Characterization and Applications of Colloidal Systems as Versatile Drug Delivery Carriers for Parenteral Formulations
Abstract
1. Introduction
2. Discussion
2.1. Necessity and Potential of Biphasic Colloidal Carriers in Parenteral Drug Delivery
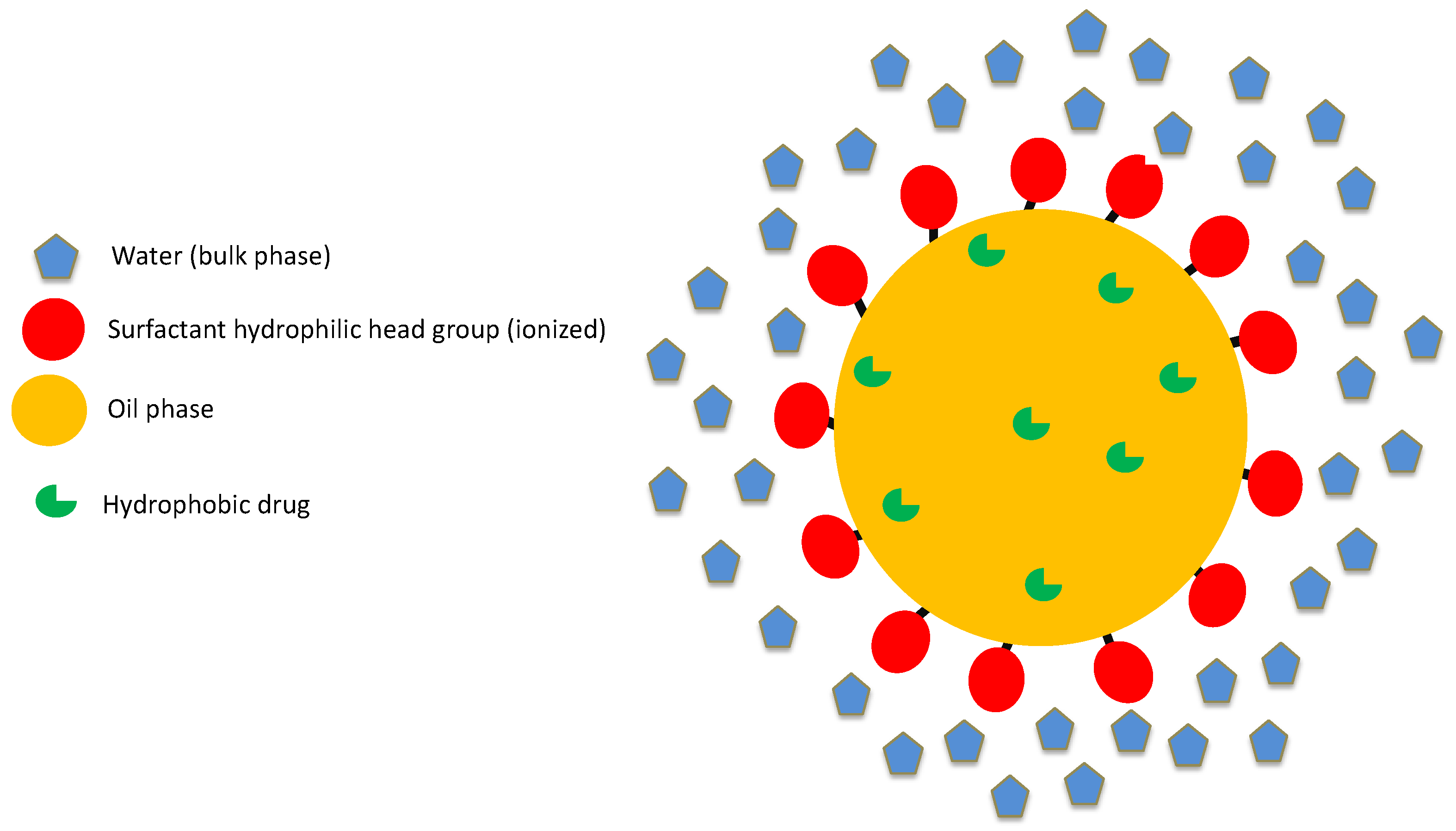
2.2. Structure of Lipid-Based Biphasic Colloidal Systems
2.3. Structure of Nanoparticulate Systems
2.4. FDA Perspective on Excipients
- Drug-excipient compatibility.
- Compatibility of the excipient with manufacturing process and container–closure system.
- Excipient impact on quality, safety, and effectiveness of the drug product.
- Route of administration.
- Dose volume and intended use of the drug product: single versus multiple dose.
- New chemical excipients: A full safety evaluation program is required for these excipients. A drug master file (DMF) must be filed with the FDA for a new excipient. The DMF contains relevant safety information.
- Existing chemical excipients—first use in man: Animal safety data are available for this class of excipients. Additional safety information is required when there is a change in dosage form, route of administration, higher dose, etc.
- New modifications or combinations of existing excipients: This class of excipients indicate a physical reaction and not a chemical reaction. Thus, no additional safety evaluation is necessary.
2.5. Physico-Chemical Barriers in Development of Submicron Drug Delivery Systems
3. Scientific and Regulatory Considerations for Approval of Biphasic Colloidal Systems
- Comprehensive comparison of physico-chemical characterization of at least three batches of test and reference products.
- In-vivo studies to demonstrate BE.
3.1. Critical Quality Attributes of Colloidal Drug Delivery Systems
3.1.1. Particle Size Distribution
3.1.2. In-vitro Dissolution Test
3.1.3. Amorphous/Crystalline Content
3.1.4. Rheology and Sterility
4. Applications of Colloidal Carriers
4.1. Total Parenteral Nutrition
4.2. Vaccine Delivery
4.3. Long-Acting Injectable (LAI) Therapy
4.4. Anti-Cancer Drugs and Diagnostic Agents
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Preparation by a size-reduction technique. Int. J. Pharm. 1998, 160, 229–237.
- Müller, R.H.; Jacobs, C.; Kayser, O. Nanosuspensions as particulate drug formulations in therapy: Rationale for development and what we can expect for the future. Adv. Drug Deliv. Rev. 2001, 47, 3–19. [Google Scholar] [CrossRef]
- Date, A.A.; Nagarsenker, M. Parenteral microemulsions: An overview. Int. J. Pharm. 2008, 355, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Marti-Mestres, G.; Nielloud, F. Emulsions in health care applications—An overview. J. Dispers. Sci. Technol. 2002, 23, 419–439. [Google Scholar] [CrossRef]
- Patravale, V.B.; Date, A.A.; Kulkarni, R.M. Nanosuspensions: A promising drug delivery strategy. J. Pharm. Pharmacol. 2004, 56, 827–840. [Google Scholar] [CrossRef]
- Morais, J.M.; Burgess, D.J. Micro-and nanoemulsions (controlled release parenteral drug delivery systems). In Long Acting Injections and Implants; Springer: Berlin/Heidelberg, Germany, 2012; pp. 221–238. [Google Scholar]
- Giri, T.K.; Goswami, N.G.; Jha, V.K. Prospective and challenges of micro-emulsion as a novel carrier for drug delivery. J. PharmaSciTech 2013, 2, 56–61. [Google Scholar]
- Rhee, Y.-S.; Park, C.; Nam, T.-Y.; Shin, Y.-S.; Chi, S.-C.; Park, E.-S. Formulation of parenteral microemulsion containing itraconazole. Arch. Pharmacal Res. 2007, 30, 114–123. [Google Scholar] [CrossRef]
- Nesamony, J.; Zachar, C.L.; Jung, R.; Williams, F.E.; Nauli, S. Preparation, characterization, sterility validation, and in vitro cell toxicity studies of microemulsions possessing potential parenteral applications. Drug Dev. Ind. Pharm. 2012, 39, 240–251. [Google Scholar] [CrossRef]
- Kolluru, L.P.; Chandran, T.; Shastri, P.N.; Rizvi, S.A.; D’Souza, M.J. Development and evaluation of polycaprolactone based docetaxel nanoparticle formulation for targeted breast cancer therapy. J. Nanopart. Res. 2020, 22, 372. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Z.; Zhang, N.; Miao, L.; Huang, G. Development of etoposide-loaded bovine serum albumin nanosuspensions for parenteral delivery. Drug Deliv. 2014, 22, 79–85. [Google Scholar] [CrossRef]
- Tian, X.; Li, H.; Zhang, D.; Liu, G.; Jia, L.; Zheng, D.; Shen, J.; Shen, Y.; Zhang, Q. Nanosuspension for parenteral delivery of a p-terphenyl derivative: Preparation, characteristics and pharmacokinetic studies. Colloids Surfaces B Biointerfaces 2013, 108, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Ganta, S.; Talekar, M.; Singh, A.; Coleman, T.P.; Amiji, M. Nanoemulsions in Translational Research—Opportunities and Challenges in Targeted Cancer Therapy. AAPS PharmSciTech 2014, 15, 694–708. [Google Scholar] [CrossRef] [PubMed]
- Lam, A.C.; Schechter, R.S. The theory of diffusion in microemulsion. J. Colloid Interface Sci. 1987, 120, 56–63. [Google Scholar] [CrossRef]
- Muzaffar, F.; Singh, U.; Chauhan, L. Review on microemulsion as futuristic drug delivery. Int. J. Pharm. Pharm. Sci. 2013, 5, 39–53. [Google Scholar]
- Kahlweit, M.; Strey, R.; Busse, G. Microemulsions: A qualitative thermodynamic approach. J. Phys. Chem. 1990, 94, 3881–3894. [Google Scholar] [CrossRef]
- Ghosh, P.; Murthy, R. Microemulsions: A potential drug delivery system. Curr. Drug Deliv. 2006, 3, 167–180. [Google Scholar] [CrossRef]
- Nigade, P.M.; Patil, S.L.; Tiwari, S.S. Self emulsifying drug delivery system (SEDDS): A review. Int. J. Pharm. Biol. Sci. 2012, 2, 42–52. [Google Scholar]
- Lawrence, M.J.; Rees, G.D. Microemulsion-based media as novel drug delivery systems. Adv. Drug Deliv. Rev. 2000, 45, 89–121. [Google Scholar] [CrossRef]
- Kumar, S.; Burgess, D.J. Nanosuspensions. In Long Acting Injections and Implants; Springer: Berlin/Heidelberg, Germany, 2012; pp. 239–261. [Google Scholar]
- Martinez, M.N.; Khan, M.A. Regulatory Issues and Challenges Associated with the Development of Per-formance Specifications for Modified Release Parenteral Products. In Long Acting Injections and Implants; Springer: Berlin/Heidelberg, Germany, 2012; pp. 505–535. [Google Scholar]
- Pramanick, S.; Singodia, D.; Chandel, V. Excipient selection in parenteral formulation development. Pharma Times 2013, 45, 65–77. [Google Scholar]
- Shrestha, H.; Bala, R.; Arora, S. Lipid-Based Drug Delivery Systems. J. Pharm. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Sharma, N.; Bansal, M.; Visht, S.; Sharma, P.K.; Kulkarni, G.T. Nanoemulsion: A new concept of delivery system. Chron. Young Sci. 2010, 1, 2. [Google Scholar]
- Tadros, T.F. Emulsion Science and Technology: A General Introduction; Wiley-VCH: Weinheim, Germany, 2009. [Google Scholar]
- Wang, Y.; Zheng, Y.; Zhang, L.; Wang, Q.; Zhang, D. Stability of nanosuspensions in drug delivery. J. Control. Release 2013, 172, 1126–1141. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.S.; York, P.; Blagden, N. Preparation of hydrocortisone nanosuspension through a bottom-up nanoprecipitation technique using microfluidic reactors. Int. J. Pharm. 2009, 375, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.W.; Mitchnick, M. Early-Stage Formulation Considerations. Curr. Protoc. Chem. Biol. 2017, 9, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Rahman, Z.; Charoo, N.A.; Akhter, S.; Beg, S.; Reddy, I.K.; Khan, M.A. Nanotechnology-based drug products: Science and regulatory considerations. In Nanoscale Fabrication, Optimization, Scale-Up and Biological Aspects of Pharmaceutical Nanotechnology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 619–655. [Google Scholar]
- Zheng, N.; Sun, D.D.; Zou, P.; Jiang, W. Scientific and regulatory considerations for generic complex drug products containing na-nomaterials. AAPS J. 2017, 19, 619–631. [Google Scholar] [CrossRef]
- Benita, S.; Levy, M. Submicron emulsions as colloidal drug carriers for intravenous administration: Comprehensive physicochemical characterization. J. Pharm. Sci. 1993, 82, 1069–1079. [Google Scholar] [CrossRef]
- Ha, W.N.; Bentz, D.P.; Kahler, B.; Walsh, L.J. D90: The Strongest Contributor to Setting Time in Mineral Trioxide Aggregate and Portland Cement. J. Endod. 2015, 41, 1146–1150. [Google Scholar] [CrossRef]
- Shen, J.; Burgess, D.J. In vitro dissolution testing strategies for nanoparticulate drug delivery systems: Recent developments and challenges. Drug Deliv. Transl. Res. 2013, 3, 409–415. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar]
- Agrawal, O.P.; Agrawal, S. An overview of new drug delivery system: Microemulsion. Asian J. Pharm. Sci. Tech. 2012, 2, 5–12. [Google Scholar]
- Nesamony, J.; Shah, I.S.; Kalra, A.; Jung, R. Nebulized oil-in-water nanoemulsion mists for pulmonary delivery: Development, physico-chemical characterization andin vitroevaluation. Drug Dev. Ind. Pharm. 2013, 40, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Jensen, G.L.; Koletzko, B.V.; Singer, P.; Wanten, G.J.A. Lipid emulsions in parenteral nutrition of intensive care patients: Current thinking and future directions. Intensiv. Care Med. 2010, 36, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Boullata, J.I.; Berlana, D.; Pietka, M.; Klek, S.; Martindale, R. Use of Intravenous Lipid Emulsions with Parenteral Nutrition: Practical Handling Aspects. J. Parenter. Enter. Nutr. 2020, 44, S74–S81. [Google Scholar] [CrossRef] [PubMed]
- Shahiwala, A.; Vyas, T.K.; Amiji, M. Nanocarriers for systemic and mucosal vaccine delivery. Recent Patents Drug Deliv. Formul. 2007, 1, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A.E.; Titball, R.W.; Ewilliamson, D. Vaccine delivery using nanoparticles. Front. Cell. Infect. Microbiol. 2013, 3, 13. [Google Scholar] [CrossRef]
- Souza, B.D.; Shastri, P.N.; Hammons, G.; Kim, E.; Kolluru, L.P.; Carlone, G.M.; Rajam, G.; Souza, M.J.D. Immune-potentiation of Pneumococcal Capsular Polysaccharide Antigen using Albumin Microparticles. J. Pharmacovigil. 2018, 6, 1–6. [Google Scholar] [CrossRef]
- D’Souza, M.J. Microparticulate Formulation for a Pneumococcal Capsular Polysaccharide Antigen. In Nanoparticulate Vaccine Delivery Systems; Jenny Stanford Publishing: Temasek Avenue, Singapore, 2015; pp. 136–147. [Google Scholar]
- Baker, J.; Bielinska, A.; Myc, A. Compositions and Methods for Human Immunodeficiency Virus Vaccination. U.S. Patent 11/786,855, 2008. [Google Scholar]
- Nkanga, C.I.; Fisch, A.; Rad-Malekshahi, M.; Romic, M.D.; Kittel, B.; Ullrich, T.; Wang, J.; Krause, R.W.M.; Adler, S.; Lammers, T.; et al. Clinically established biodegradable long acting injectables: An industry perspective. Adv. Drug Deliv. Rev. 2020, 167, 19–46. [Google Scholar] [CrossRef]
- Owen, A.; Rannard, S. Strengths, weaknesses, opportunities and challenges for long acting injectable therapies: Insights for applications in HIV therapy. Adv. Drug Deliv. Rev. 2016, 103, 144–156. [Google Scholar] [CrossRef]
- Kolluru, L.P.; Rizvi, S.A.A.; D’Souza, M.; D’Souza, M.J. Formulation development of albumin based theragnostic nanoparticles as a potential delivery system for tumor targeting. J. Drug Target. 2012, 21, 77–86. [Google Scholar] [CrossRef]
| Excipient | Function | Route of Administration | Maximum Potency Per Unit Dose |
|---|---|---|---|
| Chlorobutanol | Antimicrobial preservative | Parenteral | 0.5% w/v |
| Methylparaben | Antimicrobial preservative | IV | 5% w/v |
| Bisulfite sodium | Antioxidant | IV | 50 mg |
| Polyethyleneglycol 300 | Co-solvent | IM | 50% w/v |
| Disodium EDTA | Chelating agent | IM | 10% w/v |
| Sorbitan monolaurate | Surfactant | IM | 0.38% w/v |
| Castor oil | Lipid phase | IM | 30% w/v |
| Monothioglycerol | Tonicity modifier | IM | 0.5% w/v |
| Dosage Form | Sponsor | Indication | Active Ingredient | Route of Administration |
|---|---|---|---|---|
| Paclitaxel Injection, USP | Grand Pharma Ltd. | Anti-tumor | Paclitaxel | Intravenous |
| Diprivan® | Fresenius Kabi USA LLC | General anesthetic and sedation drug | Propofol | Intravenous |
| ZYPREXA® Intramuscular Injection | Lilly | Treatment of Schizophrenia | Olanzapine | Intramuscular |
| Phytonadione Injection, USP | International Medication Systems Ltd. | Treatment of Hypoprothrombinemia | Phytonadione | Intravenous/Intramuscular |
| Abraxane® for Injectable Suspension, USP | Abraxis Bioscience | Treatment of Metastatic Breast Cancer | Paclitaxel | Intravenous |
| Invega sustenna® | Janssen Pharms | Antipyschotic | Paliperidone Palmitate | Intramuscular |
| Methylprednisolone Acetate Injectable Suspension, USP | Sandoz Inc. | Anti-inflammatory | Methylprednisolone Acetate | Intramuscular, Intraarticular, Intralesional |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolluru, L.P.; Atre, P.; Rizvi, S.A.A. Characterization and Applications of Colloidal Systems as Versatile Drug Delivery Carriers for Parenteral Formulations. Pharmaceuticals 2021, 14, 108. https://doi.org/10.3390/ph14020108
Kolluru LP, Atre P, Rizvi SAA. Characterization and Applications of Colloidal Systems as Versatile Drug Delivery Carriers for Parenteral Formulations. Pharmaceuticals. 2021; 14(2):108. https://doi.org/10.3390/ph14020108
Chicago/Turabian StyleKolluru, Lakshmi Prasanna, Prachi Atre, and Syed A. A. Rizvi. 2021. "Characterization and Applications of Colloidal Systems as Versatile Drug Delivery Carriers for Parenteral Formulations" Pharmaceuticals 14, no. 2: 108. https://doi.org/10.3390/ph14020108
APA StyleKolluru, L. P., Atre, P., & Rizvi, S. A. A. (2021). Characterization and Applications of Colloidal Systems as Versatile Drug Delivery Carriers for Parenteral Formulations. Pharmaceuticals, 14(2), 108. https://doi.org/10.3390/ph14020108








