Simulation-Based Assessment of the Impact of Non-Adherence on Endoxifen Target Attainment in Different Tamoxifen Dosing Strategies
Abstract
1. Introduction
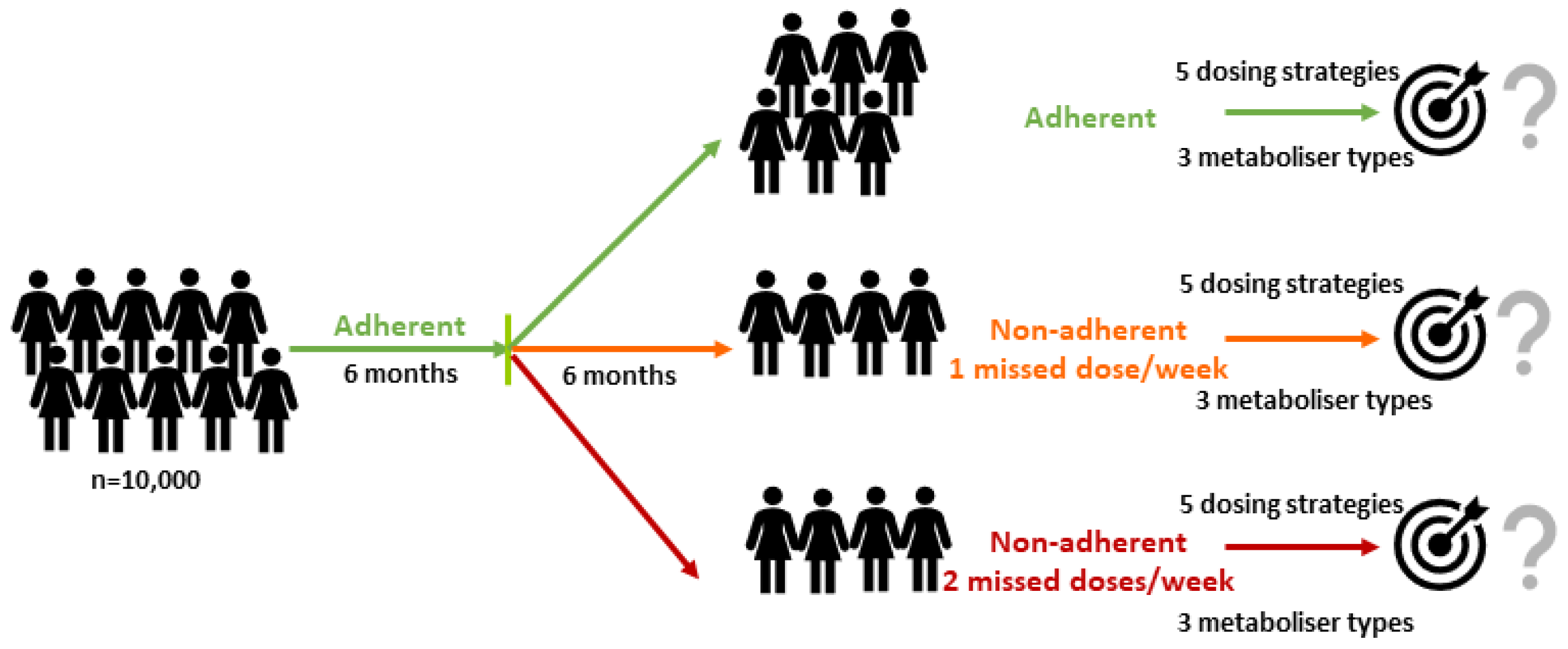
2. Results
- (i)
- Conventional dosing (20 mg QD);
- (ii)
- CYP2D6-guided dosing (gNM: 20 mg, gIM: 30 mg, gPM: 60 mg QD);
- (iii)
- MIPD targeting the proposed 5.97 ng/mL (initial CYP2D6-guided dosing for 4 weeks, collection of virtual TDM samples at 2,3 and 4 weeks after treatment start and selection of maintenance dose after week 4 using Bayesian Forecasting);
- (iv)
- MIPD targeting 5.97 ng/mL (dosing strategy (iii)) when adding 10 mg to each selected dose; and
- (v)
- MIPD (dosing strategy (iii)) but targeting the lowest reported mean CSS,min ENDX in gNM (9 ng/mL) [29].
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef] [PubMed]
- Burstein, H.J.; Lacchetti, C.; Anderson, H.; Buchholz, T.A.; Davidson, N.E.; Gelmon, K.A.; Giordano, S.H.; Hudis, C.A.; Solky, A.J.; Stearns, V.; et al. Adjuvant endocrine therapy for women with hormone receptor–positive breast cancer: ASCO clinical practice guideline focused update. J. Clin. Oncol. 2019, 37, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Mürdter, T.E.; Schroth, W.; Bacchus-Gerybadze, L.; Winter, S.; Heinkele, G.; Simon, W.; Fasching, P.A.; Fehm, T.; Eichelbaum, M.; Schwab, M.; et al. Activity levels of tamoxifen metabolites at the estrogen receptor and the impact of genetic polymorphisms of phase I and II enzymes on their concentration levels in plasma. Clin. Pharmacol. Ther. 2011, 89, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Desta, Z. Comprehensive Evaluation of Tamoxifen Sequential Biotransformation by the Human Cytochrome P450 System in Vitro: Prominent Roles for CYP3A and CYP2D6. J. Pharmacol. Exp. Ther. 2004, 310, 1062–1075. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Spitman, A.B.; Swen, J.J.; Dezentje, V.O.; Moes, D.J.A.R.; Gelderblom, H.; Guchelaar, H.J. Clinical pharmacokinetics and pharmacogenetics of tamoxifen and endoxifen. Expert Rev. Clin. Pharmacol. 2019, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Saladores, P.; Mürdter, T.; Eccles, D.; Chowbay, B.; Zgheib, N.K.; Winter, S.; Ganchev, B.; Eccles, B.; Gerty, S.; Tfayli, A.; et al. Tamoxifen metabolism predicts drug concentrations and outcome in premenopausal patients with early breast cancer. Pharm. J. 2015, 15, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Madlensky, L.; Natarajan, L.; Tchu, S.; Pu, M.; Mortimer, J.; Flatt, S.W.; Nikoloff, D.M.; Hillman, G.; Fontecha, M.R.; Lawrence, H.J.; et al. Tamoxifen metabolite concentrations, CYP2D6 genotype, and breast cancer outcomes. Clin. Pharmacol. Ther. 2011, 89, 718–725. [Google Scholar] [CrossRef]
- Murphy, C.C.; Bartholomew, L.K.; Carpentier, M.Y.; Bluethmann, S.M.; Vernon, S.W. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: A systematic review. Breast Cancer Res. Treat. 2012, 134, 459–478. [Google Scholar] [CrossRef]
- Dezentjé, V.O.; Van Blijderveen, N.J.C.; Gelderblom, H.; Putter, H.; Van Herk-Sukel, M.P.P.; Casparie, M.K.; Egberts, A.C.G.; Nortier, J.W.R.; Guchelaar, H.J. Effect of concomitant CYP2D6 inhibitor use and tamoxifen adherence on breast cancer recurrence in early-stage breast cancer. J. Clin. Oncol. 2010, 28, 2423–2429. [Google Scholar] [CrossRef]
- Chirgwin, J.H.; Giobbie-Hurder, A.; Coates, A.S.; Price, K.N.; Ejlertsen, B.; Debled, M.; Gelber, R.D.; Goldhirsch, A.; Smith, I.; Rabaglio, M.; et al. Treatment adherence and its impact on Disease-Free survival in the breast international group 1-98 trial of tamoxifen and letrozole, alone and in sequence. J. Clin. Oncol. 2016, 34, 2452–2459. [Google Scholar] [CrossRef]
- Pistilli, B.; Paci, A.; Ferreira, A.R.; Di Meglio, A.; Poinsignon, V.; Bardet, A.; Menvielle, G.; Dumas, A.; Pinto, S.; Dauchy, S.; et al. Serum Detection of Nonadherence to Adjuvant Tamoxifen and Breast Cancer Recurrence Risk. J. Clin. Oncol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Partridge, A.H.; Wang, P.S.; Winer, E.P.; Avorn, J. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J. Clin. Oncol. 2003, 21, 602–606. [Google Scholar] [CrossRef]
- Lundgren, C.; Lindman, H.; Rolander, B.; Ekholm, M. Good adherence to adjuvant endocrine therapy in early breast cancer–a population-based study based on the Swedish Prescribed Drug Register. Acta Oncol. 2018, 57, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Wigertz, A.; Ahlgren, J.; Holmqvist, M.; Fornander, T.; Adolfsson, J.; Lindman, H.; Bergkvist, L.; Lambe, M. Adherence and discontinuation of adjuvant hormonal therapy in breast cancer patients: A population-based study. Breast Cancer Res. Treat. 2012, 133, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Hershman, D.L.; Kushi, L.H.; Shao, T.; Buono, D.; Kershenbaum, A.; Tsai, W.Y.; Fehrenbacher, L.; Lin Gomez, S.; Miles, S.; Neugut, A.I. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J. Clin. Oncol. 2010, 28, 4120–4128. [Google Scholar] [CrossRef]
- Lash, T.L.; Fox, M.P.; Westrup, J.L.; Fink, A.K.; Silliman, R.A. Adherence to tamoxifen over the five-year course. Breast Cancer Res. Treat. 2006, 99, 215–220. [Google Scholar] [CrossRef]
- Hsieh, K.P.; Chen, L.C.; Cheung, K.L.; Yang, Y.H. Risks of nonadherence to hormone therapy in Asian women with breast cancer. Kaohsiung J. Med. Sci. 2015, 31, 328–334. [Google Scholar] [CrossRef]
- Kahn, K.L.; Schneider, E.C.; Malin, J.L.; Adams, J.L.; Epstein, A.M. Patient centered experiences in breast cancer: Predicting long-term adherence to tamoxifen use. Med. Care 2007, 45, 431–439. [Google Scholar] [CrossRef]
- Kimmick, G.; Anderson, R.; Camacho, F.; Bhosle, M.; Hwang, W.; Balkrishnan, R. Adjuvant hormonal therapy use among insured, low-income women with breast cancer. J. Clin. Oncol. 2009, 27, 3445–3451. [Google Scholar] [CrossRef]
- Land, S.R.; Cronin, W.M.; Wickerham, D.L.; Costantino, J.P.; Christian, N.J.; Klein, W.M.P.; Ganz, P.A. Cigarette smoking, obesity, physical activity, and alcohol use as predictors of chemoprevention adherence in the national surgical adjuvant breast and bowel project P-1 breast cancer prevention trial. Cancer Prev. Res. 2011, 4, 1393–1400. [Google Scholar] [CrossRef]
- Maurice, A.; Howell, A.; Evans, D.G.; O’Neil, A.C.; Scobie, S. Predicting compliance in a breast cancer prevention trial. Breast J. 2006, 12, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Day, R.; Ganz, P.A.; Costantino, J.P.; Cronin, W.M.; Wickerham, D.L.; Fisher, B. Health-related quality of life and tamoxifen in breast cancer prevention: A report from the National Surgical Adjuvant Breast and Bowel Project P-1 study. J. Clin. Oncol. 1999, 17, 2659–2669. [Google Scholar] [CrossRef] [PubMed]
- Demissie, S.; Silliman, R.A.; Lash, T.L. Adjuvant tamoxifen: Predictors of use side effects, and discontinuation in older women. J. Clin. Oncol. 2001, 19, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Grunfeld, E.A.; Hunter, M.S.; Sikka, P.; Mittal, S. Adherence beliefs among breast cancer patients taking tamoxifen. Patient Educ. Couns. 2005, 59, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Klopp-Schulze, L.; Mueller-Schoell, A.; Neven, P.; Koolen, S.L.; Mathijssen, R.; Joerger, M.; Kloft, C. Integrated data analysis of six clinical studies points toward model-informed precision dosing of tamoxifen. Front. Pharmacol. 2020, 11, 283. [Google Scholar] [CrossRef]
- Darwich, A.S.; Ogungbenro, K.; Vinks, A.A.; Powell, J.R.; Reny, J.L.; Marsousi, N.; Daali, Y.; Fairman, D.; Cook, J.; Lesko, L.J.; et al. Why has model-informed precision dosing not yet become common clinical reality? lessons from the past and a roadmap for the future. Clin. Pharmacol. Ther. 2017, 101, 646–656. [Google Scholar] [CrossRef]
- Kluwe, F.; Michelet, R.; Mueller-Schoell, A.; Maier, C.; Klopp-Schulze, L.; van Dyk, M.; Mikus, G.; Huisinga, W.; Kloft, C. Perspectives on Model-Informed Precision Dosing in the Digital Health Era: Challenges, Opportunities, and Recommendations. Clin. Pharmacol. Ther. 2020, 109, 29–36. [Google Scholar] [CrossRef]
- Keizer, R.J.; ter Heine, R.; Frymoyer, A.; Lesko, L.J.; Mangat, R.; Goswami, S. Model-Informed Precision Dosing at the Bedside: Scientific Challenges and Opportunities. CPT Pharmacomet. Syst. Pharmacol. 2018, 7, 785–787. [Google Scholar] [CrossRef]
- Hertz, D.L.; Deal, A.; Ibrahim, J.G.; Walko, C.M.; Weck, K.E.; Anderson, S.; Magrinat, G.; Olajide, O.; Moore, S.; Raab, R.; et al. Tamoxifen Dose Escalation in Patients With Diminished CYP2D6 Activity Normalizes Endoxifen Concentrations without Increasing Toxicity. Oncologist 2016, 21, 795–803. [Google Scholar] [CrossRef]
- Nardin, J.M.; Schroth, W.; Almeida, T.A.; Mürdter, T.; Picolotto, S.; Vendramini, E.C.L.; Hoppe, R.; Kogin, J.P.; Miqueleto, D.; de Moraes, S.D.R.; et al. The Influences of Adherence to Tamoxifen and CYP2D6 Pharmacogenetics on Plasma Concentrations of the Active Metabolite (Z)-Endoxifen in Breast Cancer. Clin. Transl. Sci. 2020, 13, 284–292. [Google Scholar] [CrossRef]
- Gallicchio, L.; Lord, G.; Tkaczuk, K.; Danton, M.; Lewis, L.M.; Lim, C.K.; Flaws, J.A. Association of tamoxifen (TAM) and TAM metabolite concentrations with self-reported side effects of TAM in women with breast cancer. Breast Cancer Res. Treat. 2004, 85, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Thorén, L.; Lindh, J.D.; Ackehed, G.; Kringen, M.K.; Hall, P.; Bergh, J.; Molden, E.; Margolin, S.; Eliasson, E. Impairment of endoxifen formation in tamoxifen-treated premenopausal breast cancer patients carrying reduced-function CYP2D6 alleles. Br. J. Clin. Pharmacol. 2020, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Khalaj, Z.; Baratieh, Z.; Nikpour, P.; Schwab, M.; Schaeffeler, E.; Mokarian, F.; Khanahmad, H.; Salehi, R.; Mürdter, T.E.; Salehi, M. Clinical Trial: CYP2D6 Related Dose Escalation of Tamoxifen in Breast Cancer Patients With Iranian Ethnic Background Resulted in Increased Concentrations of Tamoxifen and Its Metabolites. Front. Pharmacol. 2019, 10, 530. [Google Scholar] [CrossRef]
- Irvin, W.J.; Walko, C.M.; Weck, K.E.; Ibrahim, J.G.; Chiu, W.K.; Dees, E.C.; Moore, S.G.; Olajide, O.A.; Graham, M.L.; Canale, S.T.; et al. Genotype-guided tamoxifen dosing increases active metabolite exposure in women with reduced CYP2D6 metabolism: A multicenter study. J. Clin. Oncol. 2011, 29, 3232–3239. [Google Scholar] [CrossRef] [PubMed]
- Martinez de Dueñas, E.; Ochoa Aranda, E.; Blancas Lopez-Barajas, I.; Ferrer Magdalena, T.; Bandrés Moya, F.; Chicharro García, L.M.; Gómez Capilla, J.A.; Zafra Ceres, M.; de Haro, T.; Romero Llorens, R.; et al. Adjusting the dose of tamoxifen in patients with early breast cancer and CYP2D6 poor metabolizer phenotype. Breast 2014, 23, 400–406. [Google Scholar] [CrossRef]
- Dezentjé, V.O.; Opdam, F.L.; Gelderblom, H.; Hartigh den, J.; Van der Straaten, T.; Vree, R.; Maartense, E.; Smorenburg, C.H.; Putter, H.; Dieudonné, A.S.; et al. CYP2D6 genotype- and endoxifen-guided tamoxifen dose escalation increases endoxifen serum concentrations without increasing side effects. Breast Cancer Res. Treat. 2015, 153, 583–590. [Google Scholar] [CrossRef]
- Kiyotani, K.; Mushiroda, T.; Imamura, C.K.; Tanigawara, Y.; Hosono, N.; Kubo, M.; Sasa, M.; Nakamura, Y.; Zembutsu, H. Dose-adjustment study of tamoxifen based on CYP2D6 genotypes in Japanese breast cancer patients. Breast Cancer Res. Treat. 2012, 131, 137–145. [Google Scholar] [CrossRef]
- Fox, P.; Balleine, R.L.; Lee, C.; Gao, B.; Balakrishnar, B.; Menzies, A.M.; Yeap, S.H.; Ali, S.S.; Gebski, V.; Provan, P.; et al. Dose Escalation of Tamoxifen in Patients with Low Endoxifen Level: Evidence for Therapeutic Drug Monitoring—The TADE Study. Clin. Cancer Res. 2016, 22, 3164–3171. [Google Scholar] [CrossRef]
- Mueller-Schoell, A.; Klopp-Schulze, L.; Schroth, W.; Mürdter, T.; Michelet, R.; Brauch, H.; Huisinga, W.; Joerger, M.; Neven, P.; Koolen, S.L.W.; et al. Obesity Alters Endoxifen Plasma Levels in Young Breast Cancer Patients: A Pharmacometric Simulation Approach. Clin. Pharmacol. Ther. 2020, 108, 661–670. [Google Scholar] [CrossRef]
- Keizer, R.J.; Karlsson, M.O.; Hooker, A. Modeling and simulation workbench for NONMEM: Tutorial on Pirana, PsN, and Xpose. CPT Pharmacomet. Syst. Pharmacol. 2013, 2, 1–9. [Google Scholar] [CrossRef]
- Goetz, M.P.; Sangkuhl, K.; Guchelaar, H.J.; Schwab, M.; Province, M.; Whirl-Carrillo, M.; Symmans, W.F.; McLeod, H.L.; Ratain, M.J.; Zembutsu, H.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6 and Tamoxifen Therapy. Clin. Pharmacol. Ther. 2018, 103, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Gaedigk, A.; Simon, S.D.; Pearce, R.E.; Bradford, L.D.; Kennedy, M.J.; Leeder, J.S. The CYP2D6 Activity Score: Translating Genotype Information into a Qualitative Measure of Phenotype. Clin. Pharmacol. Ther. 2008, 83, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Caudle, K.E.; Sangkuhl, K.; Whirl-Carrillo, M.; Swen, J.J.; Haidar, C.E.; Klein, T.E.; Gammal, R.S.; Relling, M.V.; Scott, S.A.; Hertz, D.L.; et al. Standardizing CYP2D6 Genotype to Phenotype Translation: Consensus Recommendations from the Clinical Pharmacogenetics Implementation Consortium and Dutch Pharmacogenetics Working Group. Clin. Transl. Sci. 2020, 13, 116–124. [Google Scholar] [CrossRef]
- Barginear, M.F.; Jaremko, M.; Peter, I.; Yu, C.; Kasai, Y.; Kemeny, M.; Raptis, G.; Desnick, R.J. Increasing tamoxifen dose in breast cancer patients based on CYP2D6 genotypes and endoxifen levels: Effect on active metabolite isomers and the antiestrogenic activity score. Clin. Pharmacol. Ther. 2011, 90, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Mueller-Schoell, A.; Groenland, S.L.; Scherf-Clavel, O.; van Dyk, M.; Huisinga, W.; Michelet, R.; Jaehde, U.; Steeghs, N.; Huitema, A.D.R.; Kloft, C. Therapeutic drug monitoring of oral targeted antineoplastic drugs. Eur. J. Clin. Pharmacol. 2020. [Google Scholar] [CrossRef]
- Klopp-Schulze, L.; Joerger, M.; Wicha, S.G.; ter Heine, R.; Csajka, C.; Parra-Guillen, Z.P.; Kloft, C. Exploiting Pharmacokinetic Models of Tamoxifen and Endoxifen to Identify Factors Causing Subtherapeutic Concentrations in Breast Cancer Patients. Clin. Pharmacokinet. 2018, 57, 229–242. [Google Scholar] [CrossRef]
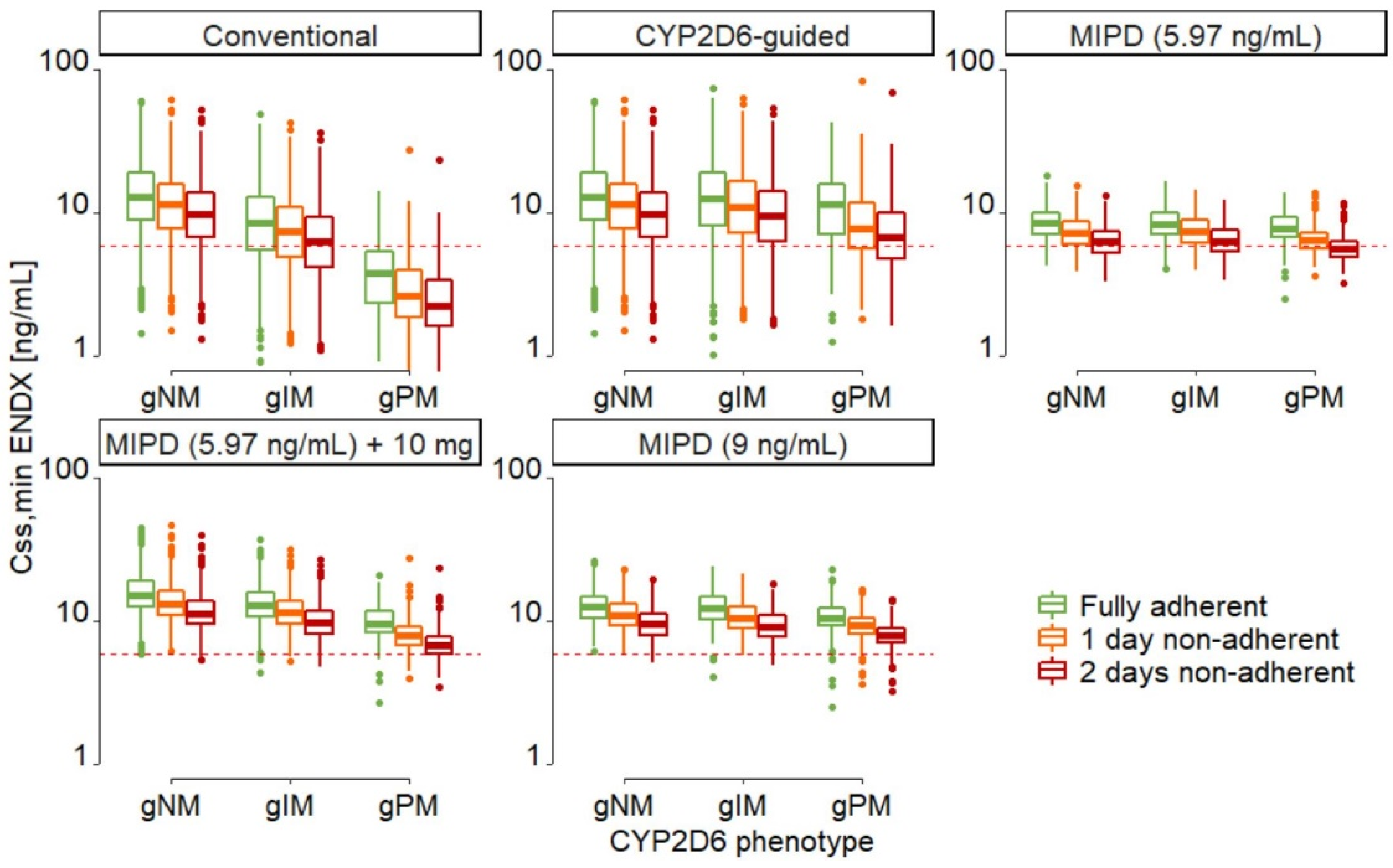
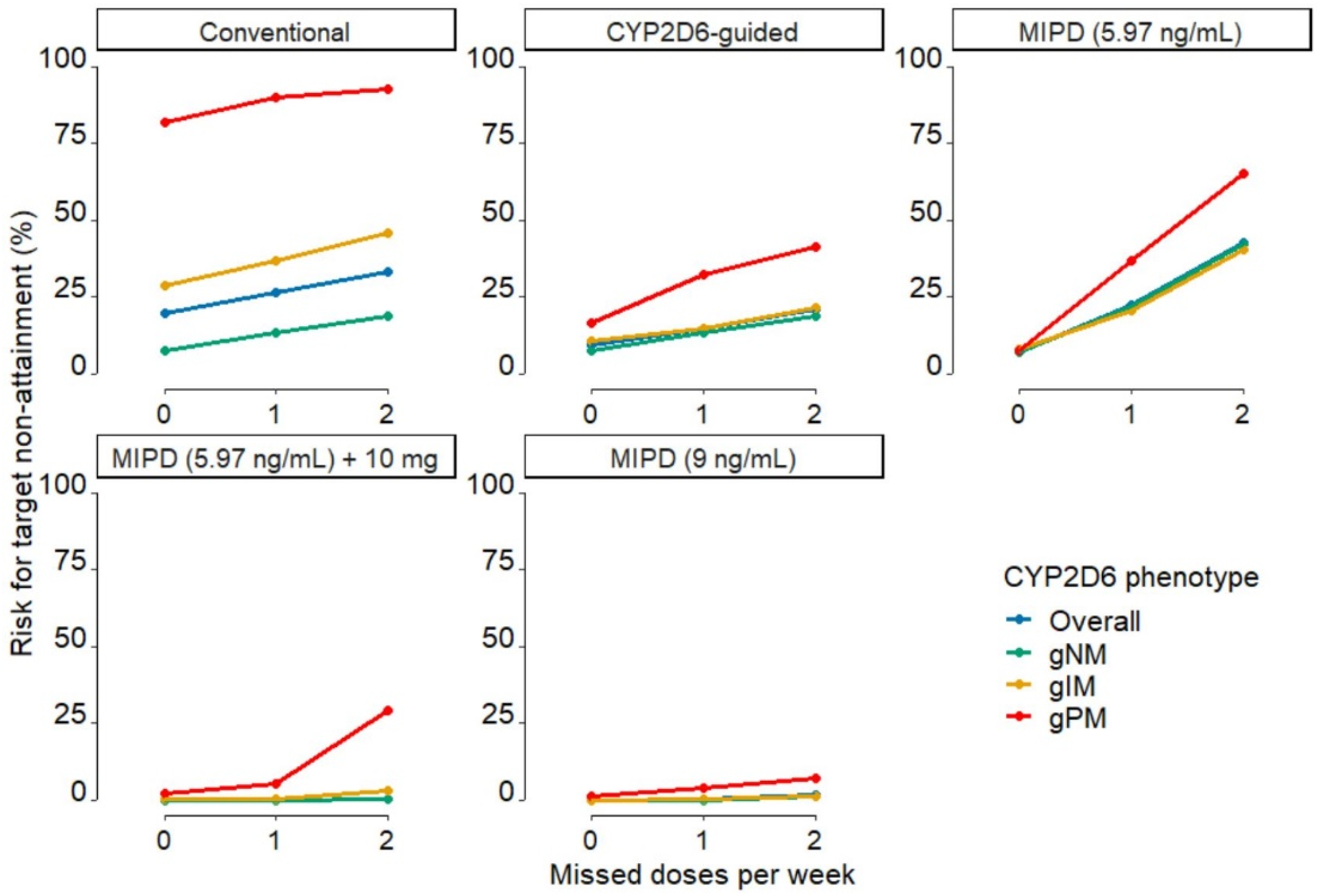
| Patient Subpopulation | Conventional Dosing | CYP2D6-Guided Dosing | MIPD (5.97 ng/mL Target) | MIPD (5.97 ng/mL Target) +10 mg | MIPD (9 ng/mL Target) |
|---|---|---|---|---|---|
| Overall † | 19.8 | 9.19 | 7.34 | 0.233 | 0.133 |
| gNM | 7.60 | 7.60 | 6.98 | 0.0294 | 0.00 |
| gIM | 28.9 | 10.5 | 7.85 | 0.220 | 0.132 |
| gPM | 81.7 | 16.5 | 7.51 | 2.40 | 1.50 |
| Conventional Dosing | CYP2D6-Guided Dosing | MIPD (5.97 ng/mL Target) | MIPD (5.97 ng/mL Target) +10 mg | MIPD (9 ng/mL Target) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of missed doses | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 |
| Overall | 26.4 | 33.3 | 14.8 | 21.1 | 22.3 | 42.8 | 0.525 | 3.02 | 0.375 | 1.55 |
| gNM | 13.2 | 19.0 | 13.2 | 19.0 | 22.1 | 42.1 | 0.00 | 0.530 | 0.132 | 1.15 |
| gIM | 36.8 | 45.8 | 14.8 | 21.3 | 20.5 | 40.4 | 0.594 | 2.91 | 0.198 | 1.32 |
| gPM | 90.1 | 92.8 | 32.4 | 41.4 | 36.9 | 65.3 | 5.41 | 29.3 | 4.05 | 7.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mueller-Schoell, A.; Klopp-Schulze, L.; Michelet, R.; van Dyk, M.; Mürdter, T.E.; Schwab, M.; Joerger, M.; Huisinga, W.; Mikus, G.; Kloft, C. Simulation-Based Assessment of the Impact of Non-Adherence on Endoxifen Target Attainment in Different Tamoxifen Dosing Strategies. Pharmaceuticals 2021, 14, 115. https://doi.org/10.3390/ph14020115
Mueller-Schoell A, Klopp-Schulze L, Michelet R, van Dyk M, Mürdter TE, Schwab M, Joerger M, Huisinga W, Mikus G, Kloft C. Simulation-Based Assessment of the Impact of Non-Adherence on Endoxifen Target Attainment in Different Tamoxifen Dosing Strategies. Pharmaceuticals. 2021; 14(2):115. https://doi.org/10.3390/ph14020115
Chicago/Turabian StyleMueller-Schoell, Anna, Lena Klopp-Schulze, Robin Michelet, Madelé van Dyk, Thomas E. Mürdter, Matthias Schwab, Markus Joerger, Wilhelm Huisinga, Gerd Mikus, and Charlotte Kloft. 2021. "Simulation-Based Assessment of the Impact of Non-Adherence on Endoxifen Target Attainment in Different Tamoxifen Dosing Strategies" Pharmaceuticals 14, no. 2: 115. https://doi.org/10.3390/ph14020115
APA StyleMueller-Schoell, A., Klopp-Schulze, L., Michelet, R., van Dyk, M., Mürdter, T. E., Schwab, M., Joerger, M., Huisinga, W., Mikus, G., & Kloft, C. (2021). Simulation-Based Assessment of the Impact of Non-Adherence on Endoxifen Target Attainment in Different Tamoxifen Dosing Strategies. Pharmaceuticals, 14(2), 115. https://doi.org/10.3390/ph14020115










