Emerging Applications of Nanotechnology in Healthcare Systems: Grand Challenges and Perspectives
Abstract
1. Introduction
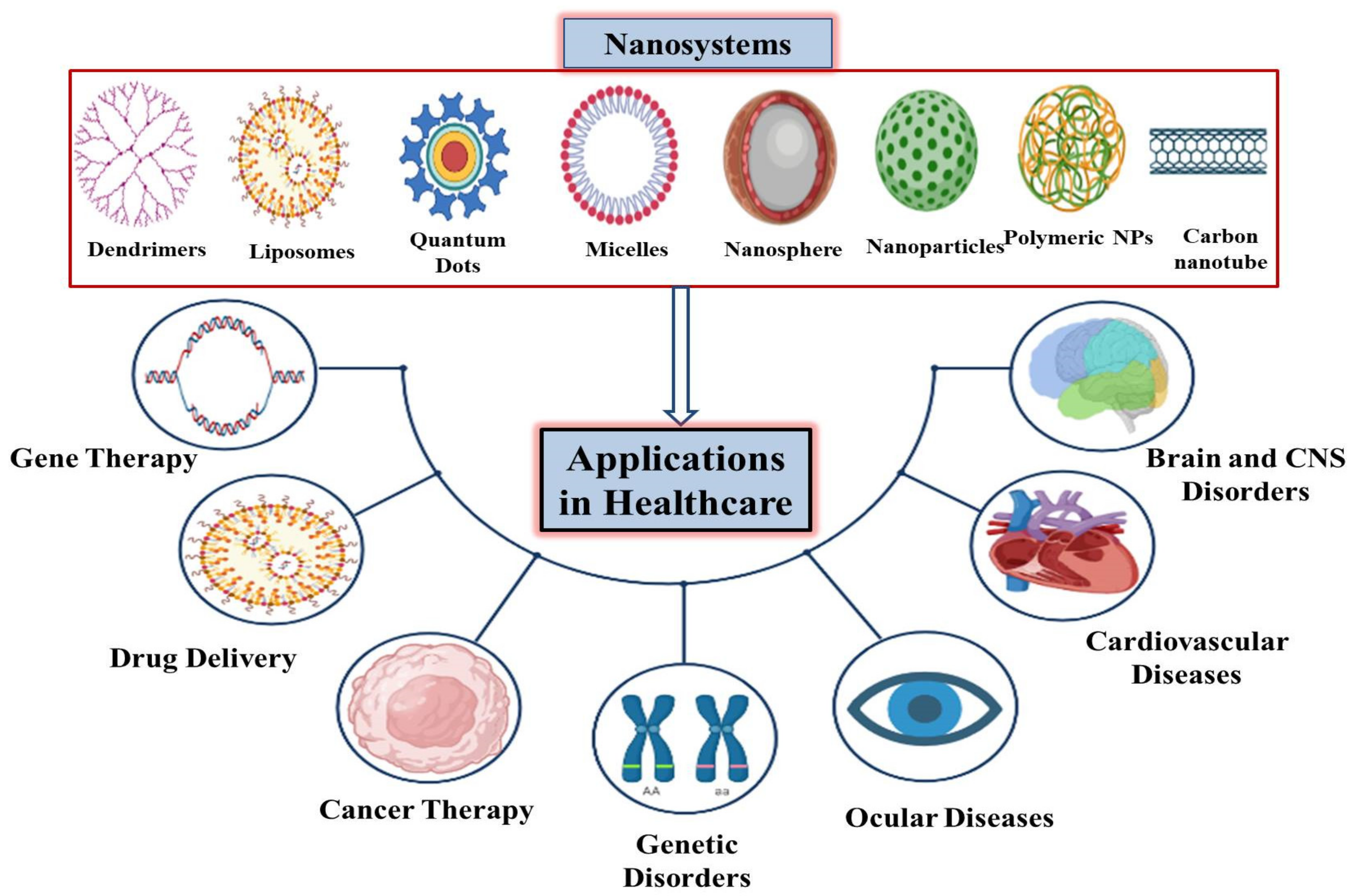
2. Nanosystems Used in Various Healthcare Sectors
| Nanostructure | Applications in Health Sector | References |
|---|---|---|
| Nanoparticles | Used as antimicrobials and antifungals; used as sensors, as catalysts, and for imaging in diagnostics | [5,21] |
| Carbon Nanotubes | Used for delivering fibrinogen and bovine protein to cells; serve as vectors for gene delivery; and in the treatment of broken bones, osteoporosis, and breast cancer | [26,27,46,47,48,49] |
| Dendrimers | Used for diagnostic applications, for gene delivery, as anti-bacterial agents, as anticancer drugs, to improve vaccine formulations by acting as carriers of antigens, and in treating ocular diseases. | [32] |
| Nano-Diamonds | Used for the treatment of bone disease by targeted drug delivery (bone regeneration); used in imaging and therapy, in the early detection of cancer, and in the treatment of brain and breast cancers | [40,50,51] |
| Quantum Dots | Useful in diagnostics, real time in vivo bio-imaging, in controlling various diseases, intracellular tracking and therapeutic drug delivery, and to deliver siRNA for RNA interference | [52,53] |
| Nanofilms | Act as useful biological, chemical and nanomechanical sensors in electrochemical devices, used for controlled drug release, used as nanopatches after open surgery to close incisions | [43,44,45,54,55,56,57,58] |
| Liposomes | Used for drug delivery, capable of containing hydrophobic and hydrophilic drugs, protect drugs from chemical and enzymatic degradation, have the ability to encapsulate anti-tumoral drugs, for example, anthracyclines such as epirubicin, daunorubicin, and Dox, etc. | [59,60,61,62,63] |
3. Applications of Nanotechnology in Healthcare Sectors
3.1. Role of Nanotechnology in Gene Therapy
3.2. The Role of Nanotechnology in Targeted Drug Delivery
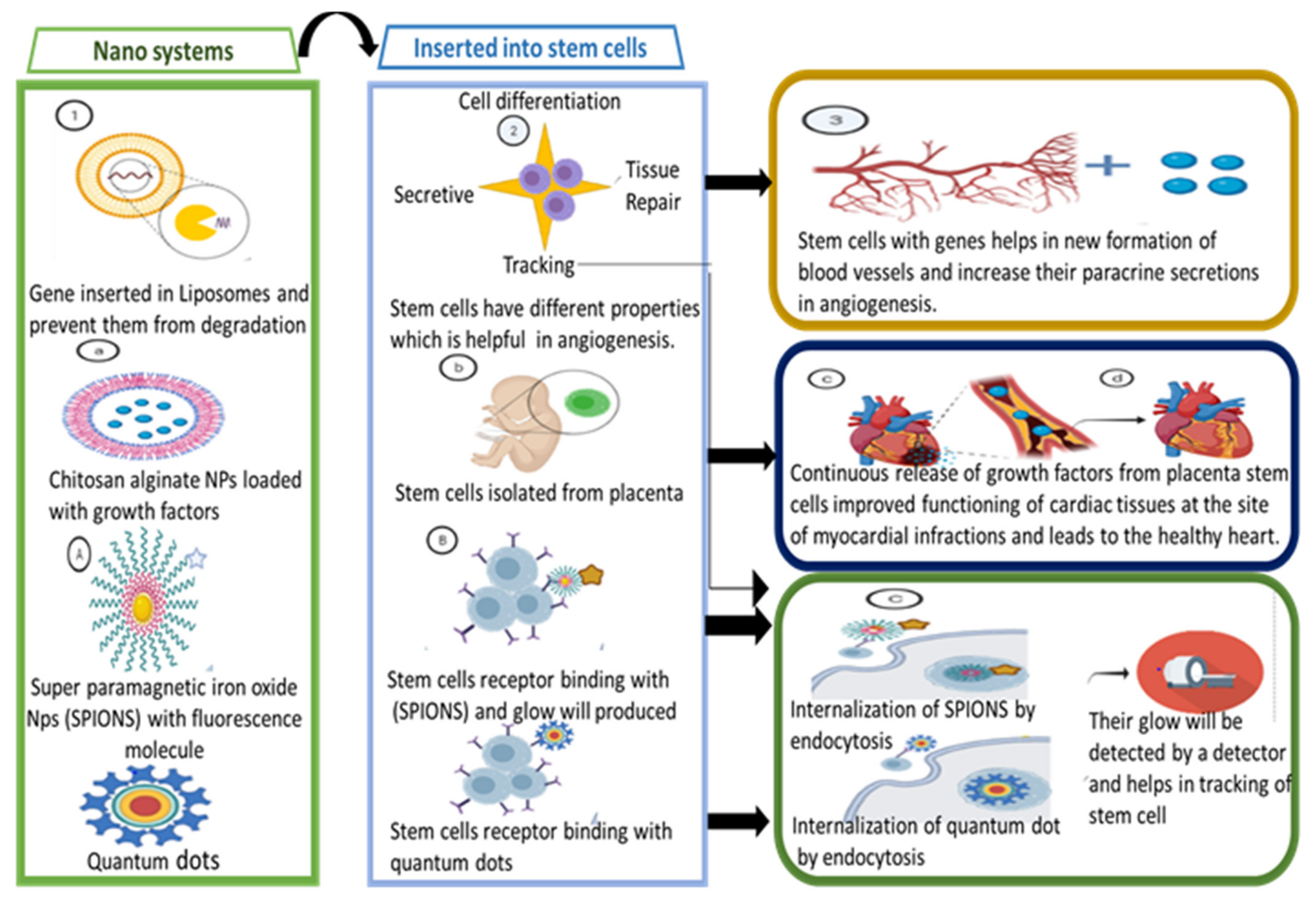
3.3. Treating Cardiovascular Diseases through Nanosystems
3.4. Nanotechnology in the Treatment of Ocular Diseases
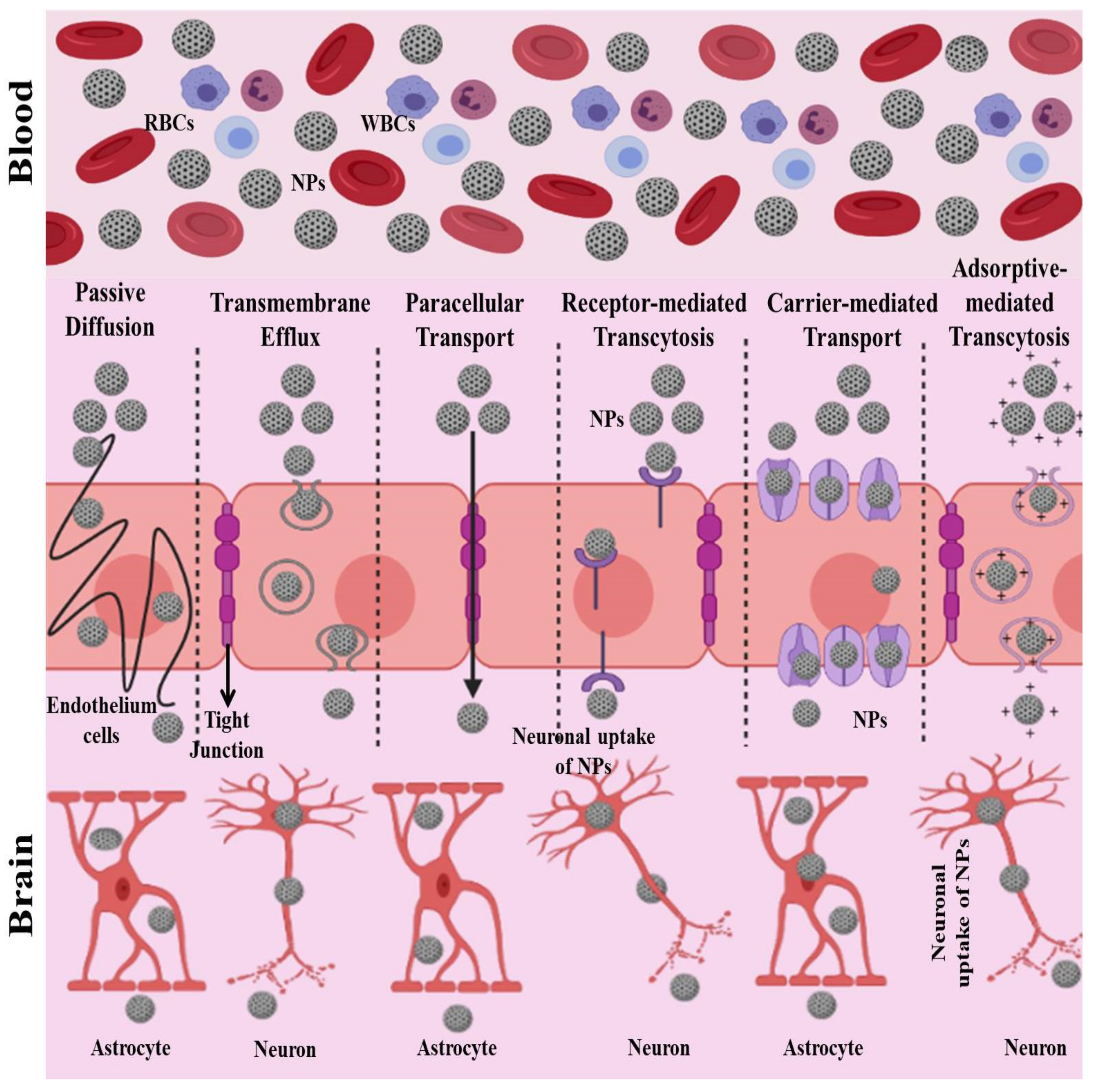
3.5. Nanotechnology in the Treatment of Brain Diseases
- The paracellular pathway and passive transmembrane diffusion;
- Transport proteins: carrier-mediated transport and efflux proteins;
- Receptor-mediated transcytosis;
- Adsorptive-mediated transcytosis.
3.6. Role of Nanotechnology in Cancer Diagnosis and Treatment
3.6.1. The Utilization of Different Methods Involving Nanotechnology in Cancer Diagnosis
3.6.2. Different Methods Used for Cancer Treatment
3.6.3. Targeting the Cancerous Micro-Environment
3.6.4. Targeting Drug-Resistant Tumors
3.6.5. Personalized Therapy for Cancer
3.6.6. Cancer Treatment through Thermal Ablation
3.7. Nanotechnology in the Treatment of Genetic Disorders
3.7.1. Alzheimer’s Disease
3.7.2. Parkinson’s Disease
3.7.3. Amyotrophic Lateral Sclerosis
3.7.4. Huntington’s Disease
3.7.5. Cystic Fibrosis
3.8. Nanotechnology in the Treatment of Nervous System Diseases
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hobson, D.W. The commercialization of medical nanotechnology for medical applications. In Intracellular Delivery III; Springer: Berlin/Heidelberg, Germany, 2016; pp. 405–449. [Google Scholar]
- Sindhwani, S.; Chan, W.C. Nanotechnology for modern medicine: Next step towards clinical translation. J. Intern. Med. 2021. [Google Scholar] [CrossRef]
- Laouini, S.; Bouafia, A.; Tedjani, M. Catalytic Activity for Dye Degradation and Characterization of Silver/Silver Oxide Nanoparticles Green Synthesized by Aqueous Leaves Extract of Phoenix Dactylifera L. Res. Sq. 2021, in press. [Google Scholar] [CrossRef]
- Agarwal, H.; Kumar, S.V.; Rajeshkumar, S. A review on green synthesis of zinc oxide nanoparticles–An eco-friendly approach. Resour. Effic. Technol. 2017, 3, 406–413. [Google Scholar] [CrossRef]
- Bogutska, K.; Sklyarov, Y.P.; Prylutskyy, Y.I. Zinc and zinc nanoparticles: Biological role and application in biomedicine. Ukr. Bioorg. Acta 2013, 1, 9–16. [Google Scholar]
- Baker, J.R.; Brent, B.W., Jr.; Thomas, T.P. Nanotechnology in Clinical and Translational Research. In Clinical and Translational Science; Elsevier: Amsterdam, The Netherlands, 2009; pp. 123–135. [Google Scholar]
- El Shafey, A.M. Green synthesis of metal and metal oxide nanoparticles from plant leaf extracts and their applications: A review. Green Process. Synth. 2020, 9, 304–339. [Google Scholar] [CrossRef]
- Madamsetty, V.S.; Mukherjee, A.; Mukherjee, S. Recent trends of the bio-inspired nanoparticles in cancer theranostics. Front. Pharmacol. 2019, 10, 1264. [Google Scholar] [CrossRef] [PubMed]
- Hatami, A.; Heydarinasab, A.; Akbarzadehkhiyavi, A.; Pajoum Shariati, F. An Introduction to Nanotechnology and Drug Delivery. Chem. Methodol. 2021, 5, 153–165. [Google Scholar]
- Rizvi, S.A.; Saleh, A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm. J. 2018, 26, 64–70. [Google Scholar] [CrossRef]
- Sahu, A.N. Nanotechnology in herbal medicines and cosmetics. Int. J. Res. Ayurveda Pharm. 2013, 4, 472–474. [Google Scholar] [CrossRef]
- Roco, M.C. National nanotechnology initiative-past, present, future. Handb. Nanosci. Eng. Technol. 2007, 2, 39. [Google Scholar]
- Subramani, K.; Ahmed, W. Emerging Nanotechnologies in Dentistry; William Andrew: Norwich, NY, USA, 2017. [Google Scholar]
- Khan, A.U.; Khan, M.; Cho, M.H.; Khan, M.M. Selected nanotechnologies and nanostructures for drug delivery, nanomedicine and cure. Bioprocess Biosyst. Eng. 2020, 43, 1339–1357. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, A.; Giuliano, E.; Eeda, V.; Fresta, M.; Bulotta, S.; Awasthi, V.; Cosco, D. Biodegradable polymeric nanoparticles for drug delivery to solid tumors. Front. Pharmacol. 2021, 12, 601626. [Google Scholar] [CrossRef]
- Shiku, H.; Wang, L.; Ikuta, Y.; Okugawa, T.; Schmitt, M.; Gu, X.; Akiyoshi, K.; Sunamoto, J.; Nakamura, H. Development of a cancer vaccine: Peptides, proteins, and DNA. Cancer Chemother. Pharmacol. 2000, 46, S77–S82. [Google Scholar] [CrossRef] [PubMed]
- Saul, J.M.; Annapragada, A.V.; Bellamkonda, R.V. A dual-ligand approach for enhancing targeting selectivity of therapeutic nanocarriers. J. Control. Release 2006, 114, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Prajnamitra, R.P.; Chen, H.-C.; Lin, C.-J.; Chen, L.-L.; Hsieh, P.C.-H. Nanotechnology approaches in tackling cardiovascular diseases. Molecules 2019, 24, 2017. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Khan, I.; Khan, S.A.; Sohail, M.; Ahmed, R.; ur Rehman, A.; Ansari, M.S.; Morsy, M.A. Electrocatalytic performance of Ni@ Pt core–shell nanoparticles supported on carbon nanotubes for methanol oxidation reaction. J. Electroanal. Chem. 2017, 795, 17–25. [Google Scholar] [CrossRef]
- Thomas, S.C.; Kumar Mishra, P.; Talegaonkar, S. Ceramic nanoparticles: Fabrication methods and applications in drug delivery. Curr. Pharm. Des. 2015, 21, 6165–6188. [Google Scholar] [CrossRef]
- So, W.C.; Kita, S.; Goldin-Meadow, S. Using the hands to identify who does what to whom: Gesture and speech go hand-in-hand. Cogn. Sci. 2009, 33, 115–125. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Vickers, N.J. Animal communication: When i’m calling you, will you answer too? Curr. Biol. 2017, 27, R713–R715. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Hu, H.; Mandal, S.K.; Haddon, R.C. A bone mimic based on the self-assembly of hydroxyapatite on chemically functionalized single-walled carbon nanotubes. Chem. Mater. 2005, 17, 3235–3241. [Google Scholar] [CrossRef]
- Dey, P.; Das, N. Carbon Nanotubes: It’s role in modern health care. Int. J. Pharm. Pharm. Sci 2013, 5, 9–13. [Google Scholar]
- Kesharwani, P.; Jain, K.; Jain, N.K. Dendrimer as nanocarrier for drug delivery. Prog. Polym. Sci. 2014, 39, 268–307. [Google Scholar] [CrossRef]
- Kannan, R.; Nance, E.; Kannan, S.; Tomalia, D.A. Emerging concepts in dendrimer-based nanomedicine: From design principles to clinical applications. J. Intern. Med. 2014, 276, 579–617. [Google Scholar] [CrossRef] [PubMed]
- Tolia, G.T.; Choi, H.H. The role of dendrimers in topical drug delivery. Pharm. Technol. 2008, 32, 88–98. [Google Scholar]
- Semwal, R.; Semwal, D.; Madan, A.; Paul, P.; Mujaffer, F.; Badoni, R. Dendrimers: A novel approach for drug targeting. J. Pharm. Res. 2010, 3, 2238–2247. [Google Scholar]
- Kalomiraki, M.; Thermos, K.; Chaniotakis, N.A. Dendrimers as tunable vectors of drug delivery systems and biomedical and ocular applications. Int. J. Nanomed. 2016, 11, 1–12. [Google Scholar]
- Madaan, K.; Kumar, S.; Poonia, N.; Lather, V.; Pandita, D. Dendrimers in drug delivery and targeting: Drug-dendrimer interactions and toxicity issues. J. Pharm. Bioallied Sci. 2014, 6, 139–150. [Google Scholar] [PubMed]
- Pillai, O.; Panchagnula, R. Polymers in drug delivery. Curr. Opin. Chem. Biol. 2001, 5, 447–451. [Google Scholar] [CrossRef]
- D’Emanuele, A.; Attwood, D. Dendrimer–drug interactions. Adv. Drug Deliv. Rev. 2005, 57, 2147–2162. [Google Scholar] [CrossRef] [PubMed]
- Twibanire, J.-d.A.K.; Grindley, T.B. Efficient and controllably selective preparation of esters using uronium-based coupling agents. Org. Lett. 2011, 13, 2988–2991. [Google Scholar] [CrossRef]
- Aulenta, F.; Hayes, W.; Rannard, S. Dendrimers: A new class of nanoscopic containers and delivery devices. Eur. Polym. J. 2003, 39, 1741–1771. [Google Scholar] [CrossRef]
- Chaudhary, A.; Welch, J.O.; Jackman, R.B. Electrical properties of monodispersed detonation nanodiamonds. Appl. Phys. Lett. 2010, 96, 242903. [Google Scholar] [CrossRef]
- Chauhan, S.; Jain, N.; Nagaich, U. Nanodiamonds with powerful ability for drug delivery and biomedical applications: Recent updates on in vivo study and patents. J. Pharm. Anal. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Slocombe, D.; Porch, A.; Bustarret, E.; Williams, O.A. Microwave properties of nanodiamond particles. Appl. Phys. Lett. 2013, 102, 244102. [Google Scholar] [CrossRef]
- Krueger, A. New carbon materials: Biological applications of functionalized nanodiamond materials. Chem. A Eur. J. 2008, 14, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.J.; Leon, L.; Rinaldi, C. Nanoparticles for Biomedical Applications: Fundamental Concepts, Biological Interactions and Clinical Applications; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Skwarczynski, M.; Toth, I. Micro- and Nanotechnology in Vaccine Development; William Andrew: Norwich, NY, USA, 2016. [Google Scholar]
- Lvov, Y.; Ariga, K.; Ichinose, I.; Kunitake, T. Assembly of multicomponent protein films by means of electrostatic layer-by-layer adsorption. J. Am. Chem. Soc. 1995, 117, 6117–6123. [Google Scholar] [CrossRef]
- Fujie, T.; Okamura, Y.; Takeoka, S. Ubiquitous transference of a free-standing polysaccharide nanosheet with the development of a nano-adhesive plaster. Adv. Mater. 2007, 19, 3549–3553. [Google Scholar] [CrossRef]
- Murugesan, S.; Mousa, S.; Vijayaraghavan, A.; Ajayan, P.M.; Linhardt, R.J. Ionic liquid-derived blood-compatible composite membranes for kidney dialysis. J. Biomed. Mater. Res. Part B 2006, 79, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Balakumar, K.; Rajkumar, M.; Raghavan, C.V.; Tamilselvan, N.; Dineshkumar, B. Carbon nanotubes: A versatile technique for drug delivery. Int. J. Nanomater. Biostruct. 2012, 2, 55–59. [Google Scholar]
- Kam, N.W.S.; Dai, H. Carbon nanotubes as intracellular protein transporters: Generality and biological functionality. J. Am. Chem. Soc. 2005, 127, 6021–6026. [Google Scholar] [CrossRef] [PubMed]
- Endo, M.; Strano, M.S.; Ajayan, P.M. Potential applications of carbon nanotubes. Carbon Nanotub. 2007, 11, 13–62. [Google Scholar]
- Gupta, C.; Prakash, D.; Gupta, S. Cancer treatment with nano-diamonds. Front. Biosci. 2017, 9, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Duan, H.; Mohs, A.M.; Nie, S. Bioconjugated quantum dots for in vivo molecular and cellular imaging. Adv. Drug Deliv. Rev. 2008, 60, 1226–1240. [Google Scholar] [CrossRef]
- Zhao, M.-X.; Zhu, B.-J. The research and applications of quantum dots as nano-carriers for targeted drug delivery and cancer therapy. Nanoscale Res. Lett. 2016, 11, 207. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Green synthesis, biomedical and biotechnological applications of carbon and gra-phene quantum dots. A review. Environ. Chem. Lett. 2020, 18, 703–727. [Google Scholar] [CrossRef]
- Tsukruk, V.; Bliznyuk, V.; Visser, D.; Campbell, A.; Bunning, T.; Adams, W. Electrostatic deposition of polyionic monolayers on charged surfaces. Macromolecules 1997, 30, 6615–6625. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, Y.; Podsiadlo, P.; Kotov, N.A. Biomedical applications of layer-by-layer assembly: From biomimetics to tissue engineering. Adv. Mater. 2006, 18, 3203–3224. [Google Scholar] [CrossRef]
- Okamura, Y.; Utsunomiya, S.; Suzuki, H.; Niwa, D.; Osaka, T.; Takeoka, S. Fabrication of free-standing nanoparticle-fused nanosheets and their hetero-modification using sacrificial film. Colloids Surf. A Physicochem. Eng. Asp. 2008, 318, 184–190. [Google Scholar] [CrossRef]
- Okamura, Y.; Kabata, K.; Kinoshita, M.; Saitoh, D.; Takeoka, S. Free-standing biodegradable poly (lactic acid) nanosheet for sealing operations in surgery. Adv. Mater. 2009, 21, 4388–4392. [Google Scholar] [CrossRef]
- Fujie, T.; Matsutani, N.; Kinoshita, M.; Okamura, Y.; Saito, A.; Takeoka, S. Adhesive, flexible, and robust polysaccharide nanosheets integrated for tissue-defect repair. Adv. Funct. Mater. 2009, 19, 2560–2568. [Google Scholar] [CrossRef]
- Felice, B.; Prabhakaran, M.P.; Rodriguez, A.P.; Ramakrishna, S. Drug delivery vehicles on a nano-engineering perspective. Mater. Sci. Eng. C 2014, 41, 178–195. [Google Scholar] [CrossRef]
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomed. 2006, 1, 297–315. [Google Scholar]
- Mohan, A.; Narayanan, S.; Sethuraman, S.; Krishnan, U.M. Novel resveratrol and 5-fluorouracil coencapsulated in PEGylated nanoliposomes improve chemotherapeutic efficacy of combination against head and neck squamous cell carcinoma. BioMed Res. Int. 2014, 2014, 424239. [Google Scholar] [CrossRef]
- Drummond, D.C.; Meyer, O.; Hong, K.; Kirpotin, D.B.; Papahadjopoulos, D. Optimizing liposomes for delivery of chemotherapeutic agents to solid tumors. Pharmacol. Rev. 1999, 51, 691–744. [Google Scholar] [PubMed]
- Lao, J.; Madani, J.; Puértolas, T.; Álvarez, M.; Hernández, A.; Pazo-Cid, R.; Artal, Á.; Antón Torres, A. Liposomal doxorubicin in the treatment of breast cancer patients: A review. J. Drug Deliv. 2013, 2013, 456409. [Google Scholar] [CrossRef] [PubMed]
- Misra, S. Human gene therapy: A brief overview of the genetic revolution. J. Assoc. Physicians India 2013, 61, 127–133. [Google Scholar] [PubMed]
- Gardlík, R.; Pálffy, R.; Hodosy, J.; Lukács, J.; Turna, J.; Celec, P. Vectors and delivery systems in gene therapy. Med Sci. Monit. 2005, 11, RA110–RA121. [Google Scholar]
- Kay, M.A. State-of-the-art gene-based therapies: The road ahead. Nat. Rev. Genet. 2011, 12, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Verma, I.M.; Weitzman, M.D. Gene therapy: Twenty-first century medicine. Annu. Rev. Biochem. 2005, 74, 711–738. [Google Scholar] [CrossRef]
- Lundstrom, K. Latest development in viral vectors for gene therapy. Trends Biotechnol. 2003, 21, 117–122. [Google Scholar] [CrossRef]
- Ye, K.; Jin, S. Potent and specific inhibition of retrovirus production by coexpression of multiple siRNAs directed against different regions of viral genomes. Biotechnol. Prog. 2006, 22, 45–52. [Google Scholar] [CrossRef]
- Oka, M.; Chang, L.-J.; Costantini, F.; Terada, N. Lentiviral vector-mediated gene transfer in embryonic stem cells. In Embryonic Stem Cell Protocols; Springer: Berlin/Heidelberg, Germany, 2006; pp. 273–281. [Google Scholar]
- Fedorova, E.; Battini, L.; Prakash-Cheng, A.; Marras, D.; Gusella, G.L. Lentiviral gene delivery to CNS by spinal intrathecal administration to neonatal mice. J. Gene Med. 2006, 8, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Dodart, J.-C.; Marr, R.A.; Koistinaho, M.; Gregersen, B.M.; Malkani, S.; Verma, I.M.; Paul, S.M. Gene delivery of human apolipoprotein E alters brain Abeta burden in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2005, 102, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Naldini, L.; Blömer, U.; Gage, F.H.; Trono, D.; Verma, I.M. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc. Natl. Acad. Sci. USA 1996, 93, 11382–11388. [Google Scholar] [CrossRef]
- Julie, H.Y.; Schaffer, D.V. Advanced targeting strategies for murine retroviral and adeno-associated viral vectors. Gene Ther. Gene Deliv. Syst. 2005, 99, 147–167. [Google Scholar]
- Roberts, D.M.; Nanda, A.; Havenga, M.J.; Abbink, P.; Lynch, D.M.; Ewald, B.A.; Liu, J.; Thorner, A.R.; Swanson, P.E.; Gorgone, D.A. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature 2006, 441, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Glasgow, J.N.; Bauerschmitz, G.J.; Curiel, D.T.; Hemminki, A. Transductional and transcriptional targeting of adenovirus for clinical applications. Curr. Gene Ther. 2004, 4, 1–14. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Sen, G.C. Tyrosine phosphorylation in Toll-like receptor signaling. Cytokine Growth Factor Rev. 2014, 25, 533–541. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Sen, G.C. RIG-I-like receptor-induced IRF3 mediated pathway of apoptosis (RIPA): A new antiviral pathway. Protein Cell 2017, 8, 165–168. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Sen, G.C. dsRNA-activation of TLR3 and RLR signaling: Gene induction-dependent and independent effects. J. Interferon Cytokine Res. 2014, 34, 427–436. [Google Scholar] [CrossRef]
- Fensterl, V.; Chattopadhyay, S.; Sen, G.C. No love lost between viruses and interferons. Annu. Rev. Virol. 2015, 2, 549–572. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, G.; Kuzmanovic, T.; Zhang, Y.; Peter, C.B.; Veleeparambil, M.; Chakravarti, R.; Sen, G.C.; Chattopadhyay, S. A new mechanism of interferon’s antiviral action: Induction of autophagy, essential for paramyxovirus replication, is inhibited by the interferon stimulated gene, TDRD7. PLoS Pathog. 2018, 14, e1006877. [Google Scholar] [CrossRef] [PubMed]
- Stolberg, S.G. The biotech death of Jesse Gelsinger. N. Y. Times Mag. 1999, 28, 136–140. [Google Scholar]
- Goswami, R.; Subramanian, G.; Silayeva, L.; Newkirk, I.; Doctor, D.; Chawla, K.; Chattopadhyay, S.; Chandra, D.; Chilukuri, N.; Betapudi, V. Gene therapy leaves a vicious cycle. Front. Oncol. 2019, 9, 297. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Wang, K.; He, X.; Zhao, X.J.; Drake, T.; Wang, L.; Bagwe, R.P. Bionanotechnology based on silica nanoparticles. Med. Res. Rev. 2004, 24, 621–638. [Google Scholar] [CrossRef]
- Singh, M.; Briones, M.; Ott, G.; O’Hagan, D. Cationic microparticles: A potent delivery system for DNA vaccines. Proc. Natl. Acad. Sci. USA 2000, 97, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Koreeda, N.; Yamashita, F.; Takakura, Y.; Hashida, M. Effect of particle size and charge on the disposition of lipid carriers after intratumoral injection into tissue-isolated tumors. Pharm. Res. 1998, 15, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Kole, R.; Krainer, A.R.; Altman, S. RNA therapeutics: Beyond RNA interference and antisense oligonucleotides. Nat. Rev. Drug Discov. 2012, 11, 125–140. [Google Scholar] [CrossRef]
- Martinez, J.; Patkaniowska, A.; Urlaub, H.; Lührmann, R.; Tuschl, T. Single-stranded antisense siRNAs guide target RNA cleavage in RNAi. Cell 2002, 110, 563–574. [Google Scholar] [CrossRef]
- Elbashir, S.M.; Harborth, J.; Lendeckel, W.; Yalcin, A.; Weber, K.; Tuschl, T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 2001, 411, 494–498. [Google Scholar] [CrossRef]
- Toub, N.; Malvy, C.; Fattal, E.; Couvreur, P. Innovative nanotechnologies for the delivery of oligonucleotides and siRNA. Biomed. Pharmacother. 2006, 60, 607–620. [Google Scholar] [CrossRef]
- Putnam, D.; Gentry, C.A.; Pack, D.W.; Langer, R. Polymer-based gene delivery with low cytotoxicity by a unique balance of side-chain termini. Proc. Natl. Acad. Sci. USA 2001, 98, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Pillé, J.-Y.; Li, H.; Blot, E.; Bertrand, J.-R.; Pritchard, L.-L.; Opolon, P.; Maksimenko, A.; Lu, H.; Vannier, J.-P.; Soria, J. Intravenous delivery of anti-RhoA small interfering RNA loaded in nanoparticles of chitosan in mice: Safety and efficacy in xenografted aggressive breast cancer. Hum. Gene Ther. 2006, 17, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Lee, A.; Hwang, S.R.; Park, J.-S.; Jang, J.; Huh, M.S.; Jo, D.-G.; Yoon, S.-Y.; Byun, Y.; Kim, S.H. TNF-α gene silencing using polymerized siRNA/thiolated glycol chitosan nanoparticles for rheumatoid arthritis. Mol. Ther. 2014, 22, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Kaul, G.; Amiji, M. Tumor-targeted gene delivery using poly (ethylene glycol)-modified gelatin nanoparticles: In vitro and in vivo studies. Pharm. Res. 2005, 22, 951–961. [Google Scholar] [CrossRef]
- Kneuer, C.; Sameti, M.; Bakowsky, U.; Schiestel, T.; Schirra, H.; Schmidt, H.; Lehr, C.-M. A nonviral DNA delivery system based on surface modified silica-nanoparticles can efficiently transfect cells in vitro. Bioconjugate Chem. 2000, 11, 926–932. [Google Scholar] [CrossRef]
- Kneuer, C.; Sameti, M.; Haltner, E.G.; Schiestel, T.; Schirra, H.; Schmidt, H.; Lehr, C.-M. Silica nanoparticles modified with aminosilanes as carriers for plasmid DNA. Int. J. Pharm. 2000, 196, 257–261. [Google Scholar] [CrossRef]
- Reszka, R.; Zhu, J.-H.; Weber, F.; Walther, W.; Greferath, R.; Dyballa, S. Liposome mediated transfer of marker and cytokine genes into rat and human glioblastoma cells in vitro and in vivo. J. Liposome Res. 1995, 5, 149–167. [Google Scholar] [CrossRef]
- Junghans, M.; Kreuter, J.; Zimmer, A. Antisense delivery using protamine–oligonucleotide particles. Nucleic Acids Res. 2000, 28, e45. [Google Scholar] [CrossRef] [PubMed]
- Schwab, G.; Chavany, C.; Duroux, I.; Goubin, G.; Lebeau, J.; Helene, C.; Saison-Behmoaras, T. Antisense oligonucleotides adsorbed to polyalkylcyanoacrylate nanoparticles specifically inhibit mutated Ha-ras-mediated cell proliferation and tumorigenicity in nude mice. Proc. Natl. Acad. Sci. USA 1994, 91, 10460–10464. [Google Scholar] [CrossRef]
- Erbacher, P.; Zou, S.; Bettinger, T.; Steffan, A.-M.; Remy, J.-S. Chitosan-based vector/DNA complexes for gene delivery: Biophysical characteristics and transfection ability. Pharm. Res. 1998, 15, 1332–1339. [Google Scholar] [CrossRef]
- Zheng, G.; Chen, J.; Li, H.; Glickson, J.D. Rerouting lipoprotein nanoparticles to selected alternate receptors for the targeted delivery of cancer diagnostic and therapeutic agents. Proc. Natl. Acad. Sci. USA 2005, 102, 17757–17762. [Google Scholar] [CrossRef]
- Hattori, Y.; Maitani, Y. Folate-linked lipid-based nanoparticle for targeted gene delivery. Curr. Drug Deliv. 2005, 2, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Maitani, Y. Enhanced in vitro DNA transfection efficiency by novel folate-linked nanoparticles in human prostate cancer and oral cancer. J. Control. Release 2004, 97, 173–183. [Google Scholar] [CrossRef]
- Kukowska-Latallo, J.F.; Candido, K.A.; Cao, Z.; Nigavekar, S.S.; Majoros, I.J.; Thomas, T.P.; Balogh, L.P.; Khan, M.K.; Baker, J.R. Nanoparticle targeting of anticancer drug improves therapeutic response in animal model of human epithelial cancer. Cancer Res. 2005, 65, 5317–5324. [Google Scholar] [CrossRef]
- Dixit, V.; Van den Bossche, J.; Sherman, D.M.; Thompson, D.H.; Andres, R.P. Synthesis and grafting of thioctic acid− PEG− folate conjugates onto Au nanoparticles for selective targeting of folate receptor-positive tumor cells. Bioconjugate Chem. 2006, 17, 603–609. [Google Scholar] [CrossRef]
- Gu, X.-G.; Schmitt, M.; Hiasa, A.; Nagata, Y.; Ikeda, H.; Sasaki, Y.; Akiyoshi, K.; Sunamoto, J.; Nakamura, H.; Kuribayashi, K. A novel hydrophobized polysaccharide/oncoprotein complex vaccine induces in vitro and in vivo cellular and humoral immune responses against HER2-expressing murine sarcomas. Cancer Res. 1998, 58, 3385–3390. [Google Scholar] [PubMed]
- Calin, M.; Stan, D.; Simion, V. Stem cell regenerative potential combined with nanotechnology and tissue engineering for myocardial regeneration. Curr. Stem Cell Res. Ther. 2013, 8, 292–303. [Google Scholar] [CrossRef][Green Version]
- Van der Spoel, T.I.; Jansen of Lorkeers, S.J.; Agostoni, P.; van Belle, E.; Gyöngyösi, M.; Sluijter, J.P.; Cramer, M.J.; Doevendans, P.A.; Chamuleau, S.A. Human relevance of pre-clinical studies in stem cell therapy: Systematic review and meta-analysis of large animal models of ischaemic heart disease. Cardiovasc. Res. 2011, 91, 649–658. [Google Scholar] [CrossRef]
- Deuse, T.; Peter, C.; Fedak, P.W.; Doyle, T.; Reichenspurner, H.; Zimmermann, W.H.; Eschenhagen, T.; Stein, W.; Wu, J.C.; Robbins, R.C. Hepatocyte growth factor or vascular endothelial growth factor gene transfer maximizes mesenchymal stem cell–based myocardial salvage after acute myocardial infarction. Circulation 2009, 120, S247–S254. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wang, J.; Yang, J.; Kong, X.; Zheng, F.; Guo, L.; Zhang, L.; Huang, Y. Mesenchymal stem cells over-expressing SDF-1 promote angiogenesis and improve heart function in experimental myocardial infarction in rats. Eur. J. Cardio Thorac. Surg. 2009, 36, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Pack, D.; Hoffman, A.; Pun, S.; Stayton, P.S. Design and development of polymers for gene delivery. Nat. Rev. Drug Discov. 2005, 4, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gu, F.; Chan, J.; Wang, A.; Langer, R.; Farokhzad, O. Clinical Pharmacol. Therapeutics 2008, 83, 761. [Google Scholar]
- Cui, Z.-K.; Fan, J.; Kim, S.; Bezouglaia, O.; Fartash, A.; Wu, B.M.; Aghaloo, T.; Lee, M. Delivery of siRNA via cationic Sterosomes to enhance osteogenic differentiation of mesenchymal stem cells. J. Control. Release 2015, 217, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Binsalamah, Z.M.; Paul, A.; Khan, A.A.; Prakash, S.; Shum-Tim, D. Intramyocardial sustained delivery of placental growth factor using nanoparticles as a vehicle for delivery in the rat infarct model. Int. J. Nanomed. 2011, 6, 2667. [Google Scholar]
- Frank, J.A.; Zywicke, H.; Jordan, E.; Mitchell, J.; Lewis, B.K.; Miller, B.; Bryant, L.H.; Bulte, J.W. Magnetic intracellular labeling of mammalian cells by combining (FDA-approved) superparamagnetic iron oxide MR contrast agents and commonly used transfection agents. Acad. Radiol. 2002, 9, S484–S487. [Google Scholar] [CrossRef]
- Ricles, L.M.; Nam, S.Y.; Sokolov, K.; Emelianov, S.Y.; Suggs, L.J. Function of mesenchymal stem cells following loading of gold nanotracers. Int. J. Nanomed. 2011, 6, 407–416. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Z.; Sun, J.; Ma, T.; Hua, F.; Shen, Z. A brief review of cytotoxicity of nanoparticles on mesenchymal stem cells in regenerative medicine. Int. J. Nanomed. 2019, 14, 3875–3892. [Google Scholar] [CrossRef] [PubMed]
- Alam, T.; Khan, S.; Gaba, B.; Haider, M.F.; Baboota, S.; Ali, J. Nanocarriers as treatment modalities for hypertension. Drug Deliv. 2017, 24, 358–369. [Google Scholar] [CrossRef]
- Sharma, M.; Sharma, R.; Jain, D.K. Nanotechnology based approaches for enhancing oral bioavailability of poorly water soluble antihypertensive drugs. Scientifica 2016, 2016, 8525679. [Google Scholar] [CrossRef]
- Gorain, B.; Choudhury, H.; Kundu, A.; Sarkar, L.; Karmakar, S.; Jaisankar, P.; Pal, T.K. Nanoemulsion strategy for olmesartan medoxomil improves oral absorption and extended antihypertensive activity in hypertensive rats. Colloids Surf. B 2014, 115, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-Y.; Hao, J.-L.; Wang, S.; Zheng, Y.; Zhang, W.-S. Nanoparticles in the ocular drug delivery. Int. J. Ophthalmol. 2013, 6, 390–396. [Google Scholar] [PubMed]
- Kaur, I.P.; Kakkar, S. Nanotherapy for posterior eye diseases. J. Control. Release 2014, 193, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.; Liu, J.; Jin, S.; Guo, W.; Liang, X.; Hu, Z. Nanotechnology-based strategies for treatment of ocular disease. Acta Pharm. Sin. B 2017, 7, 281–291. [Google Scholar] [CrossRef]
- Chew, E.Y.; Glassman, A.R.; Beck, R.W.; Bressler, N.M.; Fish, G.E.; Ferris, F.L.; Kinyoun, J.L.; Network, D.R.C.R. Ocular side effects associated with peribulbar injections of triamcinolone acetonide for diabetic macular edema. Retina 2011, 31, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Chen, J.; Gao, J. Nanocarriers as a powerful vehicle to overcome blood-brain barrier in treating neurodegenerative diseases: Focus on recent advances. Asian J. Pharm. Sci. 2019, 14, 480–496. [Google Scholar] [CrossRef]
- Saraiva, C.; Praça, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-mediated brain drug delivery: Overcoming blood–brain barrier to treat neurodegenerative diseases. J. Control. Release 2016, 235, 34–47. [Google Scholar] [CrossRef]
- Lombardo, S.M.; Schneider, M.; Türeli, A.E.; Türeli, N.G. Key for crossing the BBB with nanoparticles: The rational design. Beilstein J. Nanotechnol. 2020, 11, 866–883. [Google Scholar] [CrossRef]
- Sarmah, D.; Saraf, J.; Kaur, H.; Pravalika, K.; Tekade, R.K.; Borah, A.; Kalia, K.; Dave, K.R.; Bhattacharya, P. Stroke management: An emerging role of nanotechnology. Micromachines 2017, 8, 262. [Google Scholar] [CrossRef]
- Petri, B.; Bootz, A.; Khalansky, A.; Hekmatara, T.; Müller, R.; Uhl, R.; Kreuter, J.; Gelperina, S. Chemotherapy of brain tumour using doxorubicin bound to surfactant-coated poly (butyl cyanoacrylate) nanoparticles: Revisiting the role of surfactants. J. Control. Release 2007, 117, 51–58. [Google Scholar] [CrossRef]
- Gromnicova, R.; Davies, H.A.; Sreekanthreddy, P.; Romero, I.A.; Lund, T.; Roitt, I.M.; Phillips, J.B.; Male, D.K. Glucose-coated gold nanoparticles transfer across human brain endothelium and enter astrocytes in vitro. PLoS ONE 2013, 8, e81043. [Google Scholar] [CrossRef]
- Shilo, M.; Motiei, M.; Hana, P.; Popovtzer, R. Transport of nanoparticles through the blood–brain barrier for imaging and therapeutic applications. Nanoscale 2014, 6, 2146–2152. [Google Scholar] [CrossRef] [PubMed]
- Wiley, D.T.; Webster, P.; Gale, A.; Davis, M.E. Transcytosis and brain uptake of transferrin-containing nanoparticles by tuning avidity to transferrin receptor. Proc. Natl. Acad. Sci. USA 2013, 110, 8662–8667. [Google Scholar] [CrossRef]
- Guerrero, S.; Araya, E.; Fiedler, J.L.; Arias, J.I.; Adura, C.; Albericio, F.; Giralt, E.; Arias, J.L.; Fernández, M.S.; Kogan, M.J. Improving the brain delivery of gold nanoparticles by conjugation with an amphipathic peptide. Nanomedicine 2010, 5, 897–913. [Google Scholar] [CrossRef]
- Pautler, M.; Brenner, S. Nanomedicine: Promises and challenges for the future of public health. Int. J. Nanomed. 2010, 5, 803–809. [Google Scholar]
- Choi, C.H.J.; Alabi, C.A.; Webster, P.; Davis, M.E. Mechanism of active targeting in solid tumors with transferrin-containing gold nanoparticles. Proc. Natl. Acad. Sci. USA 2010, 107, 1235–1240. [Google Scholar] [CrossRef]
- Jain, K.K. Applications of nanobiotechnology in clinical diagnostics. Clin. Chem. 2007, 53, 2002–2009. [Google Scholar] [CrossRef]
- Jain, K. Advances in the field of nanooncology. BMC Med. 2010, 8, 1–11. [Google Scholar] [CrossRef]
- Misra, R.; Acharya, S.; Sahoo, S.K. Cancer nanotechnology: Application of nanotechnology in cancer therapy. Drug Discov. Today 2010, 15, 842–850. [Google Scholar] [CrossRef]
- Ferrari, M. Cancer nanotechnology: Opportunities and challenges. Nat. Rev. Cancer 2005, 5, 161–171. [Google Scholar] [CrossRef]
- Majumdar, A. Bioassays based on molecular nanomechanics. Dis. Markers 2002, 18, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.; Huang, D.; Zhao, Z.; Zhou, Z.; Yin, Z.; Gao, J. Nanoprobes for in vitro diagnostics of cancer and infectious diseases. Biomaterials 2012, 33, 189–206. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, F.; Dan, W.; Fu, Y.; Liu, S. Construction of carbon nanotube based nanoarchitectures for selective impedimetric detection of cancer cells in whole blood. Analyst 2014, 139, 5086–5092. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Mumper, R.J. Nanomedicinal strategies to treat multidrug-resistant tumors: Current progress. Nanomedicine 2010, 5, 597–615. [Google Scholar] [CrossRef]
- Satchi-Fainaro, R.; Mamluk, R.; Wang, L.; Short, S.M.; Nagy, J.A.; Feng, D.; Dvorak, A.M.; Dvorak, H.F.; Puder, M.; Mukhopadhyay, D. Inhibition of vessel permeability by TNP-470 and its polymer conjugate, caplostatin. Cancer Cell 2005, 7, 251–261. [Google Scholar] [CrossRef]
- Satchi-Fainaro, R.; Puder, M.; Davies, J.W.; Tran, H.T.; Sampson, D.A.; Greene, A.K.; Corfas, G.; Folkman, J. Targeting angiogenesis with a conjugate of HPMA copolymer and TNP-470. Nat. Med. 2004, 10, 255–261. [Google Scholar] [CrossRef]
- Sengupta, S.; Eavarone, D.; Capila, I.; Zhao, G.; Watson, N.; Kiziltepe, T.; Sasisekharan, R. Temporal targeting of tumour cells and neovasculature with a nanoscale delivery system. Nature 2005, 436, 568–572. [Google Scholar] [CrossRef]
- Chaudhuri, P.; Harfouche, R.; Soni, S.; Hentschel, D.M.; Sengupta, S. Shape effect of carbon nanovectors on angiogenesis. ACS Nano 2010, 4, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Harfouche, R.; Basu, S.; Soni, S.; Hentschel, D.M.; Mashelkar, R.A.; Sengupta, S. Nanoparticle-mediated targeting of phosphatidylinositol-3-kinase signaling inhibits angiogenesis. Angiogenesis 2009, 12, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Yigit, M.V.; Mazumdar, D.; Lu, Y. Molecular diagnostic and drug delivery agents based on aptamer-nanomaterial conjugates. Adv. Drug Deliv. Rev. 2010, 62, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, C.; Qian, M.; Zhao, X.S. Aptamer biosensor for protein detection using gold nanoparticles. Anal. Biochem. 2008, 373, 213–219. [Google Scholar] [CrossRef]
- Estévez, M.C.; Huang, Y.-F.; Kang, H.; O’Donoghue, M.B.; Bamrungsap, S.; Yan, J.; Chen, X.; Tan, W. Nanoparticle–aptamer conjugates for cancer cell targeting and detection. In Cancer Nanotechnology; Springer: Berlin/Heidelberg, Germany, 2010; pp. 235–248. [Google Scholar]
- Smith, J.E.; Medley, C.D.; Tang, Z.; Shangguan, D.; Lofton, C.; Tan, W. Aptamer-conjugated nanoparticles for the collection and detection of multiple cancer cells. Anal. Chem. 2007, 79, 3075–3082. [Google Scholar] [CrossRef]
- Wu, X.; Luo, L.; Yang, S.; Ma, X.; Li, Y.; Dong, C.; Tian, Y.; Zhang, L.; Shen, Z.; Wu, A. Improved SERS nanoparticles for direct detection of circulating tumor cells in the blood. ACS Appl. Mater. Interfaces 2015, 7, 9965–9971. [Google Scholar] [CrossRef]
- Gindy, M.E.; Prud’homme, R.K. Multifunctional nanoparticles for imaging, delivery and targeting in cancer therapy. Expert Opin. Drug Deliv. 2009, 6, 865–878. [Google Scholar] [CrossRef]
- Mansour, A.M.; Drevs, J.; Esser, N.; Hamada, F.M.; Badary, O.A.; Unger, C.; Fichtner, I.; Kratz, F. A new approach for the treatment of malignant melanoma: Enhanced antitumor efficacy of an albumin-binding doxorubicin prodrug that is cleaved by matrix metalloproteinase 2. Cancer Res. 2003, 63, 4062–4066. [Google Scholar] [PubMed]
- Hanahan, D.; Bergers, G.; Bergsland, E. Less is more, regularly: Metronomic dosing of cytotoxic drugs can target tumor angiogenesis in mice. J. Clin. Investig. 2000, 105, 1045–1047. [Google Scholar] [CrossRef]
- Pietras, K.; Hanahan, D. A multitargeted, metronomic, and maximum-tolerated dose “chemo-switch” regimen is antiangiogenic, producing objective responses and survival benefit in a mouse model of cancer. J. Clin. Oncol. 2005, 23, 939–952. [Google Scholar] [CrossRef]
- Gasparini, G. Metronomic scheduling: The future of chemotherapy? Lancet Oncol. 2001, 2, 733–740. [Google Scholar] [CrossRef]
- Won, Y.-Y.; Lee, H. “pH phoresis”: A new concept that can be used for improving drug delivery to tumor cells. J. Control. Release 2013, 170, 396–400. [Google Scholar] [CrossRef]
- Garg, A.; Tisdale, A.W.; Haidari, E.; Kokkoli, E. Targeting colon cancer cells using PEGylated liposomes modified with a fibronectin-mimetic peptide. Int. J. Pharm. 2009, 366, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Constantinides, P.P.; Wasan, K.M. Lipid formulation strategies for enhancing intestinal transport and absorption of P-glycoprotein (P-gp) substrate drugs: In vitro/in vivo case studies. J. Pharm. Sci. 2007, 96, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Dintaman, J.M.; Silverman, J.A. Inhibition of P-glycoprotein by D-α-tocopheryl polyethylene glycol 1000 succinate (TPGS). Pharm. Res. 1999, 16, 1550–1556. [Google Scholar] [CrossRef]
- Batrakova, E.V.; Li, S.; Vinogradov, S.V.; Alakhov, V.Y.; Miller, D.W.; Kabanov, A.V. Mechanism of pluronic effect on P-glycoprotein efflux system in blood-brain barrier: Contributions of energy depletion and membrane fluidization. J. Pharmacol. Exp. Ther. 2001, 299, 483–493. [Google Scholar]
- Batrakova, E.V.; Li, S.; Li, Y.; Alakhov, V.Y.; Kabanov, A.V. Effect of pluronic P85 on ATPase activity of drug efflux transporters. Pharm. Res. 2004, 21, 2226–2233. [Google Scholar] [CrossRef]
- Leroux, J.-C. Injectable nanocarriers for biodetoxification. Nat. Nanotechnol. 2007, 2, 679–684. [Google Scholar] [CrossRef]
- Alexis, F.; Pridgen, E.M.; Langer, R.; Farokhzad, O.C. Nanoparticle technologies for cancer therapy. Drug Deliv. 2010, 55–86. [Google Scholar] [CrossRef]
- Schmieder, A.H.; Winter, P.M.; Caruthers, S.D.; Harris, T.D.; Williams, T.A.; Allen, J.S.; Lacy, E.K.; Zhang, H.; Scott, M.J.; Hu, G. Molecular MR imaging of melanoma angiogenesis with ανβ3-targeted paramagnetic nanoparticles. Magn. Reson. Med. 2005, 53, 621–627. [Google Scholar] [CrossRef]
- Choi, Y.; Thomas, T.; Kotlyar, A.; Islam, M.T.; Baker, J.R., Jr. Synthesis and functional evaluation of DNA-assembled polyamidoamine dendrimer clusters for cancer cell-specific targeting. Chem. Biol. 2005, 12, 35–43. [Google Scholar] [CrossRef]
- Wang, M.D.; Shin, D.M.; Simons, J.W.; Nie, S. Nanotechnology for targeted cancer therapy. Expert Rev. Anticancer Ther. 2007, 7, 833–837. [Google Scholar] [CrossRef]
- Harisinghani, M.G.; Saini, S.; Weissleder, R.; Hahn, P.F.; Yantiss, R.K.; Tempany, C.; Wood, B.; Mueller, P. MR lymphangiography using ultrasmall superparamagnetic iron oxide in patients with primary abdominal and pelvic malignancies: Radiographic-pathologic correlation. Ajr. Am. J. Roentgenol. 1999, 172, 1347–1351. [Google Scholar] [CrossRef] [PubMed]
- Tsivgoulis, G.; Katsanos, A.H.; Alexandrov, A.V. Reperfusion therapies of acute ischemic stroke: Potentials and failures. Front. Neurol. 2014, 5, 215. [Google Scholar] [CrossRef] [PubMed]
- Cherukuri, P.; Glazer, E.S.; Curley, S.A. Targeted hyperthermia using metal nanoparticles. Adv. Drug Deliv. Rev. 2010, 62, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Mody, V.V.; Siwale, R.; Singh, A.; Mody, H.R. Introduction to metallic nanoparticles. J. Pharm. Bioallied Sci. 2010, 2, 282–289. [Google Scholar] [CrossRef]
- Samanta, B.; Yan, H.; Fischer, N.O.; Shi, J.; Jerry, D.J.; Rotello, V.M. Protein-passivated Fe 3 O 4 nanoparticles: Low toxicity and rapid heating for thermal therapy. J. Mater. Chem. 2008, 18, 1204–1208. [Google Scholar] [CrossRef] [PubMed]
- Jain, K. Role of nanobiotechnology in developing personalized medicine for cancer. Technol. Cancer Res. Treat. 2005, 4, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Hilger, I.; Hergt, R.; Kaiser, W. Use of Magnetic Nanoparticle Heating in the Treatment of Breast Cancer. IEE Proc. Nanobiotechnol. 2005, 152, 33–39. [Google Scholar] [CrossRef]
- Pearce, J. Mathematical models of laser-induced tissue thermal damage. Int. J. Hyperth. 2011, 27, 741–750. [Google Scholar] [CrossRef]
- Giustini, A.J.; Petryk, A.A.; Cassim, S.M.; Tate, J.A.; Baker, I.; Hoopes, P.J. Magnetic nanoparticle hyperthermia in cancer treatment. Nano Life 2010, 1, 17–32. [Google Scholar] [CrossRef]
- Pu, P.-y.; Zhang, Y.-z.; Jiang, D.-h. Apoptosis induced by hyperthermia in human glioblastoma cell line and murine glioblastoma. Chin. J. Cancer Res. 2000, 12, 257–262. [Google Scholar] [CrossRef]
- Wust, P.; Hildebrandt, B.; Sreenivasa, G.; Rau, B.; Gellermann, J.; Riess, H.; Felix, R.; Schlag, P. Hyperthermia in combined treatment of cancer. Lancet Oncol. 2002, 3, 487–497. [Google Scholar] [CrossRef]
- Poduslo, J.F.; Hultman, K.L.; Curran, G.L.; Preboske, G.M.; Chamberlain, R.; Marjańska, M.; Garwood, M.; Jack, C.R., Jr.; Wengenack, T.M. Targeting vascular amyloid in arterioles of Alzheimer disease transgenic mice with amyloid β protein antibody-coated nanoparticles. J. Neuropathol. Exp. Neurol. 2011, 70, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, S.K.; Ahmad, A.; Chinnathambi, S. Protein-capped metal nanoparticles inhibit tau aggregation in Alzheimer’s disease. ACS Omega 2019, 4, 12833–12840. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, E.; Ettcheto, M.; Egea, M.A.; Espina, M.; Cano, A.; Calpena, A.C.; Camins, A.; Carmona, N.; Silva, A.M.; Souto, E.B. Memantine loaded PLGA PEGylated nanoparticles for Alzheimer’s disease: In vitro and in vivo characterization. J. Nanobiotechnol. 2018, 16, 32. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.H.; Chang, Y.J.; Yoshiike, Y.; Chang, Y.C.; Chen, Y.R. Negatively charged gold nanoparticles inhibit Alzheimer’s amyloid-β fibrillization, induce fibril dissociation, and mitigate neurotoxicity. Small 2012, 8, 3631–3639. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Zheng, Y.; Liu, X.; Fang, W.; Chen, X.; Liao, W.; Jing, X.; Lei, M.; Tao, E.; Ma, Q. Curcumin-loaded PLGA-PEG nanoparticles conjugated with B6 peptide for potential use in Alzheimer’s disease. Drug Deliv. 2018, 25, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Palanivelu, K. The effect of curcumin (turmeric) on Alzheimer’s disease: An overview. Ann. Indian Acad. Neurol. 2008, 11, 13–19. [Google Scholar] [CrossRef]
- Garcia-Alloza, M.; Borrelli, L.; Rozkalne, A.; Hyman, B.; Bacskai, B. Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. J. Neurochem. 2007, 102, 1095–1104. [Google Scholar] [CrossRef]
- Yang, F.; Lim, G.P.; Begum, A.N.; Ubeda, O.J.; Simmons, M.R.; Ambegaokar, S.S.; Chen, P.P.; Kayed, R.; Glabe, C.G.; Frautschy, S.A. Curcumin inhibits formation of amyloid β oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 2005, 280, 5892–5901. [Google Scholar] [CrossRef]
- Ringman, J.M.; Frautschy, S.A.; Cole, G.M.; Masterman, D.L.; Cummings, J.L. A potential role of the curry spice curcumin in Alzheimer’s disease. Curr. Alzheimer Res. 2005, 2, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Cartiera, M.S.; Ferreira, E.C.; Caputo, C.; Egan, M.E.; Caplan, M.J.; Saltzman, W.M. Partial correction of cystic fibrosis defects with PLGA nanoparticles encapsulating curcumin. Mol. Pharm. 2010, 7, 86–93. [Google Scholar] [CrossRef]
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as “Curecumin”: From kitchen to clinic. Biochem. Pharmacol. 2008, 75, 787–809. [Google Scholar] [CrossRef]
- Mathew, A.; Fukuda, T.; Nagaoka, Y.; Hasumura, T.; Morimoto, H.; Yoshida, Y.; Maekawa, T.; Venugopal, K.; Kumar, D.S. Curcumin loaded-PLGA nanoparticles conjugated with Tet-1 peptide for potential use in Alzheimer’s disease. PLoS ONE 2012, 7, e32616. [Google Scholar] [CrossRef]
- Kaur, I.P.; Bhandari, R.; Bhandari, S.; Kakkar, V. Potential of solid lipid nanoparticles in brain targeting. J. Control. Release 2008, 127, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Kundu, P.; Das, M.; Tripathy, K.; Sahoo, S.K. Delivery of dual drug loaded lipid based nanoparticles across the blood–brain barrier impart enhanced neuroprotection in a rotenone induced mouse model of Parkinson’s disease. ACS Chem. Neurosci. 2016, 7, 1658–1670. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.R.; Lúcio, M.; Martins, S.; Lima, J.L.C.; Reis, S. Novel resveratrol nanodelivery systems based on lipid nanoparticles to enhance its oral bioavailability. Int. J. Nanomed. 2013, 8, 177–187. [Google Scholar]
- Neves, A.R.; Lucio, M.; Lima, J.L.C.; Reis, S. Resveratrol in medicinal chemistry: A critical review of its pharmacokinetics, drug-delivery, and membrane interactions. Curr. Med. Chem. 2012, 19, 1663–1681. [Google Scholar] [CrossRef]
- Loureiro, J.A.; Andrade, S.; Duarte, A.; Neves, A.R.; Queiroz, J.F.; Nunes, C.; Sevin, E.; Fenart, L.; Gosselet, F.; Coelho, M.A. Resveratrol and grape extract-loaded solid lipid nanoparticles for the treatment of Alzheimer’s disease. Molecules 2017, 22, 277. [Google Scholar] [CrossRef]
- Andreasen, N.; Blennow, K. β-Amyloid (Aβ) protein in cerebrospinal fluid as a biomarker for Alzheimer’s disease. Peptides 2002, 23, 1205–1214. [Google Scholar] [CrossRef]
- Suh, Y.-H.; Checler, F. Amyloid precursor protein, presenilins, and α-synuclein: Molecular pathogenesis and pharmacological applications in Alzheimer’s disease. Pharmacol. Rev. 2002, 54, 469–525. [Google Scholar] [CrossRef]
- Clippingdale, A.B.; Wade, J.D.; Barrow, C.J. The amyloid-β peptide and its role in Alzheimer’s disease. J. Pept. Sci. 2001, 7, 227–249. [Google Scholar] [CrossRef]
- Ladiwala, A.R.A.; Litt, J.; Kane, R.S.; Aucoin, D.S.; Smith, S.O.; Ranjan, S.; Davis, J.; Van Nostrand, W.E.; Tessier, P.M. Conformational differences between two amyloid β oligomers of similar size and dissimilar toxicity. J. Biol. Chem. 2012, 287, 24765–24773. [Google Scholar] [CrossRef]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer, A.; Stelzmann, R.A.; Schnitzlein, H.N.; Murtagh, F.R. An English translation of Alzheimer’s 1907 paper, Uber eine eigenartige Erkankung der Hirnrinde. Clin. Anat. 1995, 8, 429–431. [Google Scholar]
- Bi, C.; Wang, A.; Chu, Y.; Liu, S.; Mu, H.; Liu, W.; Wu, Z.; Sun, K.; Li, Y. Intranasal delivery of rotigotine to the brain with lactoferrin-modified PEG-PLGA nanoparticles for Parkinson’s disease treatment. Int. J. Nanomed. 2016, 11, 6547–6559. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A. A review on Alzheimer’s disease pathophysiology and its management: An update. Pharmacol. Rep. 2015, 67, 195–203. [Google Scholar] [CrossRef]
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef]
- Helmschrodt, C.; Höbel, S.; Schöniger, S.; Bauer, A.; Bonicelli, J.; Gringmuth, M.; Fietz, S.A.; Aigner, A.; Richter, A.; Richter, F. Polyethylenimine nanoparticle-mediated siRNA delivery to reduce α-Synuclein expression in a model of Parkinson’s disease. Mol. Ther. Nucleic Acids 2017, 9, 57–68. [Google Scholar] [CrossRef]
- Esteves, M.; Cristóvão, A.C.; Saraiva, T.; Rocha, S.M.; Baltazar, G.; Ferreira, L.; Bernardino, L. Retinoic acid-loaded polymeric nanoparticles induce neuroprotection in a mouse model for Parkinson’s disease. Front. Aging Neurosci. 2015, 7, 20. [Google Scholar] [CrossRef]
- Niu, S.; Zhang, L.-K.; Zhang, L.; Zhuang, S.; Zhan, X.; Chen, W.-Y.; Du, S.; Yin, L.; You, R.; Li, C.-H. Inhibition by multifunctional magnetic nanoparticles loaded with alpha-Synuclein RNAi plasmid in a Parkinson’s disease model. Theranostics 2017, 7, 344–356. [Google Scholar] [CrossRef]
- Bekris, L.M.; Mata, I.F.; Zabetian, C.P. The genetics of Parkinson disease. J. Geriatr. Psychiatry Neurol. 2010, 23, 228–242. [Google Scholar] [CrossRef]
- Vekrellis, K.; Rideout, H.J.; Stefanis, L. Neurobiology of α-synuclein. Mol. Neurobiol. 2004, 30, 1–21. [Google Scholar] [CrossRef]
- Desplats, P.; Patel, P.; Kosberg, K.; Mante, M.; Patrick, C.; Rockenstein, E.; Fujita, M.; Hashimoto, M.; Masliah, E. Combined exposure to Maneb and Paraquat alters transcriptional regulation of neurogenesis-related genes in mice models of Parkinson’s disease. Mol. Neurodegener. 2012, 7, 49. [Google Scholar] [CrossRef]
- Ozansoy, M.; Başak, A.N. The central theme of Parkinson’s disease: α-synuclein. Mol. Neurobiol. 2013, 47, 460–465. [Google Scholar] [CrossRef] [PubMed]
- DeCoteau, W.; Heckman, K.L.; Estevez, A.Y.; Reed, K.J.; Costanzo, W.; Sandford, D.; Studlack, P.; Clauss, J.; Nichols, E.; Lipps, J. Cerium oxide nanoparticles with antioxidant properties ameliorate strength and prolong life in mouse model of amyotrophic lateral sclerosis. Nanomedicine 2016, 12, 2311–2320. [Google Scholar] [CrossRef] [PubMed]
- Barber, S.C.; Mead, R.J.; Shaw, P.J. Oxidative stress in ALS: A mechanism of neurodegeneration and a therapeutic target. Biochim. Biophys. Acta 2006, 1762, 1051–1067. [Google Scholar] [CrossRef]
- Chen, L.; Watson, C.; Morsch, M.; Cole, N.J.; Chung, R.S.; Saunders, D.N.; Yerbury, J.J.; Vine, K.L. Improving the delivery of SOD1 antisense oligonucleotides to motor neurons using calcium phosphate-lipid nanoparticles. Front. Neurosci. 2017, 11, 476. [Google Scholar] [CrossRef]
- Debnath, K.; Pradhan, N.; Singh, B.K.; Jana, N.R.; Jana, N.R. Poly (trehalose) nanoparticles prevent amyloid aggregation and suppress polyglutamine aggregation in a Huntington’s disease model mouse. ACS Appl. Mater. Interfaces 2017, 9, 24126–24139. [Google Scholar] [CrossRef]
- Bhatt, R.; Singh, D.; Prakash, A.; Mishra, N. Development, characterization and nasal delivery of rosmarinic acid-loaded solid lipid nanoparticles for the effective management of Huntington’s disease. Drug Deliv. 2015, 22, 931–939. [Google Scholar] [CrossRef]
- Godinho, B.M.; Ogier, J.R.; Darcy, R.; O’Driscoll, C.M.; Cryan, J.F. Self-assembling modified β-cyclodextrin nanoparticles as neuronal siRNA delivery vectors: Focus on Huntington’s disease. Mol. Pharm. 2013, 10, 640–649. [Google Scholar] [CrossRef]
- Sandhir, R.; Yadav, A.; Mehrotra, A.; Sunkaria, A.; Singh, A.; Sharma, S. Curcumin nanoparticles attenuate neurochemical and neurobehavioral deficits in experimental model of Huntington’s disease. Neuromol. Med. 2014, 16, 106–118. [Google Scholar] [CrossRef]
- Tabrizi, S.; Cleeter, M.; Xuereb, J.; Taanman, J.W.; Cooper, J.; Schapira, A. Biochemical abnormalities and excitotoxicity in Huntington’s disease brain. Ann. Neurol. 1999, 45, 25–32. [Google Scholar] [CrossRef]
- Montoya, A.; Price, B.H.; Menear, M.; Lepage, M. Brain imaging and cognitive dysfunctions in Huntington’s disease. J. Psychiatry Neurosci. 2006, 31, 21–29. [Google Scholar] [PubMed]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Türeli, N.G.; Torge, A.; Juntke, J.; Schwarz, B.C.; Schneider-Daum, N.; Türeli, A.E.; Lehr, C.-M.; Schneider, M. Ciprofloxacin-loaded PLGA nanoparticles against cystic fibrosis P. aeruginosa lung infections. Eur. J. Pharm. Biopharm. 2017, 117, 363–371. [Google Scholar] [CrossRef]
- Robinson, E.; MacDonald, K.D.; Slaughter, K.; McKinney, M.; Patel, S.; Sun, C.; Sahay, G. Lipid nanoparticle-delivered chemically modified mRNA restores chloride secretion in cystic fibrosis. Mol. Ther. 2018, 26, 2034–2046. [Google Scholar] [CrossRef]
- Vij, N.; Min, T.; Marasigan, R.; Belcher, C.N.; Mazur, S.; Ding, H.; Yong, K.-T.; Roy, I. Development of PEGylated PLGA nanoparticle for controlled and sustained drug delivery in cystic fibrosis. J. Nanobiotechnol. 2010, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.L.; Burns, J.L.; Ramsey, B.W. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2003, 168, 918–951. [Google Scholar] [CrossRef]
- Davis, P.B. Cystic fibrosis since 1938. Am. J. Respir. Crit. Care Med. 2006, 173, 475–482. [Google Scholar] [CrossRef]
- Chen, J.; Patil, S.; Seal, S.; McGinnis, J.F. Rare earth nanoparticles prevent retinal degeneration induced by intracellular peroxides. Nat. Nanotechnol. 2006, 1, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Papa, S.; Ferrari, R.; De Paola, M.; Rossi, F.; Mariani, A.; Caron, I.; Sammali, E.; Peviani, M.; Dell’Oro, V.; Colombo, C. Polymeric nanoparticle system to target activated microglia/macrophages in spinal cord injury. J. Control. Release 2014, 174, 15–26. [Google Scholar] [CrossRef]
- Sato, S.; Hiruma, Y.; Nagata, H.; Takenaka, T. Excess Potassium and Microstructure Control for Producing Dense KNbO3 Ceramics. Trans. Mater. Res. Soc. Jpn. 2012, 37, 65–68. [Google Scholar] [CrossRef][Green Version]
- Liu, K.; Tedeschi, A.; Park, K.K.; He, Z. Neuronal intrinsic mechanisms of axon regeneration. Annu. Rev. Neurosci. 2011, 34, 131–152. [Google Scholar] [CrossRef]
- Nash, M.; Pribiag, H.; Fournier, A.E.; Jacobson, C. Central nervous system regeneration inhibitors and their intracellular substrates. Mol. Neurobiol. 2009, 40, 224–235. [Google Scholar] [CrossRef]
- Srikanth, M.; Kessler, J.A. Nanotechnology—Novel therapeutics for CNS disorders. Nat. Rev. Neurol. 2012, 8, 307–318. [Google Scholar] [CrossRef]
- Loane, D.J.; Byrnes, K.R. Role of microglia in neurotrauma. Neurotherapeutics 2010, 7, 366–377. [Google Scholar] [CrossRef]
- Gaudin, A.; Yemisci, M.; Eroglu, H.; Lepetre-Mouelhi, S.; Turkoglu, O.F.; Dönmez-Demir, B.; Caban, S.; Sargon, M.F.; Garcia-Argote, S.; Pieters, G. Squalenoyl adenosine nanoparticles provide neuroprotection after stroke and spinal cord injury. Nat. Nanotechnol. 2014, 9, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Klein, P.M.; Wagner, E. Bioreducible polycations as shuttles for therapeutic nucleic acid and protein transfection. Antioxid. Redox Signal. 2014, 21, 804–817. [Google Scholar] [CrossRef] [PubMed]
- Koutsopoulos, S.; Unsworth, L.D.; Nagai, Y.; Zhang, S. Controlled release of functional proteins through designer self-assembling peptide nanofiber hydrogel scaffold. Proc. Natl. Acad. Sci. USA 2009, 106, 4623–4628. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.-X.; Cheung, S.W.; Chan, K.C.; Wu, E.X.; Tay, D.K.; Ellis-Behnke, R.G. CNS regeneration after chronic injury using a self-assembled nanomaterial and MEMRI for real-time in vivo monitoring. Nanomedicine 2011, 7, 351–359. [Google Scholar] [CrossRef]
- Robinson, R.; Viviano, S.R.; Criscione, J.M.; Williams, C.A.; Jun, L.; Tsai, J.C.; Lavik, E.B. Nanospheres delivering the EGFR TKI AG1478 promote optic nerve regeneration: The role of size for intraocular drug delivery. ACS Nano 2011, 5, 4392–4400. [Google Scholar] [CrossRef]
- Nkansah, M.K.; Tzeng, S.Y.; Holdt, A.M.; Lavik, E.B. Poly (lactic-co-glycolic acid) nanospheres and microspheres for short-and long-term delivery of bioactive ciliary neurotrophic factor. Biotechnol. Bioeng. 2008, 100, 1010–1019. [Google Scholar] [CrossRef]
- Hou, Z.; Wei, H.; Wang, Q.; Sun, Q.; Zhou, C.; Zhan, C.; Tang, X.; Zhang, Q. New method to prepare mitomycin C loaded PLA-nanoparticles with high drug entrapment efficiency. Nanoscale Res. Lett. 2009, 4, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Tysseling-Mattiace, V.M.; Sahni, V.; Niece, K.L.; Birch, D.; Czeisler, C.; Fehlings, M.G.; Stupp, S.I.; Kessler, J.A. Self-assembling nanofibers inhibit glial scar formation and promote axon elongation after spinal cord injury. J. Neurosci. 2008, 28, 3814–3823. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, S.R.; Oliveira, J.M.; Silva, N.A.; Leite-Almeida, H.; Ribeiro-Samy, S.; Almeida, A.; Mano, J.F.; Sousa, N.; Salgado, A.J.; Reis, R.L. Microglia response and in vivo therapeutic potential of methylprednisolone-loaded dendrimer nanoparticles in spinal cord injury. Small 2013, 9, 738–749. [Google Scholar] [CrossRef]
- Park, J.; Zhang, Y.; Saito, E.; Gurczynski, S.J.; Moore, B.B.; Cummings, B.J.; Anderson, A.J.; Shea, L.D. Intravascular innate immune cells reprogrammed via intravenous nanoparticles to promote functional recovery after spinal cord injury. Proc. Natl. Acad. Sci. USA 2019, 116, 14947–14954. [Google Scholar] [CrossRef]
- Kanwar, J.R.; Sun, X.; Punj, V.; Sriramoju, B.; Mohan, R.R.; Zhou, S.-F.; Chauhan, A.; Kanwar, R.K. Nanoparticles in the treatment and diagnosis of neurological disorders: Untamed dragon with fire power to heal. Nanomedicine 2012, 8, 399–414. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Patel, S.; Nanda, R.; Sahoo, S. Nanotechnology in healthcare: Applications and challenges. Med. Chem. 2015, 5, 528–533. [Google Scholar] [CrossRef]
| Genetic Disease | Nanosystem | Effects | Ref. |
|---|---|---|---|
| Alzheimer’s Disease |
|
| [189,190,205] |
| Parkinson’s Disease | Retinoic acid NPs | Increased bioavailability of loaded compounds, prevented apoptosis, oxidative stress, and toxicity | [213,214] |
| Amyotrophic Lateral Sclerosis | Calcium phosphate lipid-coated NPs | able to pass through the BBB, neutralized RNS and ROS reactions | [218,219] |
| Huntington’s Disease | Curcumin SLN | Reduced the activity of intermediate complex II | [224,227] |
| Trehalose-loaded zwitterion NPs | Inhibited amyloid and polyglutamine aggregation | ||
| Cystic Fibrosis | Lipid NPs | Increased the amount of membrane-localized CFTR | [230,231] |
| PLGA NPs coated with PEG | Effectively delivered anti-inflammatory compounds |
| Disease | Nanosystems Used | Drug | Target | Effect | Reference |
|---|---|---|---|---|---|
| Optic Nerve Injury | Designed, self-assembling peptide nanofiber | None | Site of acute injury in axon and dismantled brain tissues of hamsters | Creates permissive environment for axon regeneration and knitting brain tissues | [238] |
| Optic Nerve Injury | Self-assembling peptide nanofiber scaffold | None | Chronic optic tract (OT) lesion and damaged axons of hamsters | Both show improved healing activity | [239] |
| Optic Nerve Injury | Fabricated PLGA-coated NPs | EGFR TKI 4-(3-chloroanilino)-6,7-dimethoxyquinazoline (AG1478) | Rat optic nerve crush injury | Optic nerve regeneration | [240] |
| Optic Nerve Injury (Glaucoma) | PLGA nanosphere | Ciliary neurotrophic factor (CNTF) | Retinal ganglion cells (RGCs) | Prolonged survival of RGCs in rats | [241] |
| Optic Nerve Injury | PLA nanoparticles | water soluble mitomycin C (MMC) | Tumor tissues | Inhibited scar formation | [242] |
| Spinal Cord Injury | Peptide amphiphile (PA) self-assembling nanofibers | None | Astrogliosis process | Reduced astrogliosis and cell death, increased number of oligodendroglia at the site of injury, promoted regeneration of descending motor fibers and ascending sensory fibers | [243] |
| Spinal Cord Injury | carboxymethylchitosan/polyamidoamine (CMCht/PAMAM) dendrimer | Methylprednisolone | Glial cells | significant differences in the locomotor output | [244] |
| Stroke and Spinal Cord Injury | Squalenoyl adenosine NPs | None | Fast metobolizing rate of neuroprotective adenosine | prolonged circulation of this nucleoside, to provide neuroprotection in mouse stroke and rat spinal cord injury | [236] |
| Spinal Cord Injury | Polymeric NPs | None | Circulating immune cells | immune cell infiltration is reduced, leading to decreased tissue degeneration | [245] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anjum, S.; Ishaque, S.; Fatima, H.; Farooq, W.; Hano, C.; Abbasi, B.H.; Anjum, I. Emerging Applications of Nanotechnology in Healthcare Systems: Grand Challenges and Perspectives. Pharmaceuticals 2021, 14, 707. https://doi.org/10.3390/ph14080707
Anjum S, Ishaque S, Fatima H, Farooq W, Hano C, Abbasi BH, Anjum I. Emerging Applications of Nanotechnology in Healthcare Systems: Grand Challenges and Perspectives. Pharmaceuticals. 2021; 14(8):707. https://doi.org/10.3390/ph14080707
Chicago/Turabian StyleAnjum, Sumaira, Sara Ishaque, Hijab Fatima, Wajiha Farooq, Christophe Hano, Bilal Haider Abbasi, and Iram Anjum. 2021. "Emerging Applications of Nanotechnology in Healthcare Systems: Grand Challenges and Perspectives" Pharmaceuticals 14, no. 8: 707. https://doi.org/10.3390/ph14080707
APA StyleAnjum, S., Ishaque, S., Fatima, H., Farooq, W., Hano, C., Abbasi, B. H., & Anjum, I. (2021). Emerging Applications of Nanotechnology in Healthcare Systems: Grand Challenges and Perspectives. Pharmaceuticals, 14(8), 707. https://doi.org/10.3390/ph14080707








