Activity and Safety of Immune Checkpoint Inhibitors in Neuroendocrine Neoplasms: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
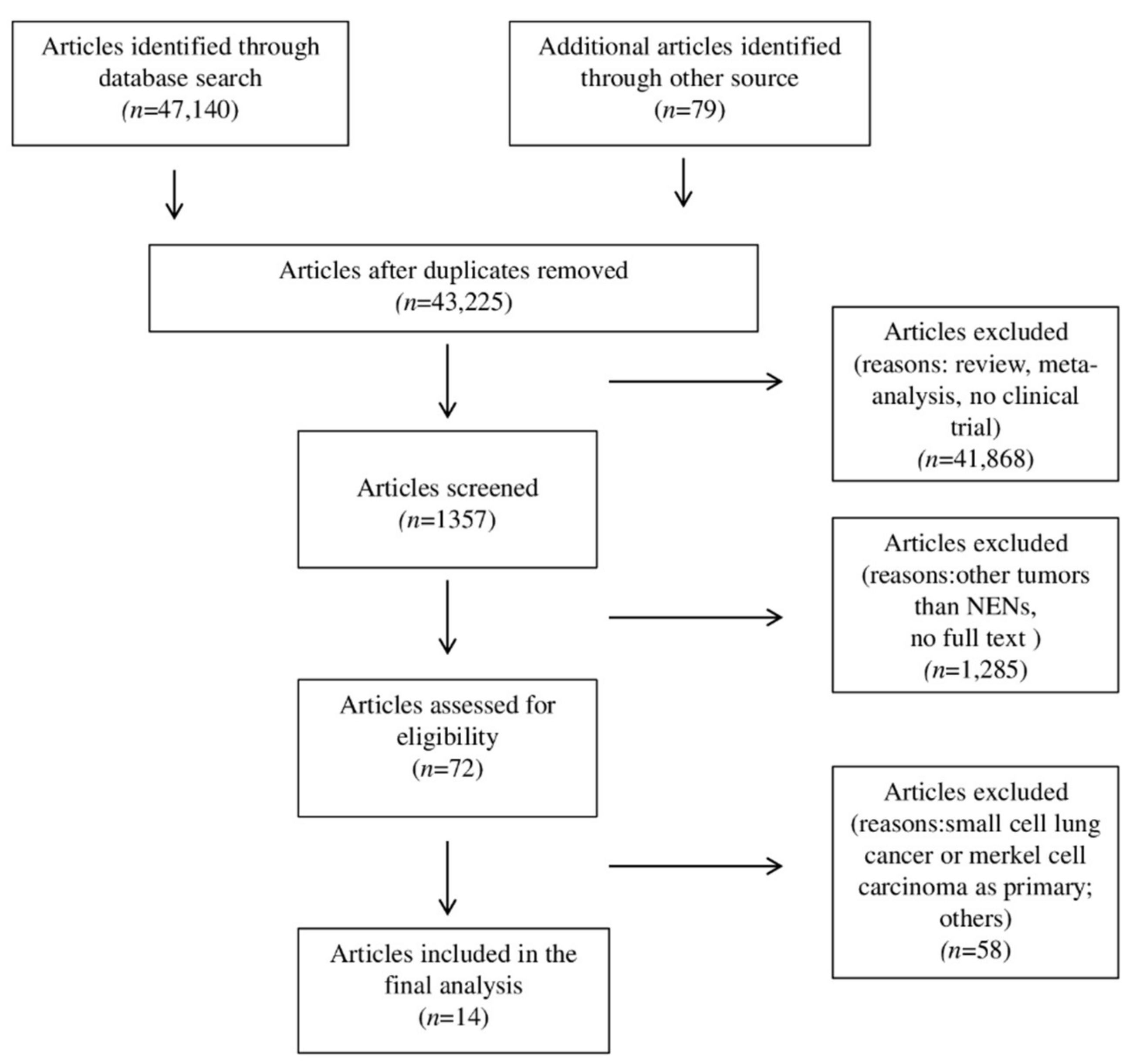
2. Materials and Methods
2.1. Data Extraction
2.2. Quality Assessment
2.3. Statistical Analysis
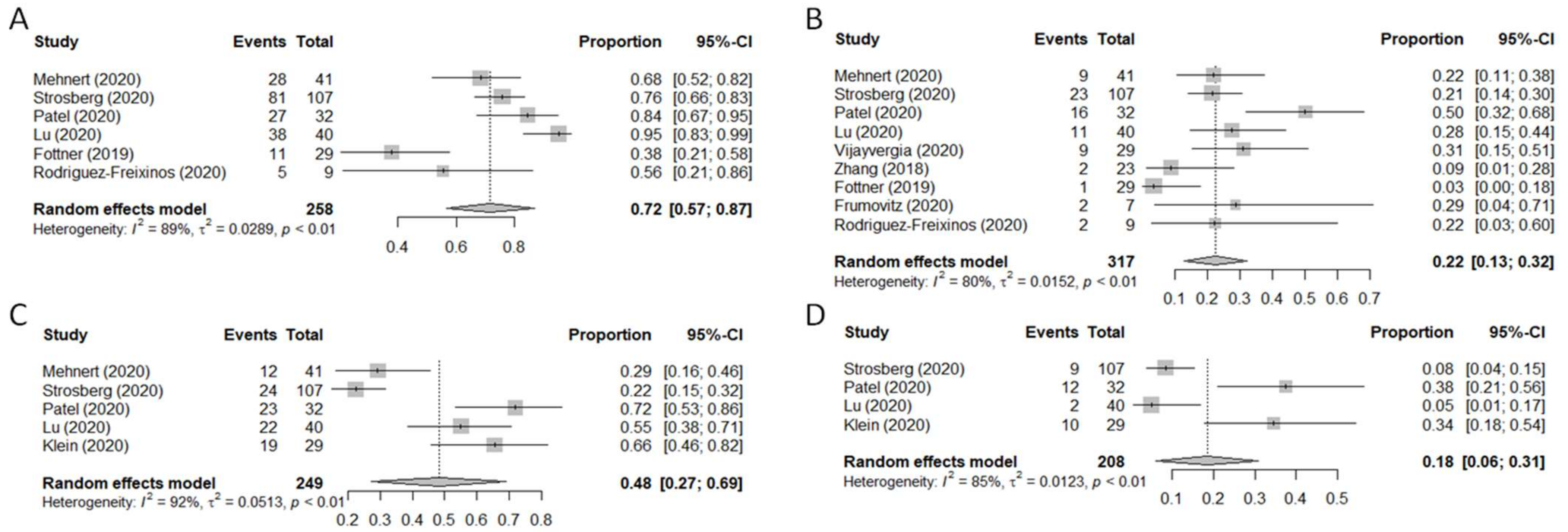
3. Results
3.1. Characteristics of Studies
3.2. Patient Characteristics at Baseline
3.3. Clinical Outcomes
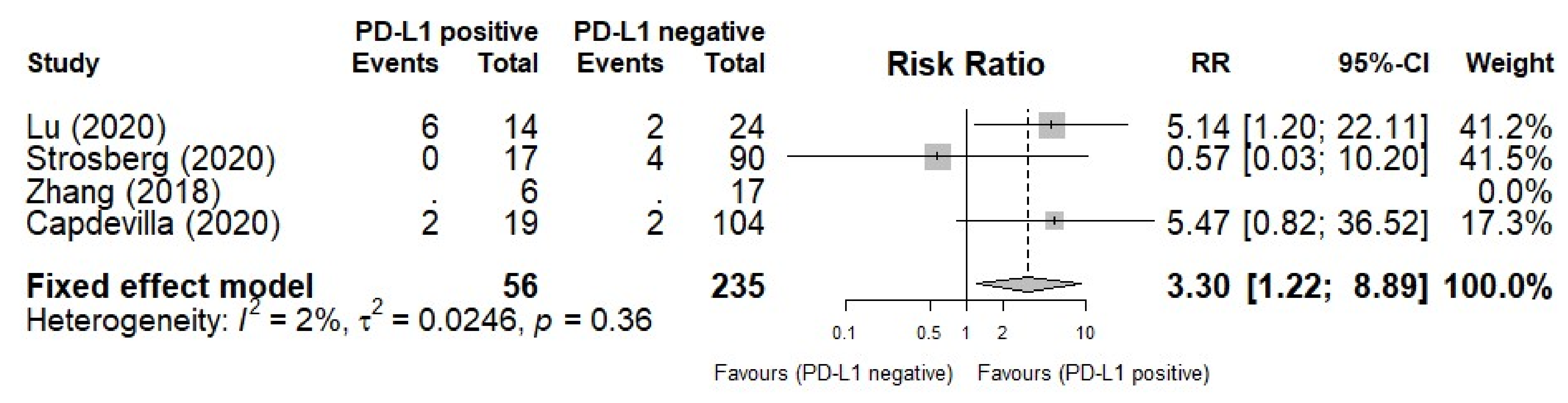
3.4. PD-L1 Biomarker for ORR
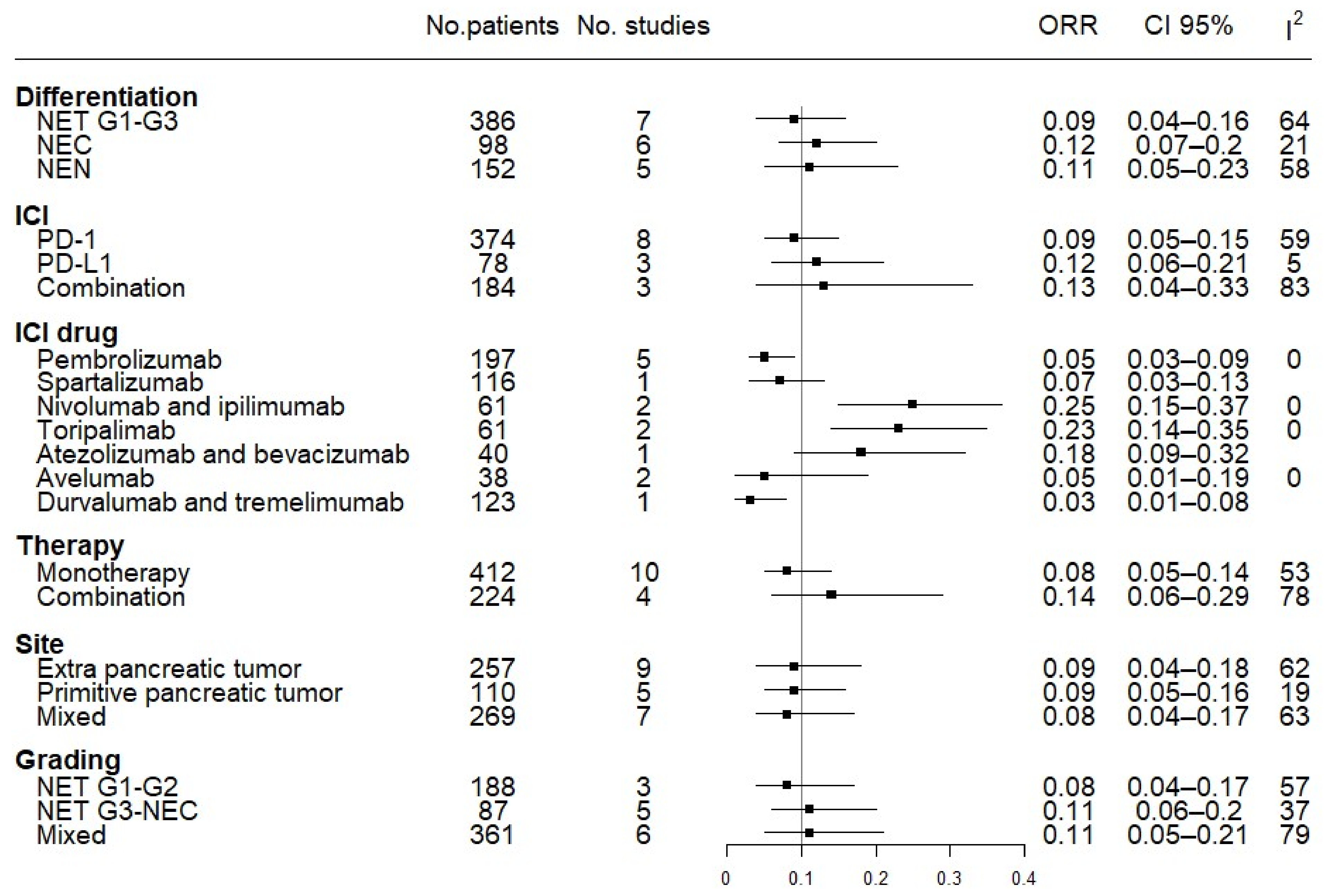
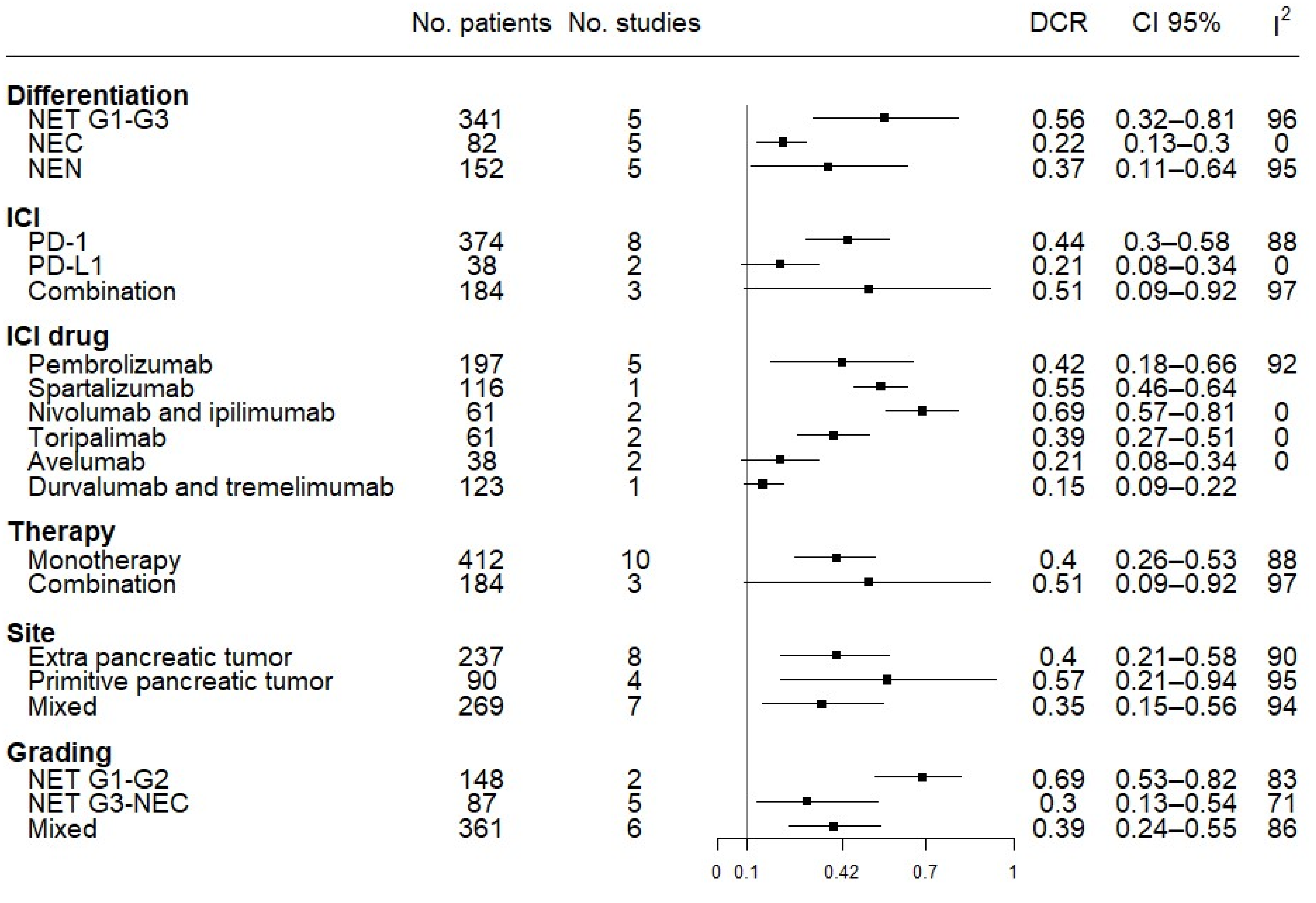
3.5. Subgroup Efficacy Analysis: ORR and DCR
3.6. PFS and OS
3.7. Safety Analysis
3.8. Risk of Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability
Acknowledgments
Conflicts of Interest
References
- Kulke, M.H.; Shah, M.H.; Benson, A.B., III; Bergsland, E.; Berlin, J.D.; Blaszkowsky, L.S.; Emerson, L.; Engstrom, P.F.; Fanta, P.; Giordano, T.; et al. Neuroendocrine tumors, version 1.2015. J. Natl. Compr. Cancer Netw. 2015, 13, 78–108. [Google Scholar] [CrossRef]
- Yao, J.C.; Hassan, M.; Phan, A.; Dagohoy, C.; Leary, C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.N.; Rashid, A.; et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar] [CrossRef]
- Fraenkel, M.; Kim, M.K.; Faggiano, A.; Valk, G.D. Epidemiology of gastroenteropancreatic neuroendocrine tumours. Best Pract. Res. Clin. Gastroenterol. 2012, 26, 691–703. [Google Scholar] [CrossRef] [PubMed]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Kaderli, R.M.; Spanjol, M.; Kollár, A.; Bütikofer, L.; Gloy, V.; Dumont, R.A.; Seiler, C.A.; Christ, E.R.; Radojewski, P.; Briel, M.; et al. Therapeutic options for neuroendocrine tumors: A systematic review and network meta-analysis. JAMA Oncol. 2019, 5, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Caplin, M.E.; Pavel, M.; Ruszniewski, P. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N. Engl. J. Med. 2014, 371, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Caplin, M.E.; Pavel, M.; Ćwikła, J.B.; Phan, A.T.; Raderer, M.; Sedláčková, E.; Cadiot, G.; Wolin, E.M.; Capdevila, J.; Wall, L.; et al. Anti-tumour effects of lanreotide for pancreatic and intestinal neuroendocrine tumours: The CLARINET open-label extension study. Endocr. Relat. Cancer 2016, 23, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Rinke, A.; Müller, H.H.; Schade-Brittinger, C.; Klose, K.J.; Barth, P.; Wied, M.; Mayer, C.; Aminossadati, B.; Pape, U.F.; Bläker, M.; et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: A report from the PROMID Study Group. J. Clin. Oncol. 2009, 27, 4656–4663. [Google Scholar] [CrossRef]
- Rinke, A.; Wittenberg, M.; Schade-Brittinger, C.; Aminossadati, B.; Ronicke, E.; Gress, T.M.; Müller, H.H.; Arnold, R.; PROMID study group. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors (PROMID): Results of long-term survival. Neuroendocrinology 2017, 104, 26–32. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Yao, J.C.; Shah, M.H.; Ito, T.; Bohas, C.L.; Wolin, E.M.; Van Cutsem, E.; Hobday, T.J.; Okusaka, T.; Capdevila, J.; de Vries, E.G.; et al. Everolimus for advanced pancreatic neuroendocrine tumors. N. Engl. J. Med. 2011, 364, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.C.; Pavel, M.; Lombard-Bohas, C.; Van Cutsem, E.; Voi, M.; Brandt, U.; He, W.; Chen, D.; Capdevila, J.; de Vries, E.; et al. Everolimus for the treatment of advanced pancreatic neuroendocrine tumors: Overall survival and circulating biomarkers from the randomized, phase III RADIANT-3 study. J. Clin. Oncol. 2016, 34, 3906–3913. [Google Scholar] [CrossRef]
- Yao, J.C.; Fazio, N.; Singh, S.; Buzzoni, R.; Carnaghi, C.; Wolin, E.; Tomasek, J.; Raderer, M.; Lahner, H.; Voi, M.; et al. RAD001 in advanced neuroendocrine tumours, fourth trial (RADIANT-4) study group. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): A randomised, placebo-controlled, phase 3 study. Lancet 2016, 387, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.E.; Hainsworth, J.D.; Baudin, E.; Peeters, M.; Hörsch, D.; Winkler, R.E.; Klimovsky, J.; Lebwohl, D.; Jehl, V.; Wolin, E.M.; et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): A randomised, placebo-controlled, phase 3 study. Lancet 2011, 378, 2005–2012. [Google Scholar] [CrossRef]
- Raymond, E.; Dahan, L.; Raoul, J.L.; Bang, Y.J.; Borbath, I.; Lombard-Bohas, C.; Valle, J.; Metrakos, P.; Smith, D.; Vinik, A.; et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N. Engl. J. Med. 2011, 364, 501–513. [Google Scholar] [CrossRef]
- Raymond, E.; Kulke, M.H.; Qin, S.; Yu, X.; Schenker, M.; Cubillo, A.; Lou, W.; Tomasek, J.; Thiis-Evensen, E.; Xu, J.M.; et al. Efficacy and safety of sunitinib in patients with well-differentiated pancreatic neuroendocrine tumours. Neuroendocrinology 2018, 107, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Mitry, E.; Baudin, E.; Ducreux, M.; Sabourin, J.C.; Rufié, P.; Aparicio, T.; Aparicio, T.; Lasser, P.; Elias, D.; Duvillard, P.; et al. Treatment of poorly differentiated neuroendocrine tumours with etoposide and cisplatin. Br. J. Cancer 1999, 81, 1351–1355. [Google Scholar] [CrossRef]
- Welin, S.; Sorbye, H.; Sebjornsen, S.; Knappskog, S.; Busch, C.; Oberg, K. Clinical effect of temozolomide-based chemotherapy in poorly differentiated endocrine carcinoma after progression on first-line chemotherapy. Cancer 2011, 117, 4617–4622. [Google Scholar] [CrossRef]
- Hentic, O.; Hammel, P.; Couvelard, A.; Rebours, V.; Zappa, M.; Palazzo, M.; Maire, F.; Goujon, G.; Gillet, A.; Lévy, P.; et al. FOLFIRI regimen: An effective second-line chemotherapy after failure of etoposide-platinum combination in patients with neuroendocrine carcinomas grade 3. Endocr. Relat. Cancer 2012, 19, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Bongiovanni, A.; Riva, N.; Ricci, M.; Liverani, C.; La Manna, F.; De Vita, A.; Foca, F.; Mercatali, L.; Severi, S.; Amadori, D.; et al. First-line chemotherapy in patients with metastatic gastroenteropancreatic neuroendocrine carcinoma. Oncol. Targets Ther. 2015, 8, 3613–3619. [Google Scholar] [CrossRef]
- Robert, C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat. Commun. 2020, 11, 3801. [Google Scholar] [CrossRef] [PubMed]
- Buqué, A.; Bloy, N.; Aranda, F.; Castoldi, F.; Eggermont, A.; Cremer, I.; Fridman, W.H.; Fucikova, J.; Galon, J.; Marabelle, A.; et al. Trial Watch: Immunomodulatory monoclonal antibodies for oncological indications. Oncoimmunology 2015, 4, e1008814. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Wei, R.; Lin, Y.; Kwok, H.F. Clinical and recent patents applications of PD-1/PD-L1 targeting immunotherapy in cancer treatment-current progress, strategy, and future perspective. Front. Immunol. 2020, 11, 1508. [Google Scholar] [CrossRef]
- Tarhini, A.; Lo, E.; Minor, D.R. Releasing the brake on the immune system: Ipilimumab in melanoma and other tumors. Cancer Biother. Radiopharm. 2010, 25, 601–613. [Google Scholar] [CrossRef]
- Rotte, A. Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J. Exp. Clin. Cancer Res. 2019, 38, 255. [Google Scholar] [CrossRef] [PubMed]
- Mehnert, J.M.; Bergsland, E.; O’Neil, B.H.; Santoro, A.; Schellens, J.; Cohen, R.B.; Doi, T.; Ott, P.A.; Pishvaian, M.J.; Puzanov, I.; et al. Pembrolizumab for the treatment of programmed death-ligand 1-positive advanced carcinoid or pancreatic neuroendocrine tumors: Results from the KEYNOTE-028 study. Cancer 2020, 126, 3021–3030. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.; Mizuno, N.; Doi, T.; Grande, E.; Delord, J.P.; Shapira-Frommer, R.; Bergsland, E.; Shah, M.; Fakih, M.; Takahashi, S.; et al. Efficacy and safety of pembrolizumab in previously treated advanced neuroendocrine tumors: Results from the phase II KEYNOTE-158 study. Clin. Cancer Res. 2020, 26, 2124–2130. [Google Scholar] [CrossRef]
- Yao, J.C.; Strosberg, J.; Fazio, N.; Pavel, M.E.; Bergsland, E.; Ruszniewski, P.; Halperin, D.M.; Li, D.; Tafuto, S.; Raj, N.; et al. Spartalizumab in metastatic, well/poorly-differentiated neuroendocrine neoplasms. Endocr. Relat. Cancer 2021. [Google Scholar] [CrossRef]
- Patel, S.P.; Othus, M.; Chae, Y.K.; Giles, F.J.; Hansel, D.E.; Singh, P.P.; Fontaine, A.; Shah, M.H.; Kasi, A.; Baghdadi, T.; et al. A phase II basket trial of dual anti-CTLA-4 and anti-PD-1 blockade in rare tumors (DART SWOG 1609) in patients with nonpancreatic neuroendocrine tumors. Clin. Cancer Res. 2020, 26, 2290–2296. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, P.; Zhang, Y.; Li, Z.; Gong, J.; Li, J.; Li, J.; Li, Y.; Zhang, X.; Lu, Z.; et al. Efficacy, safety, and biomarkers of toripalimab in patients with recurrent or metastatic neuroendocrine neoplasms: A multiple-center phase Ib trial. Clin. Cancer Res. 2020, 26, 2337–2345. [Google Scholar] [CrossRef]
- Vijayvergia, N.; Dasari, A.; Deng, M.; Litwin, S.; Al-Toubah, T.; Alpaugh, R.K.; Dotan, E.; Hall, M.J.; Ross, N.M.; Runyen, M.M.; et al. Pembrolizumab monotherapy in patients with previously treated metastatic high-grade neuroendocrine neoplasms: Joint analysis of two prospective, non-randomised trials. Br. J. Cancer 2020, 122, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Halperin, D.M.; Liu, S.; Dasari, A.; Fogelman, D.R.; Bhosale, P.; Mahvash, A.; Dervin, S.; Estrella, J.; Cortazar, P.; Maru, D.M.; et al. A phase II trial of atezolizumab and bevacizumab in patients with advanced, progressive neuroendocrine tumors (NETs). J. Clin. Oncol. 2020, 38, 619. [Google Scholar] [CrossRef]
- Zhang, P.; Lu, M.; Li, J.; Shen, L. Efficacy and safety of PD-1 blockade with JS001 in patients with advanced neuroendocrine neoplasms: A non-randomized, open-label, phase Ib trial. Ann. Oncol. 2018, 29, viii468. [Google Scholar] [CrossRef]
- Fottner, C.; Apostolidis, L.; Ferrata, M.; Krug, S.; Michl, P.; Schad, A.; Roth, W.; Jaeger, D.; Galle, P.R.; Weber, M.M. A phase II, open label, multicenter trial of avelumab in patients with advanced, metastatic high-grade neuroendocrine carcinomas NEC G3 (WHO 2010) progressive after first-line chemotherapy (AVENEC). J. Clin. Oncol. 2019, 37, 4103. [Google Scholar] [CrossRef]
- Mulvey, C.; Raj, N.P.; Chan, J.A.; Aggarwal, R.R.; Cinar, P.; Hope, T.A.; Kolli, K.; Zhang, L.; Calabrese, S.; Grabowsky, J.A.; et al. Phase II study of pembrolizumab-based therapy in previously treated extrapulmonary poorly differentiated neuroendocrine carcinomas: Results of Part A (pembrolizumab alone). J. Clin. Oncol. 2019, 37, 363. [Google Scholar] [CrossRef]
- Frumovitz, M.; Westin, S.N.; Salvo, G.; Zarifa, A.; Xu, M.; Yap, T.A.; Rodon, A.J.; Karp, D.D.; Abonofal, A.; Jazaeri, A.A.; et al. Phase II study of pembrolizumab efficacy and safety in women with recurrent small cell neuroendocrine carcinoma of the lower genital tract. Gynecol. Oncol. 2020, 158, 570–575. [Google Scholar] [CrossRef]
- Rodriguez-Freixinos, V.; Chan, D.; Doherty, M.; Wasson, K.; Iscoe, N.; Raskin, W.; Hallet, J.; Myrehaug, S.; Law, C.; Thawer, A.; et al. Avelumab in Unresectable/Metastatic, Progressive, Poorly Differentiated, Grade 3 Neuroendocrine Carcinomas (NECs). Available online: https://www.enets.org/avelumab-in-unresectable-metastatic-progressive-poorly-differentiated-grade-3-neuroendocrine-carcinomas-necs.html (accessed on 11 May 2021).
- Klein, O.; Kee, D.; Markman, B.; Michael, M.; Underhill, C.; Carlino, M.S.; Jackett, L.; Lum, C.; Scott, C.; Nagrial, A.; et al. Immunotherapy of ipilimumab and nivolumab in patients with advanced neuroendocrine tumors: A subgroup analysis of the ca209-538 clinical trial for rare cancers. Clin. Cancer Res. 2020, 26, 4454–4459. [Google Scholar] [CrossRef]
- Capdevila, J.; Teule, A.; López, C.; García-Carbonero, R.; Benavent, M.; Custodio, A.; Cubillo, A.; Alonso, V.; Alonso Gordoa, T.; Carmona-Bayonas, A.; et al. A multi-cohort phase II study of durvalumab plus tremelimumab for the treatment of patients (pts) with advanced neuroendocrine neoplasms (NENs) of gastroenteropancreatic or lung origin: The DUNE trial (GETNE 1601). Ann. Oncol. 2020, 31, S711–S724. [Google Scholar] [CrossRef]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A.; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, R.V.; Osamura, R.Y.; Klöppel, G.; Rosai, J. WHO Classification of Tumours of Endocrine Organs; IARC Press: Lyon, France, 2017. [Google Scholar]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann. Intern. Med. 2009, 151, W65–W94. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. Br. Med. J. 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- McGrath, S.; Zhao, X.; Qin, Z.Z.; Steele, R.; Benedetti, A. One-sample aggregate data meta-analysis of medians. Stat. Med. 2019, 38, 969–984. [Google Scholar] [CrossRef]
- Mittra, A.; Takebe, N.; Florou, V.; Chen, A.P.; Naqash, A.R. The emerging landscape of immune checkpoint inhibitor based clinical trials in adults with advanced rare tumors. Hum. Vaccin Immunother. 2020, 1–5. [Google Scholar] [CrossRef]
- Lamarca, A.; Nonaka, D.; Breitwieser, W.; Ashton, G.; Barriuso, J.; McNamara, M.G.; Moghadam, S.; Rogan, J.; Mansoor, W.; Hubner, R.A.; et al. PD-L1 expression and presence of TILs in small intestinal neuroendocrine tumours. Oncotarget 2018, 9, 14922–14938. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Scoazec, J.Y. Angiogenesis in neuroendocrine tumors: Therapeutic applications. Neuroendocrinology 2013, 97, 45–56. [Google Scholar] [CrossRef] [PubMed]


| Study | Phase | Design | ICI | Target | Sample Size * | ORR No. (%) | Median PFS, mos (Range) | Median OS, mos (Range) |
|---|---|---|---|---|---|---|---|---|
| Mehnert [26] (NCT02054806) | 1b | Multicohort: epNET | Pembrolizumab | PD-1 | 25 | 3 (12) | 5.6 (3.5–10.7) | 21.1 (9.1–22.4) |
| Mehnert [26] (NCT02054806) | 1b | Multicohort: pNET | Pembrolizumab | PD-1 | 16 | 1 (6) | 4.5 (3.6–8.3) | 21.0 |
| Strosberg [27] (NCT02628067) | 2 | Single cohort: mixNET | Pembrolizumab | PD-1 | 107 | 4 (4) | 4.1 (3.5–5.4) | 24.2 (15.8–32.5) |
| Yao [28] (NCT02955069) | 2 | Multicohort: epNET | Spartalizumab | PD-1 | 62 | 6 (10) | - | - |
| Yao [28] (NCT02955069) | 2 | Multicohort: pNET | Spartalizumab | PD-1 | 33 | 1 (3) | - | - |
| Yao [28] (NCT02955069) | 2 | Multicohort: mixNEC | Spartalizumab | PD-1 | 21 | 1 (5) | - | - |
| Patel [29] (NCT02834013) | 2 | Single cohort: epNEN | Nivolumab + ipilimumab | PD-1, CTLA-4 | 32 | 8 (25) | 4.0 (3.0–6.0) | 11 |
| Lu [30] (NCT03167853) | 1b | Multicohort: mixNEC, mixNET/pNEN, epNEN, mixNEN | Toripalimab | PD-1 | 40 | 8 (20) | 2.5 (1.9–3.1) | 7.8 (5.0–10.8) |
| Vijayvergia [31] (NCT02939651) | 2 | Single cohort: mixNEN | Pembrolizumab | PD-1 | 29 | 1 (3) | 2.0 (1.5–2.4) | 4.7 |
| Halperin [32] (NCT03074513) | 2 | Multicohort: pNET | Atezolizumab + bevacizumab | PD-L1, TKI | 20 | 4 (20) | 19.6 | - |
| Halperin [32] (NCT03074513) | 2 | Multicohort: epNET | Atezolizumab + bevacizumab | PD-L1, anti-VEGF | 20 | 3 (15) | 14.9 | - |
| Zhang [33] (NCT03167853) | 1b | Multicohort: mixNEC, mixNET | Toripalimab | PD-1 | 21 | 6 (29) | 2.8 (1.6–4.0) | - |
| Fottner [34] (NCT03352934) | 2 | Single cohort: mixNEN | Avelumab | PD-L1 | 29 | 2 (7) | - | 4.2 (1.0–12.0) |
| Mulvey [35] (NCT03136055) | 2 | Single cohort: epNEC | Pembrolizumab | PD-1 | 13 | 1, 8 | 2.0 | - |
| Frumovitz [6] (NCT02721732) | 2 | Single cohort: epNEC | Pembrolizumab | PD-1 | 7 | 0 (0) | 2.1 (0.8–3.3) | - |
| Rodriguez-Freixinos [37] (NCT03278405) | 2a | Single cohort: epNEC | Avelumab | PD-L1 | 9 | 0 (0) | 3.0 (1.0–10.0) | 5.0 (2.0–15.0) |
| Klein [38] (NCT02923934) | 2 | Single cohort: mixNEN | Nivolumab + ipilimumab | PD-1, CTLA-4 | 29 | 7 (24) | 4.8 (2.7–10.5) | 14.8 (4.1–21.2) |
| Capdevila [39] (NCT03095274) | 2 | Multicohort:epNET | Durvalumab + tremelimumab | PD-L1, CTLA-4 | 27 | 0 (0) | 5.3 (4.5–6.0) | - |
| Capdevila [39] (NCT03095274) | 2 | Multicohort:epNET | Durvalumab + tremelimumab | PD-L1, CTLA-4 | 31 | 0 (0) | 8 (4.9–11.1) | - |
| Capdevila [39] (NCT03095274) | 2 | Multicohort:pNET | Durvalumab + trremelimumab | PD-L1, CTLA-4 | 32 | 2 (6) | 8.1 (3.8–12.4) | - |
| Capdevila [39] (NCT03095274) | 2 | Multicohort:mixNEN | Durvalumab + tremelimumab | PD-L1, CTLA-4 | 33 | 2 (6) | 2.5 (2.1–2.7) | - |
| Adverse Events | trAEs (No. Cases) | irAEs (No. Cases) | ||
|---|---|---|---|---|
| Any Grades | ≥Grade 3 | Any Grades | ≥Grade 3 | |
| Adrenal insufficiency | 0 | 0 | 0 | 0 |
| Anorexia | 23 | 1 | 23 | 1 |
| Arthralgia/arthritis | 10 | 1 | 10 | 1 |
| Asthenia | 12 | 1 | 12 | 1 |
| Colitis/ulcerative colitis/peritonitis | 6 | 6 | 6 | 6 |
| Diarrhea | 67 | 12 | 67 | 12 |
| Dyspnea | 5 | 2 | 5 | 2 |
| Electrolyte alterations | 17 | 3 | 17 | 3 |
| Elevated alkaline phosphatase | 11 | 6 | 11 | 6 |
| Elevated AST/ALT | 46 | 11 | 46 | 11 |
| Elevated lipase/amylase, pancreatitis | 18 | 6 | 18 | 6 |
| Fatigue | 123 | 7 | 123 | 7 |
| Fever | 11 | 0 | 11 | 0 |
| Hematologic alterations (anemia, decrease in white blood cells/platelets) | 41 | 3 | 41 | 3 |
| Hepatitis | 2 | 2 | 2 | 2 |
| Hyperbilirubinemia | 16 | 2 | 16 | 2 |
| Hyperglycemia, diabetes mellitus | 18 | 5 | 18 | 5 |
| Hyperthyroidism | 0 | 0 | 0 | 0 |
| Hypoalbuminemia | 7 | 0 | 7 | 0 |
| Hypophysitis | 1 | 0 | 1 | 0 |
| Hypotension/hypertension | 4 | 2 | 4 | 2 |
| Hypothyroidism | 27 | 1 | 27 | 1 |
| Infusion-related reactions | 1 | 0 | 1 | 0 |
| Muscular adverse events (weakness, myalgia, increased CK) | 14 | 1 | 14 | 1 |
| Myocarditis | 0 | 0 | 0 | 0 |
| Nausea/vomiting | 57 | 2 | 57 | 2 |
| Pain (abdomen, head) | 7 | 0 | 7 | 0 |
| Pneumonitis | 1 | 1 | 1 | 1 |
| Proteinuria | 28 | 0 | 28 | 0 |
| Retinopathy, encephalopathy | 0 | 0 | 0 | 0 |
| Skin toxicity (rash, dermatitis, worsening psoriasis, pruritus) | 89 | 5 | 89 | 5 |
| Weight loss | 7 | 0 | 7 | 0 |
| Other gastrointestinal toxicity (dizziness, dry mouth, dysgeusia) | 7 | 0 | 7 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bongiovanni, A.; Maiorano, B.A.; Azzali, I.; Liverani, C.; Bocchini, M.; Fausti, V.; Di Menna, G.; Grassi, I.; Sansovini, M.; Riva, N.; et al. Activity and Safety of Immune Checkpoint Inhibitors in Neuroendocrine Neoplasms: A Systematic Review and Meta-Analysis. Pharmaceuticals 2021, 14, 476. https://doi.org/10.3390/ph14050476
Bongiovanni A, Maiorano BA, Azzali I, Liverani C, Bocchini M, Fausti V, Di Menna G, Grassi I, Sansovini M, Riva N, et al. Activity and Safety of Immune Checkpoint Inhibitors in Neuroendocrine Neoplasms: A Systematic Review and Meta-Analysis. Pharmaceuticals. 2021; 14(5):476. https://doi.org/10.3390/ph14050476
Chicago/Turabian StyleBongiovanni, Alberto, Brigida Anna Maiorano, Irene Azzali, Chiara Liverani, Martine Bocchini, Valentina Fausti, Giandomenico Di Menna, Ilaria Grassi, Maddalena Sansovini, Nada Riva, and et al. 2021. "Activity and Safety of Immune Checkpoint Inhibitors in Neuroendocrine Neoplasms: A Systematic Review and Meta-Analysis" Pharmaceuticals 14, no. 5: 476. https://doi.org/10.3390/ph14050476
APA StyleBongiovanni, A., Maiorano, B. A., Azzali, I., Liverani, C., Bocchini, M., Fausti, V., Di Menna, G., Grassi, I., Sansovini, M., Riva, N., & Ibrahim, T. (2021). Activity and Safety of Immune Checkpoint Inhibitors in Neuroendocrine Neoplasms: A Systematic Review and Meta-Analysis. Pharmaceuticals, 14(5), 476. https://doi.org/10.3390/ph14050476







