Apamin-Conjugated Alendronate Sodium Nanocomplex for Management of Pancreatic Cancer
Abstract
1. Introduction
2. Results and Discussion
2.1. Formulation and Optimization of APA-ALS-NC
2.2. In Vitro Cell Line Studies of APA-ALS-NC in Pancreatic Cancer Cells (PANC-1 Cells)
2.2.1. IC50 Determination Using MTT Assay
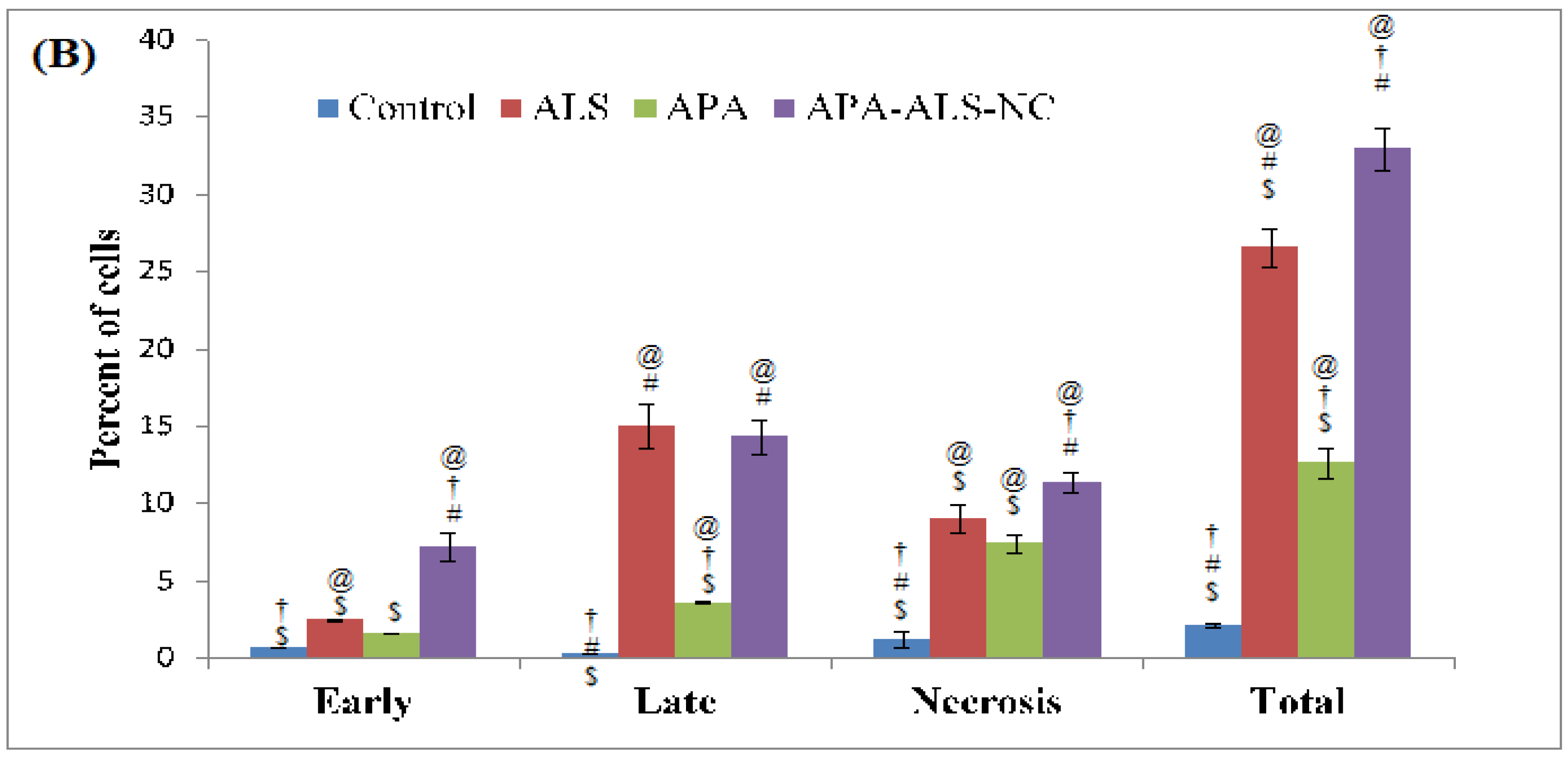
2.2.2. Apoptotic Activity
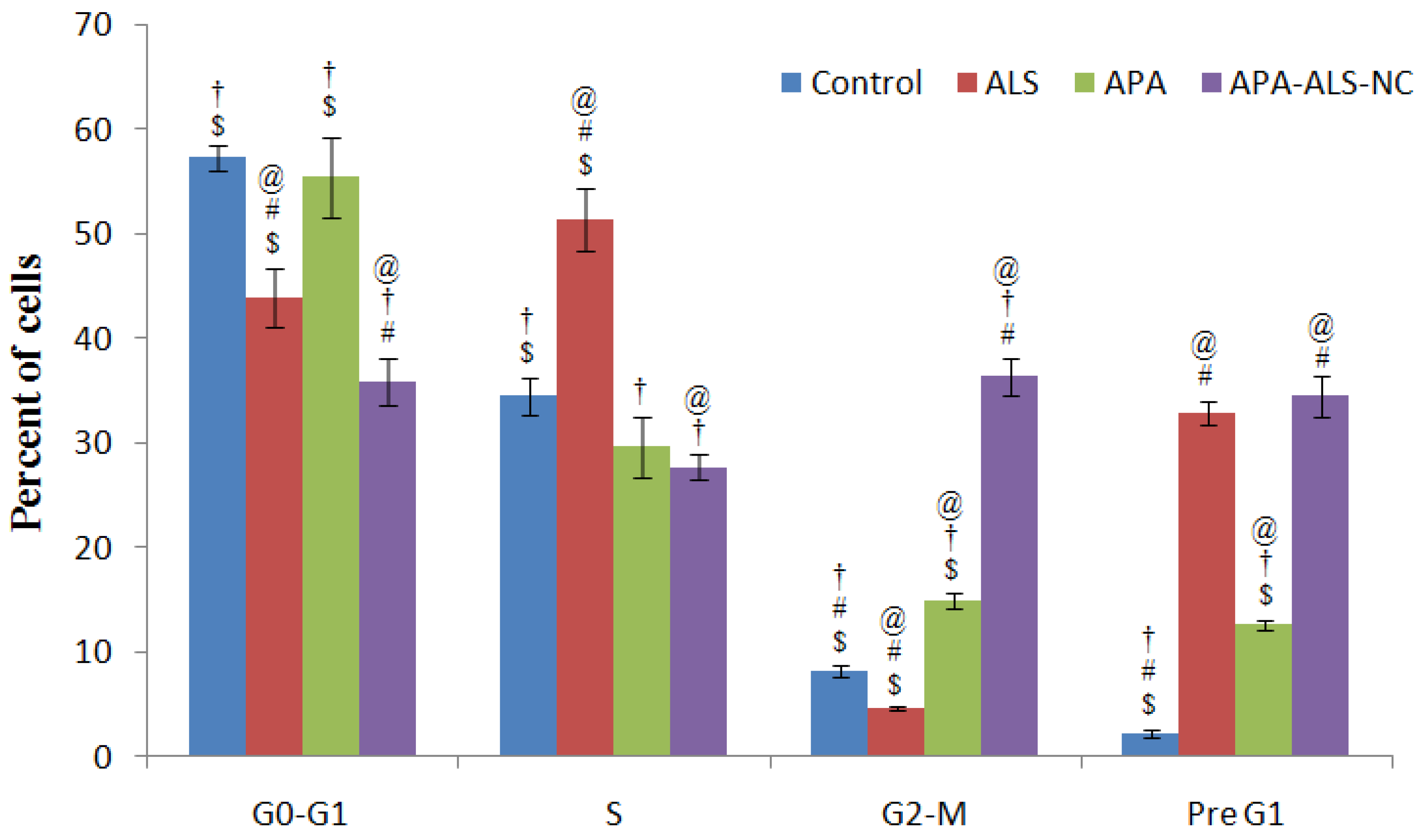
2.2.3. Cell Cycle Analysis
2.2.4. Mitochondrial Membrane Potential (MMP)
2.2.5. Determination of Marker Molecules by ELISA
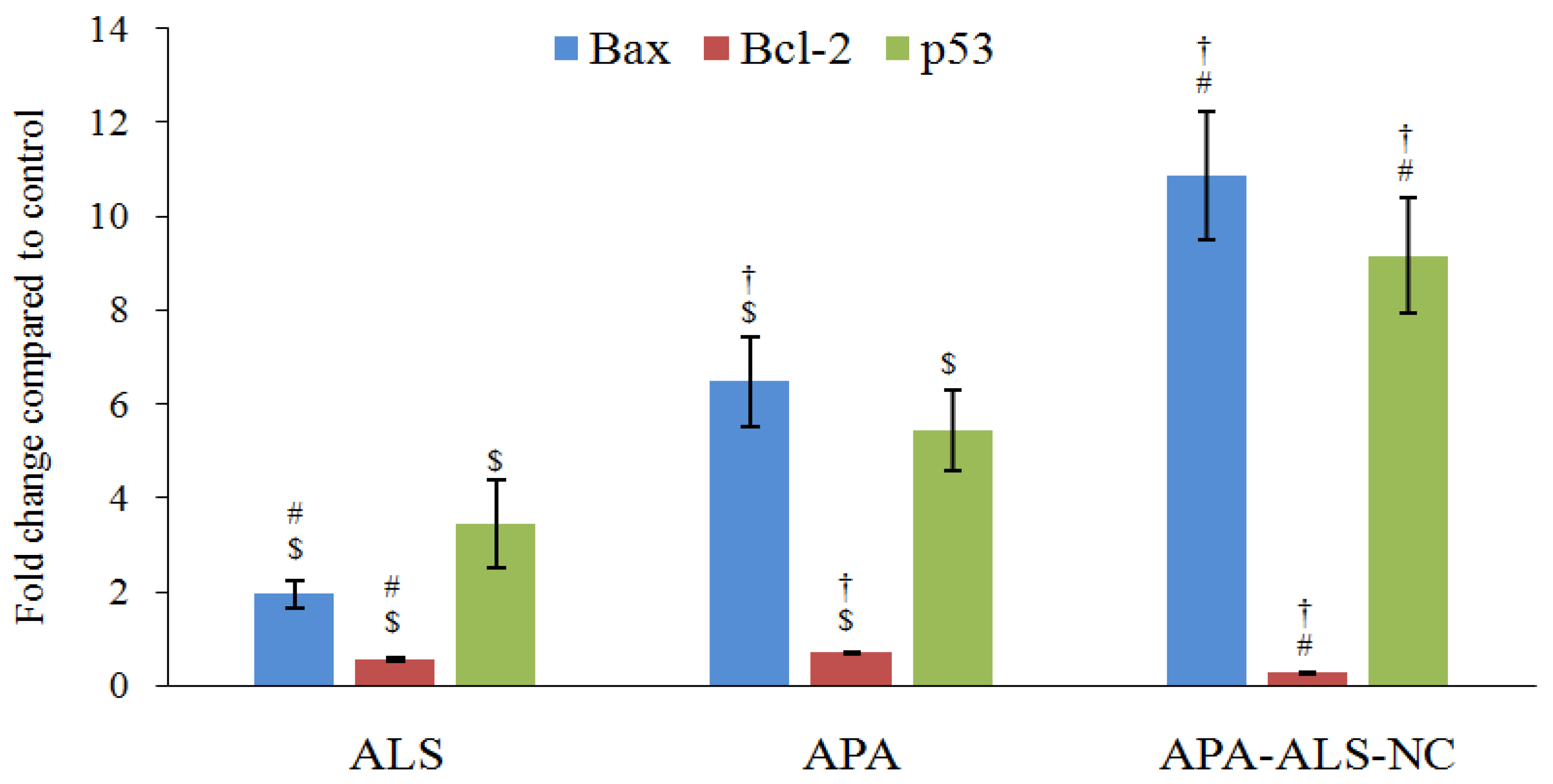
2.2.6. Estimation of Bax, Bcl-2, and p53 Gene Expressions Using RT-PCR
3. Materials and methods
3.1. Materials
3.2. Formulation and Optimization of APA-ALS Nanocomplex (APA-ALS-NC)
Determination of Particle Size, Size Distribution, and Zeta Potential
3.3. In Vitro Cell Line Studies of APA-ALS-NC in Pancreatic Cancer Cells (PANC-1 Cells)
3.3.1. IC50 Determination Using MTT Assay
3.3.2. Apoptotic Activity
3.3.3. Cell Cycle Analysis
3.3.4. Mitochondrial Membrane Potential (MMP)
3.3.5. Determination of Marker Molecules by ELISA
3.3.6. Estimation of Bax, Bcl-2, and p53 Gene Expressions Using RT-PCR
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Neoptolemos, J.P.; Kleeff, J.; Michl, P.; Costello, E.; Greenhalf, W.; Palmer, D.H. Therapeutic Developments in Pancreatic Cancer: Current and Future Perspectives. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M. Pancreatic Cancer. N. Engl. J. Med. 2010, 362, 1605–1617. [Google Scholar] [CrossRef] [PubMed]
- Neesse, A.; Michl, P.; Frese, K.K.; Feig, C.; Cook, N.; Jacobetz, M.A.; Lolkema, M.P.; Buchholz, M.; Olive, K.P.; Gress, T.M.; et al. Stromal Biology and Therapy in Pancreatic Cancer. Gut 2011, 60, 861–868. [Google Scholar] [CrossRef]
- Rossi, M.L.; Rehman, A.A.; Gondi, C.S. Therapeutic Options for the Management of Pancreatic Cancer. World J. Gastroenterol. 2014, 20, 11142–11159. [Google Scholar] [CrossRef] [PubMed]
- Amanam, I.; Chung, V. Targeted Therapies for Pancreatic Cancer. Cancers 2018, 10, 36. [Google Scholar] [CrossRef]
- Trajkovic-Arsic, M.; Kalideris, E.; Siveke, J.T. The Role of Insulin and IGF System in Pancreatic Cancer. J. Mol. Endocrinol. 2013, 50, R67–R74. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Bien, H.; Mofunanya, A.; Powers, S. Challenges in Ras Therapeutics in Pancreatic Cancer. Semin. Cancer Biol. 2019, 54, 101–108. [Google Scholar] [CrossRef]
- Cruz-Monserrate, Z.; Roland, C.L.; Deng, D.; Arumugam, T.; Moshnikova, A.; Andreev, O.A.; Reshetnyak, Y.K.; Logsdon, C.D. Targeting Pancreatic Ductal Adenocarcinoma Acidic Microenvironment. Sci. Rep. 2014, 4, 4410. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, B.; He, X.; Cao, G.; Li, Y.; Cai, K.; Yang, B.; Wu, Y. A New Fusion Peptide Targeting Pancreatic Cancer and Inhibiting Tumor Growth. Onco. Targets. Ther. 2020, 13, 7865–7875. [Google Scholar] [CrossRef]
- Montet, X.; Weissleder, R.; Josephson, L. Imaging Pancreatic Cancer with a Peptide-Nanoparticle Conjugate Targeted to Normal Pancreas. Bioconjug. Chem. 2006, 17, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Jiang, H.; Bi, Q.; Luo, Q.; Li, J.; Zhang, Y.; Chen, Z.; Li, C. Apamin-Mediated Actively Targeted Drug Delivery for Treatment of Spinal Cord Injury: More Than Just a Concept. Mol. Pharm. 2014, 11, 3210–3222. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Han, S.M.; Park, K.-K. Therapeutic Effects of Apamin As a Bee Venom Component for Non-Neoplastic Disease. Toxins 2020, 12, 195. [Google Scholar] [CrossRef]
- Moga, M.A.; Dimienescu, O.G.; Arvătescu, C.A.; Ifteni, P.; Pleş, L. Anticancer Activity of Toxins from Bee and Snake Venom-An Overview on Ovarian Cancer. Molecules 2018, 23, 692. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Jia, L.; Niu, Y.; Qi, H.; Chen, X.; Zhang, Q.; Zhang, J.; Wang, Y.; Dong, L.; Wang, C. Targeted Depletion of Tumour-Associated Macrophages by an Alendronate-Glucomannan Conjugate for Cancer Immunotherapy. Biomaterials 2014, 35, 10046–10057. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.S.; Katayama, E.; Bombana, A.C.; Marques, M.M. Cytotoxicity Analysis of Alendronate on Cultured Endothelial Cells and Subcutaneous Tissue. A pilot study. Dent. Traumatol. Off. Publ. Int. Assoc. Dent. Traumatol. 2005, 21, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zheng, C.; Li, Y.; Bian, S.; Pan, H.; Zhao, X.; Lu, W.W. Bone Targeted Delivery of SDF-1 Via Alendronate Functionalized Nanoparticles in Guiding Stem Cell Migration. ACS Appl. Mater. Interfaces 2018, 10, 23700–23710. [Google Scholar] [CrossRef]
- De Rosa, G.; Misso, G.; Salzano, G.; Caraglia, M. Bisphosphonates and Cancer: What Opportunities from Nanotechnology? J. Drug Deliv. 2013, 2013, 637976. [Google Scholar] [CrossRef]
- Ang, C.; Doyle, E.; Branch, A. Bisphosphonates as Potential Adjuvants for Patients with Cancers of the Digestive System. World J. Gastroenterol. 2016, 22, 906–916. [Google Scholar] [CrossRef]
- Green, J.R. Antitumor Effects of Bisphosphonates. Cancer 2003, 97, 840–847. [Google Scholar] [CrossRef]
- Virtanen, S.S.; Ishizu, T.; Sandholm, J.A.; Löyttyniemi, E.; Väänänen, H.K.; Tuomela, J.M.; Härkönen, P.L. Alendronate-Induced Disruption of Actin Cytoskeleton and Inhibition of Migration/Invasion are Associated with Cofilin Downregulation in PC-3 Prostate Cancer Cells. Oncotarget 2018, 9, 32593–32608. [Google Scholar] [CrossRef]
- Satoh, M.; Takano, S.; Sogawa, K.; Noda, K.; Yoshitomi, H.; Ishibashi, M.; Mogushi, K.; Takizawa, H.; Otsuka, M.; Shimizu, H.; et al. Immune-Complex Level of Cofilin-1 in Sera is Associated with Cancer Progression and Poor Prognosis in Pancreatic Cancer. Cancer Sci. 2017, 108, 795–803. [Google Scholar] [CrossRef]
- Su, J.; Zhou, Y.; Pan, Z.; Shi, L.; Yang, J.; Liao, A.; Liao, Q.; Su, Q. Downregulation of LIMK1–ADF/Cofilin by DADS Inhibits the Migration and Invasion of Colon Cancer. Sci. Rep. 2017, 7, 45624. [Google Scholar] [CrossRef] [PubMed]
- Hodgins, N.O.; Al-Jamal, W.T.; Wang, J.T.-W.; Klippstein, R.; Costa, P.M.; Sosabowski, J.K.; Marshall, J.F.; Maher, J.; Al-Jamal, K.T. Investigating In Vitro and In Vivo αvβ6 Integrin Receptor-Targeting Liposomal Alendronate for Combinatory γδ T Cell Immunotherapy. J. Control. Release 2017, 256, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, V.K.; Singh, A.; Singh, V.K.; Singh, M.P. Cancer Nanotechnology: A New Revolution for Cancer Diagnosis and Therapy. Curr. Drug Metab. 2019, 20, 416–429. [Google Scholar] [CrossRef]
- Irby, D.; Du, C.; Li, F. Lipid–Drug Conjugate for Enhancing Drug Delivery. Mol. Pharm. 2017, 14, 1325–1338. [Google Scholar] [CrossRef] [PubMed]
- Ajithkumar, K.C.; Pramod, K. Doxorubicin-DNA Adduct Entrenched and Motif Tethered Artificial Virus Encased in pH-Responsive Polypeptide Complex for Targeted Cancer Therapy. Mater. Sci. Eng. C 2018, 89, 387–400. [Google Scholar] [CrossRef]
- Abioye, A.O.; Chi, G.T.; Kola-Mustapha, A.T.; Ruparelia, K.; Beresford, K.; Arroo, R. Polymer-Drug Nanoconjugate—An Innovative Nanomedicine: Challenges and Recent Advancements in Rational Formulation Design for Effective Delivery of Poorly Soluble Drugs. Pharm. Nanotechnol. 2016, 4, 38–79. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Cheetham, A.G.; Angacian, G.; Su, H.; Xie, L.; Cui, H. Peptide–Drug Conjugates as Effective Prodrug Strategies for Targeted Delivery. Adv. Drug Deliv. Rev. 2017, 110, 112–126. [Google Scholar] [CrossRef]
- Khan, J.A.; Kudgus, R.A.; Szabolcs, A.; Dutta, S.; Wang, E.; Cao, S.; Curran, G.L.; Shah, V.; Curley, S.; Mukhopadhyay, D.; et al. Designing Nanoconjugates to Effectively Target Pancreatic Cancer Cells In Vitro and In Vivo. PLoS ONE 2011, 6, e20347. [Google Scholar] [CrossRef] [PubMed]
- Politis, S.N.; Colombo, P.; Colombo, G.; Rekkas, D.M. Design of Experiments (DoE) in Pharmaceutical Development. Drug Dev. Ind. Pharm. 2017, 43, 889–901. [Google Scholar] [CrossRef]
- Hao, Y.; Huang, Y.; He, Y.; Peng, J.; Chen, L.; Hu, X.; Qian, Z. The Evaluation of Cellular Uptake Efficiency and Tumor-Targeting Ability of MPEG–PDLLA Micelles: Effect of Particle Size. RSC Adv. 2016, 6, 13698–13709. [Google Scholar] [CrossRef]
- Ristroph, K.D.; Prud’homme, R.K. Hydrophobic Ion Pairing: Encapsulating Small Molecules, Peptides, and Proteins into Nanocarriers. Nanoscale Adv. 2019, 1, 4207–4237. [Google Scholar] [CrossRef]
- You, S.-K.; Kwon, H.-H.; Lee, J.-M.; Shin, S.-C.; Cho, C.-W. Studies on the Formation of Hydrophobic Ion-Pairing Complex of Alendronate. Arch. Pharmacal Res. 2009, 32, 1055–1060. [Google Scholar] [CrossRef]
- Spoerri, P.E.; Jentsch, J.; Glees, P. Apamin from Bee Venom. Effects of the Neurotoxin on Subcellular Particles of Neural Cultures. FEBS Lett. 1975, 53, 143–147. [Google Scholar] [CrossRef]
- Shinde, G.; Shiyani, S.; Shelke, S.; Chouthe, R.; Kulkarni, D.; Marvaniya, K. Enhanced Brain Targeting Efficiency Using 5-FU (Fluorouracil) lipid-Drug Conjugated Nanoparticles in Brain Cancer Therapy. Prog. Biomater. 2020, 9, 259–275. [Google Scholar] [CrossRef]
- Wang, L.; Song, Y.; Parikh, A.; Joyce, P.; Chung, R.; Liu, L.; Afinjuomo, F.; Hayball, J.D.; Petrovsky, N.; Barclay, T.G.; et al. Doxorubicin-Loaded Delta Inulin Conjugates for Controlled and Targeted Drug Delivery: Development, Characterization, and In Vitro Evaluation. Pharmaceutics 2019, 11, 581. [Google Scholar] [CrossRef]
- Siddiqui, A.; Alayoubi, A.; El-Malah, Y.; Nazzal, S. Modeling the Effect of Sonication Parameters on Size and Dispersion Temperature of Solid Lipid Nanoparticles (SLNs) by Response Surface Methodology (RSM). Pharm. Dev. Technol. 2014, 19, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Pourgonabadi, S.; Ghorbani, A.; Najaran, Z.T.; Mousavi, S.H. In Vitro Assessment of Alendronate Toxic and Apoptotic Effects on Human Dental Pulp Stem Cells. Iran. J. Basic Med. Sci. 2018, 21, 905–910. [Google Scholar]
- Sakamuru, S.; Attene-Ramos, M.S.; Xia, M. Mitochondrial Membrane Potential Assay. Methods Mol. Biol. 2016, 1473, 17–22. [Google Scholar] [PubMed]
- Mönkkönen, H.; Auriola, S.; Lehenkari, P.; Kellinsalmi, M.; Hassinen, I.E.; Vepsäläinen, J.; Mönkkönen, J. A New Endogenous ATP Analog (ApppI) Inhibits the Mitochondrial Adenine Nucleotide Translocase (ANT) and is Responsible for the Apoptosis Induced by Nitrogen-Containing Bisphosphonates. Br. J. Pharmacol. 2006, 147, 437–445. [Google Scholar] [CrossRef]
- Ratcliffe, N.; Azambuja, P.; Mello, C.B. Recent Advances in Developing Insect Natural Products as Potential Modern Day Medicines. Evid. Based Complement. Altern. Med. 2014, 2014, 904958. [Google Scholar] [CrossRef] [PubMed]
- Bent, R.; Moll, L.; Grabbe, S.; Bros, M. Interleukin-1 Beta-A Friend or Foe in Malignancies? Int. J. Mol. Sci. 2018, 19, 2155. [Google Scholar] [CrossRef] [PubMed]
- Yeung, M.C.; Lau, A.S. Mechanism of Tumor Necrosis Factor(TNF)-Induced Cytotoxicity: Apoptosis, Oncogene, and Interferon signaling • 934. Pediatr. Res. 1998, 43, 161. [Google Scholar] [CrossRef]
- Jerome, K.R.; Sloan, D.D.; Aubert, M. Measurement of CTL-Induced Cytotoxicity: The Caspase 3 Assay. Apoptosis 2003, 8, 563–571. [Google Scholar] [CrossRef]
- Khalil, W.K.B.; Assaf, N.; ElShebiney, S.A.; Salem, N.A. Neuroprotective Effects of Bee Venom Acupuncture Therapy against Rotenone-Induced Oxidative Stress and Apoptosis. Neurochem. Int. 2015, 80, 79–86. [Google Scholar] [CrossRef]
- Cairns, J.A.; Guy, G.R.; Tan, Y.H. Interleukin-6 Regulates the Cytotoxic Effect of Tumour Necrosis Factor on U937 Cells. Immunology 1992, 75, 669–673. [Google Scholar]
- Kim, S.-J.; Park, J.-H.; Kim, K.-H.; Lee, W.-R.; Pak, S.C.; Han, S.-M.; Park, K.-K. The Protective Effect of Apamin on LPS/Fat-Induced Atherosclerotic Mice. Evid. Based Complement. Altern. Med. 2012, 2012, 1–10. [Google Scholar] [CrossRef]
- Khanzadeh, T.; Hagh, M.F.; Talebi, M.; Yousefi, B.; Azimi, A.; Hossein Pour Feizi, A.A.; Baradaran, B. Investigation of BAX and BCL2 Expression and Apoptosis in a Resveratrol- and Prednisolone-Treated Human T-ALL Cell line, CCRF-CEM. Blood Res. 2018, 53, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Park, J.-H.; Kim, K.-H.; Lee, W.-R.; An, H.-J.; Min, B.-K.; Han, S.-M.; Kim, K.-S.; Park, K.-K. Apamin Inhibits THP-1-Derived Macrophage Apoptosis Via Mitochondria-Related Apoptotic Pathway. Exp. Mol. Pathol. 2012, 93, 129–134. [Google Scholar] [CrossRef]
- Liu, R.; Ji, P.; Liu, B.; Qiao, H.; Wang, X.; Zhou, L.; Deng, T.; Ba, Y. Apigenin enhances the Cisplatin Cytotoxic Effect through p53-Modulated Apoptosis. Oncol. Lett. 2017, 13, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, K.Y.; Wu, Y.-J.; Liu, Z.N.; Chuang, C.W.; Huang, H.H.; Kuo, S.M. Anticancer Effects of Sinulariolide-Conjugated Hyaluronan Nanoparticles on Lung Adenocarcinoma Cells. Molecules 2016, 21, 297. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Chen, Q.; Ding, T.; Sun, J. SiO2 Nanoparticle-Induced Impairment of Mitochondrial Energy Metabolism in Hepatocytes Directly and through a Kupffer Cell-Mediated Pathway In Vitro. Int. J. Nanomed. 2014, 9, 2891–2903. [Google Scholar]
- Golestani Eimani, B.; Sanati, M.H.; Houshmand, M.; Ataei, M.; Akbarian, F.; Shakhssalim, N. Expression and Prognostic Significance of bcl-2 and Bax in the Progression and Clinical Outcome of Transitional Bladder Cell Carcinoma. Cell J. 2014, 15, 356–363. [Google Scholar]
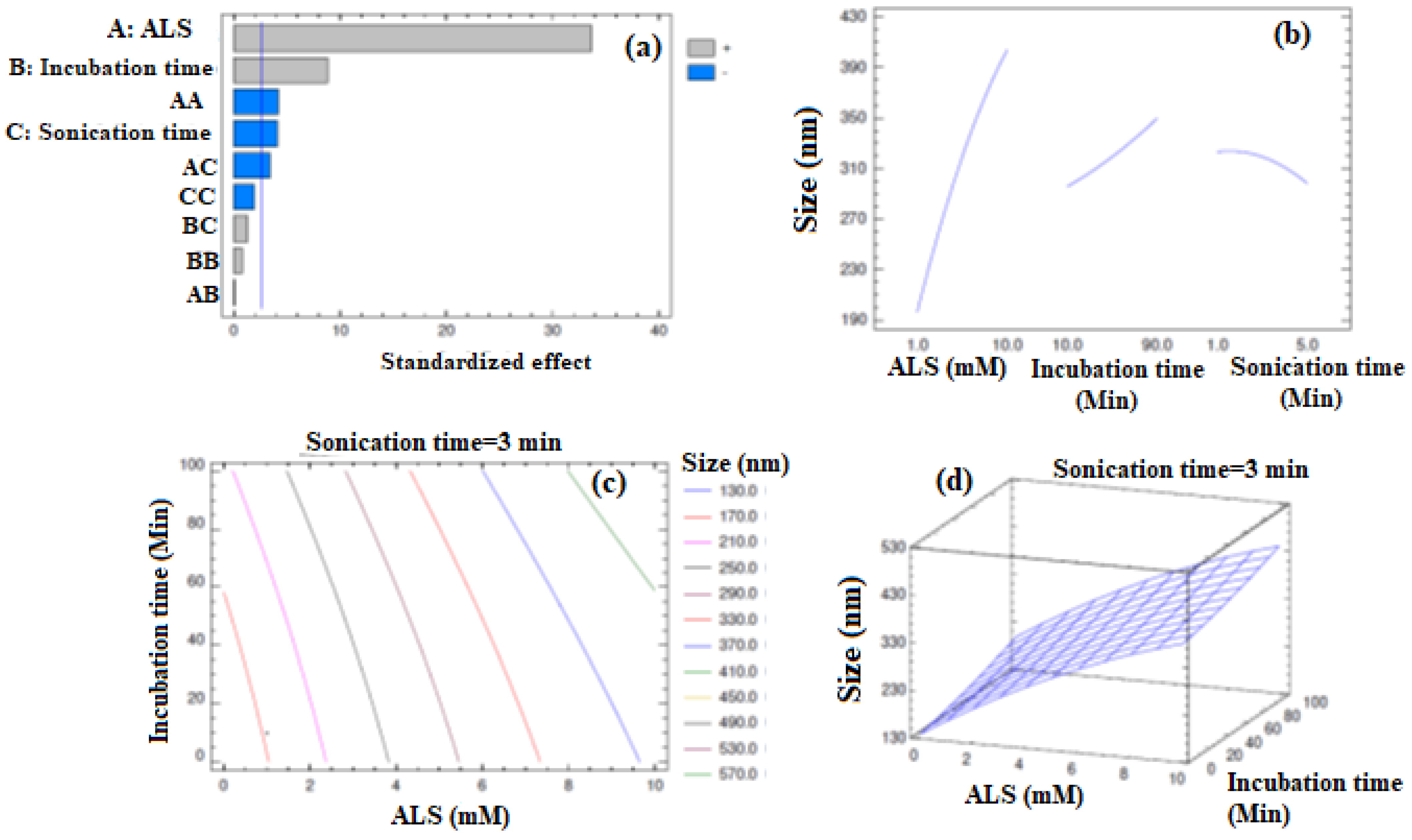




| Run | Independent Factors | Dependent Factor | |||
|---|---|---|---|---|---|
| Factor A: ALS Amount (mM) | Factor B: Incubation Time (min) | Factor C: Sonication Time (min) | Response 1: Mean Particle Size (nm) | ||
| Observed | Predicted | ||||
| 1 | 1 | 50 | 5 | 192 | 190.5 |
| 2 | 5.5 | 90 | 5 | 342 | 334.5 |
| 3 | 1 | 90 | 3 | 218 | 227.0 |
| 4 | 10 | 10 | 3 | 389 | 380.0 |
| 5 | 5.5 | 50 | 3 | 321 | 319.3 |
| 6 | 10 | 50 | 1 | 421 | 422.5 |
| 7 | 1 | 50 | 1 | 191 | 186.0 |
| 8 | 5.5 | 50 | 3 | 319 | 319.3 |
| 9 | 5.5 | 50 | 3 | 318 | 319.3 |
| 10 | 5.5 | 10 | 1 | 298 | 305.5 |
| 11 | 1 | 10 | 3 | 176 | 173.5 |
| 12 | 10 | 50 | 5 | 363 | 368.0 |
| 13 | 10 | 90 | 3 | 432 | 434.5 |
| 14 | 5.5 | 10 | 5 | 265 | 269.0 |
| 15 | 5.5 | 90 | 1 | 352 | 348.0 |
| Source | Sum of Squares | Degrees of Freedom | Mean Square | F-Ratio | p-Value |
|---|---|---|---|---|---|
| A: ALS (mM) | 85,698.0 | 1 | 85,698.0 | 1133.07 | 0.0000 |
| B: Incubation time (min) | 5832.0 | 1 | 5832.0 | 77.11 | 0.0003 |
| C: Sonication time (min) | 1250.0 | 1 | 1250.0 | 16.53 | 0.0097 |
| AA | 1338.78 | 1 | 1338.78 | 17.70 | 0.0084 |
| AB | 0.25 | 1 | 0.25 | 0.00 | 0.9564 |
| AC | 870.25 | 1 | 870.25 | 11.51 | 0.0194 |
| BB | 44.1603 | 1 | 44.1603 | 0.58 | 0.4793 |
| BC | 132.25 | 1 | 132.25 | 1.75 | 0.2433 |
| CC | 269.391 | 1 | 269.391 | 3.56 | 0.1178 |
| Total error | 378.167 | 5 | 75.6333 | -- | -- |
| Total (corr.) | 95,795.7 | 14 | -- | -- | -- |
| Factor | Low | High | Optimum |
|---|---|---|---|
| ALS (mM) | 1.0 | 10.0 | 1.00171 |
| Incubation time (min) | 10.0 | 90.0 | 10.0 |
| Sonication time (min) | 1.0 | 5.0 | 4.99857 |
| Bax F | 5′-TGGCAGCTGACATGTTTTCTGAC-3′ |
| Bax R | 5′-TCACCCAACCACCCTGGTCTT-3′ |
| Bcl-2 F | 5′-TCGCCCTGTGGATGACTGA-3′ |
| Bcl-2 R | 5′-CAGAGACAGCCAGGAGAAATCA-3′ |
| p53 F | 5′-GACGGTGACACGCTTCCCTGGATT-3′ |
| P53 R | 5′-GGGAACAAGAAGTGGAGAATGTCA-3′ |
| GAPDH F | 5′-AATGCATCCTGCACCACCAA-3′ |
| GAPDH R | 5′-GATGCCATATTCATTGTCATA-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhakamy, N.A.; Ahmed, O.A.A.; Fahmy, U.A.; Md, S. Apamin-Conjugated Alendronate Sodium Nanocomplex for Management of Pancreatic Cancer. Pharmaceuticals 2021, 14, 729. https://doi.org/10.3390/ph14080729
Alhakamy NA, Ahmed OAA, Fahmy UA, Md S. Apamin-Conjugated Alendronate Sodium Nanocomplex for Management of Pancreatic Cancer. Pharmaceuticals. 2021; 14(8):729. https://doi.org/10.3390/ph14080729
Chicago/Turabian StyleAlhakamy, Nabil A., Osama A. A. Ahmed, Usama A. Fahmy, and Shadab Md. 2021. "Apamin-Conjugated Alendronate Sodium Nanocomplex for Management of Pancreatic Cancer" Pharmaceuticals 14, no. 8: 729. https://doi.org/10.3390/ph14080729
APA StyleAlhakamy, N. A., Ahmed, O. A. A., Fahmy, U. A., & Md, S. (2021). Apamin-Conjugated Alendronate Sodium Nanocomplex for Management of Pancreatic Cancer. Pharmaceuticals, 14(8), 729. https://doi.org/10.3390/ph14080729







