Monoclonal Antibodies Targeting CGRP: From Clinical Studies to Real-World Evidence—What Do We Know So Far?
Abstract
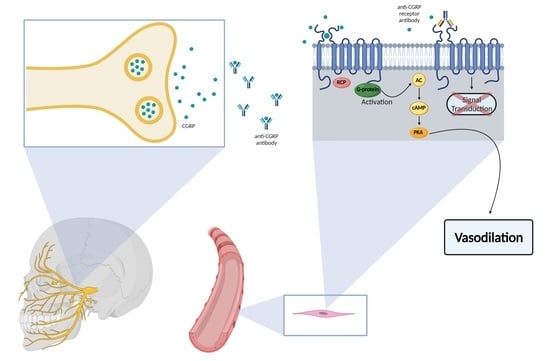
1. Introduction
2. Erenumab
2.1. Clinical Studies
2.2. Real-World Evidence
2.3. Adverse Events
2.4. Beyond Migraine and Future
3. Galcanezumab
3.1. Clinical Studies
3.2. Real-World Evidence
3.3. Adverse Events
3.4. Beyond Migraine and Future
4. Fremanezumab
4.1. Clinical Studies
4.2. Adverse Events
4.3. Beyond Migraine and Future
5. Eptinezumab
5.1. Clinical Studies
5.2. Adverse Events
5.3. Beyond Migraine and Future
6. Approvals
7. Guidelines for the Use of mAbs in Migraine (American Headache Society/European Headache Federation)
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Giamberardino, M.A.; Affaitati, G.; Costantini, R.; Cipollone, F.; Martelletti, P. Calcitonin gene-related peptide receptor as a novel target for the management of people with episodic migraine: Current evidence and safety profile of erenumab. J. Pain Res. 2017, 10, 2751–2760. [Google Scholar] [CrossRef][Green Version]
- Wrobel Goldberg, S.; Silberstein, S.D. Targeting CGRP: A New Era for Migraine Treatment. CNS Drugs 2015, 29, 443–452. [Google Scholar] [CrossRef]
- Bigal, M.E.; Walter, S.; Bronson, M.; Alibhoy, A.; Escandon, R. Cardiovascular and hemodynamic parameters in women following prolonged CGRP inhibition using LBR-101, a monoclonal antibody against CGRP. Cephalalgia 2014, 34, 968–976. [Google Scholar] [CrossRef]
- De Hoon, J.; Van Hecken, A.; Vandermeulen, C.; Yan, L.; Smith, B.; Chen, J.S.; Bautista, E.; Hamilton, L.; Waksman, J.; Vu, T.; et al. Phase I, Randomized, Double-blind, Placebo-controlled, Single-dose, and Multiple-dose Studies of Erenumab in Healthy Subjects and Patients with Migraine. Clin. Pharmacol. Ther. 2018, 103, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Lehto, S.G.; Zhu, D.X.; Sun, H.; Zhang, J.; Smith, B.P.; Immke, D.C.; Wild, K.D.; Xu, C. Pharmacologic Characterization of AMG 334, a Potent and Selective Human Monoclonal Antibody against the Calcitonin Gene-Related Peptide Receptor. J. Pharmacol. Exp. Ther. 2016, 356, 223–231. [Google Scholar] [CrossRef] [PubMed]
- De Hoon, J.; Van Hecken, A.; Vandermeulen, C.; Herbots, M.; Kubo, Y.; Lee, E.; Eisele, O.; Vargas, G.; Gabriel, K. Phase 1, randomized, parallel-group, double-blind, placebo-controlled trial to evaluate the effects of erenumab (AMG 334) and concomitant sumatriptan on blood pressure in healthy volunteers. Cephalalgia Int. J. Headache 2019, 39, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.; Ma, P.; Chen, J.S.; de Hoon, J.; Van Hecken, A.; Yan, L.; Wu, L.S.; Hamilton, L.; Vargas, G. Pharmacokinetic-Pharmacodynamic Relationship of Erenumab (AMG 334) and Capsaicin-Induced Dermal Blood Flow in Healthy and Migraine Subjects. Pharm. Res. 2017, 34, 1784–1795. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Gabriel, K.; Wang, Y.; Zhou, Y.; Eisele, O.; Vutikullird, A.; Mikol, D.D.; Lee, E. A Multi-Center, Open-Label, Pharmacokinetic Drug Interaction Study of Erenumab and a Combined Oral Contraceptive in Healthy Females. CNS Drugs 2019, 33, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Mavridis, T.; Koniari, C.; Fakas, N.; Mitsikostas, D.D.J.I. Anti-Calcitonin Gene-Related Peptide Monoclonal Antibodies: Adverse Effects. What Do We Really Know? A Literature Review. EMJ Innov. 2019, 3, 64–72. [Google Scholar]
- Favoni, V.; Giani, L.; Al-Hassany, L.; Asioli, G.M.; Butera, C.; de Boer, I.; Guglielmetti, M.; Koniari, C.; Mavridis, T.; Vaikjärv, M.; et al. CGRP and migraine from a cardiovascular point of view: What do we expect from blocking CGRP? J. Headache Pain 2019, 20, 27. [Google Scholar] [CrossRef]
- Depre, C.; Antalik, L.; Starling, A.; Koren, M.; Eisele, O.; Lenz, R.A.; Mikol, D.D. A Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Effect of Erenumab on Exercise Time During a Treadmill Test in Patients with Stable Angina. Headache 2018, 58, 715–723. [Google Scholar] [CrossRef]
- Dodick, D.W.; Ashina, M.; Brandes, J.L.; Kudrow, D.; Lanteri-Minet, M.; Osipova, V.; Palmer, K.; Picard, H.; Mikol, D.D.; Lenz, R.A. ARISE: A Phase 3 randomized trial of erenumab for episodic migraine. Cephalalgia Int. J. Headache 2018, 38, 1026–1037. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Reuter, U.; Hallström, Y.; Broessner, G.; Bonner, J.H.; Zhang, F.; Sapra, S.; Picard, H.; Mikol, D.D.; Lenz, R.A. A Controlled Trial of Erenumab for Episodic Migraine. N. Engl. J. Med. 2017, 377, 2123–2132. [Google Scholar] [CrossRef]
- Reuter, U.; Goadsby, P.J.; Lanteri-Minet, M.; Wen, S.; Hours-Zesiger, P.; Ferrari, M.D.; Klatt, J. Efficacy and tolerability of erenumab in patients with episodic migraine in whom two-to-four previous preventive treatments were unsuccessful: A randomised, double-blind, placebo-controlled, phase 3b study. Lancet 2018, 392, 2280–2287. [Google Scholar] [CrossRef]
- Sakai, F.; Takeshima, T.; Tatsuoka, Y.; Hirata, K.; Lenz, R.; Wang, Y.; Cheng, S.; Hirama, T.; Mikol, D.D. A Randomized Phase 2 Study of Erenumab for the Prevention of Episodic Migraine in Japanese Adults. Headache 2019, 59, 1731–1742. [Google Scholar] [CrossRef]
- Sun, H.; Dodick, D.W.; Silberstein, S.; Goadsby, P.J.; Reuter, U.; Ashina, M.; Saper, J.; Cady, R.; Chon, Y.; Dietrich, J.; et al. Safety and efficacy of AMG 334 for prevention of episodic migraine: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2016, 15, 382–390. [Google Scholar] [CrossRef]
- Tepper, S.; Ashina, M.; Reuter, U.; Brandes, J.L.; Doležil, D.; Silberstein, S.; Winner, P.; Leonardi, D.; Mikol, D.; Lenz, R. Safety and efficacy of erenumab for preventive treatment of chronic migraine: A randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2017, 16, 425–434. [Google Scholar] [CrossRef]
- Zhu, C.; Guan, J.; Xiao, H.; Luo, W.; Tong, R. Erenumab safety and efficacy in migraine: A systematic review and meta-analysis of randomized clinical trials. Medicine 2019, 98, e18483. [Google Scholar] [CrossRef]
- Lanteri-Minet, M.; Goadsby, P.J.; Reuter, U.; Wen, S.; Hours-Zesiger, P.; Ferrari, M.D.; Klatt, J. Effect of erenumab on functional outcomes in patients with episodic migraine in whom 2–4 preventives were not useful: Results from the LIBERTY study. J. Neurol. Neurosurg. Psychiatry 2021, 92, 466–472. [Google Scholar] [CrossRef]
- Ashina, M.; Goadsby, P.J.; Reuter, U.; Silberstein, S.; Dodick, D.; Rippon, G.A.; Klatt, J.; Xue, F.; Chia, V.; Zhang, F.; et al. Long-term safety and tolerability of erenumab: Three-plus year results from a five-year open-label extension study in episodic migraine. Cephalalgia 2019, 39, 1455–1464. [Google Scholar] [CrossRef]
- Ashina, M.; Goadsby, P.J.; Reuter, U.; Silberstein, S.; Dodick, D.W.; Xue, F.; Zhang, F.; Paiva da Silva Lima, G.; Cheng, S.; Mikol, D.D. Long-term efficacy and safety of erenumab in migraine prevention: Results from a 5-year, open-label treatment phase of a randomized clinical trial. Eur. J. Neurol. 2021, 28, 1716–1725. [Google Scholar] [CrossRef]
- Ashina, M.; Kudrow, D.; Reuter, U.; Dolezil, D.; Silberstein, S.; Tepper, S.J.; Xue, F.; Picard, H.; Zhang, F.; Wang, A.; et al. Long-term tolerability and nonvascular safety of erenumab, a novel calcitonin gene-related peptide receptor antagonist for prevention of migraine: A pooled analysis of four placebo-controlled trials with long-term extensions. Cephalalgia 2019, 39, 1798–1808. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Reuter, U.; Hallström, Y.; Broessner, G.; Bonner, J.H.; Zhang, F.; Wright, I.K.; Chou, D.E.; Klatt, J.; Picard, H.; et al. One-year sustained efficacy of erenumab in episodic migraine: Results of the STRIVE study. Neurology 2020, 95, e469–e479. [Google Scholar] [CrossRef]
- Tepper, S.J.; Ashina, M.; Reuter, U.; Brandes, J.L.; Doležil, D.; Silberstein, S.D.; Winner, P.; Zhang, F.; Cheng, S.; Mikol, D.D. Long-term safety and efficacy of erenumab in patients with chronic migraine: Results from a 52-week, open-label extension study. Cephalalgia Int. J. Headache 2020, 40, 543–553. [Google Scholar] [CrossRef]
- Barbanti, P.; Aurilia, C.; Egeo, G.; Fofi, L. Erenumab: From scientific evidence to clinical practice-the first Italian real-life data. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2019, 40, 177–179. [Google Scholar] [CrossRef] [PubMed]
- De Icco, R.; Fiamingo, G.; Greco, R.; Bottiroli, S.; Demartini, C.; Zanaboni, A.M.; Allena, M.; Guaschino, E.; Martinelli, D.; Putortì, A.; et al. Neurophysiological and biomolecular effects of erenumab in chronic migraine: An open label study. Cephalalgia Int. J. Headache 2020, 40, 1336–1345. [Google Scholar] [CrossRef]
- Lambru, G.; Hill, B.; Murphy, M.; Tylova, I.; Andreou, A.P. A prospective real-world analysis of erenumab in refractory chronic migraine. J. Headache Pain 2020, 21, 61. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, G.P. Treatment of Chronic Migraine with Erenumab Alone or as an Add on Therapy: A real-world observational study. Anesth. Pain Res. 2020, 4, 1–4. [Google Scholar] [CrossRef]
- Ornello, R.; Casalena, A.; Frattale, I.; Gabriele, A.; Affaitati, G.; Giamberardino, M.A.; Assetta, M.; Maddestra, M.; Marzoli, F.; Viola, S.; et al. Real-life data on the efficacy and safety of erenumab in the Abruzzo region, central Italy. J. Headache Pain 2020, 21, 32. [Google Scholar] [CrossRef] [PubMed]
- Raffaelli, B.; Kalantzis, R.; Mecklenburg, J.; Overeem, L.H.; Neeb, L.; Gendolla, A.; Reuter, U. Erenumab in Chronic Migraine Patients Who Previously Failed Five First-Line Oral Prophylactics and OnabotulinumtoxinA: A Dual-Center Retrospective Observational Study. Front. Neurol. 2020, 11, 417. [Google Scholar] [CrossRef]
- Russo, A.; Silvestro, M.; Scotto di Clemente, F.; Trojsi, F.; Bisecco, A.; Bonavita, S.; Tessitore, A.; Tedeschi, G. Multidimensional assessment of the effects of erenumab in chronic migraine patients with previous unsuccessful preventive treatments: A comprehensive real-world experience. J. Headache Pain 2020, 21, 69. [Google Scholar] [CrossRef]
- Kudrow, D.; Pascual, J.; Winner, P.K.; Dodick, D.W.; Tepper, S.J.; Reuter, U.; Hong, F.; Klatt, J.; Zhang, F.; Cheng, S.; et al. Vascular safety of erenumab for migraine prevention. Neurology 2020, 94, e497–e510. [Google Scholar] [CrossRef]
- Saely, S.; Croteau, D.; Jawidzik, L.; Brinker, A.; Kortepeter, C. Hypertension: A new safety risk for patients treated with erenumab. Headache 2021, 61, 202–208. [Google Scholar] [CrossRef]
- Ashina, H.; Iljazi, A.; Al-Khazali, H.M.; Eigenbrodt, A.K.; Larsen, E.L.; Andersen, A.M.; Hansen, K.J.; Bräuner, K.B.; Mørch-Jessen, T.; Chaudhry, B.; et al. Efficacy, tolerability, and safety of erenumab for the preventive treatment of persistent post-traumatic headache attributed to mild traumatic brain injury: An open-label study. J. Headache Pain 2020, 21, 62. [Google Scholar] [CrossRef] [PubMed]
- Benschop, R.J.; Collins, E.C.; Darling, R.J.; Allan, B.W.; Leung, D.; Conner, E.M.; Nelson, J.; Gaynor, B.; Xu, J.; Wang, X.F.; et al. Development of a novel antibody to calcitonin gene-related peptide for the treatment of osteoarthritis-related pain. Osteoarthr. Cartil. 2014, 22, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Tepper, S.J. History and Review of anti-Calcitonin Gene-Related Peptide (CGRP) Therapies: From Translational Research to Treatment. Headache 2018, 58 (Suppl. 3), 238–275. [Google Scholar] [CrossRef] [PubMed]
- Kielbasa, W.; Helton, D.L. A new era for migraine: Pharmacokinetic and pharmacodynamic insights into monoclonal antibodies with a focus on galcanezumab, an anti-CGRP antibody. Cephalalgia Int. J. Headache 2019, 39, 1284–1297. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Dodick, D.W.; Leone, M.; Bardos, J.N.; Oakes, T.M.; Millen, B.A.; Zhou, C.; Dowsett, S.A.; Aurora, S.K.; Ahn, A.H.; et al. Trial of Galcanezumab in Prevention of Episodic Cluster Headache. N. Engl. J. Med. 2019, 381, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Vermeersch, S.; Benschop, R.J.; Van Hecken, A.; Monteith, D.; Wroblewski, V.J.; Grayzel, D.; de Hoon, J.; Collins, E.C. Translational Pharmacodynamics of Calcitonin Gene-Related Peptide Monoclonal Antibody LY2951742 in a Capsaicin-Induced Dermal Blood Flow Model. J. Pharmacol. Exp. Ther. 2015, 354, 350–357. [Google Scholar] [CrossRef]
- Monteith, D.; Collins, E.C.; Vandermeulen, C.; Van Hecken, A.; Raddad, E.; Scherer, J.C.; Grayzel, D.; Schuetz, T.J.; de Hoon, J. Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of the CGRP Binding Monoclonal Antibody LY2951742 (Galcanezumab) in Healthy Volunteers. Front. Pharmacol. 2017, 8, 740. [Google Scholar] [CrossRef] [PubMed]
- Dodick, D.W.; Goadsby, P.J.; Spierings, E.L.; Scherer, J.C.; Sweeney, S.P.; Grayzel, D.S. Safety and efficacy of LY2951742, a monoclonal antibody to calcitonin gene-related peptide, for the prevention of migraine: A phase 2, randomised, double-blind, placebo-controlled study. Lancet Neurol. 2014, 13, 885–892. [Google Scholar] [CrossRef]
- Eli Lilly and Company. A Study of Galcanezumab (LY2951742) in Participants with Migraine Headache; Eli Lilly and Company: Indianapolis, IN, USA, 2014. [Google Scholar]
- Skljarevski, V.; Oakes, T.M.; Zhang, Q.; Ferguson, M.B.; Martinez, J.; Camporeale, A.; Johnson, K.W.; Shan, Q.; Carter, J.; Schacht, A.; et al. Effect of Different Doses of Galcanezumab vs. Placebo for Episodic Migraine Prevention: A Randomized Clinical Trial. JAMA Neurol. 2018, 75, 187–193. [Google Scholar] [CrossRef]
- Sakai, F.; Ozeki, A.; Skljarevski, V. Efficacy and safety of galcanezumab for prevention of migraine headache in Japanese patients with episodic migraine: A phase 2 randomized controlled clinical trial. Cephalalgia Rep. 2020, 3. [Google Scholar] [CrossRef]
- Stauffer, V.L.; Dodick, D.W.; Zhang, Q.; Carter, J.N.; Ailani, J.; Conley, R.R. Evaluation of Galcanezumab for the Prevention of Episodic Migraine: The EVOLVE-1 Randomized Clinical Trial. JAMA Neurol. 2018, 75, 1080–1088. [Google Scholar] [CrossRef] [PubMed]
- Stauffer, V.L.; Wang, S.; Voulgaropoulos, M.; Skljarevski, V.; Kovacik, A.; Aurora, S.K. Effect of Galcanezumab Following Treatment Cessation in Patients with Migraine: Results from 2 Randomized Phase 3 Trials. Headache 2019, 59, 834–847. [Google Scholar] [CrossRef] [PubMed]
- Skljarevski, V.; Matharu, M.; Millen, B.A.; Ossipov, M.H.; Kim, B.K.; Yang, J.Y. Efficacy and safety of galcanezumab for the prevention of episodic migraine: Results of the EVOLVE-2 Phase 3 randomized controlled clinical trial. Cephalalgia 2018, 38, 1442–1454. [Google Scholar] [CrossRef] [PubMed]
- Camporeale, A.; Kudrow, D.; Sides, R.; Wang, S.; Van Dycke, A.; Selzler, K.J.; Stauffer, V.L. A phase 3, long-term, open-label safety study of Galcanezumab in patients with migraine. BMC Neurol. 2018, 18, 188. [Google Scholar] [CrossRef] [PubMed]
- Mulleners, W.; Kim, B.-K.; Lainez, M.; Lanteri-Minet, M.; Aurora, S.; Nichols, R.; Wang, S.; Tockhorn-Heidenreich, A.; Detke, H. A Randomized, Placebo-Controlled Study of Galcanezumab in Patients with Treatment-Resistant Migraine: Double-Blind Results from the CONQUER Study (162). Neurology 2020, 94, 162. [Google Scholar]
- Detke, H.C.; Goadsby, P.J.; Wang, S.; Friedman, D.I.; Selzler, K.J.; Aurora, S.K. Galcanezumab in chronic migraine: The randomized, double-blind, placebo-controlled REGAIN study. Neurology 2018, 91, e2211–e2221. [Google Scholar] [CrossRef]
- Dodick, D.W.; Goadsby, P.J.; Lucas, C.; Jensen, R.; Bardos, J.N.; Martinez, J.M.; Zhou, C.; Aurora, S.K.; Yang, J.Y.; Conley, R.R.; et al. Phase 3 randomized, placebo-controlled study of galcanezumab in patients with chronic cluster headache: Results from 3-month double-blind treatment. Cephalalgia 2020, 40, 935–948. [Google Scholar] [CrossRef] [PubMed]
- Eli Lilly and Company. A Study of Galcanezumab (LY2951742) in Participants 6 to 17 Years of Age with Episodic Migraine; Eli Lilly and Company: Indianapolis, IN, USA, 2018. [Google Scholar]
- Allergan. Safety, Tolerability & Drug Interaction Study of Ubrogepant with Erenumab or Galcanezumab in Participants with Migraine; Allergan: Washington, DC, USA, 2019. [Google Scholar]
- Beth Israel Deaconess Medical Center. Novel Insight into Migraine Pathophysiolgy and Galcanezumab Mechanisms of Action; Beth Israel Deaconess Medical Center: Boston, MA, USA, 2020. [Google Scholar]
- Eli Lilly and Company. A Study of LY2951742 (Galcanezumab) in Participants with Cluster Headache; Eli Lilly and Company: Indianapolis, IN, USA, 2015. [Google Scholar]
- Bigal, M.E.; Rapoport, A.M.; Silberstein, S.D.; Walter, S.; Hargreaves, R.J.; Aycardi, E. From LBR-101 to Fremanezumab for Migraine. CNS Drugs 2018, 32, 1025–1037. [Google Scholar] [CrossRef] [PubMed]
- Bigal, M.E.; Escandon, R.; Bronson, M.; Walter, S.; Sudworth, M.; Huggins, J.P.; Garzone, P. Safety and tolerability of LBR-101, a humanized monoclonal antibody that blocks the binding of CGRP to its receptor: Results of the Phase 1 program. Cephalalgia 2014, 34, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Bigal, M.E.; Dodick, D.W.; Rapoport, A.M.; Silberstein, S.D.; Ma, Y.; Yang, R.; Loupe, P.S.; Burstein, R.; Newman, L.C.; Lipton, R.B. Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of high-frequency episodic migraine: A multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet Neurol. 2015, 14, 1081–1090. [Google Scholar] [CrossRef]
- Bigal, M.E.; Edvinsson, L.; Rapoport, A.M.; Lipton, R.B.; Spierings, E.L.; Diener, H.C.; Burstein, R.; Loupe, P.S.; Ma, Y.; Yang, R.; et al. Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of chronic migraine: A multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet Neurol. 2015, 14, 1091–1100. [Google Scholar] [CrossRef]
- Cohen, J.M.; Dodick, D.W.; Yang, R.; Newman, L.C.; Li, T.; Aycardi, E.; Bigal, M.E. Fremanezumab as Add-On Treatment for Patients Treated with Other Migraine Preventive Medicines. Headache 2017, 57, 1375–1384. [Google Scholar] [CrossRef]
- VanderPluym, J.; Dodick, D.W.; Lipton, R.B.; Ma, Y.; Loupe, P.S.; Bigal, M.E. Fremanezumab for preventive treatment of migraine: Functional status on headache-free days. Neurology 2018, 91, e1152–e1165. [Google Scholar] [CrossRef] [PubMed]
- Brandes, J.L.; Kudrow, D.; Yeung, P.P.; Sakai, F.; Aycardi, E.; Blankenbiller, T.; Grozinski-Wolff, M.; Yang, R.; Ma, Y. Effects of fremanezumab on the use of acute headache medication and associated symptoms of migraine in patients with episodic migraine. Cephalalgia Int. J. Headache 2020, 40, 470–477. [Google Scholar] [CrossRef]
- Silberstein, S.D.; Dodick, D.W.; Bigal, M.E.; Yeung, P.P.; Goadsby, P.J.; Blankenbiller, T.; Grozinski-Wolff, M.; Yang, R.; Ma, Y.; Aycardi, E. Fremanezumab for the Preventive Treatment of Chronic Migraine. N. Engl. J. Med. 2017, 377, 2113–2122. [Google Scholar] [CrossRef]
- Ferrari, M.D.; Diener, H.C.; Ning, X.; Galic, M.; Cohen, J.M.; Yang, R.; Mueller, M.; Ahn, A.H.; Schwartz, Y.C.; Grozinski-Wolff, M.; et al. Fremanezumab versus placebo for migraine prevention in patients with documented failure to up to four migraine preventive medication classes (FOCUS): A randomised, double-blind, placebo-controlled, phase 3b trial. Lancet 2019, 394, 1030–1040. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Silberstein, S.D.; Yeung, P.P.; Cohen, J.M.; Ning, X.; Yang, R.; Dodick, D.W. Long-term safety, tolerability, and efficacy of fremanezumab in migraine: A randomized study. Neurology 2020, 95, 2487–2499. [Google Scholar] [CrossRef]
- Cohen-Barak, O.; Weiss, S.; Rasamoelisolo, M.; Faulhaber, N.; Yeung, P.P.; Loupe, P.S.; Yoon, E.; Gandhi, M.D.; Spiegelstein, O.; Aycardi, E. A phase 1 study to assess the pharmacokinetics, safety, and tolerability of fremanezumab doses (225 mg, 675 mg and 900 mg) in Japanese and Caucasian healthy subjects. Cephalalgia Int. J. Headache 2018, 38, 1–12. [Google Scholar] [CrossRef]
- A Study Comparing the Efficacy and Safety of Fremanezumab (TEV-48125) for the Prevention of Chronic Cluster Headache (CCH). Available online: https://ClinicalTrials.gov/show/NCT02964338 (accessed on 24 May 2021).
- A Study to Evaluate the Efficacy and Safety of Fremanezumab for Preventive Treatment of Migraine in Patients with Major Depressive Disorder. Available online: https://ClinicalTrials.gov/show/NCT04041284 (accessed on 24 May 2021).
- Safety and Efficacy of Fremanezumab for Migraine in Adult CADASIL. Available online: https://ClinicalTrials.gov/show/NCT04334408 (accessed on 24 May 2021).
- A Study to Evaluate the Efficacy and Safety of TEV-48125 (Fremanezumab) for the Prevention of Episodic Cluster Headache (ECH). Available online: https://ClinicalTrials.gov/show/NCT02945046 (accessed on 24 May 2021).
- A Study to Test the Effectiveness and Safety of Fremanezumab on Patients with Fibromyalgia. Available online: https://ClinicalTrials.gov/show/NCT03965091 (accessed on 24 May 2021).
- A Study to Test if Fremanezumab Reduces Headache in Patients with Posttraumatic Headache (PTH). Available online: https://ClinicalTrials.gov/show/NCT03347188 (accessed on 24 May 2021).
- A Study to Test if Fremanezumab Reduces Pain in Patients with Interstitial Cystitis-Bladder Pain Syndrome. Available online: https://ClinicalTrials.gov/show/NCT04447729 (accessed on 24 May 2021).
- Efficacy of AJOVY (Fremanezumab-Vfrm) on Interictal Migraine Related Burden. Available online: https://ClinicalTrials.gov/show/NCT04461795 (accessed on 24 May 2021).
- A Safety Evaluation Trial of TEV-48125 Self-Administered in Migraine Patients. Available online: https://ClinicalTrials.gov/show/NCT04355117 (accessed on 24 May 2021).
- A Study to Test if Fremanezumab is Effective in Preventing Chronic Migraine in Patients 6 to 17 Years of Age. Available online: https://ClinicalTrials.gov/show/NCT04464707 (accessed on 24 May 2021).
- A Study to Test if Fremanezumab is Effective in Preventing Episodic Migraine in Patients 6 to 17 Years of Age. Available online: https://ClinicalTrials.gov/show/NCT04458857 (accessed on 24 May 2021).
- Dhillon, S. Eptinezumab: First Approval. Drugs 2020, 80, 733–739. [Google Scholar] [CrossRef]
- Smith, T.R.; Spierings, E.L.H.; Cady, R.; Hirman, J.; Schaeffler, B.; Shen, V.; Sperling, B.; Brevig, T.; Josiassen, M.K.; Brunner, E.; et al. Safety and tolerability of eptinezumab in patients with migraine: A pooled analysis of 5 clinical trials. J. Headache Pain 2021, 22, 16. [Google Scholar] [CrossRef]
- A Multicenter Assessment of ALD403 in Frequent Episodic Migraine (PROMISE 1). Available online: https://ClinicalTrials.gov/show/NCT02559895 (accessed on 24 May 2021).
- Ashina, M.; Saper, J.; Cady, R.; Schaeffler, B.A.; Biondi, D.M.; Hirman, J.; Pederson, S.; Allan, B.; Smith, J. Eptinezumab in episodic migraine: A randomized, double-blind, placebo-controlled study (PROMISE-1). Cephalalgia Int. J. Headache 2020, 40, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Alder Biopharmaceuticals, Inc. Evaluation of ALD403 (Eptinezumab) in the Prevention of Chronic Migraine; Alder Biopharmaceuticals, Inc.: Bothell, WA, USA, 2016. [Google Scholar]
- Lipton, R.B.; Goadsby, P.J.; Smith, J.; Schaeffler, B.A.; Biondi, D.M.; Hirman, J.; Pederson, S.; Allan, B.; Cady, R. Efficacy and safety of eptinezumab in patients with chronic migraine: PROMISE-2. Neurology 2020, 94, e1365–e1377. [Google Scholar] [CrossRef]
- Silberstein, S.; Diamond, M.; Hindiyeh, N.A.; Biondi, D.M.; Cady, R.; Hirman, J.; Allan, B.; Pederson, S.; Schaeffler, B.; Smith, J. Eptinezumab for the prevention of chronic migraine: Efficacy and safety through 24 weeks of treatment in the phase 3 PROMISE-2 (Prevention of migraine via intravenous ALD403 safety and efficacy-2) study. J. Headache Pain 2020, 21, 120. [Google Scholar] [CrossRef] [PubMed]
- Alder Biopharmaceuticals, Inc. An Open Label Trial of ALD403 (Eptinezumab) in Chronic Migraine; Alder Biopharmaceuticals Inc.: Bothell, WA, USA, 2016. [Google Scholar]
- Kudrow, D.; Cady, R.K.; Allan, B.; Pederson, S.M.; Hirman, J.; Mehta, L.R.; Schaeffler, B.A. Long-term safety and tolerability of eptinezumab in patients with chronic migraine: A 2-year, open-label, phase 3 trial. BMC Neurol. 2021, 21, 126. [Google Scholar] [CrossRef] [PubMed]
- Dodick, D.W.; Goadsby, P.J.; Silberstein, S.D.; Lipton, R.B.; Olesen, J.; Ashina, M.; Wilks, K.; Kudrow, D.; Kroll, R.; Kohrman, B.; et al. Safety and efficacy of ALD403, an antibody to calcitonin gene-related peptide, for the prevention of frequent episodic migraine: A randomised, double-blind, placebo-controlled, exploratory phase 2 trial. Lancet Neurol. 2014, 13, 1100–1107. [Google Scholar] [CrossRef]
- Evaluate Efficacy & Safety of Eptinezumab Administered Intravenously in Subjects Experiencing Acute Attack of Migraine (RELIEF). Available online: https://ClinicalTrials.gov/show/NCT04152083 (accessed on 24 May 2021).
- Markham, A. Erenumab: First Global Approval. Drugs 2018, 78, 1157–1161. [Google Scholar] [CrossRef] [PubMed]
- Lamb, Y.N. Galcanezumab: First Global Approval. Drugs 2018, 78, 1769–1775. [Google Scholar] [CrossRef] [PubMed]
- Giani, L.; Proietti Cecchini, A.; Leone, M. Galcanezumab for the prevention of cluster headache. Expert Opin. Biol. Ther. 2020, 20, 1133–1142. [Google Scholar] [CrossRef]
- Hoy, S.M. Fremanezumab: First Global Approval. Drugs 2018, 78, 1829–1834. [Google Scholar] [CrossRef] [PubMed]
- American Headache Society. The American Headache Society Position Statement on Integrating New Migraine Treatments into Clinical Practice. J. Head Face Pain 2019, 59, 1–18. [Google Scholar] [CrossRef]
- Sacco, S.; Bendtsen, L.; Ashina, M.; Reuter, U.; Terwindt, G.; Mitsikostas, D.D.; Martelletti, P. European headache federation guideline on the use of monoclonal antibodies acting on the calcitonin gene related peptide or its receptor for migraine prevention. J. Headache Pain 2019, 20, 6. [Google Scholar] [CrossRef] [PubMed]
- Kouremenos, E.; Arvaniti, C.; Constantinidis, T.S.; Giannouli, E.; Fakas, N.; Kalamatas, T.; Kararizou, E.; Naoumis, D.; Mitsikostas, D.D.; Hellenic Headache, S. Consensus of the Hellenic Headache Society on the diagnosis and treatment of migraine. J. Headache Pain 2019, 20, 113. [Google Scholar] [CrossRef]
- Diener, H.C.; Förderreuther, S.; Gaul, C.; Giese, F.; Hamann, T.; Holle-Lee, D.; Jürgens, T.P.; Kamm, K.; Kraya, T.; Lampl, C.; et al. Prevention of migraine with monoclonal antibodies against CGRP or the CGRP receptor: Addition to the S1 guideline: Therapy of migraine attacks and prevention of migraine. Recommendations of the Germany Society of Neurology and the German Migraine and Headache Society. Neurol. Res. Pract. 2020, 2, 11. [Google Scholar] [CrossRef]
- Kowacs, F.; Roesler, C.A.P.; Piovesan, É.J.; Sarmento, E.M.; Campos, H.C.; Maciel, J.A., Jr.; Calia, L.C.; Barea, L.M.; Ciciarelli, M.C.; Valença, M.M.; et al. Consensus of the Brazilian Headache Society on the treatment of chronic migraine. Arq. Neuropsiquiatr. 2019, 77, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Greene, K.A.; Gentile, C.P.; Szperka, C.L.; Yonker, M.; Gelfand, A.A.; Grimes, B.; Irwin, S.L. Calcitonin Gene-Related Peptide Monoclonal Antibody Use for the Preventive Treatment of Refractory Headache Disorders in Adolescents. Pediatr. Neurol. 2021, 114, 62–67. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mavridis, T.; Deligianni, C.I.; Karagiorgis, G.; Daponte, A.; Breza, M.; Mitsikostas, D.D. Monoclonal Antibodies Targeting CGRP: From Clinical Studies to Real-World Evidence—What Do We Know So Far? Pharmaceuticals 2021, 14, 700. https://doi.org/10.3390/ph14070700
Mavridis T, Deligianni CI, Karagiorgis G, Daponte A, Breza M, Mitsikostas DD. Monoclonal Antibodies Targeting CGRP: From Clinical Studies to Real-World Evidence—What Do We Know So Far? Pharmaceuticals. 2021; 14(7):700. https://doi.org/10.3390/ph14070700
Chicago/Turabian StyleMavridis, Theodoros, Christina I. Deligianni, Georgios Karagiorgis, Ariadne Daponte, Marianthi Breza, and Dimos D. Mitsikostas. 2021. "Monoclonal Antibodies Targeting CGRP: From Clinical Studies to Real-World Evidence—What Do We Know So Far?" Pharmaceuticals 14, no. 7: 700. https://doi.org/10.3390/ph14070700
APA StyleMavridis, T., Deligianni, C. I., Karagiorgis, G., Daponte, A., Breza, M., & Mitsikostas, D. D. (2021). Monoclonal Antibodies Targeting CGRP: From Clinical Studies to Real-World Evidence—What Do We Know So Far? Pharmaceuticals, 14(7), 700. https://doi.org/10.3390/ph14070700