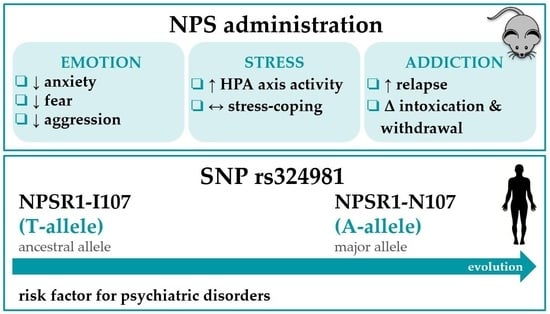
Role of the Neuropeptide S System in Emotionality, Stress Responsiveness and Addiction-Like Behaviours in Rodents: Relevance to Stress-Related Disorders
Abstract
1. Introduction
2. The NPS/NPSR1 System and Stress-Relevant Endpoints in Rodents
2.1. Role of the NPS/NPSR1 System in Anxiety- and Fear-Related Behaviours
2.2. Role of the NPS/NPSR1 System in Social Behaviours and Aggression
2.3. Role of the NPS/NPSR1 System in Stress Responsiveness and Stress-Coping Behaviour
2.4. Role of the NPS/NPSR1 System in Animal Models of Psychiatric Disorders
2.4.1. The NPS/NPSR1 System and Pathological Anxiety and Fear
2.4.2. The NPS/NPSR1 System and the Exposure to Acute Stressors
| Drug | Site | Test | Behaviour | Findings | Ref. |
|---|---|---|---|---|---|
| NPS | i.c.v. | EP/ZM, DaLi, MBT, DBT | Anxiety | ↓ ♂/♀ | [1,21,22,23,24,25,26,27,28,29,31] |
| EPM, ETM | ↔ | [32,33,34] | |||
| LA, BLA | EPM | ↔ | [51,58] | ||
| LA/BLA | EPM, DaLi | ↓ | [41] | ||
| MeA, VMH, PVN | EPM | ↓ | [21,40,43] | ||
| VH | EPM | ↓ | [44] | ||
| DaLi | ↔ | ||||
| intranasal | EPM | ↓ | [23,53] | ||
| NPSR1-A | i.p. (SHA68) | EPM | Anxiety | ↔ | [43] |
| Anxiolytic effects of intra-VMH NPS | X | ||||
| NPS | i.c.v. | FST, TST | Stress-coping | ↔ ♂/♀ | [22,28] |
| NPS | i.c.v. | SPAT | Sociability | ↔ | [23,34,48] |
| Social recognition | ↔ | [23] | |||
| RI | Aggression | ↓ ↔ | [48,49] | ||
| intranasal | SPAT | Sociability | ↔ | [23] | |
| NPSR1-A | i.c.v. ((d-Cys(tBu)5)NPS) | SPAT | Sociability | ↔ | [23] |
| i.p. (SHA68) | RI | Anti-aggressive effects of i.c.v. NPS | Ø | [49] | |
| i.c.v. ((tBu-D-Gly5)NPS) | Anti-aggressive effects of i.c.v. NPS | X | |||
| NPS | i.c.v. | CuFC | EXT | ↑ | [34] |
| EXT recall | ↔ | ||||
| LA | CuFC | EXP, EXT | ↔ | [58] | |
| Contextual fear renewal | ↔ | ||||
| BLA | ASR | Magnitude | ↔ | [51] | |
| trauma cue | Freezing | ↔ | [51] | ||
| LA/BLA | CuFC | EXP | ↔ | [41] | |
| EXT | ↑ | ||||
| Contextual fear renewal | ↓ | ||||
| EXT recall | ↔ | ||||
| NPSR1-A | i.c.v. ((d-Cys(tBu)5)NPS) | SFC | EXP | ↔ | [34] |
| LA (SHA68) | CuFC | EXP, EXT | ↔ | [58] | |
| Contextual fear renewal | ↔ | ||||
| LA/BLA (SHA68) | CuFC | EXT | ↓ | [41] | |
| Contextual fear renewal | ↑ |
| Strain Background | Sex | Test | Behaviour | Findings | Ref. |
|---|---|---|---|---|---|
| C57/BL6J mice | ♂/♀ | EPM, DaLi, MBT | Anxiety | ↔ | [30,36,37,38] |
| FST | Passive stress-coping | ↑ ♂ | [30] | ||
| TST | ↔ | ||||
| SPAT | Sociability | ↔ | [39] | ||
| Social novelty | ↔ | ||||
| SFC | EXP | ↔ | [39] | ||
| EXT | ↑ | ||||
| CFC | EXP | ↔ | [37] | ||
| Context discrimination | ↔ | [37,38] | |||
| ↑ | [37] | ||||
| CuFC | EXP, EXT | ↔ | [36] | ||
| Safety learning | Freezing | ↔ | [38] | ||
| ASR | Magnitude, Vmax | ↔ | [38,41] | ||
| ↓♂ | [30,36] | ||||
| - | Basal CORT | ↔ | [37,38] | ||
| Stress-induced CORT | ↔ | [30,38] | |||
| Locomotion | Cocaine-induced | ↔ | [36] | ||
| CD1 mice | ♂ | EPM, ETM | Anxiety | ↔ | [26,33] |
| FST | Stress-coping | ↔ | [26] | ||
| RI | Aggression | ↑ | [49] | ||
| SIH | Stress response | ↔ | [36] |
3. Role of the NPS/NPSR1 System in Addiction-Like Behaviours
3.1. Role of the NPS/NPSR1 System in Drug-Induced Conditioned Place Preference
3.2. Role of the NPS/NPSR1 System in Drug Self-Administration
3.2.1. The NPS/NPSR1 System and Operant Self-Administration
3.2.2. The NPS/NPSR1 System and Oral Self-Administration
3.3. Role of the NPS/NPSR1 System in Drug Intoxication and Withdrawal
| Drug | Site | Test | Drug of Abuse | Behaviour | Findings | Ref. |
|---|---|---|---|---|---|---|
| NPS | i.c.v. | CPP | Cocaine | EXP, EXT | ↔ | [62] |
| RST | ↑ | |||||
| Morphine | EXP | ↓ | [61] | |||
| NPS | EXP | ↔ | [61,65] | |||
| ↑ | [64] | |||||
| NPSR1-A | i.p. (SHA68) | CPP | Cocaine | RST | ↔ | [62] |
| RST (stress) | X | |||||
| RST (i.c.v. NPS) | X | |||||
| NPS | i.c.v. | oral | Ethanol | Intake | ↓ | [65] |
| Anxiety | ↓ | |||||
| Stress-coping | ↓ | |||||
| BLA | Anxiety | ↓ | ||||
| i.c.v. | IG | Ethanol | Anxiety (WD) | ↓ | [73] | |
| s.c. | Morphine | Anxiety (WD) | ↓ | [74] | ||
| NPS | i.c.v., LH, PeF | SA | Cocaine | SA (FR) | ↔ | [66] |
| RST (cue) | ↑ | [24,66] | ||||
| DMH, CeA | RST (cue) | ↔ | [66] | |||
| i.c.v. | SA | Ethanol | SA (FR) | ↔ | [32,70] | |
| RST (cue, NPS) | ↑ | |||||
| TBC | Intake | ↔ ♀ | [71] | |||
| LH | SA | RST (cue) | ↑ | [70] | ||
| NPS | i.c.v., LH, PVN | - | Palatable food | Intake | ↓ | [69] |
| CeA | Intake | ↔ | ||||
| i.c.v. | SA | NPS | SA (FR) | ↑ | [64] | |
| NPSR1-A | i.p. (SHA68) | SA | Cocaine | SA (FR) | ↔ | [66] |
| ↓ | [68] | |||||
| RST (cue) | ↓ | [66] | ||||
| i.p. (NPSR1-QA1) | SA (FR) | ↔ | [67] | |||
| RST (cue) | ↓ | |||||
| i.p. (RTI-118) | SA (FR) | ↓ | [68] | |||
| RST (cue, stress, cocaine) | ↓ | |||||
| i.c.v., CeA ((d-Cys(tBu)5)NPS) | RST (cue) | ↔ | [67] | |||
| LH, PeF ((d-Cys(tBu)5)NPS) | RST (cue) | ↓ | [66,67] | |||
| i.p. (NCGC84) | Ethanol | SA (FR, PR) | ↓ | [72] | ||
| Motivation | ↓ | |||||
| RST (cue) | ↔ | |||||
| i.c.v. ((d-Cys(tBu)5)NPS) | - | Palatable food | Intake | ↔ | [69] | |
| Anorectic effects of i.c.v. NPS | X |
4. Alterations in the NPS/NPSR1 System in Stress-Related Disorders
4.1. Role of the NPS/NPSR1 System in Affective Disorders
4.2. Role of the NPS/NPSR1 System in Substance Use Disorders
4.3. Role of the NPS/NPSR1 System in Anxiety Symptom Severity
5. Role of the NPS/NPSR1 System in Emotion Regulation and Stress Responses in Healthy Individuals
5.1. SNP rs324981, Anxiety Sensitivity and Fear Rating
5.2. SNP rs324981, Coping Abilities and Experiences of Life Stress
5.3. SNP rs324981 and Neuroendocrine Stress Responsiveness
| Parameter | TT | AT | AA | Ref. | |
|---|---|---|---|---|---|
| Emotional symptoms | |||||
| Anxiety sensitivity (emotional task) | ↔ | ↔ | ↔//↑ | [88]//[95,96,97,98] | |
| Anxiety sensitivity (attentional task) | ↔ | ↔ | ↔ | [99] | |
| Anxiety sensitivity (experience of life stress) | ↑ | ↔ | ↔ | [101] | |
| Panic agoraphobia | ↔ | ↔ | ↔ | [96] | |
| Baseline panic symptoms | ↑ | ↔ | ↔ | [100] | |
| CCK-4-induced panic symptoms | ↔ | ↔ | ↔ | [100] | |
| Depression symptoms | ↔ | ↔ | ↔ | [95,96] | |
| Fear rating (fear conditioning) | ↑ | ↑ | ↔ | [89] | |
| Startle magnitude to positive stimuli | ↔ | ↔ | ↔ | [90] | |
| Startle magnitude to negative stimuli | ↓//↑$ | ↓//↑$ | ↔ | [88]//[90] | |
| Startle magnitude to neutral stimuli | ↑ | ↑ | ↔ | [88,90] | |
| Coping abilities and personality traits | |||||
| Trait anxiety if low self-efficacy | ↑ | ↑ | ↑ | [102] | |
| Trait anxiety if high self-efficacy | ↑ | ↓ | ↓ | ||
| Adaptive impulsivity | ↓ | ↔ | ↑ | [103] | |
| Adaptive impulsivity (experience of life stress) | ↑ | ↔ | ↓ | ||
| Maladaptive impulsivity (experience of life stress) | ↑ | ↔ | ↑ | ||
| Hyperactivity | ↑ | ↔ | ↔ | ||
| Hyperactivity (experience of life stress) | ↑ | ↔ | ↔ | ||
| Openness to experiences | ↓ | ↔ | ↑ | ||
| Neuroticism (experience of life stress) | ↔ | ↔ | ↑ | ||
| Extraversion (experience of life stress) | ↔ | ↔ | ↓ | ||
| Stress responses | |||||
| Baseline CORT | ↔ | ↔ | ↔ | [104,105] | |
| ↑ CORT (stress or CCK-4 challenge) | ↔//↑♂ | ↔ | ↔ | [100]//[104,105,106] | |
| ↑ ACTH (stress or CCK-4 challenge) | ↔ | ↔ | ↔ | [106] | |
| ↑ Heart rate (stress or CCK-4 challenge) | ↔ | ↔ | ↔ | [100,106] | |
| Skin conductance level (emotional task) | ↔ | ↔ | ↔ | [89,97] | |
| Subjective stress rating (TSST) | ↔ | ↔ | ↔ | [105] | |
5.4. Neurobiological Mechanisms in Healthy Individuals
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, Y.-L.; Reinscheid, R.K.; Huitron-Resendiz, S.; Clark, S.D.; Wang, Z.; Lin, S.H.; A Brucher, F.; Zeng, J.; Ly, N.K.; Henriksen, S.J.; et al. Neuropeptide S: A neuropeptide promoting arousal and anxiolytic-like effects. Neuron 2004, 43, 487–497. [Google Scholar] [CrossRef]
- Camarda, V.; Rizzi, A.; Ruzza, C.; Zucchini, S.; Marzola, G.; Marzola, E.; Guerrini, R.; Salvadori, S.; Reinscheid, R.K.; Regoli, D.; et al. In Vitro and in Vivo Pharmacological Characterization of the Neuropeptide S Receptor Antagonist [d-Cys(tBu)5]Neuropeptide S. J. Pharmacol. Exp. Ther. 2009, 328, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, F.; Kügler, S.; Blaesse, P.; Lange, M.D.; Skryabin, B.V.; Pape, H.-C.; Jüngling, K. Neuronal Expression of the Human Neuropeptide S Receptor NPSR1 Identifies NPS-Induced Calcium Signaling Pathways. PLoS ONE 2015, 10, e0117319. [Google Scholar] [CrossRef]
- Liao, Y.; Lu, B.; Ma, Q.; Wu, G.; Lai, X.; Zang, J.; Shi, Y.; Liu, D.; Han, F.; Zhou, N. Human Neuropeptide S Receptor Is Activated via a Gαq Protein-biased Signaling Cascade by a Human Neuropeptide S Analog Lacking the C-terminal 10 Residues. J. Biol. Chem. 2016, 291, 7505–7516. [Google Scholar] [CrossRef]
- Reinscheid, R.K.; Xu, Y.-L. Neuropeptide S as a novel arousal promoting peptide transmitter. FEBS J. 2005, 272, 5689–5693. [Google Scholar] [CrossRef]
- Reinscheid, R.K. Phylogenetic appearance of neuropeptide S precursor proteins in tetrapods. Peptides 2007, 28, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-L.; Gall, C.M.; Jackson, V.R.; Civelli, O.; Reinscheid, R.K. Distribution of neuropeptide S receptor mRNA and neurochemical characteristics of neuropeptide S-expressing neurons in the rat brain. J. Comp. Neurol. 2007, 500, 84–102. [Google Scholar] [CrossRef]
- Adori, C.; Barde, S.; Bogdanovic, N.; Uhlén, M.; Reinscheid, R.; Kovacs, G.G.; Hökfelt, T. Neuropeptide S- and Neuropeptide S receptor-expressing neuron populations in the human pons. Front. Neuroanat. 2015, 9, 126. [Google Scholar] [CrossRef]
- Clark, S.D.; Duangdao, D.M.; Schulz, S.; Zhang, L.; Liu, X.; Xu, Y.-L.; Reinscheid, R.K. Anatomical characterization of the neuropeptide S system in the mouse brain by in situ hybridization and immunohistochemistry. J. Comp. Neurol. 2011, 519, 1867–1893. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zeng, J.; Zhou, A.; Theodorsson, E.; Fahrenkrug, J.; Reinscheid, R.K. Molecular fingerprint of neuropeptide s-producing neurons in the mouse brain. J. Comp. Neurol. 2011, 519, 1847–1866. [Google Scholar] [CrossRef]
- Leonard, S.; Ring, R. Immunohistochemical localization of the neuropeptide S receptor in the rat central nervous system. Neuroscience 2011, 172, 153–163. [Google Scholar] [CrossRef]
- Okamura, N.; Reinscheid, R.K. Neuropeptide S: A novel modulator of stress and arousal. Stress 2007, 10, 221–226. [Google Scholar] [CrossRef]
- Pape, H.-C.; Jüngling, K.; Seidenbecher, T.; Lesting, J.; Reinscheid, R.K. Neuropeptide S: A transmitter system in the brain regulating fear and anxiety. Neuropharmacology 2010, 58, 29–34. [Google Scholar] [CrossRef]
- Reinscheid, R.K.; Xu, Y.L.; Civelli, O. Neuropeptide S: A New Player in the Modulation of Arousal and Anxiety. Mol. Interv. 2005, 5, 42–46. [Google Scholar] [CrossRef]
- Botticelli, L.; Di Bonaventura, E.M.; Ubaldi, M.; Ciccocioppo, R.; Cifani, C.; Di Bonaventura, M.M. The Neural Network of Neuropeptide S (NPS): Implications in Food Intake and Gastrointestinal Functions. Pharmaceuticals 2021, 14, 293. [Google Scholar] [CrossRef] [PubMed]
- Cannella, N.; Kallupi, M.; Ruggeri, B.; Ciccocioppo, R.; Ubaldi, M. The role of the neuropeptide S system in addiction: Focus on its interaction with the CRF and hypocretin/orexin neurotransmission. Prog. Neurobiol. 2013, 100, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Grund, T.; Neumann, I.D. Brain neuropeptide S: Via GPCR activation to a powerful neuromodulator of socio-emotional behaviors. Cell Tissue Res. 2018, 375, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Schank, J.R.; Ryabinin, A.E.; Giardino, W.; Ciccocioppo, R.; Heilig, M. Stress-Related Neuropeptides and Addictive Behaviors: Beyond the Usual Suspects. Neuron 2012, 76, 192–208. [Google Scholar] [CrossRef]
- WHO (World Health Organization). The ICD-10 Classification of Mental and Behavioral Disorders: Clinical Description and Diagnostic Guidelines (ICD-10); World Health Organization: Geneva, Switzerland, 1992. [Google Scholar]
- Ghazal, P. The Physio-Pharmacological Role of the NPS/NPSR System in Psychiatric Disorders: A Translational Overview. Curr. Protein Pept. Sci. 2016, 17, 380–397. [Google Scholar] [CrossRef]
- Grund, T.; Goyon, S.; Li, Y.; Eliava, M.; Liu, H.; Charlet, A.; Grinevich, V.; Neumann, I.D. Neuropeptide S Activates Paraventricular Oxytocin Neurons to Induce Anxiolysis. J. Neurosci. 2017, 37, 12214–12225. [Google Scholar] [CrossRef]
- Leonard, S.K.; Dwyer, J.M.; Ring, R.H.; Rizzo, S.J.S.; Platt, B.; Logue, S.F.; Neal, S.J.; Malberg, J.E.; Beyer, C.E.; Schechter, L.E.; et al. Pharmacology of neuropeptide S in mice: Therapeutic relevance to anxiety disorders. Psychopharmacology 2008, 197, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Lukas, M.; Neumann, I.D. Nasal application of neuropeptide S reduces anxiety and prolongs memory in rats: Social versus non-social effects. Neuropharmacology 2012, 62, 398–405. [Google Scholar] [CrossRef]
- Pañeda, C.; Huitron-Resendiz, S.; Frago, L.M.; Chowen, J.A.; Picetti, R.; De Lecea, L.; Roberts, A.J. Neuropeptide S Reinstates Cocaine-Seeking Behavior and Increases Locomotor Activity through Corticotropin-Releasing Factor Receptor 1 in Mice. J. Neurosci. 2009, 29, 4155–4161. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rizzi, A.; Vergura, R.; Marzola, G.; Ruzza, C.; Guerrini, R.; Salvadori, S.; Regoli, D.; Calo, G. Neuropeptide S is a stimulatory anxiolytic agent: A behavioural study in mice. Br. J. Pharmacol. 2008, 154, 471–479. [Google Scholar] [CrossRef]
- Ruzza, C.; Pulga, A.; Rizzi, A.; Marzola, G.; Guerrini, R.; Calo’, G. Behavioural phenotypic characterization of CD-1 mice lacking the neuropeptide S receptor. Neuropharmacology 2012, 62, 1999–2009. [Google Scholar] [CrossRef]
- Vitale, G.; Filaferro, M.; Ruggieri, V.; Pennella, S.; Frigeri, C.; Rizzi, A.; Guerrini, R.; Calò, G. Anxiolytic-like effect of neuropeptide S in the rat defensive burying. Peptides 2008, 29, 2286–2291. [Google Scholar] [CrossRef]
- Wegener, G.; Finger, B.C.; Elfving, B.; Keller, K.; Liebenberg, N.; Fischer, C.W.; Singewald, N.; Slattery, D.A.; Neumann, I.D.; Mathé, A.A. Neuropeptide S alters anxiety, but not depression-like behaviour in Flinders Sensitive Line rats: A genetic animal model of depression. Int. J. Neuropsychopharmacol. 2012, 15, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Jin, X.; You, Z.; Wang, S.; Lim, G.; Yang, J.; McCabe, M.; Li, N.; Marota, J.; Chen, L.; et al. Persistent nociception induces anxiety-like behavior in rodents: Role of endogenous neuropeptide S. Pain 2014, 155, 1504–1515. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Mingler, M.K.; McBride, M.L.; Murphy, A.J.; Valenzuela, D.M.; Yancopoulos, G.D.; Williams, M.; Vorhees, C.V.; Rothenberg, M.E. Abnormal response to stress and impaired NPS-induced hyperlocomotion, anxiolytic effect and corticosterone increase in mice lacking NPSR1. Psychoneuroendocrinology 2010, 35, 1119–1132. [Google Scholar] [CrossRef]
- Slattery, D.A.; Naik, R.R.; Grund, T.; Yen, Y.-C.; Sartori, S.B.; Füchsl, A.; Finger, B.C.; Elfving, B.; Nordemann, U.; Guerrini, R.; et al. Selective Breeding for High Anxiety Introduces a Synonymous SNP That Increases Neuropeptide S Receptor Activity. J. Neurosci. 2015, 35, 4599–4613. [Google Scholar] [CrossRef]
- Cannella, N.; Kallupi, M.; Li, H.W.; Stopponi, S.; Cifani, C.; Ciccocioppo, R.; Ubaldi, M. Neuropeptide S differently modulates alcohol-related behaviors in alcohol-preferring and non-preferring rats. Psychopharmacol. 2016, 233, 2915–2924. [Google Scholar] [CrossRef]
- Pulga, A.; Ruzza, C.; Rizzi, A.; Guerrini, R.; Calo, G. Anxiolytic- and panicolytic-like effects of Neuropeptide S in the mouse elevated T-maze. Eur. J. Neurosci. 2012, 36, 3531–3537. [Google Scholar] [CrossRef] [PubMed]
- Zoicas, I.; Menon, R.; Neumann, I.D. Neuropeptide S reduces fear and avoidance of con-specifics induced by social fear conditioning and social defeat, respectively. Neuropharmacology 2016, 108, 284–291. [Google Scholar] [CrossRef]
- Liu, X.; Si, W.; Garau, C.; Jüngling, K.; Pape, H.-C.; Schulz, S.; Reinscheid, R.K. Neuropeptide S precursor knockout mice display memory and arousal deficits. Eur. J. Neurosci. 2017, 46, 1689–1700. [Google Scholar] [CrossRef]
- Fendt, M.; Buchi, M.; Bürki, H.; Imobersteg, S.; Ricoux, B.; Suply, T.; Sailer, A. Neuropeptide S receptor deficiency modulates spontaneous locomotor activity and the acoustic startle response. Behav. Brain Res. 2011, 217, 1–9. [Google Scholar] [CrossRef]
- Germer, J.; Kahl, E.; Fendt, M. Memory generalization after one-trial contextual fear conditioning: Effects of sex and neuropeptide S receptor deficiency. Behav. Brain Res. 2019, 361, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Kolodziejczyk, M.H.; Fendt, M. Corticosterone Treatment and Incubation Time After Contextual Fear Conditioning Synergistically Induce Fear Memory Generalization in Neuropeptide S Receptor-Deficient Mice. Front. Neurosci. 2020, 14, 128. [Google Scholar] [CrossRef]
- Kolodziejczyk, M.H.; Faesel, N.; Koch, M.; Fendt, M. Sociability and extinction of conditioned social fear is affected in neuropeptide S receptor-deficient mice. Behav. Brain Res. 2020, 393, 112782. [Google Scholar] [CrossRef] [PubMed]
- Grund, T.; Neumann, I.D. Neuropeptide S Induces Acute Anxiolysis by Phospholipase C-Dependent Signaling within the Medial Amygdala. Neuropsychopharmacology 2017, 43, 1156–1163. [Google Scholar] [CrossRef]
- Jüngling, K.; Seidenbecher, T.; Sosulina, L.; Lesting, J.; Sangha, S.; Clark, S.D.; Okamura, N.; Duangdao, D.M.; Xu, Y.-L.; Reinscheid, R.K.; et al. Neuropeptide S-Mediated Control of Fear Expression and Extinction: Role of Intercalated GABAergic Neurons in the Amygdala. Neuron 2008, 59, 298–310. [Google Scholar] [CrossRef]
- Tillmann, S.; Skibdal, H.E.; Christiansen, S.H.; Gøtzsche, C.; Hassan, M.; Mathé, A.A.; Wegener, G.; Woldbye, D.P. Sustained overexpression of neuropeptide S in the amygdala reduces anxiety-like behavior in rats. Behav. Brain Res. 2019, 367, 28–34. [Google Scholar] [CrossRef]
- Jiang, J.H.; Peng, Y.L.; Zhang, P.J.; Xue, H.X.; He, Z.; Liang, X.Y.; Chang, M. The ventromedial hypothalamic nucleus plays an important role in anxiolytic-like effect of neuropeptide S. Neuropeptides 2018, 67, 36–44. [Google Scholar] [CrossRef]
- Dine, J.; Ionescu, I.A.; Stepan, J.; Yen, Y.-C.; Holsboer, F.; Landgraf, R.; Eder, M.; Schmidt, U. Identification of a Role for the Ventral Hippocampus in Neuropeptide S-Elicited Anxiolysis. PLoS ONE 2013, 8, e60219. [Google Scholar] [CrossRef] [PubMed]
- Okamura, N.; Garau, C.; Duangdao, D.M.; Clark, S.D.; Jüngling, K.; Pape, H.-C.; Reinscheid, R.K. Neuropeptide S Enhances Memory During the Consolidation Phase and Interacts with Noradrenergic Systems in the Brain. Neuropsychopharmacology 2011, 36, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Han, R.-W.; Yin, X.-Q.; Chang, M.; Peng, Y.-L.; Li, W.; Wang, R. Neuropeptide S facilitates spatial memory and mitigates spatial memory impairment induced by N-methyl-d-aspartate receptor antagonist in mice. Neurosci. Lett. 2009, 455, 74–77. [Google Scholar] [CrossRef]
- Bengoetxea, X.; Goedecke, L.; Remmes, J.; Blaesse, P.; Grosch, T.; Lesting, J.; Pape, H.-C.; Jüngling, K. Human-Specific Neuropeptide S Receptor Variants Regulate Fear Extinction in the Basal Amygdala of Male and Female Mice Depending on Threat Salience. Biol. Psychiatry 2021. [Google Scholar] [CrossRef]
- Beiderbeck, D.I.; Lukas, M.; Neumann, I.D. Anti-aggressive effects of neuropeptide S independent of anxiolysis in male rats. Front. Behav. Neurosci. 2014, 8, 185. [Google Scholar] [CrossRef] [PubMed]
- Ruzza, C.; Asth, L.; Guerrini, R.; Trapella, C.; Gavioli, E. Neuropeptide S reduces mouse aggressiveness in the resident/intruder test through selective activation of the neuropeptide S receptor. Neuropharmacology 2015, 97, 1–6. [Google Scholar] [CrossRef]
- Smith, K.L.; Patterson, M.; Dhillo, W.; Patel, S.R.; Semjonous, N.M.; Gardiner, J.; Ghatei, M.A.; Bloom, S.R. Neuropeptide S Stimulates the Hypothalamo-Pituitary-Adrenal Axis and Inhibits Food Intake. Endocrinol. 2006, 147, 3510–3518. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.; Vainer, E.; Zeev, K.; Zohar, J.; Mathé, A.A. Neuropeptide S in the basolateral amygdala mediates an adaptive behavioral stress response in a rat model of posttraumatic stress disorder by increasing the expression of BDNF and the neuropeptide YY1 receptor. Eur. Neuropsychopharmacol. 2018, 28, 159–170. [Google Scholar] [CrossRef]
- Duangdao, D.M.; Clark, S.D.; Okamura, N.; Reinscheid, R.K. Behavioral phenotyping of Neuropeptide S receptor knockout mice. Behav. Brain Res. 2009, 205, 1–9. [Google Scholar] [CrossRef][Green Version]
- Ionescu, I.A.; Dine, J.; Yen, Y.-C.; Buell, D.R.; Herrmann, L.; Holsboer, F.; Eder, M.; Landgraf, R.; Schmidt, U. Intranasally Administered Neuropeptide S (NPS) Exerts Anxiolytic Effects Following Internalization into NPS Receptor-Expressing Neurons. Neuropsychopharmacology 2012, 37, 1323–1337. [Google Scholar] [CrossRef][Green Version]
- Krömer, S.A.; Keßler, M.S.; Milfay, D.; Birg, I.N.; Bunck, M.; Czibere, L.; Panhuysen, M.; Pütz, B.; Deussing, J.; Holsboer, F.; et al. Identification of Glyoxalase-I as a Protein Marker in a Mouse Model of Extremes in Trait Anxiety. J. Neurosci. 2005, 25, 4375–4384. [Google Scholar] [CrossRef]
- Landgraf, R.; Wigger, A. High vs low anxiety-related behavior rats: An animal model of extremes in trait anxiety. Behav. Genet. 2002, 32, 301–314. [Google Scholar] [CrossRef]
- Rodriguez, G.; Moore, S.; Neff, R.; Glass, E.; Stevenson, T.; Stinnett, G.; Seasholtz, A.; Murphy, G.; Cazares, V. Deficits across multiple behavioral domains align with susceptibility to stress in 129S1/SvImJ mice. Neurobiol. Stress 2020, 13, 100262. [Google Scholar] [CrossRef] [PubMed]
- Sartori, S.B.; Maurer, V.; Murphy, C.; Schmuckermair, C.; Muigg, P.; Neumann, I.D.; Whittle, N.; Singewald, N. Combined Neuropeptide S and D-Cycloserine Augmentation Prevents the Return of Fear in Extinction-Impaired Rodents: Advantage of Dual versus Single Drug Approaches. Int. J. Neuropsychopharmacol. 2016, 19, 1–11. [Google Scholar] [CrossRef]
- Chauveau, F.; Lange, M.D.; Jüngling, K.; Lesting, J.; Seidenbecher, T.; Pape, H.-C. Prevention of Stress-Impaired Fear Extinction Through Neuropeptide S Action in the Lateral Amygdala. Neuropsychopharmacol. 2012, 37, 1588–1599. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.; Kozlovsky, N.; Alona, C.; Matar, M.A.; Joseph, Z. Animal model for PTSD: From clinical concept to translational research. Neuropharmacology 2012, 62, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Lisieski, M.J.; Eagle, A.; Conti, A.C.; Liberzon, I.; Perrine, S.A. Single-Prolonged Stress: A Review of Two Decades of Progress in a Rodent Model of Post-traumatic Stress Disorder. Front. Psychiatry 2018, 9, 196. [Google Scholar] [CrossRef]
- Li, W.; Gao, Y.-H.; Chang, M.; Peng, Y.-L.; Yao, J.; Han, R.-W.; Wang, R. Neuropeptide S inhibits the acquisition and the expression of conditioned place preference to morphine in mice. Peptides 2009, 30, 234–240. [Google Scholar] [CrossRef]
- Chou, Y.H.; Hor, C.C.; Lee, M.T.; Lee, H.J.; Guerrini, R.; Calo, G.; Chiou, L.C. Stress induces reinstatement of extinguished cocaine conditioned place preference by a sequential signaling via neuropeptide S, orexin, and endocannabinoid. Addict. Biol. 2021, 26, e12971. [Google Scholar] [CrossRef] [PubMed]
- Tyree, S.; De Lecea, L. Lateral Hypothalamic Control of the Ventral Tegmental Area: Reward Evaluation and the Driving of Motivated Behavior. Front. Syst. Neurosci. 2017, 11, 50. [Google Scholar] [CrossRef]
- Cao, J.; de Lecea, L.; Ikemoto, S. Intraventricular administration of neuropeptide S has reward-like effects. Eur. J. Pharmacol. 2011, 658, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Enquist, J.; Ferwerda, M.; Madhavan, A.; Hok, D.; Whistler, J.L. Chronic Ethanol Potentiates the Effect of Neuropeptide S in the Basolateral Amygdala and Shows Increased Anxiolytic and Anti-Depressive Effects. Neuropsychopharmacology 2012, 37, 2436–2445. [Google Scholar] [CrossRef] [PubMed]
- Kallupi, M.; Cannella, N.; Economidou, D.; Ubaldi, M.; Ruggeri, B.; Weiss, F.; Massi, M.; Marugan, J.; Heilig, M.; Bonnavion, P.; et al. Neuropeptide S facilitates cue-induced relapse to cocaine seeking through activation of the hypothalamic hypocretin system. Proc. Natl. Acad. Sci. USA 2010, 107, 19567–19572. [Google Scholar] [CrossRef]
- Kallupi, M.; de Guglielmo, G.; Cannella, N.; Li, H.W.; Caló, G.; Guerrini, R.; Ubaldi, M.; Renger, J.J.; Uebele, V.N.; Ciccocioppo, R. Hypothalamic Neuropeptide S receptor blockade decreases discriminative cue-induced reinstatement of cocaine seeking in the rat. Psychopharmacology 2012, 226, 347–355. [Google Scholar] [CrossRef]
- Schmoutz, C.D.; Zhang, Y.; Runyon, S.P.; Goeders, N.E. Antagonism of the neuropeptide S receptor with RTI-118 decreases cocaine self-administration and cocaine-seeking behavior in rats. Pharmacol. Biochem. Behav. 2012, 103, 332–337. [Google Scholar] [CrossRef]
- Fedeli, A.; Braconi, S.; Economidou, D.; Cannella, N.; Kallupi, M.; Guerrini, R.; Calò, G.; Cifani, C.; Massi, M.; Ciccocioppo, R. The paraventricular nucleus of the hypothalamus is a neuroanatomical substrate for the inhibition of palatable food intake by neuropeptide S. Eur. J. Neurosci. 2009, 30, 1594–1602. [Google Scholar] [CrossRef]
- Cannella, N.; Economidou, D.; Kallupi, M.; Stopponi, S.; Heilig, M.; Massi, M.; Ciccocioppo, R. Persistent Increase of Alcohol-Seeking Evoked by Neuropeptide S: An Effect Mediated by the Hypothalamic Hypocretin System. Neuropsychopharmacology 2009, 34, 2125–2134. [Google Scholar] [CrossRef]
- Badia-Elder, N.E.; Henderson, A.N.; Bertholomey, M.L.; Dodge, N.C.; Stewart, R.B. The Effects of Neuropeptide S on Ethanol Drinking and Other Related Behaviors in Alcohol-Preferring and -Nonpreferring Rats. Alcohol. Clin. Exp. Res. 2008, 32, 1380–1387. [Google Scholar] [CrossRef]
- Thorsell, A.; Tapocik, J.D.; Liu, K.; Zook, M.; Bell, L.; Flanigan, M.; Patnaik, S.; Marugan, J.; Damadzic, R.; Dehdashti, S.J.; et al. A novel brain penetrant NPS receptor antagonist, NCGC00185684, blocks alcohol-induced ERK-phosphorylation in the central amygdala and decreases operant alcohol self-administration in rats. J. Neurosci. 2013, 33, 10132–10142. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, B.; Braconi, S.; Cannella, N.; Kallupi, M.; Soverchia, L.; Ciccocioppo, R.; Ubaldi, M. Neuropeptide S Receptor Gene Expression in Alcohol Withdrawal and Protracted Abstinence in Postdependent Rats. Alcohol. Clin. Exp. Res. 2010, 34, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Ghazal, P.; Ciccocioppo, R.; Ubaldi, M. Morphine dependence is associated with changes in neuropeptide S receptor expression and function in rat brain. Peptides 2013, 46, 6–12. [Google Scholar] [CrossRef]
- Okamura, N.; Hashimoto, K.; Iyo, M.; Shimizu, E.; Dempfle, A.; Friedel, S.; Reinscheid, R.K. Gender-specific association of a functional coding polymorphism in the Neuropeptide S receptor gene with panic disorder but not with schizophrenia or attention-deficit/hyperactivity disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2007, 31, 1444–1448. [Google Scholar] [CrossRef]
- Donner, J.; Haapakoski, R.; Ezer, S.; Melén, E.; Pirkola, S.; Gratacòs, M.; Zucchelli, M.; Anedda, F.; Johansson, L.E.; Söderhäll, C.; et al. Assessment of the Neuropeptide S System in Anxiety Disorders. Biol. Psychiatry 2010, 68, 474–483. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Shen, Z.; Ren, L.; Wang, X.; Qian, M.; Zhu, J.; Shen, X. Association of NPSR1 rs324981 polymorphism and treatment response to antidepressants in Chinese Han population with generalized anxiety disorder. Biochem. Biophys. Res. Commun. 2018, 504, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Haxhibeqiri, S.; Haxhibeqiri, V.; Agani, F.; Uka, A.G.; Hoxha, B.; Kulenovic, A.D.; Kucukalic, A.; Avdibegovic, E.; Sinanovic, O.; Babic, D.; et al. Association of Neuropeptide S Receptor 1 and Glutamate Decarboxylase 1 Gene Polymorphisms with Posttraumatic Stress Disorder. Psychiatr. Danub. 2019, 31, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Baykan, H.; Baykan, O.; Durmaz, O.; Kara, H.; Hismiogullari, A.A.; Karlidere, T. Plasma Neuropeptide-S Levels in Population with Generalized Anxiety Disorder: A Controlled Study. Arch. Neuropsychiatry 2018, 56, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Baykan, H.; Durmaz, O.; Baykan, O.; Can, M.S.; Kara, H.; Hismiogullari, A.A.; Karlidere, T. Investigating the relationship between plasma neuropeptide-S levels and clinical depression. Nord. J. Psychiatry 2018, 72, 292–295. [Google Scholar] [CrossRef]
- Laas, K.; Reif, A.; Akkermann, K.; Kiive, E.; Domschke, K.; Lesch, K.-P.; Veidebaum, T.; Harro, J. Neuropeptide S receptor gene variant and environment: Contribution to alcohol use disorders and alcohol consumption. Addict. Biol. 2014, 20, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Kotov, R.; Gamez, W.; Schmidt, F.; Watson, D. Linking “big” personality traits to anxiety, depressive, and substance use disorders: A meta-analysis. Psychol. Bull. 2010, 136, 768–821. [Google Scholar] [CrossRef]
- Laas, K.; Reif, A.; Akkermann, K.; Kiive, E.; Domschke, K.; Lesch, K.-P.; Veidebaum, T.; Harro, J. Interaction of the neuropeptide S receptor gene Asn107Ile variant and environment: Contribution to affective and anxiety disorders, and suicidal behaviour. Int. J. Neuropsychopharmacol. 2014, 17, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Camisa, K.M.; Bockbrader, M.A.; Lysaker, P.; Rae, L.L.; Brenner, C.A.; O’Donnell, B.F. Personality traits in schizophrenia and related personality disorders. Psychiatry Res. 2005, 133, 23–33. [Google Scholar] [CrossRef]
- Tomasi, J.; Zai, C.C.; Zai, G.; Herbert, D.; King, N.; Freeman, N.; Kennedy, J.L.; Tiwari, A.K. The effect of polymorphisms in startle-related genes on anxiety symptom severity. J. Psychiatr. Res. 2020, 125, 144–151. [Google Scholar] [CrossRef]
- Alshogran, O.Y.; Al-Eitan, L.N.; Altawalbeh, S.M.; Khalil, A.A.; Alqudah, M.A.Y.; Oweis, A.; Aman, H.A.; Alhawari, H.H. Investigating the Contribution of NPSR1, IL-6 and BDNF Polymorphisms to Depressive and Anxiety Symptoms in Hemodialysis Patients. Prog. Neuro-Psychopharmacology Biol. Psychiatry 2019, 94, 109657. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, M.; Zhang, Y.; Shen, X.; Yuan, Y. Correlation of 5-HTT, BDNF and NPSR1 gene polymorphisms with anxiety and depression in asthmatic patients. Int. J. Mol. Med. 2016, 38, 65–74. [Google Scholar] [CrossRef]
- Glotzbach-Schoon, E.; Andreatta, M.; Reif, A.; Ewald, H.; Tröger, C.; Baumann, C.R.; Deckert, J.; Mühlberger, A.; Pauli, P. Contextual fear conditioning in virtual reality is affected by 5HTTLPR and NPSR1 polymorphisms: Effects on fear-potentiated startle. Front. Behav. Neurosci. 2013, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- A Raczka, K.; Gartmann, N.; Mechias, M.-L.; Reif, A.; Büchel, C.; Deckert, J.; Kalisch, R. A neuropeptide S receptor variant associated with overinterpretation of fear reactions: A potential neurogenetic basis for catastrophizing. Mol. Psychiatry 2010, 15, 1067–1074. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Domschke, K.; Klauke, B.; Nienhaus, K.; Fobker, M.; Jacob, C.; Arolt, V.; Pauli, P.; Reif, A.; Zwanzger, P.; Deckert, J.; et al. Modification of caffeine effects on the affect-modulated startle by neuropeptide S receptor gene variation. Psychopharmacology 2012, 222, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Waters, A.M.; Craske, M.G.; Bergman, R.L.; Naliboff, B.D.; Negoro, H.; Ornitz, E.M. Developmental changes in startle reactivity in school-age children at risk for and with actual anxiety disorder. Int. J. Psychophysiol. 2008, 70, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Ray, W.J.; Molnar, C.; Aikins, D.; Yamasaki, A.; Newman, M.G.; Castonguay, L.; Borkovec, T.D. Startle response in generalized anxiety disorder. Depression Anxiety 2008, 26, 147–154. [Google Scholar] [CrossRef]
- McMillan, K.A.; Asmundson, G.J.G.; Zvolensky, M.J.; Carleton, R.N. Startle response and anxiety sensitivity: Subcortical indices of physiologic arousal and fear responding. Emotion 2012, 12, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- Grillon, C.; Ameli, R.; Foot, M.; Davis, M. Fear-potentiated startle: Relationship to the level of state/trait anxiety in healthy subjects. Biol. Psychiatry 1993, 33, 566–574. [Google Scholar] [CrossRef]
- Dannlowski, U.; Kugel, H.; Franke, F.; Stuhrmann, A.; Hohoff, C.; Zwanzger, P.; Lenzen, T.; Grotegerd, D.; Suslow, T.; Arolt, V.; et al. Neuropeptide-S (NPS) Receptor Genotype Modulates Basolateral Amygdala Responsiveness to Aversive Stimuli. Neuropsychopharmacology 2011, 36, 1879–1885. [Google Scholar] [CrossRef]
- Tupak, S.V.; Reif, A.; Pauli, P.; Dresler, T.; Herrmann, M.J.; Domschke, K.; Jochum, C.; Haas, E.; Baumann, C.; Weber, H.; et al. Neuropeptide S receptor gene: Fear-specific modulations of prefrontal activation. NeuroImage 2013, 66, 353–360. [Google Scholar] [CrossRef]
- Guhn, A.; Domschke, K.; Müller, L.D.; Dresler, T.; Eff, F.; Kopf, J.; Deckert, J.; Reif, A.; Herrmann, M.J. Neuropeptide S receptor gene variation and neural correlates of cognitive emotion regulation. Soc. Cogn. Affect. Neurosci. 2015, 10, 1730–1737. [Google Scholar] [CrossRef] [PubMed]
- Domschke, K.; Akhrif, A.; Romanos, M.; Bajer, C.; Mainusch, M.; Winkelmann, J.; Zimmer, C.; Neufang, S. Neuropeptide S Receptor Gene Variation Differentially Modulates Fronto-Limbic Effective Connectivity in Childhood and Adolescence. Cereb. Cortex 2015, 27, 554–566. [Google Scholar] [CrossRef] [PubMed]
- Neufang, S.; Geiger, M.J.; Homola, G.A.; Mahr, M.; Akhrif, A.; Nowak, J.; Reif, A.; Romanos, M.; Deckert, J.; Solymosi, L.; et al. Modulation of prefrontal functioning in attention systems by NPSR1 gene variation. NeuroImage 2015, 114, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Ruland, T.; Domschke, K.; Schutte, V.; Zavorotnyy, M.; Kugel, H.; Notzon, S.; Vennewald, N.; Ohrmann, P.; Arolt, V.; Pfleiderer, B.; et al. Neuropeptide S receptor gene variation modulates anterior cingulate cortex Glx levels during CCK-4 induced panic. Eur. Neuropsychopharmacol. 2015, 25, 1677–1682. [Google Scholar] [CrossRef]
- Klauke, B.; Deckert, J.; Zwanzger, P.; Baumann, C.; Arolt, V.; Pauli, P.; Reif, A.; Domschke, K. Neuropeptide S receptor gene (NPSR) and life events: G × E effects on anxiety sensitivity and its subdimensions. World J. Biol. Psychiatry 2014, 15, 17–25. [Google Scholar] [CrossRef]
- Schiele, M.A.; Herzog, K.; Kollert, L.; Schartner, C.; Leehr, E.J.; Böhnlein, J.; Repple, J.; Rosenkranz, K.; Lonsdorf, T.B.; Dannlowski, U.; et al. Extending the vulnerability–stress model of mental disorders: Three-dimensional NPSR1 × environment × coping interaction study in anxiety. Br. J. Psychiatry 2020, 217, 645–650. [Google Scholar] [CrossRef]
- Laas, K.; Reif, A.; Kiive, E.; Domschke, K.; Lesch, K.-P.; Veidebaum, T.; Harro, J. A functional NPSR1 gene variant and environment shape personality and impulsive action: A longitudinal study. J. Psychopharmacol. 2014, 28, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Streit, F.; Akdeniz, C.; Haddad, L.; Kumsta, R.; Entringer, S.; Frank, J.; Yim, I.S.; Zänkert, S.; Witt, S.H.; Kirsch, P.; et al. Sex-specific association between functional neuropeptide S receptor gene (NPSR1) variants and cortisol and central stress responses. Psychoneuroendocrinology 2017, 76, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Kumsta, R.; Chen, F.S.; Pape, H.-C.; Heinrichs, M. Neuropeptide S receptor gene is associated with cortisol responses to social stress in humans. Biol. Psychol. 2013, 93, 304–307. [Google Scholar] [CrossRef]
- Streit, F.; Haddad, L.; Paul, T.; Frank, J.; Schäfer, A.; Nikitopoulos, J.; Akdeniz, C.; Lederbogen, F.; Treutlein, J.; Witt, S.; et al. A functional variant in the neuropeptide S receptor 1 gene moderates the influence of urban upbringing on stress processing in the amygdala. Stress 2014, 17, 352–361. [Google Scholar] [CrossRef]
- Gechter, J.; Liebscher, C.; Geiger, M.J.; Wittmann, A.; Schlagenhauf, F.; Lueken, U.; Wittchen, H.-U.; Pfleiderer, B.; Arolt, V.; Kircher, T.; et al. Association of NPSR1 gene variation and neural activity in patients with panic disorder and agoraphobia and healthy controls. NeuroImage Clin. 2019, 24, 102029. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.B.; Chartoff, E. Sex differences in neural mechanisms mediating reward and addiction. Neuropsychopharmacol. 2019, 44, 166–183. [Google Scholar] [CrossRef] [PubMed]
- Donner, N.C.; Lowry, C.A. Sex differences in anxiety and emotional behavior. Pflügers Archiv Eur. J. Physiol. 2013, 465, 601–626. [Google Scholar] [CrossRef]
- Wellman, C.L.; Bangasser, D.A.; Bollinger, J.L.; Coutellier, L.; Logrip, M.L.; Moench, K.M.; Urban, K.R. Sex Differences in Risk and Resilience: Stress Effects on the Neural Substrates of Emotion and Motivation. J. Neurosci. 2018, 38, 9423–9432. [Google Scholar] [CrossRef]
- Reinscheid, R.K.; Mafessoni, F.; Lüttjohann, A.; Jüngling, K.; Pape, H.-C.; Schulz, S. Neandertal introgression and accumulation of hypomorphic mutations in the neuropeptide S (NPS) system promote attenuated functionality. Peptides 2021, 138, 170506. [Google Scholar] [CrossRef]
- Heberlein, A.; Bleich, S.; Kornhuber, J.; Hillemacher, T. Neuroendocrine pathways in benzodiazepine dependence: New targets for research and therapy. Hum. Psychopharmacol. Clin. Exp. 2008, 23, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Cooper, S.J. Palatability-dependent appetite and benzodiazepines: New directions from the pharmacology of GABAA receptor subtypes. Appetite 2005, 44, 133–150. [Google Scholar] [CrossRef] [PubMed]
- McGonigle, P. Peptide therapeutics for CNS indications. Biochem. Pharmacol. 2012, 83, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Dhuria, S.V.; Hanson, L.R.; Frey, W.H. Intranasal delivery to the central nervous system: Mechanisms and experimental considerations. J. Pharm. Sci. 2010, 99, 1654–1673. [Google Scholar] [CrossRef]

| Brain Area | TT | AT | AA | Ref. |
|---|---|---|---|---|
| Neural activation to stress or CCK-4 challenge | ||||
| Parahippocampal gyrus | ↑♂, ↓♀ | ↔ | ↔ | [104] |
| Amygdala | ↔ | ↔ | ↔ | [104,105] |
| Anterior cingulate cortex | ↔ | ↔ | ↔//↑ | [100]//[104,106] |
| Cerebellum | ↑ | ↔ | ↔ | [106] |
| Neural activation in an emotional task | ||||
| Dorsolateral prefrontal cortex | ||||
| Positive stimuli | ↓ | ↓ | ↔ | [96,97] |
| Negative stimuli | ↑ | ↑ | ↑ | |
| Neutral stimuli | ↔ | ↔ | ↔ | |
| Medial prefrontal cortex | ||||
| Positive stimuli | ↓ | ↓ | ↔ | [96,97] |
| Negative stimuli | ↑ | ↑ | ↑ | |
| Neutral stimuli | ↔ | ↔ | ↔ | |
| Rostro dorsal anterior cingulate cortex/Dorsomedial prefrontal cortex | ↑ | ↑ | ↔ | [89] |
| Amygdala | ↑ | ↑ | ↔ | [95] |
| Neural activation in a cognitive task | ||||
| Dorsolateral prefrontal cortex | ↔ | ↔ | ↑ | [96] |
| Medial prefrontal cortex | ↔ | ↔ | ↔ | |
| Neural activation in an attentional task | ||||
| Superior parietal lobule | ↑ | ↔ | ↔ | [99] |
| (Dorsolateral) Prefrontal cortex | ↑ | ↔ | ↔ | |
| Locus coeruleus | ↑ | ↔ | ↔ | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tobinski, A.-M.; Rappeneau, V. Role of the Neuropeptide S System in Emotionality, Stress Responsiveness and Addiction-Like Behaviours in Rodents: Relevance to Stress-Related Disorders. Pharmaceuticals 2021, 14, 780. https://doi.org/10.3390/ph14080780
Tobinski A-M, Rappeneau V. Role of the Neuropeptide S System in Emotionality, Stress Responsiveness and Addiction-Like Behaviours in Rodents: Relevance to Stress-Related Disorders. Pharmaceuticals. 2021; 14(8):780. https://doi.org/10.3390/ph14080780
Chicago/Turabian StyleTobinski, Ann-Marie, and Virginie Rappeneau. 2021. "Role of the Neuropeptide S System in Emotionality, Stress Responsiveness and Addiction-Like Behaviours in Rodents: Relevance to Stress-Related Disorders" Pharmaceuticals 14, no. 8: 780. https://doi.org/10.3390/ph14080780
APA StyleTobinski, A.-M., & Rappeneau, V. (2021). Role of the Neuropeptide S System in Emotionality, Stress Responsiveness and Addiction-Like Behaviours in Rodents: Relevance to Stress-Related Disorders. Pharmaceuticals, 14(8), 780. https://doi.org/10.3390/ph14080780