3-Hydroxypropane-1,2-Diyl Dipalmitoleate—A Natural Compound with Dual Roles (CB1 Agonist/FAAH1 Blocker) in Inhibiting Ovarian Cancer Cell Line
Abstract
1. Introduction
2. Results
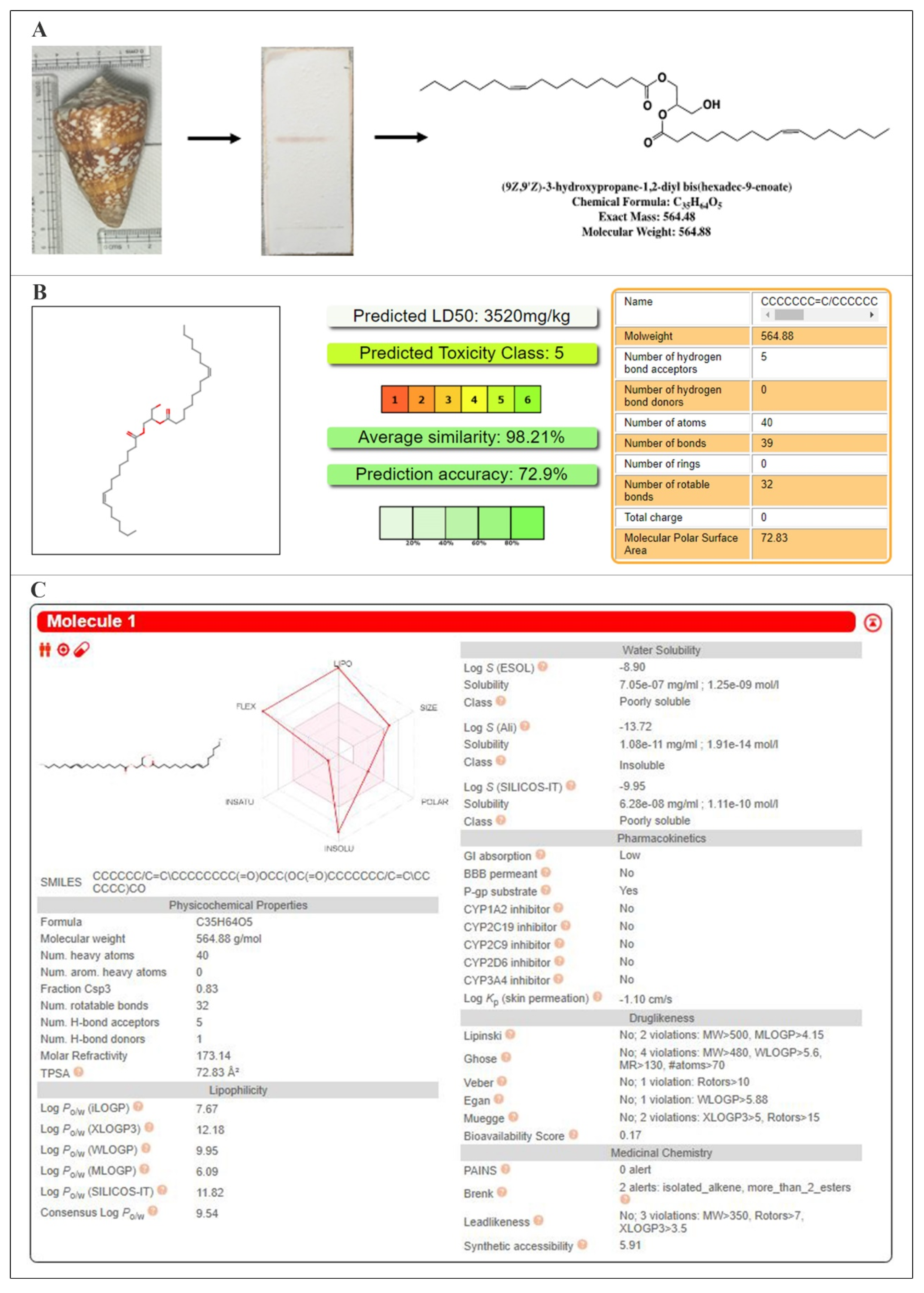
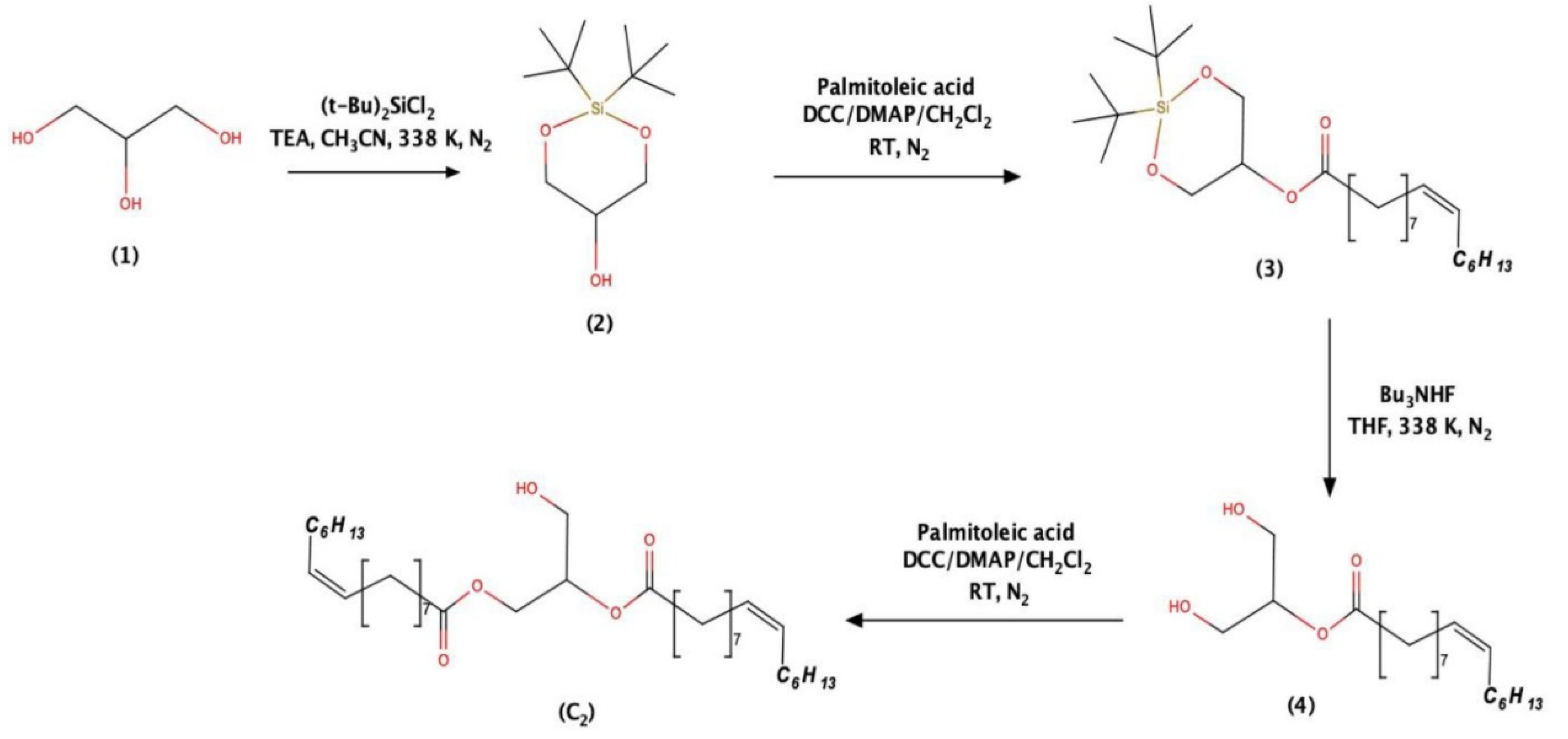
2.1. Structural Investigations and Mass Spectral Analysis of C2 and Preliminary Bioactivities
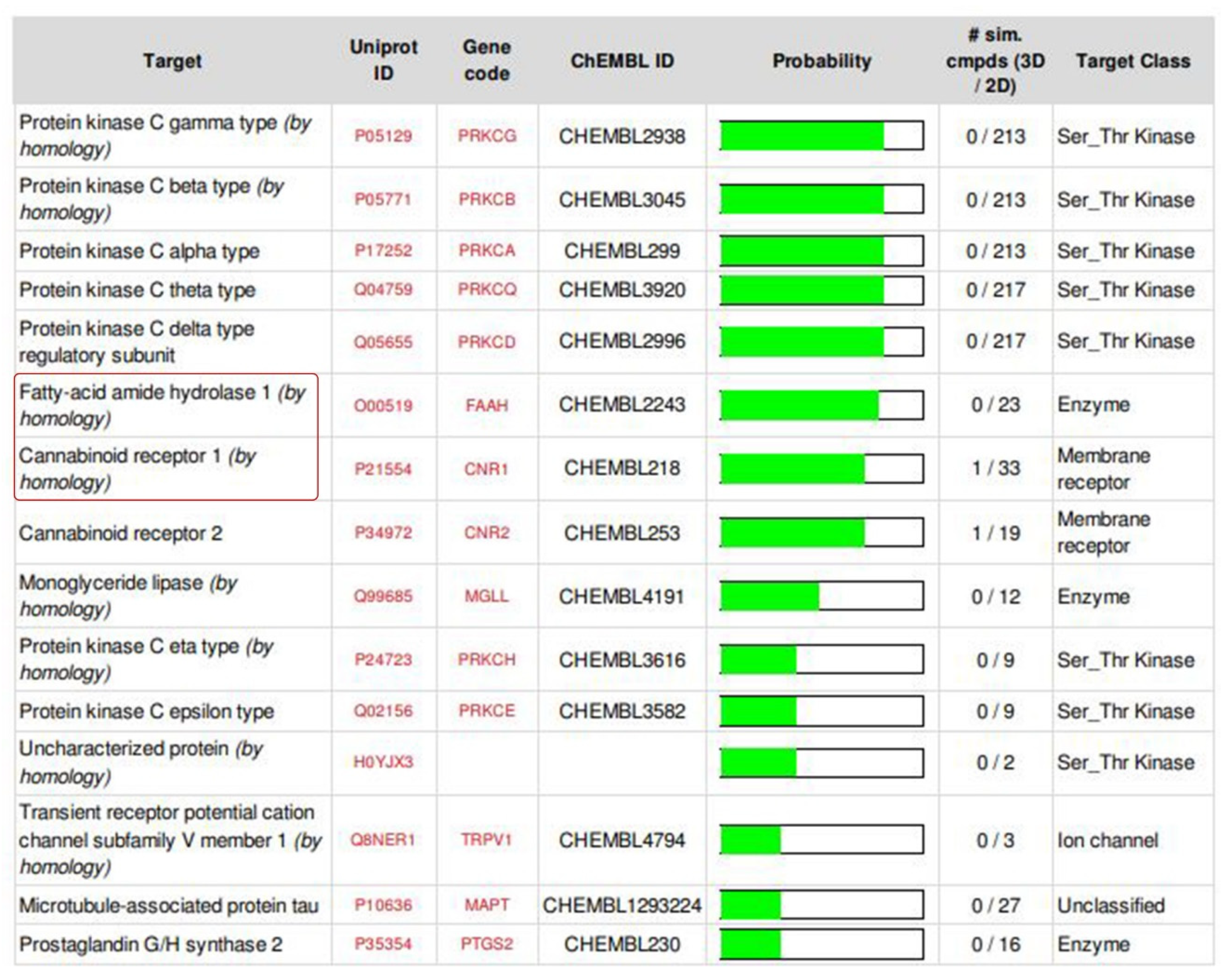
2.2. ADMET Analysis, Target Prediction, Preparation of Protein and Ligand Structures
2.3. Molecular Docking, Molecular Dynamics Simulations and Density Functional Theory (DFT) Calculations
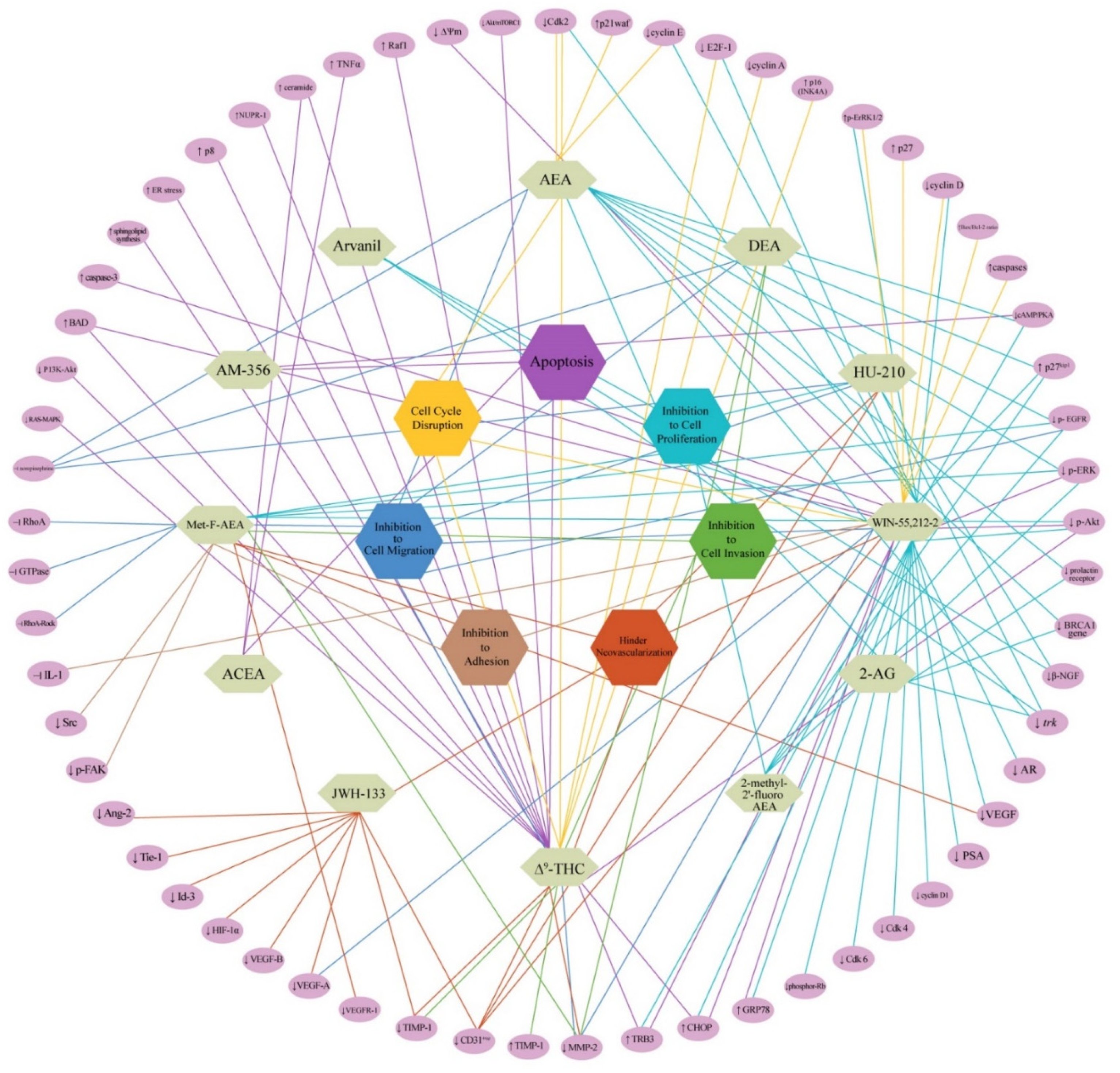
3. Discussion
4. Materials and Methods
4.1. Processing of the Biological Material and Isolation of the Bioactive Compound
4.2. Structural Elucidation and Preliminary Bioassays of the Anticancer Molecule
4.3. Bioinformatics: Tools and Methods
4.3.1. ADMET Analysis, Target Prediction of the Isolated Compound and Structurally Similar Molecules, Preparation of Protein and Ligand Structures
4.3.2. Molecular Docking, Molecular Dynamics Simulations and Density Functional Theory (DFT) Calculations
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hampson, R.E.; Deadwyler, S.A. Cannabinoids, hippocampal function and memory. Life Sci. 1999, 65, 715–723. [Google Scholar] [CrossRef]
- Jiang, W.; Zhang, Y.; Xiao, L.; Van Cleemput, J.; Ji, S.P.; Bai, G.; Zhang, X. Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic- and antidepressant-like effects. J. Clin. Investig. 2005, 115, 3104–3116. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, T.C.; Tucci, S.A. Endocannabinoids in appetite control and the treatment of obesity. CNS Neurol. Disord. Drug Targets 2006, 5, 272–292. [Google Scholar] [CrossRef] [PubMed]
- Bellocchio, L.; Cervino, C.; Pasquali, R.; Pagotto, U. The endocannabinoid system and energy metabolism. J. Neuroendocrinol. 2008, 20, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.N.; McLaughlin, R.J.; Bingham, B.; Shrestha, L.; Lee, T.T.; Gray, J.M.; Hillard, C.J.; Gorzalka, B.B.; Viau, V. Endogenous cannabinoid signaling is essential for stress adaptation. Proc. Natl. Acad. Sci. USA 2010, 107, 9406–9411. [Google Scholar] [CrossRef]
- El-Talatini, M.R.; Taylor, A.H.; Elson, J.C.; Brown, L.; Davidson, A.C.; Konje, J.C. Localisation and function of the endocannabinoid system in the human ovary. PLoS ONE 2009, 4, e4579. [Google Scholar] [CrossRef]
- Pertwee, R.G. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol. Ther. 1997, 74, 129–180. [Google Scholar] [CrossRef]
- Cianchi, F.; Papucci, L.; Schiavone, N.; Lulli, M.; Magnelli, L.; Vinci, M.C.; Messerini, L.; Manera, C.; Ronconi, E.; Romagnani, P.; et al. Cannabinoid receptor activation induces apoptosis through tumor necrosis factor alpha-mediated ceramide de novo synthesis in colon cancer cells. Clin. Cancer Res. 2008, 14, 7691–7700. [Google Scholar] [CrossRef]
- Ramer, R.; Hinz, B. Inhibition of cancer cell invasion by cannabinoids via increased expression of tissue inhibitor of matrix metalloproteinases-1. J. Natl. Cancer Inst. 2008, 100, 59–69. [Google Scholar] [CrossRef]
- Fernandez-Ruiz, J.; Romero, J.; Velasco, G.; Tolon, R.; Ramos, J.; Guzman, M. Cannabinoid CB2 receptor: A new target for controlling neural cell survival. Trends Pharmacol. Sci. 2007, 28, 39–45. [Google Scholar] [CrossRef]
- Sarfaraz, S.; Afaq, F.; Adhami, V.M.; Mukhtar, H. Cannabinoid receptor as a novel target for the treatment of prostate cancer. Cancer Res. 2005, 65, 1635–1641. [Google Scholar] [CrossRef]
- Bifulco, M.; Laezza, C.; Pisanti, S.; Gazzerro, P. Cannabinoids and cancer: Pros and cons of an antitumor strategy. Br. J. Pharmacol. 2006, 148, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Batkai, S.; Kunos, G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol. Rev. 2006, 58, 389–462. [Google Scholar] [CrossRef] [PubMed]
- Harkany, T.; Horvath, T.L. (S)Pot on mitochondria: Cannabinoids disrupt cellular respiration to limit neuronal activity. Cell Metab. 2017, 25, 8–10. [Google Scholar] [CrossRef]
- Ramalho-Santos, J.; Varum, S.; Amaral, S.; Mota, P.C.; Sousa, A.P.; Amaral, A. Mitochondrial functionality in reproduction: From gonads and gametes to embryos and embryonic stem cells. Hum. Reprod. Update 2009, 15, 553–572. [Google Scholar] [CrossRef]
- Cecconi, S.; Rossi, G.; Castellucci, A.; D’Andrea, G.; Maccarrone, M. Endocannabinoid signaling in mammalian ovary. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 178, 6–11. [Google Scholar] [CrossRef]
- Gammon, C.M.; Freeman, G.M.; Xie, W.; Petersen, S.L.; Wetsel, W.C. Regulation of gonadotropin-releasing hormone secretion by cannabinoids. Endocrinology 2005, 146, 4491–4499. [Google Scholar] [CrossRef] [PubMed]
- Valeria, T.; Maria, G.C.; Valentina, D.N.; Dionigi, L.; Nicola, V.; Isabella, G.; Maria, N.; Gianluigi, G. Down-Regulation of Cannabinoid Type 1 (CB1) Receptor and its Downstream Signaling Pathways in Metastatic Colorectal Cancer. Cancers 2019, 11, 708–721. [Google Scholar]
- Winkler, K.; Ramer, R.; Dithmer, S.; Ivanov, I.; Merkord, J.; Hinz, B. Fatty acid amide hydrolase inhibitors confer anti-invasive and antimetastatic effects on lung cancer cells. Oncotarget 2016, 7, 15048–15064. [Google Scholar] [CrossRef]
- Thors, L.; Bergh, A.; Persson, E.; Hammarsten, P.; Stattin, P.; Egevad, L.; Granfors, T.; Fowler, C.J. Fatty Acid Amide Hydrolase in Prostate Cancer: Association with Disease Severity and Outcome, CB1 Receptor Expression and Regulation by IL-4. PLoS ONE 2010, 5, e12275. [Google Scholar] [CrossRef]
- Messalli, E.M.; Grauso, F.; Luise, R.; Angelini, A.; Rossiello, R. Cannabinoid receptor type 1 immunoreactivity and disease severity in human epithelial ovarian tumors. Am. J. Obstet. Gynecol. 2014, 234, 234.e1–234.e6. [Google Scholar] [CrossRef]
- Tayo, L.L.; Lu, B.W.; Cruz, L.J.; Yates, J.R., 3rd. Proteomic analysis provides insights on venom processing in Conus textile. J. Proteome Res. 2010, 9, 2292–2301. [Google Scholar] [CrossRef]
- Norton, R.S.; Olivera, B.M. Conotoxins down under. Toxicon 2006, 48, 780–798. [Google Scholar] [CrossRef] [PubMed]
- Lebbe, E.K.M.; Tytgat, J. In the picture: Disulfide-poor conopeptides, a class of pharmacologically interesting compounds. J. Venom. Anim. Toxins. Incl. Trop. Dis. 2016, 22, 30. [Google Scholar] [CrossRef] [PubMed]
- Puillandre, N.; Koua, D.; Favreau, P.; Olivera, B.M.; Stöcklin, R. Molecular phylogeny, classification and evolution of conopeptides. J. Mol. Evol. 2012, 74, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Miljanich, G.P. Ziconotide: Neuronal calcium channel blocker for treating severe chronic pain. Curr. Med. Chem. 2004, 11, 3029–3040. [Google Scholar] [CrossRef]
- Sharpe, I.A.; Gehrmann, J.; Loughnan, M.L.; Thomas, L.; Adams, D.A.; Atkins, A.; Palant, E.; Craik, D.J.; Adams, D.J.; Alewood, P.F.; et al. Two new classes of conopeptides inhibit the alpha1-adrenoceptor and noradrenaline transporter. Nat. Neurosci. 2001, 4, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, M.A.; Abdel-Nabi, I.M.; El-Naggar, M.S.; Abbas, O.A.; Peter, N.S. Conus vexillum venom induces oxidative stress in Ehrlich’s ascites carcinoma cells: An insight into the mechanism of induction. J. Venom. Anim. Toxins. Incl. Trop. Dis. 2013, 19, 10. [Google Scholar] [CrossRef]
- Dave, K.; Lahiry, A. Conotoxins: Review and docking studies to determine potentials of conotoxin as an anticancer drug molecule. Curr. Top. Med. Chem. 2012, 12, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.C.; Hammarsten, P.; Josefsson, A.; Stattin, P.; Granfors, T.; Egevad, L.; Mancini, G.; Lutz, B.; Bergh, A.; Fowler, C.J. A high cannabinoid CB (1) receptor immunoreactivity is associated with disease severity and outcome in prostate cancer. Eur. J. Cancer 2009, 45, 174–182. [Google Scholar] [CrossRef]
- Ravi, J.; Sneh, A.; Shilo, K.; Nasser, M.W.; Ganju, R.K. FAAH inhibition enhances anandamide mediated anti-tumorigenic effects in non-small cell lung cancer by downregulating the EGF/EGFR pathway. Oncotarget 2014, 5, 2475–2486. [Google Scholar] [CrossRef] [PubMed]
- Butini, S.; Gemma, S.; Brindisi, M.; Maramai, S.; Minetti, P.; Celona, D.; Napolitano, R.; Borsini, F.; Cabri, W.; Fezza, F.; et al. Identification of a novel arylpiperazine scaffold for fatty acid amide hydrolase inhibition with improved drug disposition properties. Bioorg. Med. Chem. Lett. 2013, 23, 492–495. [Google Scholar] [CrossRef] [PubMed]
- Brindisi, M.; Borrelli, G.; Brogi, S.; Grillo, A.; Maramai, S.; Paolino, S.; Benedusi, M.; Pecorelli, A.; Valacchi, G.; Di Cesare, M.L.; et al. Development of potent inhibitors of fatty acid amide hydrolase useful for the treatment of neuropathic pain. ChemMedChem 2018, 13, 2090–2103. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Substance Record for SID 404333224, 1,2-di-(9Z-Hexadecenoyl)-sn-Glycerol, Source: Elizabeth’s Lab, Sri Ramachandra Institute of Higher Education and Research (SRIHER) (DU). PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/substance/404333224 (accessed on 7 February 2021).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 14275348, 1,2-Di-[(9Z)-hexadecenoyl]glycerol. In PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/14275348 (accessed on 7 February 2021).
- Pires, D.E.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting Small-Molecule Pharmacokinetic and Toxicity Properties Using Graph-Based Signatures. J. Med. Chem. 2015, 14, 4066–4072. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Banerjee, P.; Eckert, O.A.; Schrey, A.K.; Preissner, R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018, 46, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Gfeller, D.; Grosdidier, A.; Wirth, M.; Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: A web server for target prediction of bioactive small molecules. Nucleic Acids Res. 2014, 42, 32–38. [Google Scholar] [CrossRef]
- Riley, R.J.; Parker, A.J.; Trigg, S.; Manners, C.N. Development of a generalized, quantitative physicochemical model of CYP3A4 inhibition for use in early drug discovery. Pharm. Res. 2001, 18, 652–655. [Google Scholar] [CrossRef]
- Blázquez, C.; Carracedo, A.; Salazar, M.; Lorente, M.; Egia, A.; González-Feria, L.; Haro, A.; Velasco, G.; Guzmán, M. Down-regulation of tissue inhibitor of metalloproteinases-1 in gliomas: A new marker of cannabinoid antitumoral activity. Neuropharmacology 2008, 54, 235–243. [Google Scholar] [CrossRef]
- Li, K.; Yao, F.; Xue, Q.; Fan, H.; Yang, L.; Li, X.; Sun, L.; Liu, Y. Inhibitory effects against α-glucosidase and α-amylase of the flavonoids-rich extract from Scutellaria baicalensis shoots and interpretation of structure-activity relationship of its eight flavonoids by a refined assign-score method. Chem. Cent. J. 2018, 12, 82. [Google Scholar] [CrossRef]
- Wang, H.; Du, Y.J.; Song, H.C. α-Glucosidase and a-amylase inhibitory activities of guava leaves. Food Chem. 2010, 123, 6–13. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Kim, S.M. α-Glucosidase Inhibitory Activities of Fatty Acids Purified from the Internal Organ of Sea Cucumber, Stichopus japonicas. J. Food Sci. 2015, 80, 841–847. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information “PubChem”. Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 5 March 2020).
- Peter Wuts, G.M.; Theodora Greene, W. Protection for the Hydroxyl Group, Including 1,2- and 1,3-Diols. In Greene’s Protective Groups in Organic Synthesis, 4th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; Chapter 2; p. 306. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Shao, Z.; Yin, J.; Chapman, K.; Grzemska, M.; Clark, L.; Wang, J.; Rosenbaum, D.M. High-resolution crystal structure of the human CB1 cannabinoid receptor. Nature 2016, 540, 602–606. [Google Scholar] [CrossRef]
- Biasini, M.; Bienert, S.; Waterhouse, A.; Arnold, K.; Studer, G.; Schmidt, T.; Kiefer, F.; Gallo Cassarino, T.; Bertoni, M.; Bordoli, L.; et al. SWISS-MODEL: Modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014, 42, 252–258. [Google Scholar] [CrossRef]
- Ahn, K.; Johnson, D.S.; Mileni, M.; Beidler, D.; Long, J.Z.; Mckinney, M.K.; Weerapana, E.; Sadagopan, N.; Liimatta, M.; Smith, S.E.; et al. Discovery and Characterization of a Highly Selective Faah Inhibitor that Reduces Inflammatory Pain. Chem. Biol. 2009, 16, 411–420. [Google Scholar] [CrossRef]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef]
- Malde, A.K.; Zuo, L.; Breeze, M.; Stroet, M.; Poger, D.; Nair, P.C.; Oostenbrink, C.; Mark, A.E. An Automated Force Field Topology Builder (ATB) and Repository: Version 1.0. J. Chem. Theory Comput. 2011, 7, 4026–4037. [Google Scholar] [CrossRef]
- Panwar, U.; Singh, S.K. Structure-based virtual screening toward the discovery of novel inhibitors for impeding the protein-protein interaction between HIV-1 integrase and human lens epithelium-derived growth factor (LEDGF/p75). J. Biomol. Struct. Dyn. 2017, 36, 3199–3217. [Google Scholar] [CrossRef]

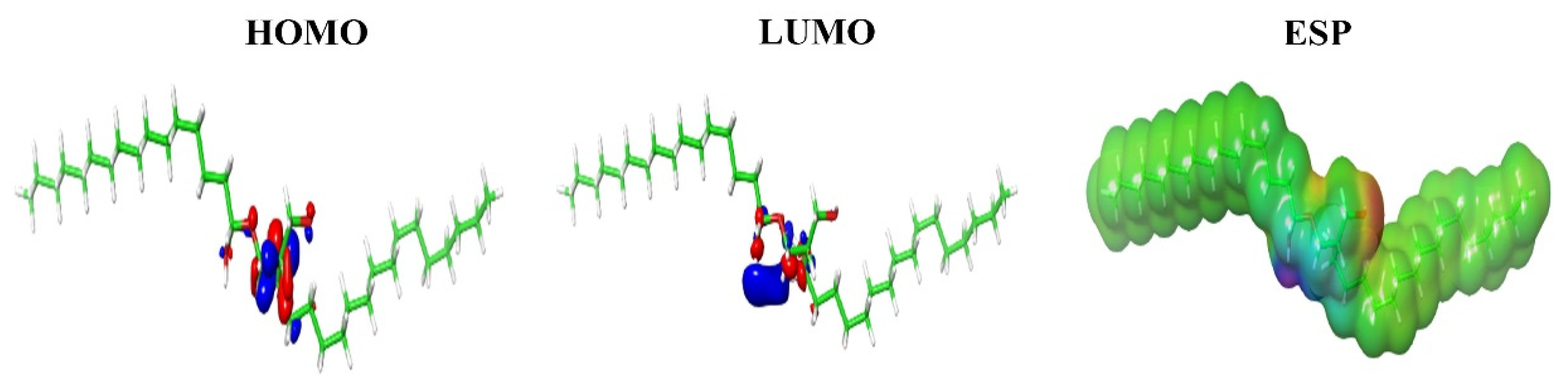
| ID | HOMO (eV) | LUMO (eV) | HLG (eV) | ESP kCal/Mol |
|---|---|---|---|---|
| C2 | −0.25527 | 0.05390 | −0.30917 | 62.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sathynathan, C.V.; Raman, L.S.; Vajiravelu, S.; Kumar, T.D.; Panchatcharam, T.S.; Narasimhan, G.; Doss, G.C.P.; Krishnan, M.E.G. 3-Hydroxypropane-1,2-Diyl Dipalmitoleate—A Natural Compound with Dual Roles (CB1 Agonist/FAAH1 Blocker) in Inhibiting Ovarian Cancer Cell Line. Pharmaceuticals 2021, 14, 255. https://doi.org/10.3390/ph14030255
Sathynathan CV, Raman LS, Vajiravelu S, Kumar TD, Panchatcharam TS, Narasimhan G, Doss GCP, Krishnan MEG. 3-Hydroxypropane-1,2-Diyl Dipalmitoleate—A Natural Compound with Dual Roles (CB1 Agonist/FAAH1 Blocker) in Inhibiting Ovarian Cancer Cell Line. Pharmaceuticals. 2021; 14(3):255. https://doi.org/10.3390/ph14030255
Chicago/Turabian StyleSathynathan, Christina Vijayaraghavan, Lakshmi Sundaram Raman, Sivamurugan Vajiravelu, Thirumal D. Kumar, Thyagarajan Sadras Panchatcharam, Gopinathan Narasimhan, George C. Priya Doss, and Mary Elizabeth Gnanambal Krishnan. 2021. "3-Hydroxypropane-1,2-Diyl Dipalmitoleate—A Natural Compound with Dual Roles (CB1 Agonist/FAAH1 Blocker) in Inhibiting Ovarian Cancer Cell Line" Pharmaceuticals 14, no. 3: 255. https://doi.org/10.3390/ph14030255
APA StyleSathynathan, C. V., Raman, L. S., Vajiravelu, S., Kumar, T. D., Panchatcharam, T. S., Narasimhan, G., Doss, G. C. P., & Krishnan, M. E. G. (2021). 3-Hydroxypropane-1,2-Diyl Dipalmitoleate—A Natural Compound with Dual Roles (CB1 Agonist/FAAH1 Blocker) in Inhibiting Ovarian Cancer Cell Line. Pharmaceuticals, 14(3), 255. https://doi.org/10.3390/ph14030255








