |
| 15 | 7o | 8-(PO(OiPr)2) | 3,4-F2-C6H3 | F | 19.26 ± 1.79 | 19.65 ± 1.73 | 18.60 ± 1.10 | 0.9 | 73.27 |
| 16 | 7p | 8-(PO(OiPr)2) | 4-F-C6H4 | Me | 5.01 ± 0.35 | 24.32 ± 2.53 | 21.23 ± 3.5 | 0.9 | 41.62 |
| 17 | 7q | 8-(PO(OiPr)2) | 3,4-F2-C6H3 | Me | 7.46 ± 0.76 | 13.91 ± 3.65 | 28.33 ± 9.16 | 2 | 52.65 |
| 18 | 7r | 6-(PO(OEt)2) | 4-F-C6H4 | H | 15.22 ± 1.14 | 29.23 ± 14 | 28.07 ± 1.23 | 1 | 1.94 |
| 19 | 7s | 6-(PO(OEt)2) | 3,4-F2-C6H3 | H | 14.72 ± 1.78 | 14.20± 2.83 | 26.23 ± 0.51 | 1.8 | 14.56 |
| 20 | 7t | 6-(PO(OEt)2) | 4-F-C6H4 | F | 16.73 ± 0.72 | 31.46 ± 2.71 | 44.97 ± 10.24 | 1.4 | 37.86 |
| 21 | 7u | 6-(PO(OEt)2) | 3,4-F2-C6H3 | F | 7.07 ± 0.61 | 26.36 ± 2.2 | >100 | >3.8 | 50.24 |
| 22 | 7v | 6-(PO(OEt)2) | 4-F-C6H4 | Me | 7.02 ± 0.94 | 17.56 ± 7.81 | 11.18 ± 4.42 | 0.6 | 43.25 |
| 23 | 7x | 6-(PO(OEt)2) | 3,4-F2-C6H3 | Me | 21.29 ± 2.34 | 32.95 ± 6.55 | 32.33 ± 3.36 | 1 | 43.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selas, A.; Fuertes, M.; Melcón-Fernández, E.; Pérez-Pertejo, Y.; Reguera, R.M.; Balaña-Fouce, R.; Knudsen, B.R.; Palacios, F.; Alonso, C. Hybrid Quinolinyl Phosphonates as Heterocyclic Carboxylate Isosteres: Synthesis and Biological Evaluation against Topoisomerase 1B (TOP1B). Pharmaceuticals 2021, 14, 784. https://doi.org/10.3390/ph14080784
Selas A, Fuertes M, Melcón-Fernández E, Pérez-Pertejo Y, Reguera RM, Balaña-Fouce R, Knudsen BR, Palacios F, Alonso C. Hybrid Quinolinyl Phosphonates as Heterocyclic Carboxylate Isosteres: Synthesis and Biological Evaluation against Topoisomerase 1B (TOP1B). Pharmaceuticals. 2021; 14(8):784. https://doi.org/10.3390/ph14080784
Chicago/Turabian StyleSelas, Asier, María Fuertes, Estela Melcón-Fernández, Yolanda Pérez-Pertejo, Rosa M. Reguera, Rafael Balaña-Fouce, Birgitta R. Knudsen, Francisco Palacios, and Concepcion Alonso. 2021. "Hybrid Quinolinyl Phosphonates as Heterocyclic Carboxylate Isosteres: Synthesis and Biological Evaluation against Topoisomerase 1B (TOP1B)" Pharmaceuticals 14, no. 8: 784. https://doi.org/10.3390/ph14080784
APA StyleSelas, A., Fuertes, M., Melcón-Fernández, E., Pérez-Pertejo, Y., Reguera, R. M., Balaña-Fouce, R., Knudsen, B. R., Palacios, F., & Alonso, C. (2021). Hybrid Quinolinyl Phosphonates as Heterocyclic Carboxylate Isosteres: Synthesis and Biological Evaluation against Topoisomerase 1B (TOP1B). Pharmaceuticals, 14(8), 784. https://doi.org/10.3390/ph14080784









