Comparative EPR Study on the Scavenging Effect of Methotrexate with the Isomers of Its Photoswitchable Derivative
Abstract
:1. Introduction
2. Results
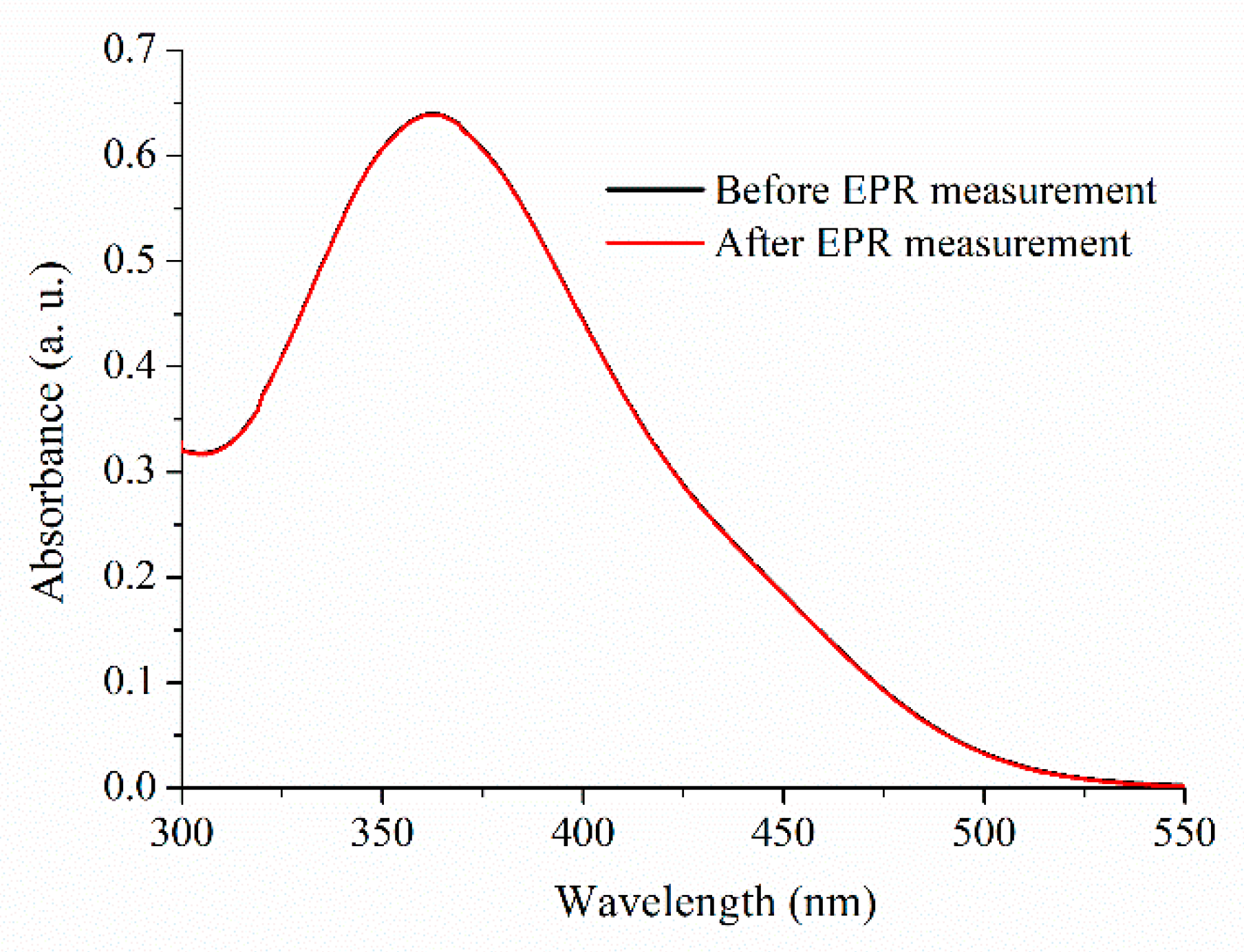
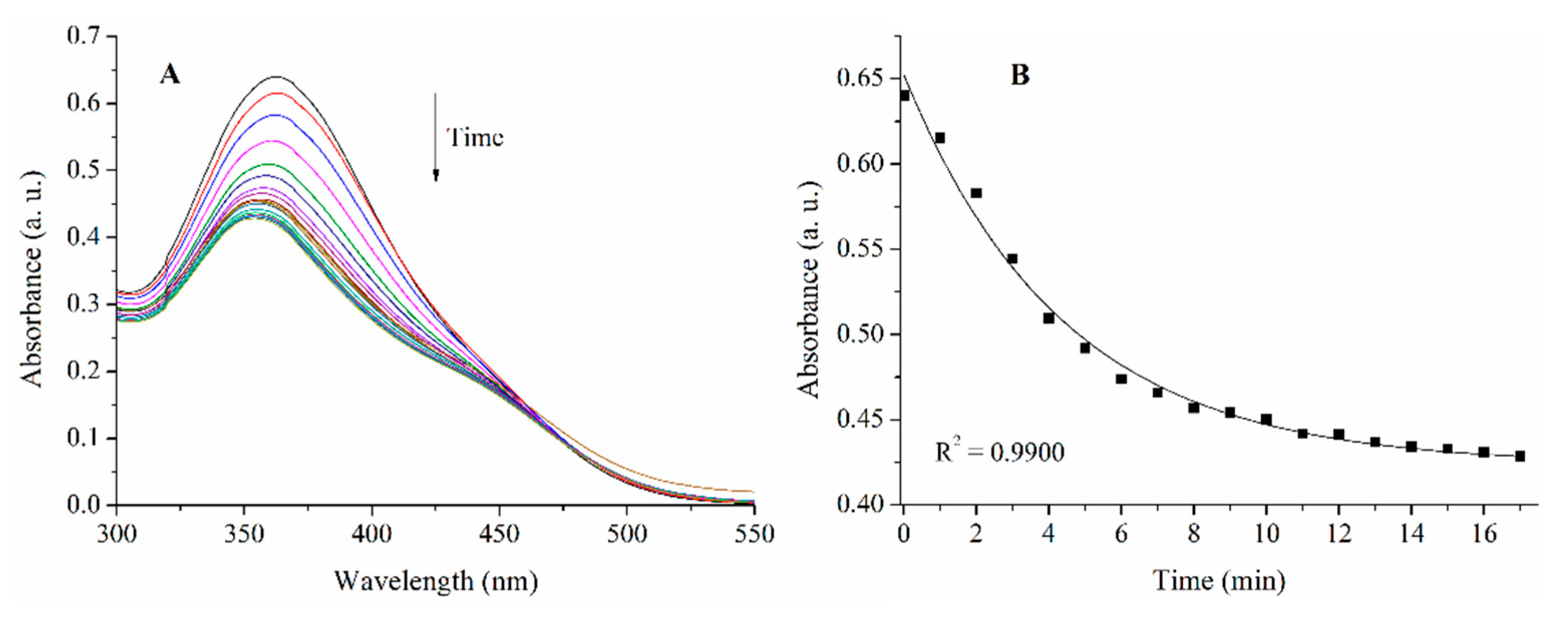
2.1. Absorbance Spectra of Trans-PHX before and after EPR Measurements
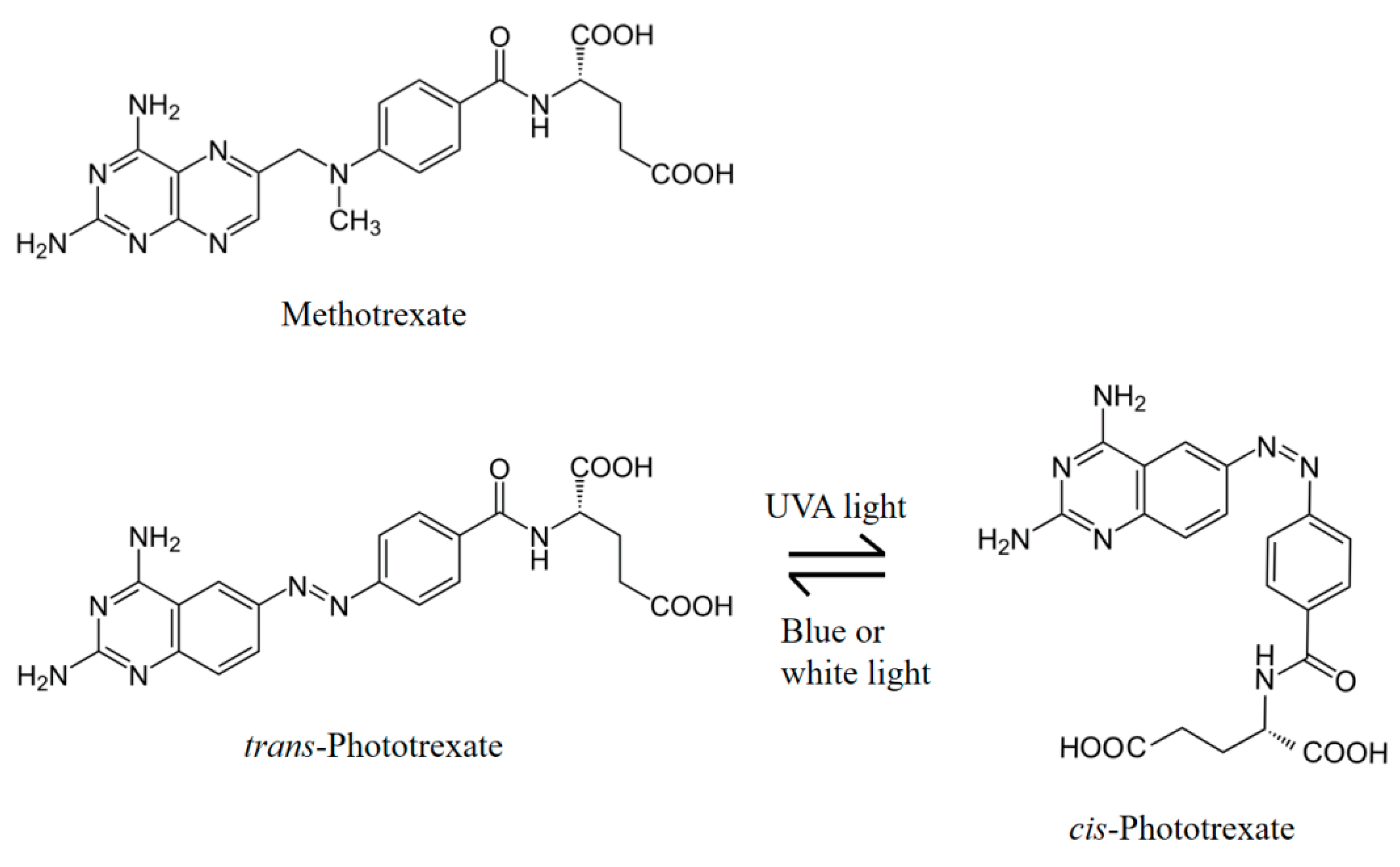
2.2. Isomerization of Trans-PHX
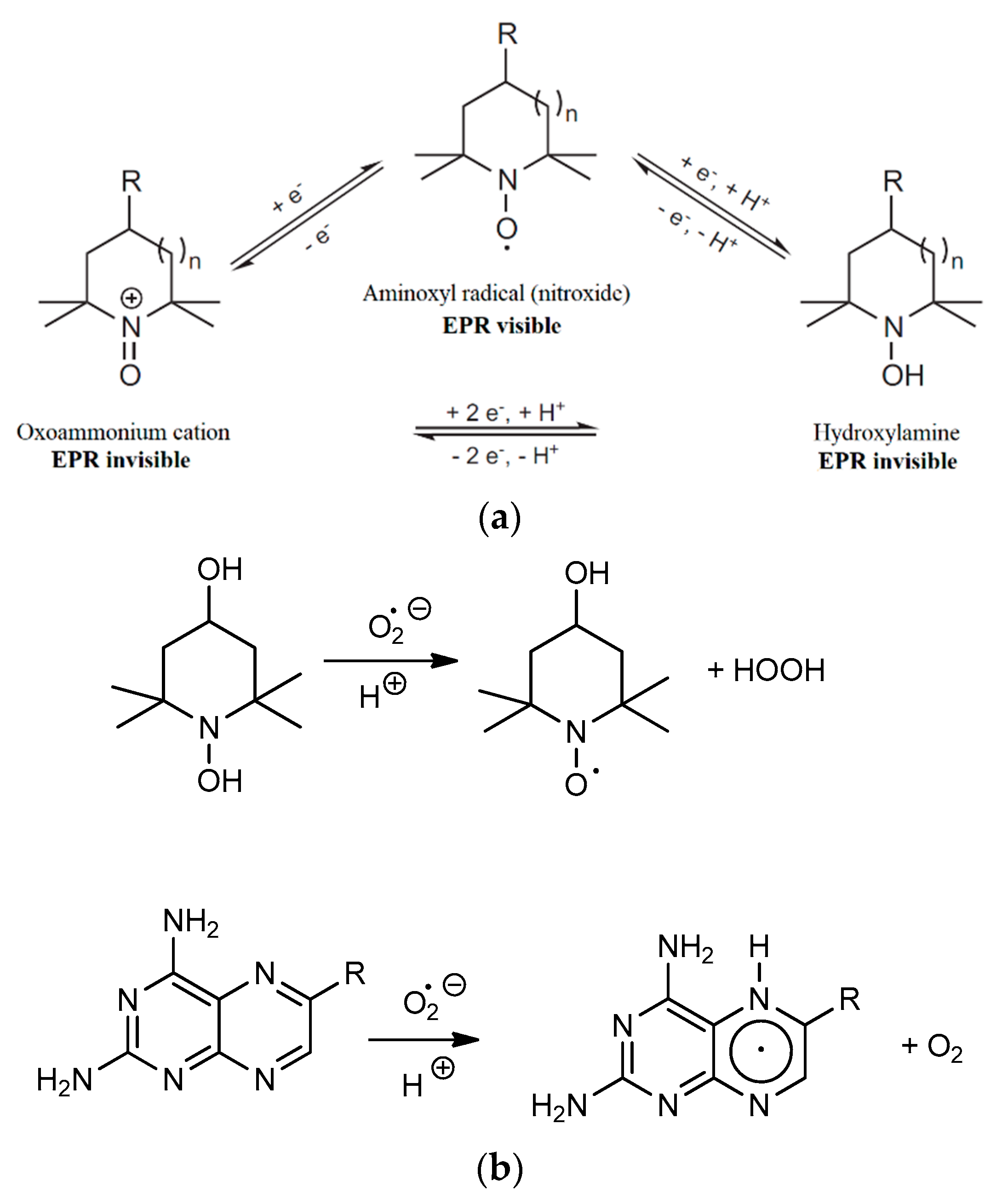
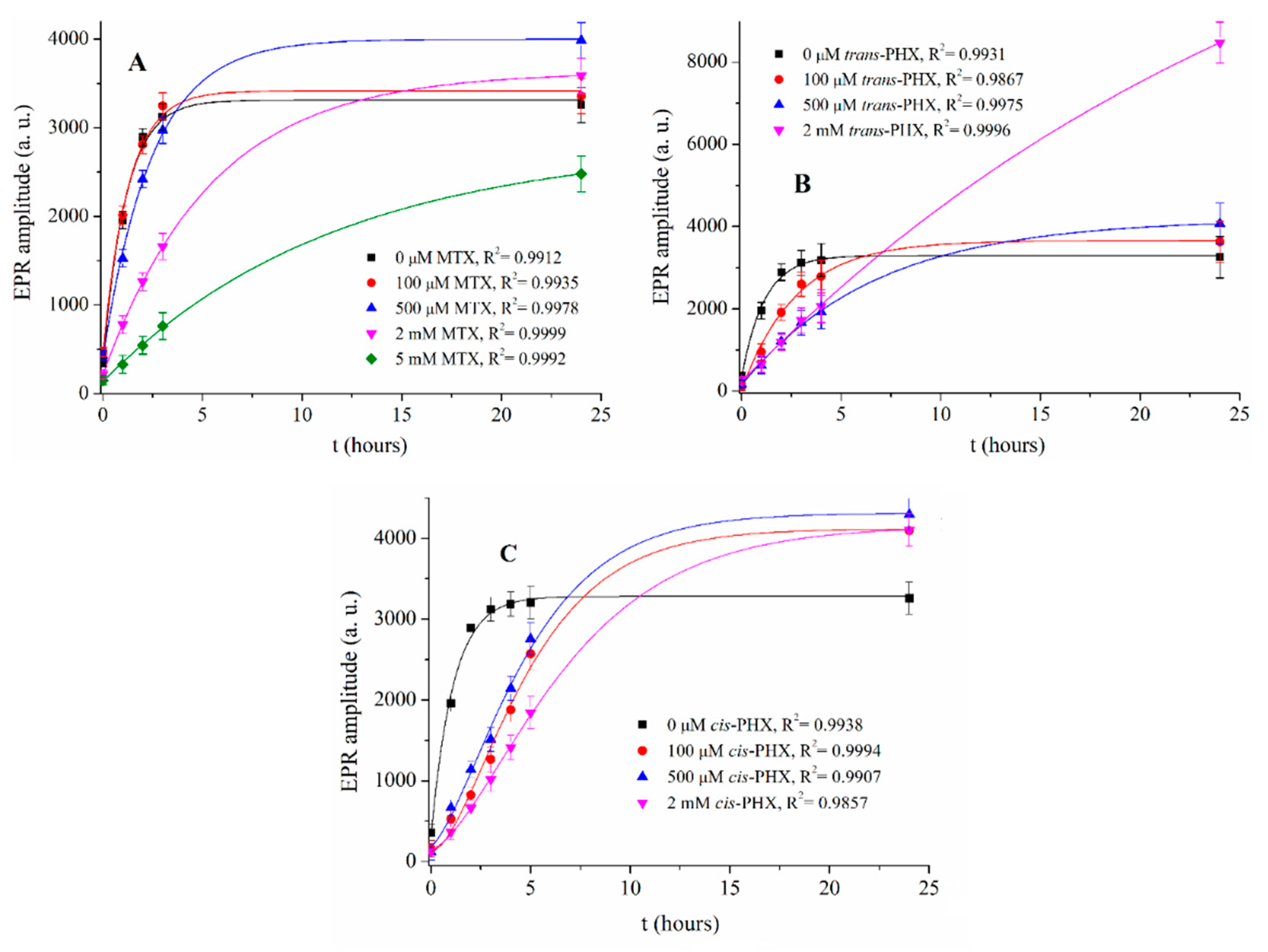
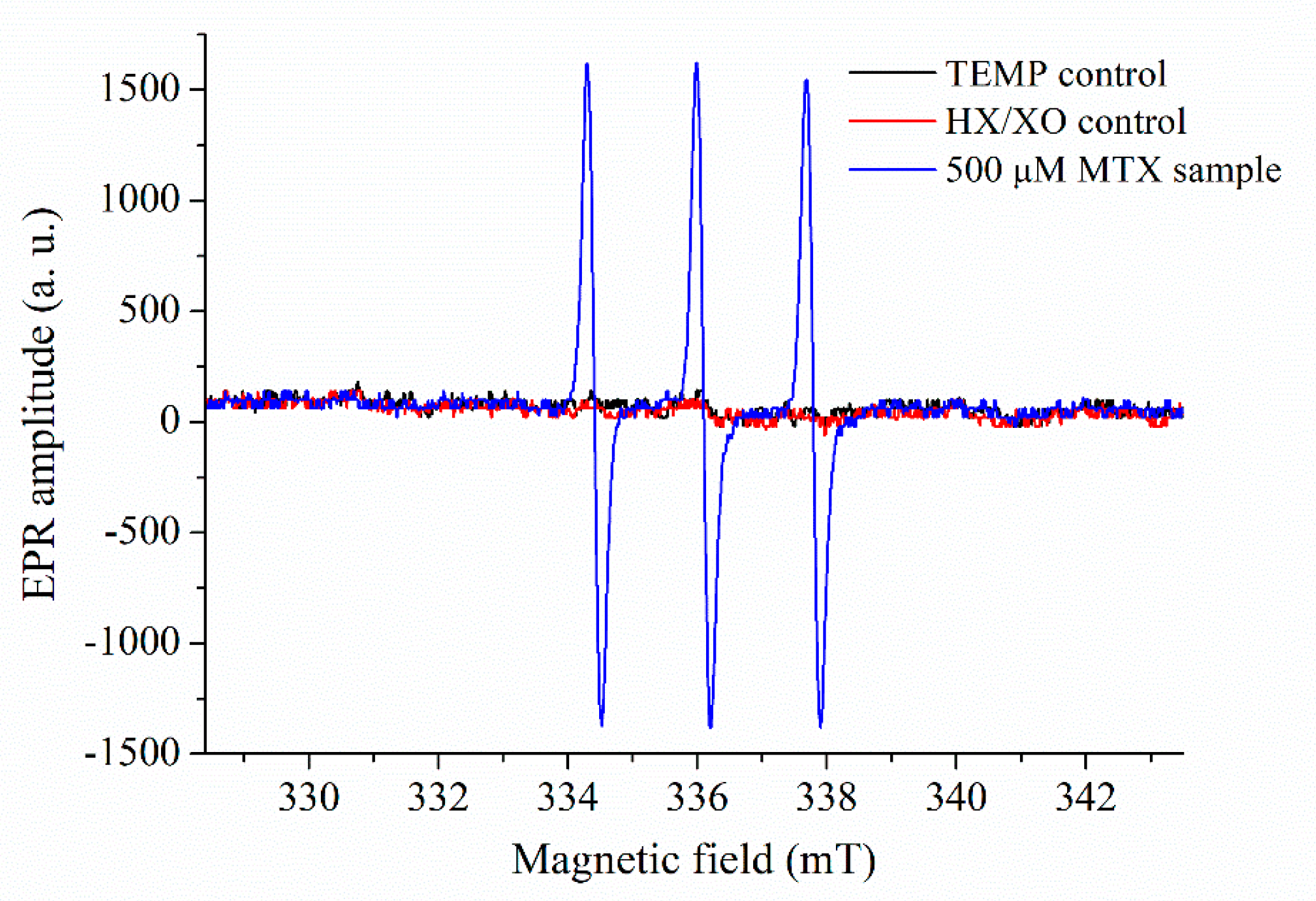
2.3. Results of EPR Measurements
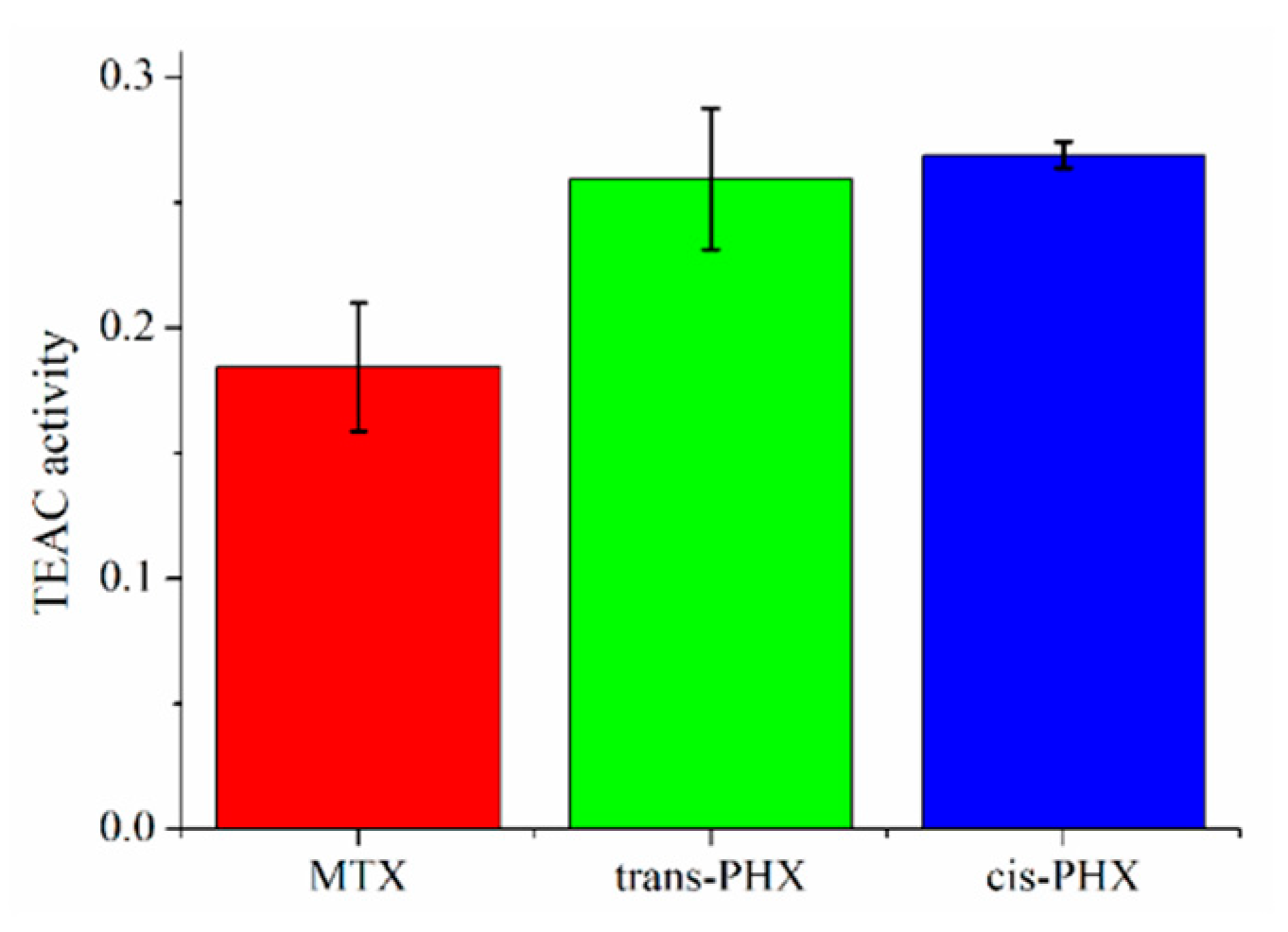
2.4. Results of ABTS Scavenging Assay
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Tian, H.; Cronstein, B.N. Understanding the mechanisms of action of methotrexate. Bull. BYU Hosp. Jt. Dis. 2007, 65, 168–173. [Google Scholar]
- Mulatihan, D.; Guo, T.; Zhao, Y. Azobenzene Photoswitch for Isomerization-Dependent Cancer Therapy via Azo-Combretastatin A4 and Phototrexate. Photochem. Photobiol. 2020, 96, 1163–1168. [Google Scholar] [CrossRef] [PubMed]
- Chane, E.S.L.; Cronstein, B.N. Molecularaction of methotrexate in inflammatory diseases. Arthritis Res. Ther. 2002, 4, 266–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cutolo, M.; Sulli, A.; Pizzorni, C.; Seriolo, B. Anti-inflammatory mechanisms of methotrexate in rheumatoid arthritis. Ann. Rheum. Dis. 2001, 60, 729–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matera, C.; Gomila, A.M.J.; Camarero, N.; Libergoli, M.; Soler, C.; Gorostiza, P. Photoswitchable Antimetabolite for Targeted Photoactivated Chemotherapy. J. Am. Chem. Soc. 2018, 140, 15764–15773. [Google Scholar] [CrossRef] [Green Version]
- Castillo, V.S.; Moyano, L.A. Methotrexate: Pharmacology, Clinical Uses and Adverse Effects; Nova Science Publishers: Hauppauge, NY, USA, 2012; ISBN 9781621005964. [Google Scholar]
- Shiroky, J.B.; Neville, C.; Esdaile, J.M.; Choquette, D.; Zummer, M.; Hazeltine, M.; Bykerk, V.; Kanji, M.; St-Pierre, A.; Robidoux, L.; et al. Low-dose methotrexate with leucovorin (folinic acid) in the management of rheumatoid arthritis. Arthritis Rheum. 1993, 36, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Broichhagen, J.; Frank, J.A.; Trauner, D. A Roadmap to Success in Photopharmacology. Acc. Chem. Res. 2015, 48, 1947–1960. [Google Scholar] [CrossRef]
- Szymanski, W.; Beierle, J.M.; Kistemaker, H.A.; Velema, W.A.; Feringa, B.L. Reversible photocontrol of biological systems by the incorporation of molecular photoswitches. Chem. Rev. 2013, 113, 6114–6178. [Google Scholar] [CrossRef] [Green Version]
- Banghart, M.R.; Mourot, A.; Fortin, D.L.; Yao, J.Z.; Kramer, R.H.; Trauner, D. Photochromic blockers of voltage-gated potassium channels. Angew. Chem. Int. Ed. 2009, 48, 9097–10001. [Google Scholar] [CrossRef] [Green Version]
- Mashita, T.; Kowada, T.; Takahashi, H.; Matsui, T.; Mizukami, S. Light-wavelength based Quantitative Control of Dihydrofolate Reductase Activity Using Photochromic Isostere of Inhibitor. ChemBioChem 2019, 20, 1382–1386. [Google Scholar] [CrossRef] [PubMed]
- Micha, R.; Imamura, F.; von Ballmoos, M.W.; Solomon, D.H.; Hernán, M.A.; Ridker, P.M.; Mozaffarian, D. Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am. J. Cardiol. 2011, 108, 1362–1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Libby, P.; Ridker, M.; Hansson, G.K. Inflammation in atherosclerosis: From pathophysiology to practice. J. Am. Coll. Cardiol. 2009, 54, 2129–2138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, R. The pathogenesis of atherosclerosis: A perspective for the 1990s. Nature 1993, 362, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Andreson, D.R.; Duryee, M.J.; Shurmur, S.W.; Um, J.Y.; Bussey, W.D.; Hunter, C.D.; Garvin, R.P.; Sayles, H.R.; Mikuls, T.R.; Klassen, L.W.; et al. Unique antibody responses to malondialdehyde-acetaldehyde (MAA)-protein adducts predict coronary artery disease. PLoS ONE 2014, 9, e107440. [Google Scholar] [CrossRef]
- Heinecke, J.W. Oxidants and antioxidants in the pathogenesis of atherosclerosis: Implications for the oxidized low density lipoprotein hypothesis. Atherosclerosis 1998, 141, 1–15. [Google Scholar] [CrossRef]
- Zimmerman, M.C.; Clemens, D.L.; Duryee, M.J.; Sarmiento, C.; Chiou, A.; Hunter, C.D.; Tian, J.; Klassen, L.W.; O’Dell, J.R.; Thiele, G.M.; et al. Direct antioxidant properties of methotrexate: Inhibition of malondialdehyde-acetaldehyde-protein adduct formation and superoxide scavenging. Redox Biol. 2017, 13, 588–593. [Google Scholar] [CrossRef]
- Babić, N.; Peyrot, F. Molecular Probes for Evaluation of Oxidative Stress by In Vivo EPR Spectroscopy and Imaging: State-of-the-Art and Limitations. Magnetochemistry 2019, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Bačić, G.; Pavićević, A.; Peyrot, F. In vivo evaluation of different alterations of redox status by studying pharmacokinetics of nitroxides using magnetic resonance techniques. Redox Biol. 2016, 8, 226–242. [Google Scholar] [CrossRef] [Green Version]
- Dikalov, S.I.; Polienko, Y.F.; Kirilyuk, I. Electron Paramagnetic Resonance Measurements of Reactive Oxygen Species by Cyclic Hydroxylamine Spin Probes. Antioxid. Redox Signal. 2018, 28. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Saura-Calixto, F. Anti-oxidant capacity of dietary polyphenols determined by ABTS assay: A kinetic expression of the results. Int. J. Food Sci. Technol. 2006, 43, 185–191. [Google Scholar] [CrossRef]
- Bartoloni, E.; Alunno, A.; Valentini, V.; Luccioli, F.; Valentini, E.; La Paglia, G.M.; Leone, M.C.; Cafaro, G.; Marcucci, E.; Gerli, R. Targeting Inflammation to Prevent Cardiovascular Disease in Chronic Rheumatic Diseases: Myth or Reality? Front. Cardiovasc. Med. 2018, 5, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thanigaimani, S.; Phie, J.; Krishna, S.M.; Moxon, J.; Golledge, J. Effect of disease modifying anti-rheumatic drugs on major cardiovascular events: A meta-analysis of randomized controlled trials. Sci. Rep. 2021, 11, 6627. [Google Scholar] [CrossRef] [PubMed]
- Lems, W.; Boers, M.; van Vollenhoven, R.F.; Nurmohamed, M. Antirheumatic drugs for cardiovascular disease prevention: The case for colchicine. RMD Open 2021, 7, e001560. [Google Scholar] [CrossRef] [PubMed]
- Tardif, J.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef]
- Avina-Zubieta, J.A.; Thomas, J.; Sadatsafavi, M.; Lehman, A.J.; Lacaille, D. Risk of incident cardiovascular events in patients with rheumatoid arthritis: A meta-analysis of observational studies. Ann. Rheum. Dis. 2012, 71, 1524–1529. [Google Scholar] [CrossRef]
- Solomon, D.H.; Karlson, E.W.; Rimm, E.B.; Cannuscio, C.C.; Mandl, L.A.; Manson, J.E.; Stampfer, M.J.; Curhan, G.C. Cardiovascular Morbidity and Mortality in Women Diagnosed With Rheumatoid Arthritis. Circulation 2003, 107, 1303–1307. [Google Scholar] [CrossRef] [Green Version]
- Roubille, C.; Richer, V.; Starnino, T.; McCourt, C.; McFarlane, A.; Fleming, P.; Siu, S.; Kraft, J.; Lynde, C.; Pope, J.; et al. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: A systematic review and meta-analysis. Ann. Rheum. Dis. 2015, 74, 480–489. [Google Scholar] [CrossRef]
- Rosenbaugh, E.G.; Roat, J.W.; Gao, L.; Yang, R.-F.; Manickam, D.S.; Yin, J.-X.; Schultz, H.D.; Bronich, T.K.; Batrakova, E.V.; Kabanov, A.V.; et al. The attenuation of central angiotensin II-dependent pressor response and intra-neuronal signaling by intracarotid injection of nanoformulated copper/zinc superoxide dismutase. Biomaterials 2010, 31, 5218–5226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dikalov, S.; Griendling, K.K.; Harrison, D.G. Measurement of Reactive Oxygen Species in Cardiovascular Studies. Hypertension 2007, 49, 717–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Concentration (µM) | MTX | trans-PHX | cis-PHX |
|---|---|---|---|
| ln k | ln k | ln k | |
| 0 | −8.70 ± 0.16 | −8.69 ± 0.18 | −8.68 ± 0.15 |
| 100 | −8.79 ± 0.23 | −9.56 ± 0.15 | −9.16 ± 0.25 |
| 500 | −9.46 ± 0.21 | −10.46 ± 0.28 | −9.19 ± 0.26 |
| 2000 | −10.25 ± 0.25 | −11.96 ± 0.23 | −9.51 ± 0.29 |
| 5000 | −11.01 ± 0.20 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Preisz, Z.; Hartvig, N.; Bognár, B.; Kálai, T.; Kunsági-Máté, S. Comparative EPR Study on the Scavenging Effect of Methotrexate with the Isomers of Its Photoswitchable Derivative. Pharmaceuticals 2021, 14, 665. https://doi.org/10.3390/ph14070665
Preisz Z, Hartvig N, Bognár B, Kálai T, Kunsági-Máté S. Comparative EPR Study on the Scavenging Effect of Methotrexate with the Isomers of Its Photoswitchable Derivative. Pharmaceuticals. 2021; 14(7):665. https://doi.org/10.3390/ph14070665
Chicago/Turabian StylePreisz, Zsolt, Nóra Hartvig, Balázs Bognár, Tamás Kálai, and Sándor Kunsági-Máté. 2021. "Comparative EPR Study on the Scavenging Effect of Methotrexate with the Isomers of Its Photoswitchable Derivative" Pharmaceuticals 14, no. 7: 665. https://doi.org/10.3390/ph14070665
APA StylePreisz, Z., Hartvig, N., Bognár, B., Kálai, T., & Kunsági-Máté, S. (2021). Comparative EPR Study on the Scavenging Effect of Methotrexate with the Isomers of Its Photoswitchable Derivative. Pharmaceuticals, 14(7), 665. https://doi.org/10.3390/ph14070665






