Signaling Pathway and Transcriptional Regulation in Osteoblasts during Bone Healing: Direct Involvement of Hydroxyapatite as a Biomaterial
Abstract
1. Introduction
2. HA
3. Osteoblasts, Their Transcription Factors, and Other Marker Proteins
3.1. Runx2
3.2. Osterix (Osx)
3.3. ATF4
3.4. Dlx5
3.5. Msx
3.6. Alkaline Phosphatase
3.7. COL1
3.8. Osteopontin
3.9. Osteocalcin
3.10. Osteonectin (ON)
3.11. Osteoprotegerin
3.12. Bone Sialoprotein 2
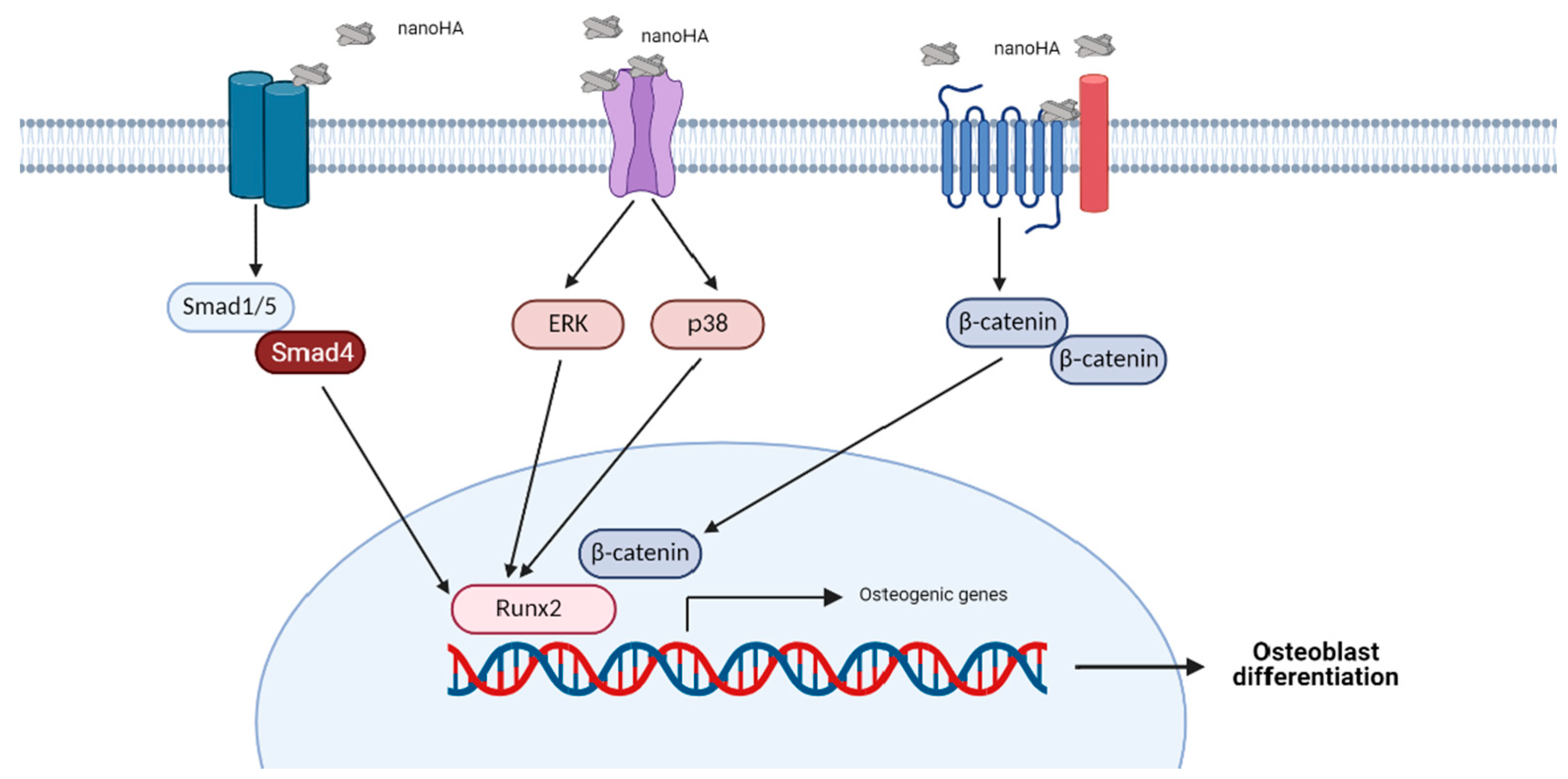
4. HA-Induced Signaling Pathways in Osteoblasts
4.1. Extracellular Signal-Regulated Kinase (ERK) Signaling Pathway
4.2. p38 Signaling Pathway
4.3. Wnt Signaling Pathway
4.4. BMP Signaling Pathway
5. How Do Bone Cells Produce HA?
6. Other Cellular Events Induced by HA
7. Event on the Cell Membrane: Direct Interaction as Intact Ligand or through Ions Release?
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liang, H.; Xu, X.; Feng, X.; Ma, L.; Deng, X.; Wu, S.; Liu, X.; Yang, C. Gold nanoparticles-loaded hydroxyapatite composites guide osteogenic differentiation of human mesenchymal stem cells through Wnt/β-catenin signaling pathway. Int. J. Nanomed. 2019, 14, 6151–6163. [Google Scholar] [CrossRef] [PubMed]
- Budiatin, A.S.; Samirah, N.; Gani, M.A.; Nilamsari, W.P.; Ardianto, C.; Khotib, J. The characterization of bovine bone-derived hydroxyapatite isolated using novel non-hazardous method. J. Biomim. Biomater. Biomed. Eng. 2020, 45, 49–56. [Google Scholar] [CrossRef]
- Budiatin, A.S.; Mahyudin, F.; Khotib, J. Fabrication and characterization of bovine hydroxyapatitegelatin- alendronate scaffold cross-linked by glutaraldehyde for bone regeneration. J. Basic Clin. Physiol. Pharmacol. 2021, in press. [Google Scholar]
- Chen, B.; Lin, T.; Yang, X.; Li, Y.; Xie, D.; Zheng, W.; Cui, H.; Deng, W.; Tan, X. Low-magnitude, high-frequency vibration promotes the adhesion and the osteogenic differentiation of bone marrow-derived mesenchymal stem cells cultured on a hydroxyapatite-coated surface: The direct role of Wnt/catenin signaling pathway activation. Int. J. Mol. Med. 2016, 38, 1531–1540. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhao, L.; Li, B.; Han, Y. Nanorod diameter modulated osteogenic activity of hierarchical micropore/nanorod-patterned coatings via a Wnt/β-catenin pathway. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1719–1731. [Google Scholar] [CrossRef]
- Budiatin, A.S.; Zainuddin, M.; Khotib, J. In vitro gentamicin release from bioactive BHAG (ELENA) implant against staphylococcus aureus. J. Biol. Res. 2013, 18, 116–118. [Google Scholar] [CrossRef]
- Lebre, F.; Sridharan, R.; Sawkins, M.J.; Kelly, D.J.; O’Brien, F.J.; Lavelle, E.C. The shape and size of hydroxyapatite particles dictate inflammatory responses following implantation. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, G.; Wu, J.; Zhang, Y.; Liu, J.; Luo, H.; Shao, L. Insights into the angiogenic effects of nanomaterials: Mechanisms involved and potential applications. J. Nanobiotechnol. 2020, 18, 1–22. [Google Scholar] [CrossRef]
- Budiatin, A.S.; Gani, M.A.; Samirah, N.; Ardianto, C.; Raharjanti, A.M.; Septiani, I.; Putri, N.P.K.P.; Khotib, J. Bovine hydroxyapatite-based bone scaffold with gentamicin accelerates vascularization and remodeling of bone defect. Int. J. Biomater. 2021, 2021, 1–7. [Google Scholar] [CrossRef]
- Germaini, M.-M.; Detsch, R.; Grünewald, A.; Magnaudeix, A.; Lalloue, F.; Boccaccini, A.R.; Champion, E. Osteoblast and osteoclast responses to A/B type carbonate-substituted hydroxyapatite ceramics for bone regeneration this. Biomed. Mater. 2017, 12, 035008. [Google Scholar] [CrossRef]
- Khotib, J.; Lasandara, C.S.; Samirah, N.; Budiatin, A.S. Acceleration of bone fracture healing through the use of natural bovine hydroxyapatite implant on bone defect animal model. Folica Med. Indones. 2019, 55, 176–187. [Google Scholar] [CrossRef]
- Raggatt, L.J.; Partridge, N.C. Cellular and molecular mechanisms of bone remodeling. J. Biol. Chem. 2010, 285, 25103–25108. [Google Scholar] [CrossRef]
- Yoshida, C.A.; Komori, H.; Maruyama, Z.; Miyazaki, T.; Kawasaki, K.; Furuichi, T.; Fukuyama, R.; Mori, M.; Yamana, K.; Nakamura, K.; et al. Sp7 inhibits osteoblast differentiation at a late stage in mice. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Komori, T. Runx2, an inducer of osteoblast and chondrocyte differentiation. Histochem. Cell Biol. 2018, 149, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lin, T.; Lian, N.; Tao, H.; Li, C.; Li, L.; Yang, X. Hop2 Interacts with ATF4 to promote osteoblast differentiation. J. Bone Miner. Res. 2019, 34, 2287–2300. [Google Scholar] [CrossRef]
- Samee, N.; Geoffroy, V.; Marty, C.; Schiltz, C.; Vieux-Rochas, M.; Levi, G.; De Vernejoul, M.C. Dlx5, a positive regulator of osteoblastogenesis, is essential for osteoblast-osteoclast coupling. Am. J. Pathol. 2008, 173, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Goto, N.; Fujimoto, K.; Fujii, S.; Ida-Yonemochi, H.; Ohshima, H.; Kawamoto, T.; Noshiro, M.; Shukunami, C.; Kozai, K.; Kato, Y. Role of MSX1 in osteogenic differentiation of human dental pulp stem cells. Stem Cells Int. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Ichida, F.; Nishimura, R.; Hata, K.; Matsubara, T.; Ikeda, F.; Hisada, K.; Yatani, H.; Cao, X.; Komori, T.; Yamaguchi, A.; et al. Reciprocal roles of Msx2 in regulation of osteoblast and adipocyte differentiation. J. Biol. Chem. 2004, 279, 34015–34022. [Google Scholar] [CrossRef]
- Vimalraj, S. Alkaline phosphatase: Structure, expression and its function in bone mineralization. Gene 2020, 754, 144855. [Google Scholar] [CrossRef]
- Neve, A.; Corrado, A.; Cantatore, F.P. Osteocalcin: Skeletal and extra-skeletal effects. J. Cell. Physiol. 2013, 228, 1149–1153. [Google Scholar] [CrossRef]
- Kruger, T.E.; Miller, A.H.; Godwin, A.K.; Wang, J. Bone sialoprotein and osteopontin in bone metastasis of osteotropic cancers. Crit. Rev. Oncol. Hematol. 2014, 89, 330–341. [Google Scholar] [CrossRef]
- Rosset, E.M.; Bradshaw, A.D. SPARC/osteonectin in mineralized tissue. Matrix Biol. 2016, 52, 78–87. [Google Scholar] [CrossRef]
- Licini, C.; Vitale-Brovarone, C.; Mattioli-Belmonte, M. Collagen and non-collagenous proteins molecular crosstalk in the pathophysiology of osteoporosis. Cytokine Growth Factor Rev. 2019, 49, 59–69. [Google Scholar] [CrossRef]
- Luukkonen, J.; Hilli, M.; Nakamura, M.; Ritamo, I.; Valmu, L.; Kauppinen, K.; Tuukkanen, J.; Lehenkari, P. Osteoclasts secrete osteopontin into resorption lacunae during bone resorption. Histochem. Cell Biol. 2019, 151, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Cawley, K.M.; Bustamante-Gomez, N.C.; Guha, A.G.; MacLeod, R.S.; Xiong, J.; Gubrij, I.; Liu, Y.; Mulkey, R.; Palmieri, M.; Thostenson, J.D.; et al. Local production of osteoprotegerin by osteoblasts suppresses bone resorption. Cell Rep. 2020, 32, 108052. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Lin, K.; Jiang, X.; Xu, Y.; Zhang, M.; Chang, J.; Zhang, Z. Enhanced osteogenesis through nano-structured surface design of macroporous hydroxyapatite bioceramic scaffolds via activation of ERK and p38 MAPK signaling pathways. J. Mater. Chem. B 2013, 1, 5403–5416. [Google Scholar] [CrossRef]
- Mao, L.; Liu, J.; Zhao, J.; Chang, J.; Xia, L.; Jiang, L.; Wang, X.; Lin, K.; Fang, B. Effect of micro-nano-hybrid structured hydroxyapatite bioceramics on osteogenic and cementogenic differentiation of human periodontal ligament stem cell via Wnt signaling pathway. Int. J. Nanomed. 2015, 10, 7031–7044. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.W.; Park, J.; Habib, M.M.; Beck, G.R. Nano-hydroxyapatite stimulation of gene expression requires fgf receptor, phosphate transporter, and Erk1/2 signaling. ACS Appl. Mater. Interfaces 2017, 9, 39185–39196. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, N.; Moratti, S.C.; Dias, G.J. Hydroxyapatite-polymer biocomposites for bone regeneration: A review of current trends. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 106, 2046–2057. [Google Scholar] [CrossRef]
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium phosphates in biomedical applications: Materials for the future? Mater. Today 2016, 19, 69–87. [Google Scholar] [CrossRef]
- Lin, X.; Patil, S.; Gao, Y.G.; Qian, A. The bone extracellular matrix in bone formation and regeneration. Front. Pharmacol. 2020, 11, 757. [Google Scholar] [CrossRef]
- Kartikasari, N.; Yuliati, A.; Kriswandini, I.L. Compressive strength and porosity tests on bovine hydroxyapatite-gelatin-chitosan scaffolds. Dent. J. Maj. Kedokt. Gigi 2016, 49, 153. [Google Scholar] [CrossRef]
- Zhou, M.; Geng, Y.M.; Li, S.Y.; Yang, X.B.; Che, Y.J.; Pathak, J.L.; Wu, G. Nanocrystalline hydroxyapatite-based scaffold adsorbs and gives sustained release of osteoinductive growth factor and facilitates bone regeneration in mice ectopic model. J. Nanomater. 2019, 2019. [Google Scholar] [CrossRef]
- Anghelescu, V.M.; Neculae, I.; Dincă, O.; Vlădan, C.; Socoliuc, C.; Cioplea, M.; Nichita, L.; Popp, C.; Zurac, S.; Bucur, A. Inflammatory-driven angiogenesis in bone augmentation with bovine hydroxyapatite, B-tricalcium phosphate, and bioglasses: A comparative study. J. Immunol. Res. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Okanoue, Y.; Ikeuchi, M.; Takemasa, R.; Tani, T.; Matsumoto, T.; Sakamoto, M.; Nakasu, M. Comparison of in vivo bioactivity and compressive strength of a novel superporous hydroxyapatite with beta-tricalcium phosphates. Arch. Orthop. Trauma Surg. 2012, 132, 1603–1610. [Google Scholar] [CrossRef]
- Lee, H.R.; Kim, H.J.; Ko, J.S.; Choi, Y.S.; Ahn, M.W.; Kim, S.; Do, S.H. Comparative characteristics of porous bioceramics for an osteogenic response in vitro and in vivo. PLoS ONE 2013, 8, e84272. [Google Scholar] [CrossRef]
- Maenhoudt, W.; Hallaert, G.; Kalala, J.P.; Baert, E.; Dewaele, F.; Bauters, W.; Van Roost, D. Hydroxyapatite cranioplasty: A retrospective evaluation of osteointegration in 17 cases. Acta Neurochir. 2018, 160, 2117–2124. [Google Scholar] [CrossRef] [PubMed]
- Kato, E.; Yamada, M.; Sakurai, K. Retrospective clinical outcome of nanopolymorphic crystalline hydroxyapatite-coated and anodic oxidized titanium implants for 10 years. J. Prosthodont. Res. 2015, 59, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Pieske, O.; Kaltenhauser, F.; Pichlmaier, L.; Schramm, N.; Trentzsch, H.; Löffler, T.; Greiner, A.; Piltz, S. Clinical benefit of hydroxyapatite-coated pins compared with stainless steel pins in external fixation at the wrist: A randomised prospective study. Injury 2010, 41, 1031–1036. [Google Scholar] [CrossRef]
- Chitsazi, M.T.; Shirmohammadi, A.; Faramarzie, M.; Pourabbas, R.; Rostamzadeh, A.N. A clinical comparison of nano-crystalline hydroxyapatite (Ostim) and autogenous bone graft in the treatment of periodontal intrabony defects. Med. Oral. Patol. Oral. Cir. Bucal. 2011, 16, 448–453. [Google Scholar] [CrossRef]
- Mohd Pu’ad, N.A.S.; Abdul Haq, R.H.; Mohd Noh, H.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Synthesis method of hydroxyapatite: A review. Mater. Today Proc. 2020, 29, 233–239. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphates as bioceramics: State of the art. J. Funct. Biomater. 2010, 1, 22–107. [Google Scholar] [CrossRef]
- Hokmabad, V.R.; Davaran, S.; Aghazadeh, M.; Alizadeh, E.; Salehi, R.; Ramazani, A. A Comparison of the Effects of Silica and Hydroxyapatite Nanoparticles on Poly(ε-caprolactone)-Poly(ethylene glycol)-Poly(ε-caprolactone)/Chitosan Nanofibrous Scaffolds for Bone Tissue Engineering. Tissue Eng. Regen. Med. 2018, 15, 735–750. [Google Scholar] [CrossRef]
- Mohd Pu’ad, N.A.S.; Koshy, P.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Syntheses of hydroxyapatite from natural sources. Heliyon 2019, 5, e01588. [Google Scholar] [CrossRef]
- Rana, M.; Akhtar, N.; Rahman, S.; Jamil, H.M.; Asaduzzaman, S.M. Extraction of Hydroxyapatite from Bovine and Human Cortical Bone by Thermal Decomposition and Effect of Gamma Radiation: A Comparative Study. Int. J. Complement. Altern. Med. 2017, 8. [Google Scholar] [CrossRef]
- Chen, F.; Wang, M.; Wang, J.; Chen, X.; Li, X.; Xiao, Y.; Zhang, X. Effects of hydroxyapatite surface nano/micro-structure on osteoclast formation and activity. J. Mater. Chem. B 2019, 7, 7574–7587. [Google Scholar] [CrossRef]
- Liu, J.; Mao, K.; Liu, Z.; Wang, X.; Cui, F.; Guo, W.; Mao, K.; Yang, S. Injectable Biocomposites for Bone Healing in Rabbit Femoral Condyle Defects. PLoS ONE 2013, 8. [Google Scholar] [CrossRef][Green Version]
- Chandran, S.; Suresh Babu, S.; Hari Krishnan, V.S.; Varma, H.K.; John, A. Osteogenic efficacy of strontium hydroxyapatite micro-granules in osteoporotic rat model. J. Biomater. Appl. 2016, 31, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Schlagenhauf, U.; Kunzelmann, K.H.; Hannig, C.; May, T.W.; Hösl, H.; Gratza, M.; Viergutz, G.; Nazet, M.; Schamberger, S.; Proff, P. Impact of a non-fluoridated microcrystalline hydroxyapatite dentifrice on enamel caries progression in highly caries-susceptible orthodontic patients: A randomized, controlled 6-month trial. J. Investig. Clin. Dent. 2019, 10, e12399. [Google Scholar] [CrossRef]
- Paszynska, E.; Pawinska, M.; Gawriolek, M.; Kaminska, I.; Otulakowska-Skrzynska, J.; Marczuk-Kolada, G.; Rzatowski, S.; Sokolowska, K.; Olszewska, A.; Schlagenhauf, U.; et al. Impact of a toothpaste with microcrystalline hydroxyapatite on the occurrence of early childhood caries: A 1-year randomized clinical trial. Sci. Rep. 2021, 11, 1–15. [Google Scholar] [CrossRef]
- Harks, I.; Jockel-Schneider, Y.; Schlagenhauf, U.; May, T.W.; Gravemeier, M.; Prior, K.; Petersilka, G.; Ehmke, B. Impact of the daily use of a microcrystal hydroxyapatite dentifrice on de novo plaque formation and clinical/microbiological parameters of periodontal health. A randomized trial. PLoS ONE 2016, 11, e0160142. [Google Scholar] [CrossRef]
- Hatakeyama, W.; Taira, M.; Chosa, N.; Kihara, H.; Ishisaki, A.; Kondo, H. Effects of apatite particle size in two apatite/collagen composites on the osteogenic differentiation profile of osteoblastic cells. Int. J. Mol. Med. 2013, 32, 1255–1261. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Liu, X.; Zhang, R.; Feng, Q. In vitro uptake of hydroxyapatite nanoparticles and their effect on osteogenic differentiation of human mesenchymal stem cells. Stem Cells Int. 2018, 2018. [Google Scholar] [CrossRef]
- Weißenböck, M.; Stein, E.; Undt, G.; Ewers, R.; Lauer, G.; Turhani, D. Particle size of hydroxyapatite granules calcified from red algae affects the osteogenic potential of human mesenchymal stem cells in vitro. Cells Tissues Organs 2006, 182, 79–88. [Google Scholar] [CrossRef]
- Shapoff, C.A.; Bowers, G.M.; Levy, B.; Mellonig, J.T.; Yukna, R.A. The Effect of Particle Size on the Osteogenic Activity of Composite Grafts of Allogeneic Freeze-Dried Bone and Autogenous Marrow. J. Periodontol. 1980, 51, 625–630. [Google Scholar] [CrossRef]
- Daugela, P.; Pranskunas, M.; Juodzbalys, G.; Liesiene, J.; Baniukaitiene, O.; Afonso, A.; Sousa Gomes, P. Novel cellulose/hydroxyapatite scaffolds for bone tissue regeneration: In vitro and in vivo study. J. Tissue Eng. Regen. Med. 2018, 12, 1195–1208. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, F.; Ferreira, M.R.; Fontes, G.N.; da Costa Fernandes, C.J.; Andia, D.C.; Cruz, N.C.; da Silva, R.A.; Zambuzzi, W.F. Nano hydroxyapatite-blasted titanium surface affects pre-osteoblast morphology by modulating critical intracellular pathways. Biotechnol. Bioeng. 2017, 114, 1888–1898. [Google Scholar] [CrossRef]
- Parisi, L.; Toffoli, A.; Ghezzi, B.; Mozzoni, B.; Lumetti, S.; Macaluso, G.M. A glance on the role of fibronectin in controlling cell response at biomaterial interface. Jpn. Dent. Sci. Rev. 2020, 56, 50–55. [Google Scholar] [CrossRef]
- Mahato, A.; Sandy, Z.; Bysakh, S.; Hupa, L.; Das, I.; Bhattacharjee, P.; Kundu, B.; De, G.; Nandi, S.K.; Vallittu, P.; et al. Development of nano-porous hydroxyapatite coated e-glass for potential bone-tissue engineering application: An in vitro approach. Mater. Sci. Eng. C 2020, 111, 110764. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, M.; Chen, F.; Wei, Y.; Chen, X.; Zhou, Y.; Yang, X.; Zhu, X.; Tu, C.; Zhang, X. Nano-hydroxyapatite coating promotes porous calcium phosphate ceramic-induced osteogenesis via BMP/SMAD signaling pathway. Int. J. Nanomed. 2019, 14, 7987–8000. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.N.Y.; Genge, B.R.; Dunkelberger, D.G.; LeGeros, R.Z.; Concannon, B.; Wuthier, R.E. Physicochemical characterization of the nucleational core of matrix vesicles. J. Biol. Chem. 1997, 272, 4404–4411. [Google Scholar] [CrossRef] [PubMed]
- Anderson, H.C.; Garimella, R.; Taugue, S.E. The role of matrix vesicles in growth plate development and biomineralization. Bone 2005, 10, 822–837. [Google Scholar] [CrossRef] [PubMed]
- Rutkovskiy, A.; Stensløkken, K.-O.; Vaage, I.J. Osteoblast Differentiation at a Glance. Med. Sci. Monit. Basic Res. 2016, 22, 95–106. [Google Scholar] [CrossRef]
- Komori, T.; Yagi, H.; Nomura, S.; Yamaguchi, A.; Sasaki, K.; Deguchi, K.; Shimizu, Y.; Bronson, R.T.; Gao, Y.H.; Inada, M.; et al. Targeted Disruption of Cbfa1 Results in a Complete Lack of Bone Formation owing to Maturational Arrest of Osteoblasts. Cell 1997, 89, 755–764. [Google Scholar] [CrossRef]
- Otto, F.; Thornell, A.P.; Crompton, T.; Denzel, A.; Gilmour, K.C.; Rosewell, I.R.; Stamp, G.W.H.; Beddington, R.S.P.; Mundlos, S.; Olsen, B.R.; et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell 1997, 89, 765–771. [Google Scholar] [CrossRef]
- Maeno, T.; Moriishi, T.; Yoshida, C.A.; Komori, H.; Kanatani, N.; Izumi, S.-I.; Takaoka, K.; Komori, T. Early onset of Runx2 expression caused craniosynostosis, ectopic bone formation, and limb defects. Bone 2011, 49, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Beck, G.R. Inorganic phosphate as a signaling molecule in osteoblast differentiation. J. Cell. Biochem. 2003, 90, 234–243. [Google Scholar] [CrossRef]
- Liu, W.; Toyosawa, S.; Furuichi, T.; Kanatani, N.; Yoshida, C.; Liu, Y.; Himeno, M.; Narai, S.; Yamaguchi, A.; Komori, T. Overexpression of Cbfa1 in osteoblasts inhibits osteoblast maturation and causes osteopenia with multiple fractures. J. Cell Biol. 2001, 155, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; Crombrugghe, B. de The Novel Zinc Finger-Containing Transcription Factor Osterix Is Required for Osteoblast Differentiation and Bone Formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef]
- Tang, W.; Li, Y.; Osimiri, L.; Zhang, C. Osteoblast-specific transcription factor osterix (Osx) is an upstream regulator of Satb2 during bone formation. J. Biol. Chem. 2011, 286, 32995–33002. [Google Scholar] [CrossRef] [PubMed]
- Baek, W.Y.; de Crombrugghe, B.; Kim, J.E. Postnatally induced inactivation of Osterix in osteoblasts results in the reduction of bone formation and maintenance. Bone 2010, 46, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Park, S.Y.; Lee, S.J.; Boo, Y.C.; Choi, J.Y.; Kim, J.E. Ucma, a direct transcriptional target of Runx2 and Osterix, promotes osteoblast differentiation and nodule formation. Osteoarthr. Cartil. 2015, 23, 1421–1431. [Google Scholar] [CrossRef]
- Chen, Q.; Shou, P.; Zheng, C.; Jiang, M.; Cao, G.; Yang, Q.; Cao, J.; Xie, N.; Velletri, T.; Zhang, X.; et al. Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death Differ. 2016, 23, 1128–1139. [Google Scholar] [CrossRef]
- Yu, S.; Zhu, K.; Lai, Y.; Zhao, Z.; Fan, J.; Im, H.J.; Chen, D.; Xiao, G. ATF4 promotes β-catenin expression and osteoblastic differentiation of bone marrow mesenchymal stem cells. Int. J. Biol. Sci. 2013, 9, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Jiang, D.; Ge, C.; Zhao, Z.; Lai, Y.; Boules, H.; Phimphilai, M.; Yang, X.; Karsenty, G.; Franceschi, R.T. Cooperative interactions between activating transcription factor 4 and Runx2/Cbfa1 stimulate osteoblast-specific osteocalcin gene expression. J. Biol. Chem. 2005, 280, 30689–30696. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Karsenty, G. ATF4, the osteoblast accumulation of which is determined post-translationally, can induce osteoblast-specific gene expression in non-osteoblastic cells. J. Biol. Chem. 2004, 279, 47109–47114. [Google Scholar] [CrossRef]
- Zhao, G.Q.; Zhao, S.; Zhou, X.; Eberspaecher, H.; Solursh, M.; de Crombrugghe, B. rDlx, a novel distal-less-like homeoprotein is expressed in developing cartilages and discrete neuronal tissue. Dev. Biol. 1994, 164, 37–51. [Google Scholar] [CrossRef]
- Li, H.; Marijanovic, I.; Kronenberg, M.S.; Erceg, I.; Stover, M.L.; Velonis, D.; Mina, M.; Heinrich, J.G.; Harris, S.E.; Upholt, W.B.; et al. Expression and function of Dlx genes in the osteoblast lineage. Dev. Biol. 2008, 316, 458–470. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Park, K.H.; Hwang, J.S.; Lee, M.; Yoon, D.S.; Ryu, H.A.; Jung, H.S.; Park, K.W.; Kim, J.; Park, S.W.; et al. Inhibition of STAT5A promotes osteogenesis by DLX5 regulation. Cell Death Dis. 2018, 9. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, Y.J.; Yoon, W.J.; Kim, J.I.; Kim, B.G.; Hwang, Y.S.; Wozney, J.M.; Chi, X.Z.; Bae, S.C.; Choi, K.Y.; et al. Dlx5 specifically regulates Runx2 type II expression by binding to homeodomain-response elements in the Runx2 distal promoter. J. Biol. Chem. 2005, 280, 35579–35587. [Google Scholar] [CrossRef]
- Bennett, C.N.; Longo, K.A.; Wright, W.S.; Suva, L.J.; Lane, T.F.; Hankenson, K.D.; MacDougald, O.A. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc. Natl. Acad. Sci. USA 2005, 102, 3324–3329. [Google Scholar] [CrossRef]
- Towler, A.; Rutledge, S.J.; Rodan, G.A. Regulator of the rat osteocalcin promoter. Mol. Endocrinol. 1994, 8, 1484–1493. [Google Scholar]
- Orestes-Cardoso, S.; Nefussi, J.R.; Lezot, F.; Oboeuf, M.; Pereira, M.; Mesbah, M.; Robert, B.; Berdal, A. Msx1 is a regulator of bone formation during development and postnatal growth: In vivo investigations in a transgenic mouse model. Connect. Tissue Res. 2002, 43, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Jabs, E.W.; Müller, U.; Li, X.; Ma, L.; Luo, W.; Haworth, I.S.; Klisak, I.; Sparkes, R.; Warman, M.L.; Mulliken, J.B.; et al. A mutation in the homeodomain of the human MSX2 gene in a family affected with autosomal dominant craniosynostosis. Cell 1993, 75, 443–450. [Google Scholar] [CrossRef]
- Gersch, R.P.; Lombardo, F.; McGovern, S.C.; Hadjiargyrou, M. Reactivation of Hox gene expression during bone regeneration. J. Orthop. Res. 2005, 23, 882–890. [Google Scholar] [CrossRef]
- Burns, J.S.; Rasmussen, P.L.; Larsen, K.H.; Schrøder, H.D.; Kassem, M. Parameters in three-dimensional osteospheroids of telomerized human mesenchymal (Stromal) stem cells grown on osteoconductive scaffolds that predict in vivo bone-forming potential. Tissue Eng. Part A 2010, 16, 2331–2342. [Google Scholar] [CrossRef]
- Bhargav, A.; Min, K.S.; Wen Feng, L.; Fuh, J.Y.H.; Rosa, V. Taguchi’s methods to optimize the properties and bioactivity of 3D printed polycaprolactone/mineral trioxide aggregate scaffold: Theoretical predictions and experimental validation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 108, 629–637. [Google Scholar] [CrossRef]
- Orimo, H. The mechanism of mineralization and the role of alkaline phosphatase in health and disease. J. Nippon Med. Sch. 2010, 77, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Blair, H.C.; Larrouture, Q.C.; Li, Y.; Lin, H.; Beer-Stoltz, D.; Liu, L.; Tuan, R.S.; Robinson, L.J.; Schlesinger, P.H.; Nelson, D.J. Osteoblast differentiation and bone matrix formation in vivo and in vitro. Tissue Eng. Part B Rev. 2017, 23, 268–280. [Google Scholar] [CrossRef]
- Wennberg, C.; Hessle, L.; Lundberg, P.; Mauro, S.; Narisawa, S.; Lerner, U.H.; Millán, J.L. Functional characterization of osteoblasts and osteoclasts from alkaline phosphatase knockout mice. J. Bone Miner. Res. 2000, 15, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Fedde, K.N.; Blair, L.; Silverstein, J.; Coburn, S.P.; Ryan, L.M.; Weinstein, R.S.; Waymire, K.; Narisawa, S.; Millán, J.L.; Macgregor, G.R.; et al. Alkaline Phosphatase Knock-Out Mice Recapitulate the Metabolic and Skeletal Defects of Infantile Hypophosphatasia. J. Bone Miner. Res. 1999, 14, 2015–2026. [Google Scholar] [CrossRef]
- Stamov, D.R.; Pompe, T. Structure and function of ECM-inspired composite collagen type i scaffolds. Soft Matter 2012, 8, 10200–10212. [Google Scholar] [CrossRef]
- Kern, B.; Shen, J.; Starbuck, M.; Karsenty, G. Cbfa1 Contributes to the Osteoblast-specific Expression of type I collagen Genes. J. Biol. Chem. 2001, 276, 7101–7107. [Google Scholar] [CrossRef] [PubMed]
- Bosetti, M.; Zanardi, L.; Hench, L.; Cannas, M. Type I collagen production by osteoblast-like cells cultured in contact with different bioactive glasses. J. Biomed. Mater. Res. Part A 2002, 64, 189–195. [Google Scholar] [CrossRef]
- Mizuno, M.; Kuboki, Y. Osteoblast-related gene expression of bone marrow cells during the osteoblastic differentiation induced by type I collagen. J. Biochem. 2001, 129, 133–138. [Google Scholar] [CrossRef]
- Hunter, G.K. Role of osteopontin in modulation of hydroxyapatite formation. Calcif. Tissue Int. 2013, 93, 348–354. [Google Scholar] [CrossRef]
- Singh, A.; Gill, G.; Kaur, H.; Amhmed, M.; Jakhu, H. Role of osteopontin in bone remodeling and orthodontic tooth movement: A review. Prog. Orthod. 2018, 19. [Google Scholar] [CrossRef]
- Inman, C.K.; Shore, P. The osteoblast transcription factor Runx2 is expressed in mammary epithelial cells and mediates osteopontin expression. J. Biol. Chem. 2003, 278, 48684–48689. [Google Scholar] [CrossRef]
- Chellaiah, M.A.; Hruska, K.A. The integrin αvβ3 and CD44 regulate the actions of osteopontin on osteoclast motility. Calcif. Tissue Int. 2003, 72, 197–205. [Google Scholar] [CrossRef]
- Ducy, P.; Desbois, C.; Boyce, B.; Pinero, G.; Story, B.; Dunstan, C.; Smith, E.; Bonadio, J.; Goldstein, S.; Gundberg, C.; et al. Increased bone formation in osteocalcin-deficient mice. Nature 1996, 382, 448–452. [Google Scholar] [CrossRef]
- Moser, S.C.; van der Eerden, B.C.J. Osteocalcin—A versatile bone-derived hormone. Front. Endocrinol. 2019, 10, 4–9. [Google Scholar] [CrossRef]
- Delany, A.M.; Amling, M.; Priemel, M.; Howe, C.; Baron, R.; Canalis, E. Osteopenia and decreased bone formation in osteonectin-deficient mice. J. Clin. Investig. 2000, 105, 915–923. [Google Scholar] [CrossRef]
- Mundlos, S.; Schwahn, B.; Reichert, T.; Zabel, B. Distribution of osteonectin mRNA and protein during human embryonic and fetal development. J. Histochem. Cytochem. 1992, 40, 283–291. [Google Scholar] [CrossRef]
- Delany, A.M.; Kalajzic, I.; Bradshaw, A.D.; Sage, E.H.; Canalis, E. Osteonectin-null mutation compromises osteoblast formation, maturation, and survival. Endocrinology 2003, 144, 2588–2596. [Google Scholar] [CrossRef]
- Jang, H.J.; Lee, E.C.; Kwon, G.J.; Seo, Y.K. The effect of coated nano-hydroxyapatite concentration on scaffolds for osteogenesis. J. Biomater. Appl. 2019, 34, 827–839. [Google Scholar] [CrossRef]
- Li, Y.; Toraldo, G.; Li, A.; Yang, X.; Zhang, H.; Qian, W.P.; Weitzmann, M.N. B cells and T cells are critical for the preservation of bone homeostasis and attainment of peak bone mass in vivo. Blood 2007, 109, 3839–3848. [Google Scholar] [CrossRef] [PubMed]
- Kramer, I.; Halleux, C.; Keller, H.; Pegurri, M.; Gooi, J.H.; Weber, P.B.; Feng, J.Q.; Bonewald, L.F.; Kneissel, M. Osteocyte Wnt/β-catenin signaling is required for normal bone homeostasis. Mol. Cell. Biol. 2010, 30, 3071–3085. [Google Scholar] [CrossRef]
- Rochette, L.; Meloux, A.; Rigal, E.; Zeller, M.; Cottin, Y.; Vergely, C. The role of osteoprotegerin and its ligands in vascular function. Int. J. Mol. Sci. 2019, 20, 705. [Google Scholar] [CrossRef]
- Thomas, G.P.; Baker, S.U.K.; Eisman, J.A.; Gardiner, E.M. Changing RANKL/OPG mRNA expression in differentiating murine primary osteoblasts. J. Endocrinol. 2001, 170, 451–460. [Google Scholar] [CrossRef]
- Bolon, B.; Grisanti, M.; Villasenor, K.; Morony, S.; Feige, U.; Simonet, W.S. Generalized degenerative joint disease in osteoprotegerin (Opg) null mutant mice. Vet. Pathol. 2015, 52, 873–882. [Google Scholar] [CrossRef]
- Prahasanti, C.; Subrata, L.H.; Saskianti, T.; Suardita, K.; Ernawati, D.S. Combined hydroxyapatite scaffold and stem cell from human exfoliated deciduous teeth modulating alveolar bone regeneration via regulating receptor activator of nuclear factor-Κb and osteoprotegerin system. Iran. J. Med. Sci. 2019, 44, 415–421. [Google Scholar] [CrossRef]
- Hunter, G.K.; Goldberg, H.A. Modulation of crystal formation by bone phosphoproteins: Role of glutamic acid-rich sequences in the nucleation of hydroxyapatite by bone sialoprotein. Biochem. J. 1994, 302, 175–179. [Google Scholar] [CrossRef]
- Gordon, J.A.R.; Tye, C.E.; Sampaio, A.V.; Underhill, T.M.; Hunter, G.K.; Goldberg, H.A. Bone sialoprotein expression enhances osteoblast differentiation and matrix mineralization in vitro. Bone 2007, 41, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Malaval, L.; Wade-Guéye, N.M.; Boudiffa, M.; Fei, J.; Zirngibl, R.; Chen, F.; Laroche, N.; Roux, J.P.; Burt-Pichat, B.; Duboeuf, F.; et al. Bone sialoprotein plays a functional role in bone formation and osteoclastogenesis. J. Exp. Med. 2008, 205, 1145–1153. [Google Scholar] [CrossRef]
- Johnson, G.L.; Lapadat, R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002, 298, 1911–1912. [Google Scholar] [CrossRef]
- Song, J.H.; Kim, J.H.; Park, S.; Kang, W.; Kim, H.W.; Kim, H.E.; Jang, J.H. Signaling responses of osteoblast cells to hydroxyapatite: The activation of ERK and SOX9. J. Bone Miner. Metab. 2008, 26, 138–142. [Google Scholar] [CrossRef]
- Xu, D.; Wan, Y.; Li, Z.; Wang, C.; Zou, Q.; Du, C.; Wang, Y. Tailorable hierarchical structures of biomimetic hydroxyapatite micro/nano particles promoting endocytosis and osteogenic differentiation of stem cells. Biomater. Sci. 2020, 8, 3286–3300. [Google Scholar] [CrossRef]
- Hiragami, F.; Akiyama, J.; Koike, Y.; Kano, Y. Enhancement of hydroxyapatite-mediated three-dimensional-like proliferation of mouse fibroblasts by heat treatment: Effects of heat shock-induced p38 MAPK pathway. J. Biomed. Mater. Res. Part A 2005, 74, 705–711. [Google Scholar] [CrossRef]
- Gong, L.; Liu, Y.; Qian, J.; Ni, Z.; Fang, W. Hydroxyapatite nanocrystals stimulate osteogenic differentiation in primary human aortic smooth muscle cells by activation of oxidative stress and the ERK pathway. Int. J. Clin. Exp. Pathol. 2017, 10, 7726–7733. [Google Scholar]
- Hu, Y.; Chan, E.; Wang, S.X.; Li, B. Activation of p38 mitogen-activated protein kinase is required for osteoblast differentiation. Endocrinology 2003, 144, 2068–2074. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.S.; Gu, Y.; Jiang, C.; Chen, L. Osteonectin regulates the extracellular matrix mineralization of osteoblasts through P38 signaling pathway. J. Cell. Physiol. 2019, 235, 2220–2231. [Google Scholar] [CrossRef]
- Suto, M.; Nemoto, E.; Kanaya, S.; Suzuki, R.; Tsuchiya, M.; Shimauchi, H. Nanohydroxyapatite increases BMP-2 expression via a p38 MAP kinase dependent pathway in periodontal ligament cells. Arch. Oral Biol. 2013, 58, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Liu, D.; Fong, C.-C.; Zhang, J.; Yang, M. Gold nanoparticles promote osteogenic differentiation of mesenchymal stem cells through p38 MAPK pathway. ACS Nano 2010, 4, 6439–6448. [Google Scholar] [CrossRef]
- Gong, W.; Dong, Y.; Wang, S.; Gao, X.; Chen, X. A novel nano-sized bioactive glass stimulates osteogenesis via the MAPK pathway. RSC Adv. 2017, 7, 13760–13767. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, D.; Zhang, J.; Fong, C.; Yang, M. Gold nanoparticles stimulate differentiation and mineralization of primary osteoblasts through the ERK/MAPK signaling pathway. Mater. Sci. Eng. C 2014, 42, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.; Kneissel, M. WNT signaling in bone homeostasis and disease: From human mutations to treatments. Nat. Med. 2013, 19, 179–192. [Google Scholar] [CrossRef]
- Hu, L.; Yin, C.; Zhao, F.; Ali, A.; Ma, J.; Qian, A. Mesenchymal stem cells: Cell fate decision to osteoblast or adipocyte and application in osteoporosis treatment. Int. J. Mol. Sci. 2018, 19, 360. [Google Scholar] [CrossRef]
- Zhang, R.; Oyajobi, B.O.; Harris, S.E.; Chen, D.; Tsao, C.; Deng, H.-W.; Zhao, M. Wnt/β-catenin signaling activates bone morphogenetic protein 2 expression in osteoblasts. Bone 2013, 52, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.C.; Hsu, H.C.; Hsu, S.K.; Chang, Y.C.; Ho, W.F. Synthesis of hydroxyapatite from eggshell powders through ball milling and heat treatment. J. Asian Ceram. Soc. 2016, 4, 85–90. [Google Scholar] [CrossRef]
- Gani, M.A.; Nurhan, A.D.; Budiatin, A.S.; Siswodihardjo, S.; Khotib, J. Predicting the molecular mechanism of glucosamine in accelerating bone defect repair by stimulating osteogenic proteins. J. Basic Clin. Physiol. Pharmacol. 2021, in press. [Google Scholar]
- Tang, Z.; Wang, Z.; Qing, F.; Ni, Y.; Fan, Y.; Tan, Y.; Zhang, X. Bone morphogenetic protein Smads signaling in mesenchymal stem cells affected by osteoinductive calcium phosphate ceramics. J. Biomed. Mater. Res. Part A 2014, 103, 1001–1010. [Google Scholar] [CrossRef]
- Nahar-Gohad, P.; Gohad, N.; Tsai, C.C.; Bordia, R.; Vyavahare, N. Rat Aortic Smooth Muscle Cells Cultured on Hydroxyapatite Differentiate into Osteoblast-Like Cells via BMP-2–SMAD-5 Pathway. Calcif. Tissue Int. 2015, 96, 359–369. [Google Scholar] [CrossRef]
- Michigami, T. Skeletal mineralization: Mechanisms and diseases. Ann. Pediatr. Endocrinol. Metab. 2019, 24, 213–219. [Google Scholar] [CrossRef]
- Bunocci, E. The locus of initial calcification in cartilage. Clin. Orthop. Relat. Res. 1971, 78, 108–139. [Google Scholar] [CrossRef]
- Martin, K.J.; González, E.A. Metabolic bone disease in chronic kidney disease. J. Am. Soc. Nephrol. 2007, 18, 875–885. [Google Scholar] [CrossRef]
- Remya, N.S.; Syama, S.; Gayathri, V.; Varma, H.K.; Mohanan, P.V. An in vitro study on the interaction of hydroxyapatite nanoparticles and bone marrow mesenchymal stem cells for assessing the toxicological behaviour. Colloids Surf. B Biointerfaces 2014, 117, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Liu, C.; Wei, J.; Sun, J. Effects of four types of hydroxyapatite nanoparticles with different nanocrystal morphologies and sizes on apoptosis in rat osteoblasts. J. Appl. Toxicol. 2012, 32, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.W.; Jang, H.L.; Nam, K.T.; Beck, G.R. Nano-hydroxyapatite modulates osteoblast lineage commitment by stimulation of DNA methylation and regulation of gene expression. Biomaterials 2015, 65, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Nosouhian, S.; Razavi, M.; Jafaripozve, N.; Rismanchian, M. Comparative evaluation of hydroxyapatite and nano-bioglass in two forms of conventional micro- and nano-particles in repairing bone defects (an animal study). Indian J. Dent. Res. 2015, 26, 366–371. [Google Scholar]
- Weiner, S.; Price, P.A. Disaggregation of bone into crystals. Calcif. Tissue Int. 1986, 39, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Guisbiers, G.; Mejía-Rosales, S.; Leonard Deepak, F. Nanomaterial properties: Size and shape dependencies. J. Nanomater. 2012, 2012, 2012–2014. [Google Scholar] [CrossRef]
- Jung, G.Y.; Park, Y.J.; Han, J.S. Effects of HA released calcium ion on osteoblast differentiation. J. Mater. Sci. Mater. Med. 2010, 21, 1649–1654. [Google Scholar] [CrossRef] [PubMed]
- Agell, N.; Bachs, O.; Rocamora, N.; Villalonga, P. Modulation of the Ras/Raf/MEK/ERK pathway by Ca2+, and calmodulin. Cell. Signal. 2002, 14, 649–654. [Google Scholar] [CrossRef]
| Signaling Pathway | Upregulated Proteins/Genes | HA Characteristics | Technique | References |
|---|---|---|---|---|
| ERK | ALP | Micro/nano flake-like HA | qRT-PCR, Western blot | [117] |
| BMP-2 | Micro-nano-hybrid surface | Pharmacologic inhibitors, Western blot | [26] | |
| BSP | Micro-nano-hybrid surface | Pharmacologic inhibitors, Western blot | [26] | |
| OCN | Micro-nano-hybrid surface, micro/nano flake-like HA | Pharmacologic inhibitors, qRT-PCR, Western blot | [26,117] | |
| OPN | Rod-like shaped (10 nm in width and 100 nm in length) | Pharmacologic inhibitors, Western blot | [28] | |
| Osx | Nanosized HA (<200 nm) | Pharmacologic inhibitors, Western blot | [119] | |
| Runx2 | Micro-nano-hybrid surface, nanosized HA (<200 nm), micro/nano flake-like HA | Pharmacologic inhibitors, qRT-PCR, Western blot | [26,117,119] | |
| COL1 | Nanosized HA (<200 nm), micro/nano flake-like HA | Pharmacologic inhibitors, qRT-PCR, Western blot | [117,119] | |
| p38 | BMP-2 | Micro-nano-hybrid surface, nanosized HA (<200 nm) | Pharmacologic inhibitors, Western blot | [26,122] |
| BSP | Micro-nano-hybrid surface | Pharmacologic inhibitors, Western blot | [26] | |
| OCN | Micro-nano-hybrid surface, nanosized HA (<200 nm) | Pharmacologic inhibitors, Western blot | [26,119] | |
| Runx2 | Micro-nano-hybrid surface | Pharmacologic inhibitors, Western blot | [26] | |
| Wnt | ALP | Micro-nano-hybrid surface, nanorod-patterned strontium-doped HA-coated surface (Sr1-HA), HA-Au nanocomposites | Pharmacologic inhibitors, Western blot | [1,5,27] |
| CAP | Micro-nano-hybrid surface | Pharmacologic inhibitors | [27] | |
| CEMP | Micro-nano-hybrid surface | Pharmacologic inhibitors | [27] | |
| LRP5 | Micro-nano-hybrid surface | Pharmacologic inhibitors | [27] | |
| OCN | Micro-nano-hybrid surface, nanorod-patterned strontium-doped HA-coated surface (Sr1-HA), HA-Au nanocomposites | Pharmacologic inhibitors, Western blot | [1,5,27] | |
| OPN | Nanorod-patterned strontium-doped HA-coated surface (Sr1-HA), HA-Au nanocomposites | Pharmacologic inhibitors, Western blot | [1,5] | |
| Osx | HA-coated surface (100 μm in thickness) | Pharmacologic inhibitors, Western blot | [4] | |
| Runx2 | Micro-nano-hybrid surface, HA-coated surface (100 μm in thickness), HA-Au nanocomposites | Pharmacologic inhibitors, Western blot | [1,4,27] | |
| COL1 | Nanorod-patterned strontium-doped HA-coated surface (Sr1-HA) | Pharmacologic inhibitors, Western blot | [5] | |
| Wnt10b | HA-coated surface (100 μm in thickness) | Pharmacologic inhibitors, Western blot | [4] | |
| β-catenin | Micro-nano-hybrid surface, HA-coated surface (100 μm in thickness), HA-Au nanocomposites | Pharmacologic inhibitors, Western blot | [1,4,27] | |
| BMP | ALP | NanoHA-coated surface | qRT-PCR | [60] |
| BMP-2 | NanoHA-coated surface, HA | qRT-PCR, pharmacologic inhibitors | [60,131,132] | |
| BMP-4 | NanoHA-coated surface, HA | qRT-PCR | [60,131] | |
| BMPRI | NanoHA-coated surface | qRT-PCR | [60] | |
| BSP | NanoHA-coated surface, HA | qRT-PCR | [60,131] | |
| Dlx5 | HA | qRT-PCR | [131] | |
| OCN | HA | qRT-PCR | [131] | |
| OPN | NanoHA-coated surface, HA | qRT-PCR | [60,131] | |
| Osx | NanoHA-coated surface, HA | qRT-PCR | [60,131] | |
| Runx2 | NanoHA-coated surface, HA | qRT-PCR | [60,131] | |
| Smad1 | HA | qRT-PCR | [131] | |
| Smad4 | HA | qRT-PCR | [131] | |
| Smad5 | HA | qRT-PCR, pharmacologic inhibitors | [131,132] | |
| COL1 | HA | qRT-PCR | [131] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khotib, J.; Gani, M.A.; Budiatin, A.S.; Lestari, M.L.A.D.; Rahadiansyah, E.; Ardianto, C. Signaling Pathway and Transcriptional Regulation in Osteoblasts during Bone Healing: Direct Involvement of Hydroxyapatite as a Biomaterial. Pharmaceuticals 2021, 14, 615. https://doi.org/10.3390/ph14070615
Khotib J, Gani MA, Budiatin AS, Lestari MLAD, Rahadiansyah E, Ardianto C. Signaling Pathway and Transcriptional Regulation in Osteoblasts during Bone Healing: Direct Involvement of Hydroxyapatite as a Biomaterial. Pharmaceuticals. 2021; 14(7):615. https://doi.org/10.3390/ph14070615
Chicago/Turabian StyleKhotib, Junaidi, Maria Apriliani Gani, Aniek Setiya Budiatin, Maria Lucia Ardhani Dwi Lestari, Erreza Rahadiansyah, and Chrismawan Ardianto. 2021. "Signaling Pathway and Transcriptional Regulation in Osteoblasts during Bone Healing: Direct Involvement of Hydroxyapatite as a Biomaterial" Pharmaceuticals 14, no. 7: 615. https://doi.org/10.3390/ph14070615
APA StyleKhotib, J., Gani, M. A., Budiatin, A. S., Lestari, M. L. A. D., Rahadiansyah, E., & Ardianto, C. (2021). Signaling Pathway and Transcriptional Regulation in Osteoblasts during Bone Healing: Direct Involvement of Hydroxyapatite as a Biomaterial. Pharmaceuticals, 14(7), 615. https://doi.org/10.3390/ph14070615






