HER2 Aberrations in Non-Small Cell Lung Cancer: From Pathophysiology to Targeted Therapy
Abstract
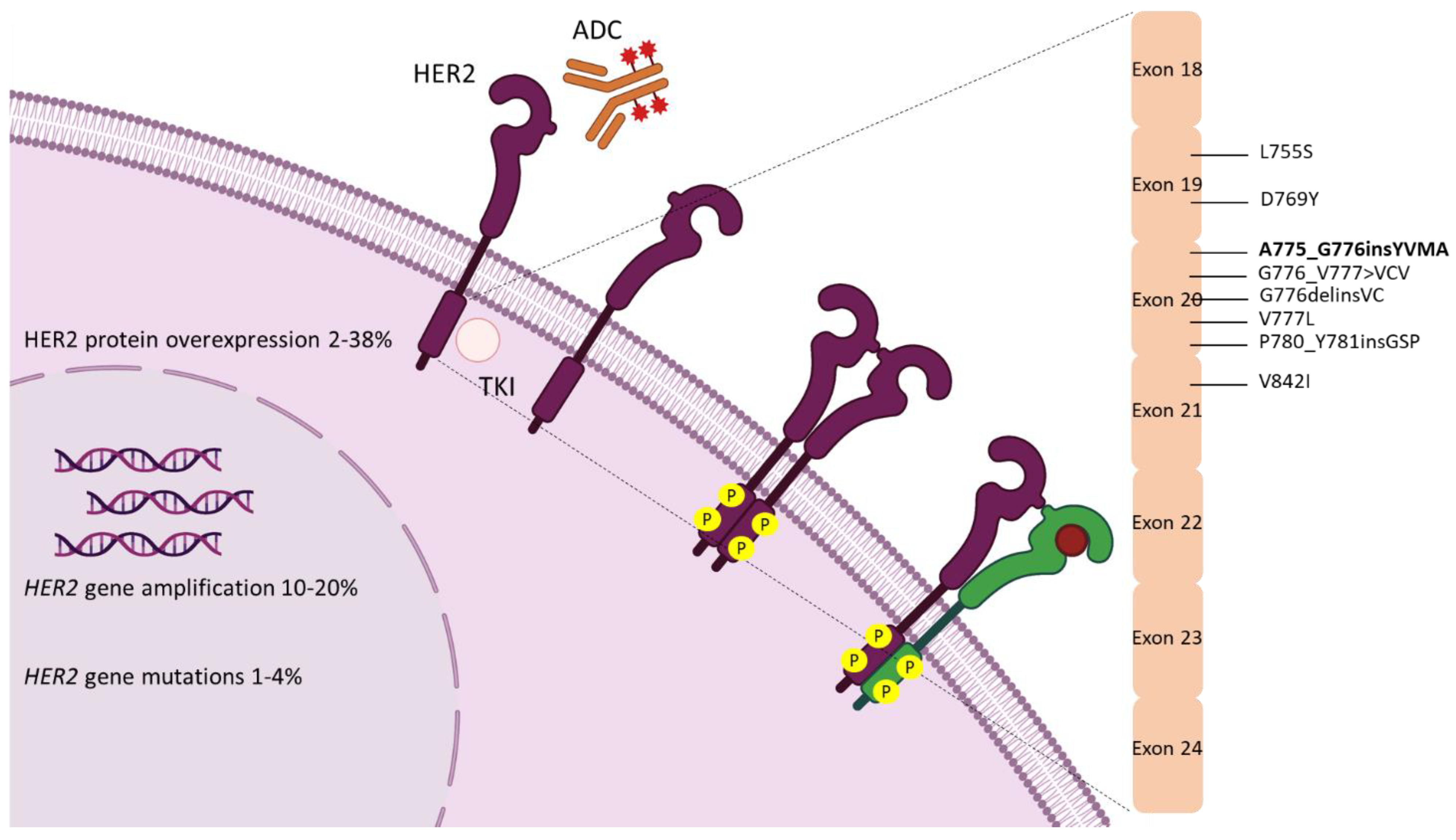
:1. Introduction
2. Biology
3. Epidemiology
4. HER2 Diagnostic Assays
5. Targeting HER2 in NSCLC
| Reference | TKI | Study | N | Previous Treatment Type; N (%) | HER2 Positivity Definition (Method) | ORR N (%) | PFS Median (95% CI) | OS Median (95% CI) | All-Grade AEs (%) |
|---|---|---|---|---|---|---|---|---|---|
| De Grève et al. [54] | Afatinib | Phase II, Basket | 7 | Chemotherapy; 5 (71.4) | Exon 20 mutation (PCR; central) | 0/7 (0) | 17 weeks (NA) | – | Diarrhea (95.0), rash/acne (80.0), stomatitis (46.0) |
| Peters et al. [55] | Afatinib | Phase II, Single-arm | 28 | Systemic therapy; 26 (92.9) | Activating mutation (NA; local) | 3/16 (18.8) | – | – | Diarrhea (35.7), skin disorders (28.6), stomatitis (14.3) |
| Dziadziuszko et al. [56] | Afatinib | Phase II, Single-arm | 13 | Chemotherapy; 13 (100.0) | Exon 20 mutation (Various; local) | 1/13 (7.7) | 15.9 weeks (6.0–35.4) | 56.0 weeks (16.3- NR) | Diarrhea (NA), skin disorders (NA), stomatitis (NA) |
| Fan et al. [57] | Afatinib | Phase II, Single-arm | 18 | Chemotherapy; 18 (100.0) | Exon 19, 20 mutation (RT-PCR; central) | 0/18 (0) | 2.8 months (1.9–4.6) | 10.0 months (8.5–10.1) | Diarrhea (66.7), rash (33.3), stomatitis (11.1) |
| Kris et al. [60] | Dacomitinib | Phase II, Single-arm | 26 | Chemotherapy; 18 (100.0) | Exon 20 mutation (NGS; central) | 3/26 (11.5) | 3 months (2.0–4.0) | 9 months (7.0–21.0) | Diarrhea (90.0), rash (73.0), fatigue (57.0) |
| Hyman et al. [63] | Neratinib | Phase II, Single-arm | 26 | Systemic therapy; 26 (100.0) | Activating mutation (NGS; local) | 1/26 (3.8) | 5.5 months (NA) | – | Diarrhea (73.8), nausea (43.3), vomiting (41.1) |
| Robichaux et al. [66] | Poziotinib | Phase II, Single-arm | 12 | Systemic therapy; 11 (91.7) | Exon 20 mutation (NA; NA) | 5/12 (41.7) | 5.6 months (NA) | – | Rash (100.0), diarrhea (91.7), paronychia (91.7) |
| Elamin et al. [52] | Poziotinib | Phase II, Single-arm | 30 | Systemic therapy; 28 (93.0) | Exon 20 mutation (NGS; local) | 8/30 (26.7) | 5.5 months (4.0–7.0) | 15.0 months (9.0-NR) | Skin rash (83.0), Diarrhea (80.0) Paronychia (70.0) |
| Cornelissen et al. [69] | Poziotinib | Phase II, Randomized | 48 | No; 0 (0) | Exon 20 mutation (NGS; local) | 21/48 (43.8) | 5.6 months (NA) | – | NA |
| Wang et al. [72] | Pyrotinib | Phase II, Single-arm | 15 | Systemic therapy; 15 (100.0) | Exon 20 mutation (NGS; local) | 8/15 (53.3) | 6.4 months (1.6–11.2) | – | Diarrhea (26.7), anemia (26.7), hypocalcemia (26.7) |
| Zhou et al. [73] | Pyrotinib | Phase II, Single-arm | 60 | Chemotherapy 60 (100.0) | Exon 19, 20 mutation (RT-PCR; central) | 18/60 (30.0) | 6.9 months (5.5–8.3) | 14.4 months (12.3–21.3) | Diarrhea (91.7), elevated creatinine (30.0), vomiting (28.3) |
| Liu et al. [75] | Tarloxitinb | Phase II, Basket | 11 | Chemotherapy 11 (100.0) | Activating mutation (NA; local) | 2/9 (22.2) | – | – | QTc prolongation (60.9), rash (43.5), diarrhea (21.7) |
| Reference | Agent | Study | N | Previous Treatment Type; N (%) | HER2 Positivity Definition (Method) | ORR N (%) | PFS Median (95% CI) | OS Median (95% CI) | All-Grade AEs (%) |
|---|---|---|---|---|---|---|---|---|---|
| Kinoshita et al. [79] | Trastuzumab | Phase II, Single-arm | 10 | Yes, systemic therapy; 10 (100.0) | Overexpression/ amplification (IHC3+, IHC2+/FISH+; NA), activating mutation (NA; NA) | 0/10 (0) | 5.2 months (1.4–6.3) | – | – |
| Gatzemeier et al. [80] | Cisplatin, gemcitabine ± trastuzumab | Phase II, Randomized | 101 (50/101 cisplatin, gemcitabine and trastuzumab; 51/101 cisplatin, gemcitabine) | No; 0 (0) | Overexpression/ amplification (IHC2-3+, FISH+, ELISA+; local) | 18/50 (36.0); 21/51 (41.2) | 6.1 months (0.1–19.6); 7.0 months (6.0–7.7) | 12.2 months (0.1–19.6); NR | Nausea (74.0), vomiting (46.0), fatigue (42.0) |
| Langer et al. [81] | Carboplatin, paclitaxel, trastuzumab | Phase II, Single-arm | 53 | No; 0 (0) | Overexpression/ amplification (IHC1-3+; local) | 13/53 (24.5) | 3.3 months (NA) | 10.1 months (6.7–14.6) | Anemia (99.0), fatigue (71.0), sensory neuropathy (65.0) |
| Hainsworth et al. [82] | Trastuzumab, pertuzumab | Phase II, Basket | 30 (16/30 HER2 overexpression/ amplification; 14/30 HER2 mutation) | Yes, systemic therapy; NA | Overexpression/ amplification (IHC3+, FISH+; local); exon 20 mutation (NGS; local) | 5/30 (16.7) [2/13 (15.4); 3/14 (21.4)] | – | – | – |
| Hotta et al. [84] | T-DM1 | Phase II, Single-arm | 15 | Yes, systemic therapy; 15 (100.0) | Overexpression/ amplification (IHC3+, IHC2+/FISH+; central), exon 20 mutation (NGS; central) | 1/15 (6.7) | 2 months (1.4–4.0) | 10.9 months (4.4–12.0) | – |
| Li et al. [85] | T-DM1 | Phase II, Single-arm | 49 (11/49 HER2 amplification; 28/49 HER2 mutation) | Yes, systemic therapy; 49 (100.0) | Activating mutation (NGS; local) | 25/49 (51.0) [6/11 (54.5); 14/28 (50.0)] | 5 months (3.5–5.9) | – | Elevated LFTs (63.3), thrombocytopenia (30.6), nausea (28.6) |
| Peters et al. [87] | T-DM1 | Phase II, Single-arm | 49 (29/49 HER2 IHC2+; 20/49 HER2 IHC3+) | Yes, systemic therapy; 49 (100.0) | Overexpression (IHC3+, IHC2+; central) | 4/49 (8.2) [0/29 (0); 4/20 (20.0)] | 2.6 months (1.4–2.8) | 12.2 (4.7–23.6) | Infusion reaction (14.3), peripheral neuropathy (14.3), hemorrhage (14.3) |
| Tsurutani et al. [89] | T-Dxd | Phase I, Single-arm | 18 | Yes, systemic therapy; 18 (100.0) | Overexpression/ amplification (IHC1+, FISH+, NGS; local) | 10/18 (55.6) | 11.3 months (5.5–14.1) | 17.3 months (17.3-NR) | Nausea (74.6), vomiting (52.6), anemia (39) |
| Li et al. [53] | T-Dxd | Phase II, Two arms | 49 HER2 overexpression; 91 HER2 mutations | Yes, systemic therapy; 140 (100.0) | Activating mutation (NGS; local) | 12/49 (24.5); 50/91 (54.9) | 5.4 months (2.8–7.0); 8.2 months (6.0–11.9) | NA; 17.8 months (13.8–22.1) | Nausea (73.0), fatigue (53.0), alopecia (46.0) |
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bargmann, C.; Hung, M.-C.; Weinberg, R.A. The neu oncogene encodes an epidermal growth factor receptor-related protein. Nature 1986, 319, 226–230. [Google Scholar] [CrossRef]
- Schechter, A.L.; Stern, D.F.; Vaidyanathan, L.; Decker, S.J.; Drebin, J.A.; Greene, M.I.; Weinberg, R.A. The neu oncogene: An erb-B-related gene encoding a 185,000-Mr tumour antigen. Nature 1984, 312, 513–516. [Google Scholar] [CrossRef]
- Slamon, D.J.; Clark, G.M.; Wong, S.G.; Levin, W.J.; Ullrich, A.; McGuire, W.L. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987, 235, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Hynes, N.E.; MacDonald, G. ErbB receptors and signaling pathways in cancer. Curr. Opin. Cell Biol. 2009, 21, 177–184. [Google Scholar] [CrossRef]
- Seshadri, R.; Firgaira, F.; Horsfall, D.; McCaul, K.; Setlur, V.; Kitchen, P. Clinical significance of HER-2/neu oncogene amplification in primary breast cancer. The South Australian Breast Cancer Study Group. J. Clin. Oncol. 1993, 11, 1936–1942. [Google Scholar] [CrossRef]
- Press, M.F.; Bernstein, L.; Thomas, P.A.; Meisner, L.F.; Zhou, J.-Y.; Ma, Y.; Hung, G.; Robinson, R.A.; Harris, C.; El-Naggar, A. HER-2/neu gene amplification characterized by fluorescence in situ hybridization: Poor prognosis in node-negative breast carcinomas. J. Clin. Oncol. 1997, 15, 2894–2904. [Google Scholar] [CrossRef] [PubMed]
- Eiermann, W.; Group IHS. Trastuzumab combined with chemotherapy for the treatment of HER2-positive metastatic breast cancer: Pivotal trial data. Ann. Oncol. 2001, 12, S57–S62. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Tripathy, D.; Mendelsohn, J.; Baughman, S.; Benz, C.C.; Dantis, L.; Sklarin, N.T.; Seidman, A.D.; Hudis, C.A.; Moore, J.; et al. Phase II study of weekly intravenous recombinant humanized anti-p185HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. J. Clin. Oncol. 1996, 14, 737–744. [Google Scholar] [CrossRef]
- Bang, Y.-J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Yan, M.; Schwaederle, M.; Arguello, D.; Millis, S.Z.; Gatalica, Z.; Kurzrock, R. HER2 expression status in diverse cancers: Review of results from 37,992 patients. Cancer Metastasis Rev. 2015, 34, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Oh, D.-Y.; Bang, Y.-J. HER2-targeted therapies—A role beyond breast cancer. Nat. Rev. Clin. Oncol. 2020, 17, 33–48. [Google Scholar] [CrossRef]
- Chmielecki, J.; Ross, J.S.; Wang, K.; Frampton, G.M.; Palmer, G.A.; Ali, S.M.; Palma, N.; Morosini, D.; Miller, V.A.; Yelensky, R.; et al. Oncogenic alterations in ERBB2/HER2 represent potential therapeutic targets across tumors from diverse anatomic sites of origin. Oncologist 2015, 20, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Burgess, A.W.; Cho, H.-S.; Eigenbrot, C.; Ferguson, K.M.; Garrett, T.P.J.; Leahy, D.J.; Lemmon, M.A.; Sliwkowski, M.X.; Ward, C.W.; Yokoyama, S. An Open-and-Shut Case? Recent Insights into the Activation of EGF/ErbB Receptors. Mol. Cell 2003, 12, 541–552. [Google Scholar] [CrossRef]
- Kovacs, E.; Zorn, J.A.; Huang, Y.; Barros, T.; Kuriyan, J. A Structural Perspective on the Regulation of the Epidermal Growth Factor Receptor. Annu. Rev. Biochem. 2015, 84, 739–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yarden, Y.; Sliwkowski, M.X. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2001, 2, 127–137. [Google Scholar] [CrossRef]
- Citri, A.; Yarden, Y. EGF–ERBB signalling: Towards the systems level. Nat. Rev. Mol. Cell Biol. 2006, 7, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-S.; Mason, K.; Ramyar, K.X.; Stanley, A.M.; Gabelli, S.B.; Denney, D.W., Jr.; Leahy, D.J. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature 2003, 421, 756–760. [Google Scholar] [CrossRef]
- Garrett, T.P.; McKern, N.M.; Lou, M.; Elleman, T.C.; Adams, T.; Lovrecz, G.O.; Kofler, M.; Jorissen, R.N.; Nice, E.C.; Burgess, A.W.; et al. The Crystal Structure of a Truncated ErbB2 Ectodomain Reveals an Active Conformation, Poised to Interact with Other ErbB Receptors. Mol. Cell 2003, 11, 495–505. [Google Scholar] [CrossRef]
- Citri, A.; Skaria, K.B.; Yarden, Y. The deaf and the dumb: The biology of ErbB-2 and ErbB-3. Exp. Cell Res. 2003, 284, 54–65. [Google Scholar] [CrossRef]
- Jura, N.; Shan, Y.; Cao, X.; Shaw, D.E.; Kuriyan, J. Structural analysis of the catalytically inactive kinase domain of the human EGF receptor 3. Proc. Natl. Acad. Sci. USA 2009, 106, 21608–21613. [Google Scholar] [CrossRef] [Green Version]
- Burstein, H.J. The Distinctive Nature of HER2-Positive Breast Cancers. N. Engl. J. Med. 2005, 353, 1652–1654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olayioye, M.A. Intracellular signaling pathways of ErbB2/HER-2 and family members. Breast Cancer Res. 2001, 3, 385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moasser, M.M. The oncogene HER2: Its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene 2007, 26, 6469–6487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirsch, F.R.; Varella-Garcia, M.; Franklin, W.A.; Veve, R.; Chen, L.; Helfrich, B.; Zeng, C.; Baron, A.; Bunn, P.A., Jr. Evaluation of HER-2/neu gene amplification and protein expression in non-small cell lung carcinomas. Br. J. Cancer 2002, 86, 1449–1456. [Google Scholar] [CrossRef] [Green Version]
- Arcila, M.E.; Chaft, J.; Nafa, K.; Roy-Chowdhuri, S.; Lau, C.; Zaidinski, M.; Paik, P.K.; Zakowski, M.F.; Kris, M.G.; Ladanyi, M. Prevalence, Clinicopathologic Associations, and Molecular Spectrum of ERBB2 (HER2) Tyrosine Kinase Mutations in Lung Adenocarcinomas. Clin. Cancer Res. 2012, 18, 4910–4918. [Google Scholar] [CrossRef] [Green Version]
- Rolfo, C.; Russo, A. HER2 Mutations in Non–Small Cell Lung Cancer: A Herculean Effort to Hit the Target. Cancer Discov. 2020, 10, 643–645. [Google Scholar] [CrossRef] [PubMed]
- Li, B.T.; Ross, D.S.; Aisner, D.L.; Chaft, J.E.; Hsu, M.; Kako, S.L.; Kris, M.G.; Varella-Garcia, M.; Arcila, M.E. HER2 Amplification and HER2 Mutation Are Distinct Molecular Targets in Lung Cancers. J. Thorac. Oncol. 2016, 11, 414–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.E.; Narasanna, A.; Perez-Torres, M.; Xiang, B.; Wu, F.Y.; Yang, S.; Carpenter, G.; Gazdar, A.F.; Muthuswamy, S.K.; Arteaga, C.L. HER2 kinase domain mutation results in constitutive phosphorylation and activation of HER2 and EGFR and resistance to EGFR tyrosine kinase inhibitors. Cancer Cell 2006, 10, 25–38. [Google Scholar] [CrossRef] [Green Version]
- Cappuzzo, F.; Varella-Garcia, M.; Shigematsu, H.; Domenichini, I.; Bartolini, S.; Ceresoli, G.L.; Rossi, E.; Ludovini, V.; Gregorc, V.; Toschi, L.; et al. Increased HER2 Gene Copy Number Is Associated with Response to Gefitinib Therapy in Epidermal Growth Factor Receptor–Positive Non–Small-Cell Lung Cancer Patients. J. Clin. Oncol. 2005, 23, 5007–5018. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.A.; Arcila, M.E.; Rekhtman, N.; Sima, C.S.; Zakowski, M.F.; Pao, W.; Kris, M.G.; Miller, V.A.; Ladanyi, M.; Riely, G.J. Analysis of Tumor Specimens at the Time of Acquired Resistance to EGFR-TKI Therapy in 155 Patients with EGFR-Mutant Lung Cancers. Clin. Cancer Res. 2013, 19, 2240–2247. [Google Scholar] [CrossRef] [Green Version]
- Friedlaender, A.; Subbiah, V.; Russo, A.; Banna, G.L.; Malapelle, U.; Rolfo, C.; Addeo, A. EGFR and HER2 exon 20 insertions in solid tumours: From biology to treatment. Nat. Rev. Clin. Oncol. 2021, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Shigematsu, H.; Takahashi, T.; Nomura, M.; Majmudar, K.; Suzuki, M.; Lee, H.; Wistuba, I.I.; Fong, K.M.; Toyooka, S.; Shimizu, N.; et al. Somatic mutations of the HER2 kinase domain in lung adenocarcinomas. Cancer Res. 2005, 65, 1642–1646. [Google Scholar] [CrossRef] [Green Version]
- Peters, S.; Zimmermann, S. Targeted therapy in NSCLC driven by HER2 insertions. Transl. Lung Cancer Res. 2014, 3, 84–88. [Google Scholar] [CrossRef]
- Stephens, P.; Hunter, C.; Bignell, G.; Edkins, S.; Davies, H.; Teague, J.; Stevens, C.; O’Meara, S.; Smith, R.; Parker, A.; et al. Lung cancer: Intragenic ERBB2 kinase mutations in tumours. Nature 2004, 431, 525–526. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Xia, Y. Targeting HER2 Alterations in Non–Small-Cell Lung Cancer: A Comprehensive Review. JCO Precis. Oncol. 2020, 411–425. [Google Scholar] [CrossRef]
- Perera, S.A.; Li, D.; Shimamura, T.; Raso, M.G.; Ji, H.; Chen, L.; Borgman, C.L.; Zaghlul, S.; Brandstetter, K.A.; Kubo, S.; et al. HER2YVMA drives rapid development of adenosquamous lung tumors in mice that are sensitive to BIBW2992 and rapamycin combination therapy. Proc. Natl. Acad. Sci. USA 2009, 106, 474–479. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, H.; Higasa, K.; Sakaguchi, M.; Shien, K.; Soh, J.; Ichimura, K.; Furukawa, M.; Hashida, S.; Tsukuda, K.; Takigawa, N.; et al. Novel Germline Mutation in the Transmembrane Domain of HER2 in Familial Lung Adenocarcinomas. J. Natl. Cancer Inst. 2013, 106, djt338. [Google Scholar] [CrossRef]
- Greulich, H.; Kaplan, B.; Mertins, P.; Chen, T.-H.; Tanaka, K.E.; Yun, C.-H.; Zhang, X.; Lee, S.-H.; Cho, J.; Ambrogio, L.; et al. Functional analysis of receptor tyrosine kinase mutations in lung cancer identifies oncogenic extracellular domain mutations of ERBB2. Proc. Natl. Acad. Sci. USA 2012, 109, 14476. [Google Scholar] [CrossRef] [Green Version]
- Collisson, E.A.C.J.; Campbell, J.; Brooks, A.; Berger, A.; Lee, W.; Chmielecki, J.; Beer, D.; Cope, L.; Creighton, C.; Danilova, L.; et al. Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014, 511, 543–550. [Google Scholar]
- Heinmöller, P.; Gross, C.; Beyser, K.; Schmidtgen, C.; Maass, G.; Pedrocchi, M.; Rüschoff, J. HER2 status in non-small cell lung cancer: Results from patient screening for enrollment to a phase II study of herceptin. Clin. Cancer Res. 2003, 9, 5238–5243. [Google Scholar] [CrossRef]
- Takeda, M.; Sakai, K.; Hayashi, H.; Tanaka, K.; Tanizaki, J.; Takahama, T.; Haratani, K.; Nishio, K.; Nakagawa, K. Clinical characteristics of non-small cell lung cancer harboring mutations in exon 20 of EGFR or HER2. Oncotarget 2018, 9, 21132–21140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ninomiya, K.; Hata, T.; Yoshioka, H.; Ohashi, K.; Bessho, A.; Hosokawa, S.; Ishikawa, N.; Yamasaki, M.; Shibayama, T.; Aoe, K.; et al. A Prospective Cohort Study to Define the Clinical Features and Outcome of Lung Cancers Harboring HER2 Aberration in Japan (HER2-CS STUDY). Chest 2019, 156, 357–366. [Google Scholar] [CrossRef]
- Takezawa, K.; Pirazzoli, V.; Arcila, M.E.; Nebhan, C.A.; Song, X.; De Stanchina, E.; Ohashi, K.; Janjigian, Y.Y.; Spitzler, P.J.; Melnick, M.A.; et al. HER2 Amplification: A Potential Mechanism of Acquired Resistance to EGFR Inhibition in EGFR-Mutant Lung Cancers That Lack the Second-Site EGFRT790M Mutation. Cancer Discov. 2012, 2, 922–933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohashi, K.; Maruvka, Y.E.; Michor, F.; Pao, W. Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor–Resistant Disease. J. Clin. Oncol. 2013, 31, 1070–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohashi, K.; Ninomiya, K.; Yoshioka, H.; Bessho, A.; Shibayama, T.; Aoe, K.; Ishikawa, N.; Kozuki, T.; Kawai, H.; Kuyama, S.; et al. Impact of HER2 expression on EGFR-TKI treatment outcomes in lung tumors harboring EGFR mutations: A HER2-CS study subset analysis. Lung Cancer 2020, 150, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Arch. Pathol. Lab. Med. 2018, 142, 1364–1382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartley, A.N.; Washington, M.K.; Ventura, C.B.; Ismaila, N.; Colasacco, C.; Benson, A.B., III; Carrato, A.; Gulley, M.L.; Jain, D.; Kakar, S.; et al. HER2 Testing and Clinical Decision Making in Gastroesophageal Adenocarcinoma: Guideline from the College of American Pathologists, American Society for Clinical Pathology, and the American Society of Clinical Oncology. J. Clin. Oncol. 2017, 35, 446–464. [Google Scholar] [CrossRef] [Green Version]
- Jørgensen, J.T.; Winther, H.; Askaa, J.; Andresen, L.; Olsen, D.; Mollerup, J. A Companion Diagnostic with Significant Clinical Impact in Treatment of Breast and Gastric Cancer. Front. Oncol. 2021, 11, 676939. [Google Scholar] [CrossRef] [PubMed]
- Hotta, K.; Yanai, H.; Ohashi, K.; Ninomiya, K.; Nakashima, H.; Kayatani, H.; Takata, M.; Kiura, K. Pilot evaluation of a HER2 testing in non-small-cell lung cancer. J. Clin. Pathol. 2019, 73, 353–357. [Google Scholar] [CrossRef]
- Yoshizawa, A.; Sumiyoshi, S.; Sonobe, M.; Kobayashi, M.; Uehara, T.; Fujimoto, M.; Tsuruyama, T.; Date, H.; Haga, H. HER2 status in lung adenocarcinoma: A comparison of immunohistochemistry, fluorescence in situ hybridization (FISH), dual-ISH, and gene mutations. Lung Cancer 2020, 85, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Shiraishi, K.; Yoshida, A.; Shimada, Y.; Suzuki, K.; Asamura, H.; Furuta, K.; Kohno, T.; Tsuta, K. HER2 gene mutations in non-small cell lung carcinomas: Concurrence with her2 gene amplification and her2 protein expression and phosphorylation. Lung Cancer 2015, 87, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Elamin, Y.Y.; Robichaux, J.P.; Carter, B.W.; Altan, M.; Gibbons, D.L.; Fossella, F.V.; Lam, V.K.; Patel, A.B.; Negrao, M.V.; Le, X.; et al. Poziotinib for Patients with HER2 Exon 20 Mutant Non–Small-Cell Lung Cancer: Results from a Phase II Trial. J. Clin. Oncol. 2021. [Google Scholar] [CrossRef]
- Li, B.T.; Smit, E.F.; Goto, Y.; Nakagawa, K.; Udagawa, H.; Mazières, J.; Nagasaka, M.; Bazhenova, L.; Saltos, A.N.; Felip, E.; et al. Trastuzumab Deruxtecan in HER2-Mutant Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- De Grève, J.; Moran, T.; Graas, M.P.; Galdermans, D.; Vuylsteke, P.; Canon, J.L.; Schallier, D.; Decoster, L.; Teugels, E.; Massey, D.; et al. Phase II study of afatinib, an irreversible ErbB family blocker, in demographically and genotypically defined lung adenocarcinoma. Lung Cancer 2015, 88, 63–69. [Google Scholar] [CrossRef]
- Peters, S.; Curioni-Fontecedro, A.; Nechushtan, H.; Shih, J.-Y.; Liao, W.-Y.; Gautschi, O.; Spataro, V.; Unk, M.; Yang, J.C.-H.; Lorence, R.M.; et al. Activity of Afatinib in Heavily Pretreated Patients with ERBB2 Mutation–Positive Advanced NSCLC: Findings From a Global Named Patient Use Program. J. Thorac. Oncol. 2018, 13, 1897–1905. [Google Scholar] [CrossRef] [Green Version]
- Dziadziuszko, R.; Smit, E.F.; Dafni, U.; Wolf, J.; Wasag, B.; Biernat, W.; Finn, S.P.; Kammler, R.; Tsourti, Z.; Rabaglio, M.; et al. Afatinib in NSCLC With HER2 Mutations: Results of the Prospective, Open-Label Phase II NICHE Trial of European Thoracic Oncology Platform (ETOP). J. Thorac. Oncol. 2019, 14, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Chen, J.; Zhou, C.; Wang, H.; Shu, Y.; Zhang, J.; Hua, H.; Huang, D.C.-L.; Zhou, C. Afatinib in patients with advanced non-small cell lung cancer harboring HER2 mutations, previously treated with chemotherapy: A phase II trial. Lung Cancer 2020, 147, 209–213. [Google Scholar] [CrossRef]
- Engelman, J.A.; Zejnullahu, K.; Gale, C.-M.; Lifshits, E.; Gonzales, A.J.; Shimamura, T.; Zhao, F.; Vincent, P.W.; Naumov, G.N.; Bradner, J.E.; et al. PF00299804, an Irreversible Pan-ERBB Inhibitor, Is Effective in Lung Cancer Models with EGFR and ERBB2 Mutations that Are Resistant to Gefitinib. Cancer Res. 2007, 67, 11924–11932. [Google Scholar] [CrossRef] [Green Version]
- Jänne, P.A.; Boss, D.S.; Camidge, D.R.; Britten, C.D.; Engelman, J.A.; Garon, E.B.; Guo, F.; Wong, S.; Liang, J.; Letrent, S.; et al. Phase I Dose-Escalation Study of the Pan-HER Inhibitor, PF299804, in Patients with Advanced Malignant Solid Tumors. Clin. Cancer Res. 2011, 17, 1131–1139. [Google Scholar] [CrossRef] [Green Version]
- Kris, M.G.; Camidge, D.R.; Giaccone, G.; Hida, T.; Li, B.; O’Connell, J.; Taylor, I.; Zhang, H.; Arcila, M.E.; Goldberg, Z.; et al. Targeting HER2 aberrations as actionable drivers in lung cancers: Phase II trial of the pan-HER tyrosine kinase inhibitor dacomitinib in patients with HER2-mutant or amplified tumors. Ann. Oncol. 2015, 26, 1421–1427. [Google Scholar] [CrossRef]
- Nagano, M.; Kohsaka, S.; Ueno, T.; Kojima, S.; Saka, K.; Iwase, H.; Kawazu, M.; Mano, H. High-Throughput Functional Evaluation of Variants of Unknown Significance in ERBB2. Clin. Cancer Res. 2018, 24, 5112–5122. [Google Scholar] [CrossRef] [Green Version]
- Ivanova, E.; Kuraguchi, M.; Xu, M.; Portell, A.; Taus, L.; Diala, I.; Lalani, A.S.; Choi, J.; Chambers, E.S.; Li, S.; et al. Use of ExVivo Patient-Derived Tumor Organotypic Spheroids to Identify Combination Therapies for HER2 Mutant Non–Small Cell Lung Cancer. Clin. Cancer Res. 2020, 26, 2393–2403. [Google Scholar] [CrossRef] [Green Version]
- Hyman, D.M.; Piha-Paul, S.A.; Won, H.; Rodon, J.; Saura, C.; Shapiro, G.I.; Juric, D.; Quinn, D.I.; Moreno, V.; Doger, B.; et al. HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature 2018, 554, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Robichaux, J.P.; Elamin, Y.Y.; Tan, Z.; Carter, B.W.; Zhang, S.; Liu, S.; Li, S.; Chen, T.; Poteete, A.; Estrada-Bernal, A.; et al. Mechanisms and clinical activity of an EGFR and HER2 exon 20–selective kinase inhibitor in non–small cell lung cancer. Nat. Med. 2018, 24, 638–646. [Google Scholar] [CrossRef]
- Kim, T.M.; Lee, K.-W.; Oh, D.-Y.; Lee, J.-S.; Im, S.-A.; Kim, D.-W.; Han, S.-W.; Kim, T.-Y.; Kim, J.H.; Han, H.; et al. Phase 1 Studies of Poziotinib, an Irreversible Pan-HER Tyrosine Kinase Inhibitor in Patients with Advanced Solid Tumors. Cancer Res. Treat. 2018, 50, 835–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robichaux, J.P.; Elamin, Y.Y.; Vijayan, R.S.K.; Nilsson, M.B.; Hu, L.; He, J.; Zhang, F.; Pisegna, M.; Poteete, A.; Sun, H.; et al. Pan-Cancer Landscape and Analysis of ERBB2 Mutations Identifies Poziotinib as a Clinically Active Inhibitor and Enhancer of T-DM1 Activity. Cancer Cell 2019, 36, 444–457.e7. [Google Scholar] [CrossRef] [PubMed]
- Socinski, M.A.; Cornelissen, R.; Garassino, M.C.; Clarke, J.; Tchekmedyian, N.; Molina, J.; Goldman, J.W.; Bhat, G.; Lebel, F.; Le, X. LBA60—ZENITH20, a multinational, multi-cohort phase II study of poziotinib in NSCLC patients with EGFR or HER2 exon 20 insertion mutations. Ann. Oncol. 2020, 31, S1142–S1215. [Google Scholar] [CrossRef]
- Le, X.; Cornelissen, R.; Garassino, M.; Clarke, J.M.; Tchekmedyian, N.; Goldman, J.W.; Leu, S.Y.; Bhat, G.; Lebel, F.; Heymach, J.V.; et al. Poziotinib in Non-Small-Cell Lung Cancer Harboring HER2 Exon 20 Insertion Mutations After Prior Therapies: ZENITH20-2 Trial. J. Clin. Oncol. 2021. [Google Scholar] [CrossRef]
- Cornelissen, R.; Sun, S.; Wollner, M.; Garassino, M.C.C.; Prelaj, A.; Haura, E.B.; Piotrowska, Z.; Goldman, J.W.; Socinski, M.; Dreling, L.; et al. LBA46—Efficacy and safety of poziotinib in treatment-naïve NSCLC harboring HER2 exon 20 mutations: A multinational phase II study (ZENITH20-4). Ann. Oncol. 2021, 32, S1283–S1346. [Google Scholar] [CrossRef]
- Prelaj, A.; Bottiglieri, A.; Proto, C.; Russo, G.L.; Signorelli, D.; Ferrara, R.; Galli, G.; De Toma, A.; Viscardi, G.; Brambilla, M.; et al. Poziotinib for EGFR and HER2 exon 20 insertion mutation in advanced NSCLC: Results from the expanded access program. Eur. J. Cancer 2021, 149, 235–248. [Google Scholar] [CrossRef]
- Kosaka, T.; Tanizaki, J.; Paranal, R.M.; Endoh, H.; Lydon, C.; Capelletti, M.; Repellin, C.E.; Choi, J.; Ogino, A.; Calles, A.; et al. Response heterogeneity of EGFR and HER2 exon 20 insertions to covalent EGFR and HER2 inhibitors. Cancer Res. 2017, 77, 2712–2721. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Jiang, T.; Qin, Z.; Jiang, J.; Wang, Q.; Yang, S.; Rivard, C.; Gao, G.; Ng, T.; Tu, M.; et al. HER2 exon 20 insertions in non-small-cell lung cancer are sensitive to the irreversible pan-HER receptor tyrosine kinase inhibitor pyrotinib. Ann. Oncol. 2019, 30, 447–455. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Li, X.; Wang, Q.; Gao, G.; Zhang, Y.; Chen, J.; Shu, Y.; Hu, Y.; Fan, Y.; Fang, J.; et al. Pyrotinib in HER2-Mutant Advanced Lung Adenocarcinoma After Platinum-Based Chemotherapy: A Multicenter, Open-Label, Single-Arm, Phase II Study. J. Clin. Oncol. 2020, 38, 2753–2761. [Google Scholar] [CrossRef]
- Estrada-Bernal, A.; Le, A.T.; Doak, A.E.; Tirunagaru, V.G.; Silva, S.; Bull, M.R.; Smaill, J.B.; Patterson, A.V.; Kim, C.; Liu, S.V.; et al. Tarloxotinib Is a Hypoxia-Activated Pan-HER Kinase Inhibitor Active Against a Broad Range of HER-Family Oncogenes. Clin. Cancer Res. 2021, 27, 1463–1475. [Google Scholar] [CrossRef]
- Liu, S.V.; Villaruz, L.C.; Lee, V.H.F.; Zhu, V.W.; Baik, C.S.; Sacher, A.; McCoach, C.E.; Nguyen, D.; Li, J.C.; Pacheco, J.M.; et al. LBA61 First analysis of RAIN-701: Study of tarloxotinib in patients with non-small cell lung cancer (NSCLC) EGFR Exon 20 insertion, HER2-activating mutations & other solid tumours with NRG1/ERBB gene fusions. Ann. Oncol. 2020, 31, S1189. [Google Scholar]
- Han, H.; Li, S.; Chen, T.; Fitzgerald, M.; Liu, S.; Peng, C.; Tang, K.H.; Cao, S.; Chouitar, J.; Wu, J.; et al. Targeting HER2 Exon 20 Insertion–Mutant Lung Adenocarcinoma with a Novel Tyrosine Kinase Inhibitor Mobocertinib. Cancer Res. 2021, 81, 5311–5324. [Google Scholar] [CrossRef]
- Zhou, C.; Ramalingam, S.S.; Kim, T.M.; Kim, S.W.; Yang, J.C.; Riely, G.J.; Mekhail, T.; Nguyen, D.; Garcia Campelo, M.R.; Felip, E.; et al. Treatment Outcomes and Safety of Mobocertinib in Platinum-Pretreated Patients with EGFR Exon 20 Insertion-Positive Metastatic Non-Small Cell Lung Cancer: A Phase 1/2 Open-label Nonrandomized Clinical Trial. JAMA Oncol. 2021, 7, e214761. [Google Scholar] [CrossRef]
- Hudis, C.A. Trastuzumab—Mechanism of Action and Use in Clinical Practice. N. Engl. J. Med. 2007, 357, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Kinoshita, I.; Goda, T.; Watanabe, K.; Maemondo, M.; Oizumi, S.; Amano, T.; Hatanaka, Y.; Matsuno, Y.; Nishihara, H.; Asahina, H.; et al. A phase II study of trastuzumab monotherapy in pretreated patientswith non-small cell lung cancers (NSCLCs) harboring HER2 alterations:HOT1303-B trial. Ann. Oncol. 2018, 29, VIII540. [Google Scholar] [CrossRef]
- Gatzemeier, U.; Groth, G.; Butts, C.; Van Zandwijk, N.; Shepherd, F.; Ardizzoni, A.; Barton, C.; Ghahramani, P.; Hirsh, V. Randomized phase II trial of gemcitabine–cisplatin with or without trastuzumab in HER2-positive non-small-cell lung cancer. Ann. Oncol. 2004, 15, 19–27. [Google Scholar] [CrossRef]
- Langer, C.J.; Stephenson, P.; Thor, A.; Vangel, M.; Johnson, D.H. Trastuzumab in the Treatment of Advanced Non-Small-Cell Lung Cancer: Is There a Role? Focus on Eastern Cooperative Oncology Group Study 2598. J. Clin. Oncol. 2004, 22, 1180–1187. [Google Scholar] [CrossRef]
- Hainsworth, J.D.; Meric-Bernstam, F.; Swanton, C.; Hurwitz, H.; Spigel, D.R.; Sweeney, C.; Burris, H.A.; Bose, R.; Yoo, B.; Stein, A.; et al. Targeted Therapy for Advanced Solid Tumors on the Basis of Molecular Profiles: Results from MyPathway, an Open-Label, Phase IIa Multiple Basket Study. J. Clin. Oncol. 2018, 36, 536–542. [Google Scholar] [CrossRef]
- Lewis Phillips, G.D.; Li, G.; Dugger, D.L.; Crocker, L.M.; Parsons, K.L.; Mai, E.; Blättler, W.A.; Lambert, J.M.; Chari, R.V.; Lutz, R.J.; et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008, 68, 9280–9290. [Google Scholar] [CrossRef] [Green Version]
- Hotta, K.; Aoe, K.; Kozuki, T.; Ohashi, K.; Ninomiya, K.; Ichihara, E.; Kubo, T.; Ninomiya, T.; Chikamori, K.; Harada, D.; et al. A Phase II Study of Trastuzumab Emtansine in HER2-Positive Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2018, 13, 273–279. [Google Scholar] [CrossRef] [Green Version]
- Li, B.T.; Shen, R.; Buonocore, D.; Olah, Z.T.; Ni, A.; Ginsberg, M.S.; Ulaner, G.A.; Offin, M.; Feldman, D.; Hembrough, T.; et al. Ado-Trastuzumab Emtansine for Patients with HER2-Mutant Lung Cancers: Results from a Phase II Basket Trial. J. Clin. Oncol. 2018, 36, 2532–2537. [Google Scholar] [CrossRef]
- Li, B.T.; Michelini, F.; Misale, S.; Cocco, E.; Baldino, L.; Cai, Y.; Shifman, S.; Tu, H.-Y.; Myers, M.L.; Xu, C.; et al. HER2-Mediated Internalization of Cytotoxic Agents in ERBB2 Amplified or Mutant Lung Cancers. Cancer Discov. 2020, 10, 674–687. [Google Scholar] [CrossRef] [Green Version]
- Peters, S.; Stahel, R.; Bubendorf, L.; Bonomi, P.; Villegas, A.; Kowalski, D.M.; Baik, C.S.; Isla, D.; Carpeno, J.C.; Garrido, P.; et al. Trastuzumab Emtansine (T-DM1) in Patients with Previously Treated HER2-Overexpressing Metastatic Non-Small Cell Lung Cancer: Efficacy, Safety, and Biomarkers. Clin. Cancer Res. 2019, 25, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Ogitani, Y.; Aida, T.; Hagihara, K.; Yamaguchi, J.; Ishii, C.; Harada, N.; Soma, M.; Okamoto, H.; Oitate, M.; Arakawa, S.; et al. DS-8201a, A Novel HER2-Targeting ADC with a Novel DNA Topoisomerase I Inhibitor, Demonstrates a Promising Antitumor Efficacy with Differentiation from T-DM1. Clin. Cancer Res. 2016, 22, 5097–5108. [Google Scholar] [CrossRef] [Green Version]
- Tsurutani, J.; Iwata, H.; Krop, I.; Jänne, P.A.; Doi, T.; Takahashi, S.; Park, H.; Redfern, C.; Tamura, K.; Wise-Draper, T.M.; et al. Targeting HER2 with Trastuzumab Deruxtecan: A Dose-Expansion, Phase I Study in Multiple Advanced Solid Tumors. Cancer Discov. 2020, 10, 688–701. [Google Scholar] [CrossRef] [Green Version]
- Riudavets, M.; Sullivan, I.; Abdayem, P.; Planchard, D. Targeting HER2 in non-small-cell lung cancer (NSCLC): A glimpse of hope? An updated review on therapeutic strategies in NSCLC harbouring HER2 alterations. ESMO Open 2021, 6. [Google Scholar] [CrossRef]
- Dong, Z.-Y.; Zhang, J.-T.; Liu, S.-Y.; Su, J.; Zhang, C.; Xie, Z.; Zhou, Q.; Tu, H.-Y.; Xu, C.-R.; Si-Yang, L.; et al. EGFR mutation correlates with uninflamed phenotype and weak immunogenicity, causing impaired response to PD-1 blockade in non-small cell lung cancer. OncoImmunology 2017, 6, e1356145. [Google Scholar] [CrossRef] [Green Version]
- Reuben, A.; Zhang, J.; Chiou, S.-H.; Gittelman, R.M.; Li, J.; Lee, W.-C.; Fujimoto, J.; Behrens, C.; Liu, X.; Wang, F.; et al. Comprehensive T cell repertoire characterization of non-small cell lung cancer. Nat. Commun. 2020, 11, 603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, W.V.; Feldman, D.L.; Buonocore, D.J.; Brzostowski, E.B.; Rizvi, H.; Plodkowski, A.J.; Ni, A.; Sabari, J.K.; Offin, M.D.; Kris, M.G.; et al. PD-L1 expression, tumor mutation burden and response to immune checkpoint blockade in patients with HER2-mutant lung cancers. J. Clin. Oncol. 2018, 36, 9060. [Google Scholar] [CrossRef]
- Negrao, M.V.; Skoulidis, F.; Montesion, M.; Schulze, K.; Bara, I.; Shen, V.; Xu, H.; Hu, S.; Sui, D.; Elamin, Y.Y.; et al. Oncogene-specific differences in tumor mutational burden, PD-L1 expression, and outcomes from immunotherapy in non-small cell lung cancer. J. Immunother. Cancer 2021, 9, e002891. [Google Scholar] [CrossRef]
- Mazieres, J.; Drilon, A.; Lusque, A.; Mhanna, L.; Cortot, A.; Mezquita, L.; Thai, A.; Mascaux, C.; Couraud, S.; Veillon, R.; et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: Results from the Immunotarget registry. Ann. Oncol. 2019, 30, 1321–1328. [Google Scholar] [CrossRef]
- Guisier, F.; Dubos-Arvis, C.; Viñas, F.; Doubre, H.; Ricordel, C.; Ropert, S.; Janicot, H.; Bernardi, M.; Fournel, P.; Lamy, R.; et al. Efficacy and Safety of Anti-PD-1 Immunotherapy in Patients with Advanced NSCLC With BRAF, HER2, or MET Mutations or RET Translocation: GFPC 01-2018. J. Thorac. Oncol. 2020, 15, 628–636. [Google Scholar] [CrossRef]
- Lau, S.C.; Fares, A.F.; Le, L.W.; Mackay, K.M.; Soberano, S.; Chan, S.W.; Smith, E.; Ryan, M.; Tsao, M.S.; Bradbury, P.A.; et al. Subtypes of EGFR- and HER2-Mutant Metastatic NSCLC Influence Response to Immune Checkpoint Inhibitors. Clin. Lung Cancer 2021, 22, 253–259. [Google Scholar] [CrossRef]
- Saalfeld, F.C.; Wenzel, C.; Christopoulos, P.; Merkelbach-Bruse, S.; Reissig, T.M.; Laßmann, S.; Thiel, S.; Stratmann, J.A.; Marienfeld, R.; Berger, J.; et al. Efficacy of Immune Checkpoint Inhibitors Alone or in Combination with Chemotherapy in NSCLC Harboring ERBB2 Mutations. J. Thorac. Oncol. 2021, 16, 1952–1958. [Google Scholar] [CrossRef]
| Reference | Study | N | Line of Treatment, ICI Regimen | PD-L1 Expression ≥1/≥50 (%) | ORR N (%) | PFS Median (95% CI) | OS Median (95% CI) |
|---|---|---|---|---|---|---|---|
| Lai et al. [93] | Retrospective | 26 | NA | NA/8.7 | 3/26 (11.5) | 1.9 months (1.5–4.0) | 10.4 months (5.9-NR) |
| Negrao et al. [94] | Retrospective | 16 | NA | NA/NA | 1/16 (6.3) | 1.8 months (NA) | 17.1 months (NA) |
| Mazieres et al. [95] | Retrospective | 29 | >1, monotherapy | 53.3/0 | 2/27 (7.4) | 2.5 months (1.8–3.5) | 20.3 months (7.8-NR) |
| Guisier et al. [96] | Retrospective | 23 | >1, monotherapy | 17.4/4.3 | 6/23 (27.3) | 2.2 months (1.7–15.2) | 20.4 months (9.3-NR) |
| Lau et al. [97] | Retrospective | 14 | >1, monotherapy | 61.5/23.1 | 4/14 (28.6) | 3.6 months (1.6-NR) | NA |
| Saalfeld et al. [98] | Retrospective | 61 | 1, monotherapy (5/61); 1, combination with chemotherapy (22/61); >1, monotherapy (34/61) | 53.4/15.5 | 1/5 (20.0); 11/21 (52.4); 5/31 (16.1) | NA; 6 months (6.0–14.0); 4 months (4.0–6.0) | NA; NR; 10 months (6.0-NR) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vathiotis, I.A.; Charpidou, A.; Gavrielatou, N.; Syrigos, K.N. HER2 Aberrations in Non-Small Cell Lung Cancer: From Pathophysiology to Targeted Therapy. Pharmaceuticals 2021, 14, 1300. https://doi.org/10.3390/ph14121300
Vathiotis IA, Charpidou A, Gavrielatou N, Syrigos KN. HER2 Aberrations in Non-Small Cell Lung Cancer: From Pathophysiology to Targeted Therapy. Pharmaceuticals. 2021; 14(12):1300. https://doi.org/10.3390/ph14121300
Chicago/Turabian StyleVathiotis, Ioannis A., Andriani Charpidou, Niki Gavrielatou, and Konstantinos N. Syrigos. 2021. "HER2 Aberrations in Non-Small Cell Lung Cancer: From Pathophysiology to Targeted Therapy" Pharmaceuticals 14, no. 12: 1300. https://doi.org/10.3390/ph14121300
APA StyleVathiotis, I. A., Charpidou, A., Gavrielatou, N., & Syrigos, K. N. (2021). HER2 Aberrations in Non-Small Cell Lung Cancer: From Pathophysiology to Targeted Therapy. Pharmaceuticals, 14(12), 1300. https://doi.org/10.3390/ph14121300






