The Hypothermic Effect of Hydrogen Sulfide Is Mediated by the Transient Receptor Potential Ankyrin-1 Channel in Mice
Abstract
:1. Introduction
2. Results
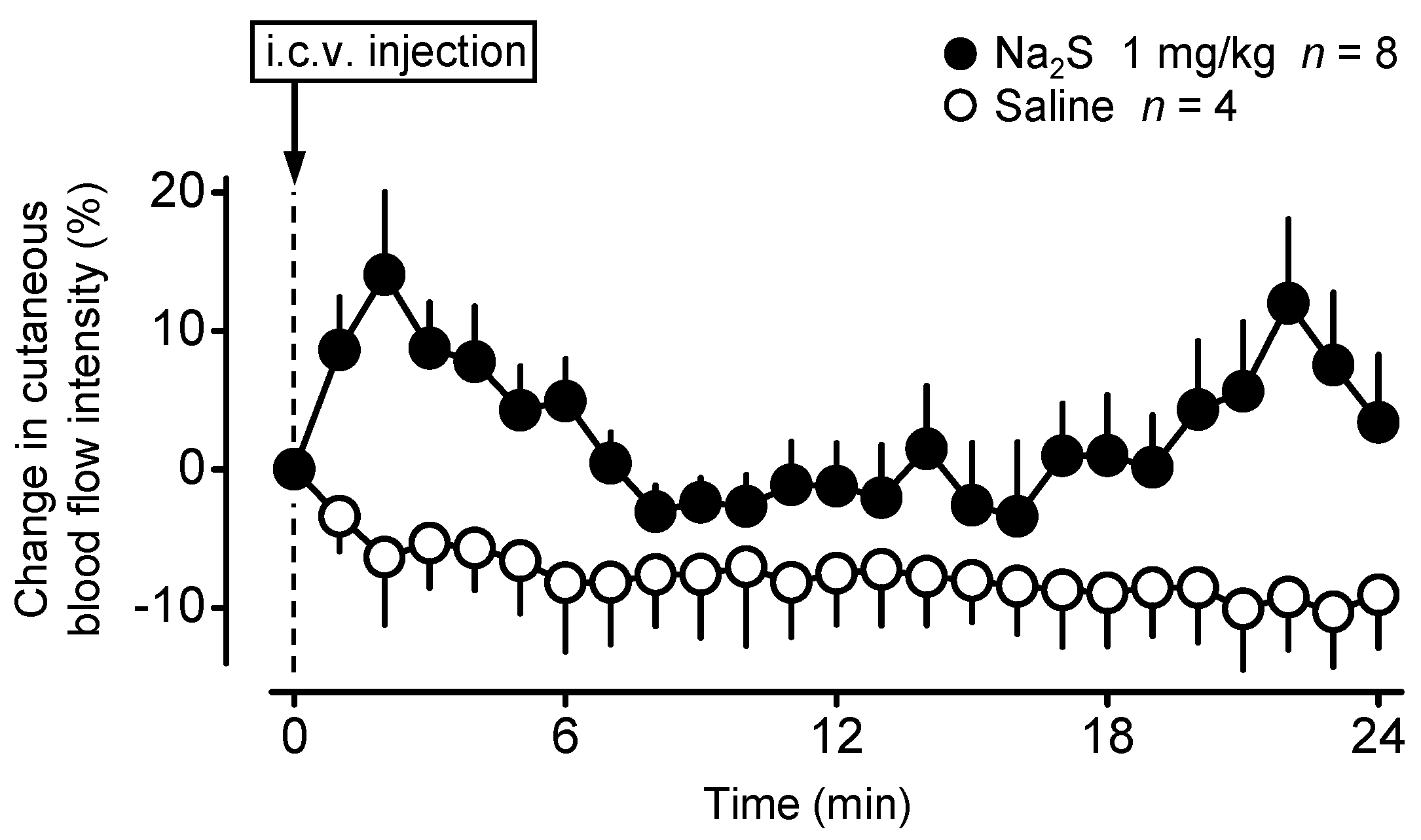
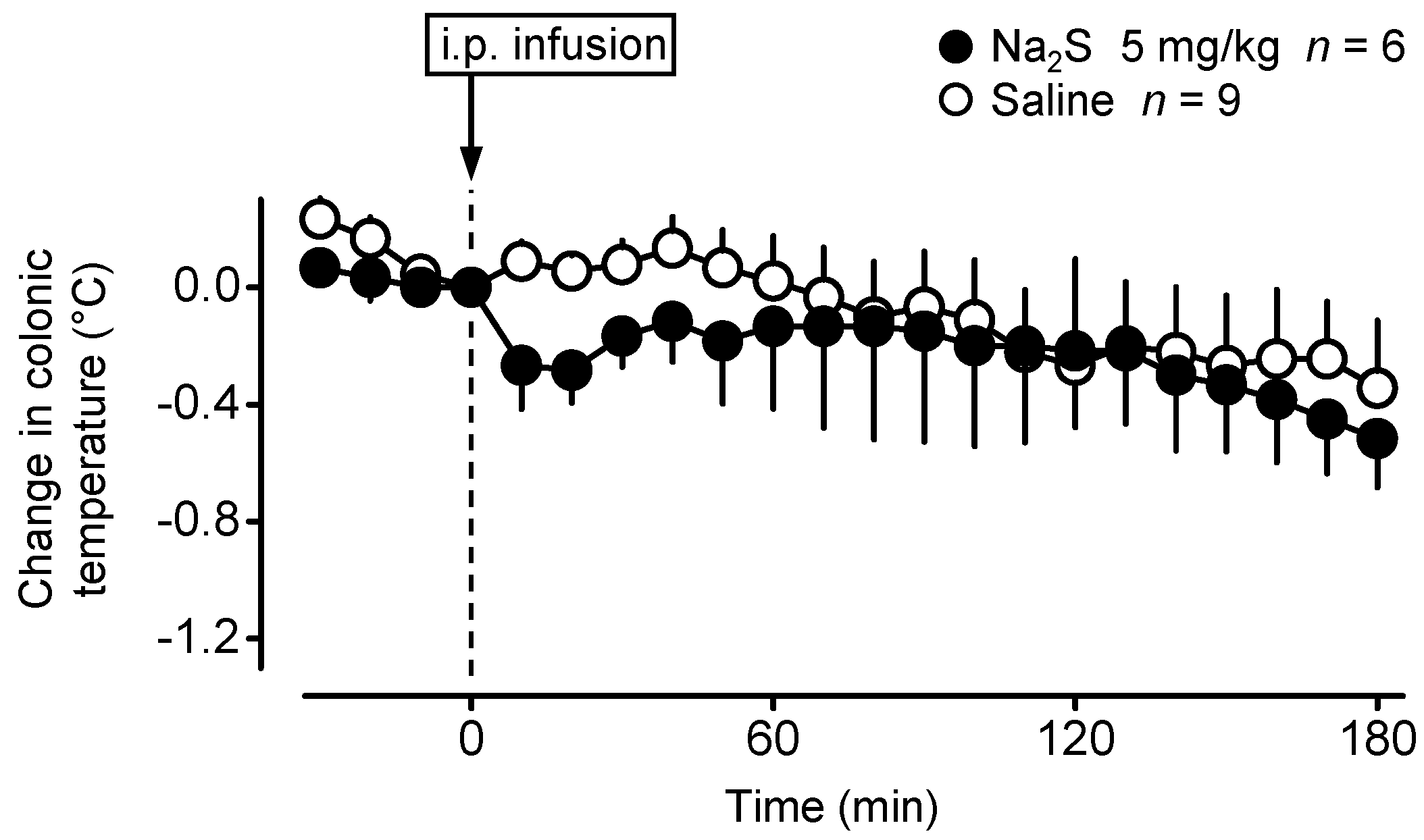
2.1. Central Administration of Na2S Decreases Deep Tb in Mice via Inhibition of Thermogenesis and Induction of Vasodilation in the Skin
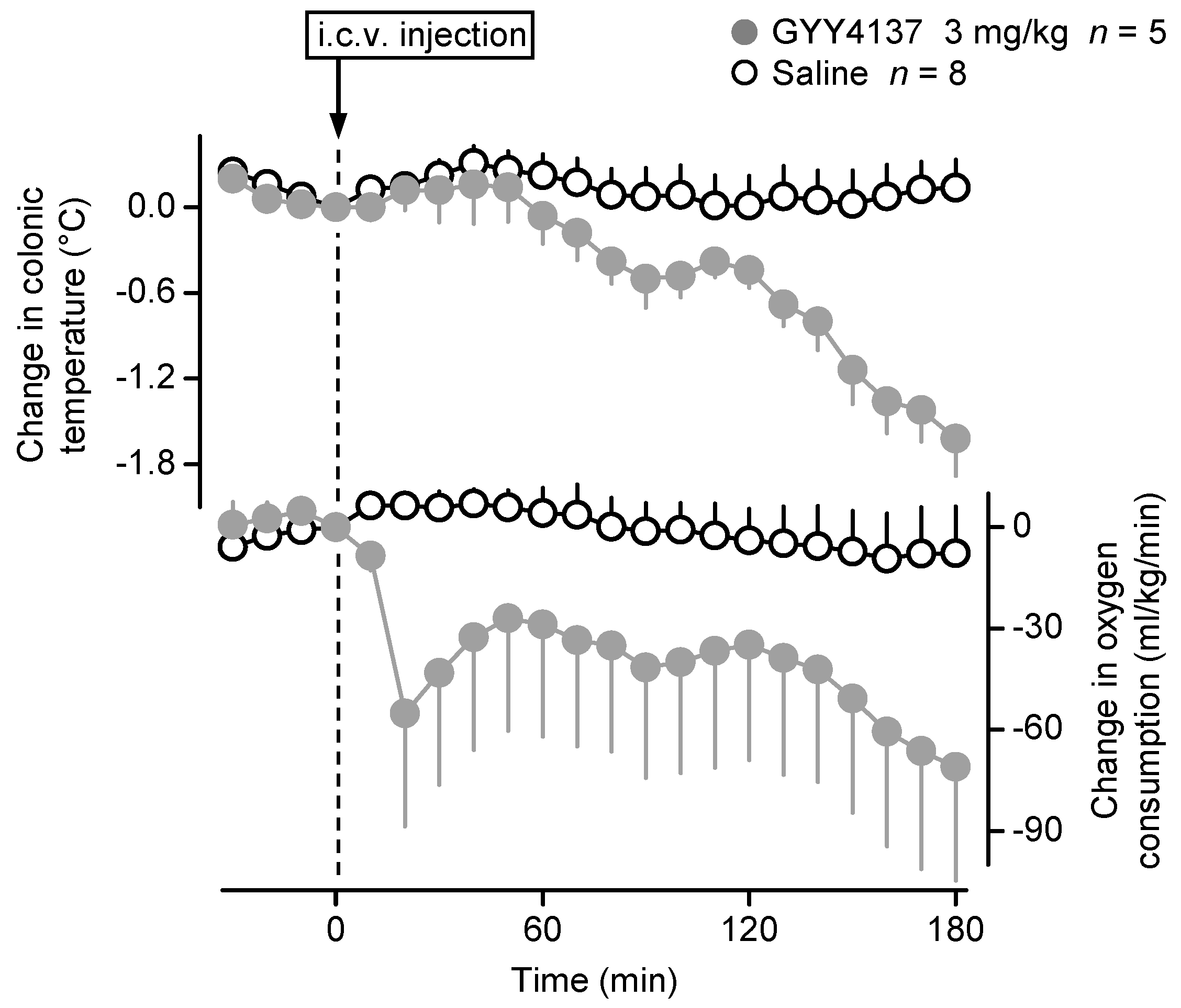
2.2. Central Administration of GYY4137 Decreases Deep Tb in Mice
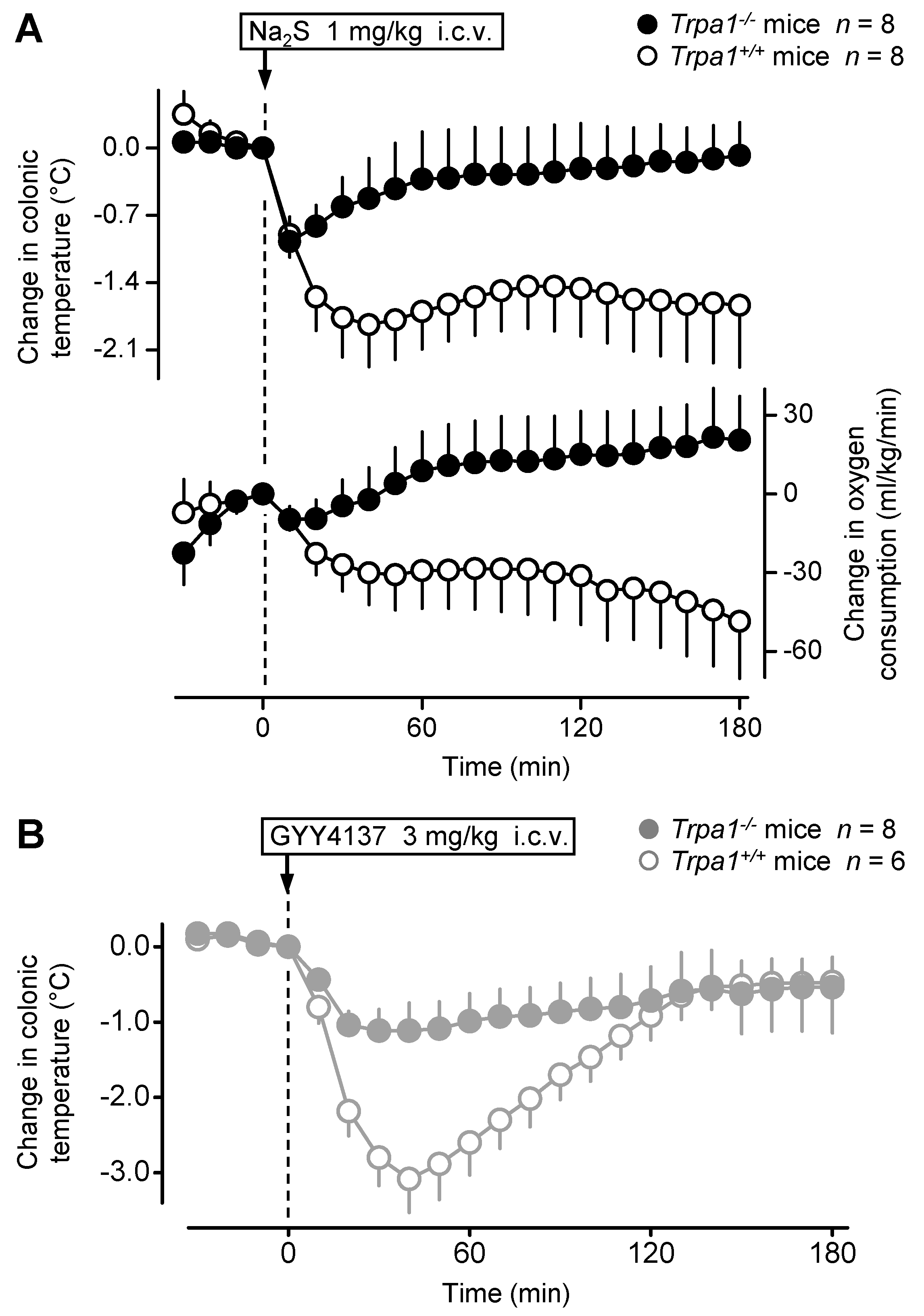
2.3. The Hypothermic Response to Na2S and GYY4137 Is Attenuated in the Absence of the TRPA1 Channel
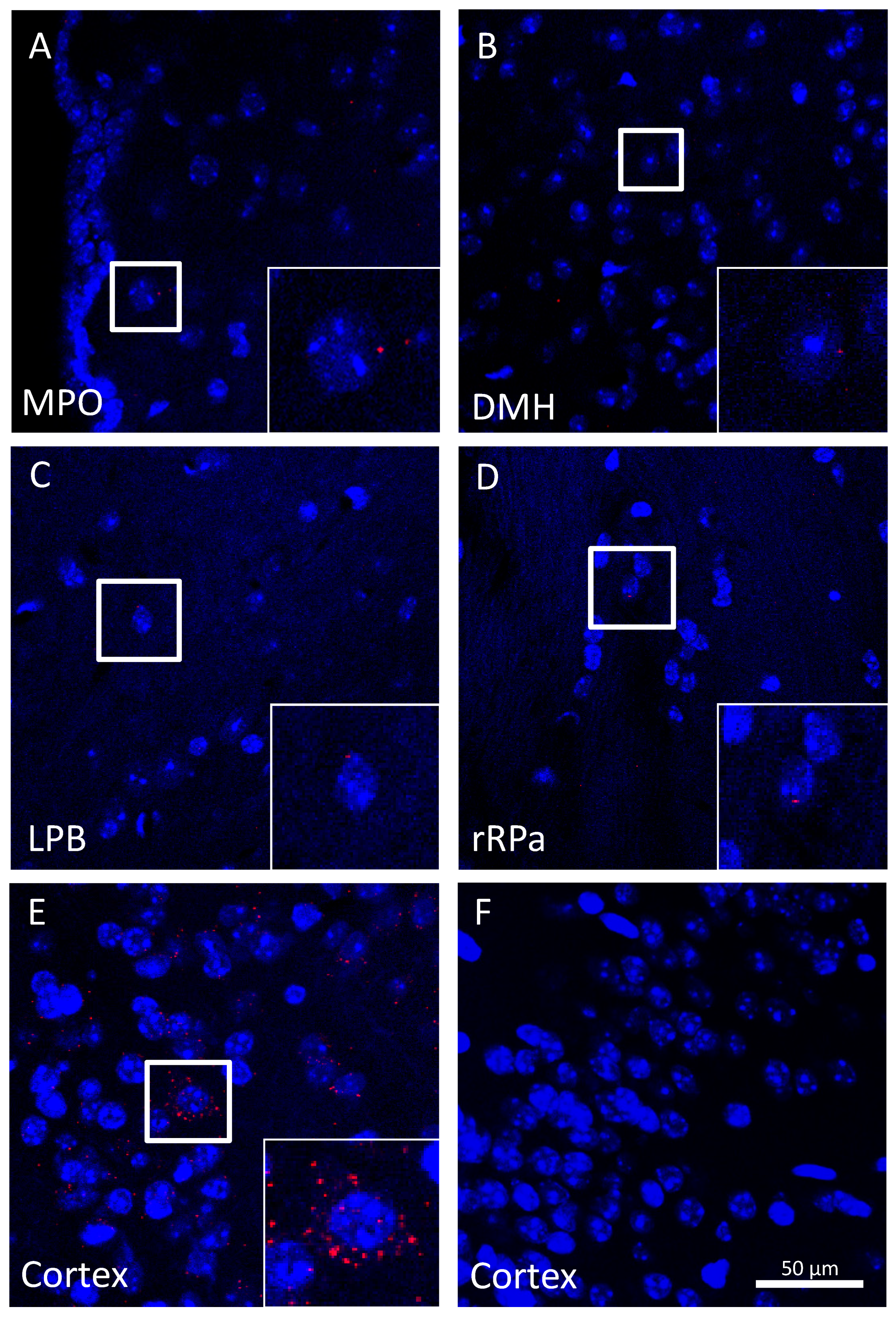
2.4. Trpa1 mRNA Is Modestly Expressed in Brain Neurons within Autonomic Thermoeffector Pathways
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Surgeries
4.2.1. Anesthesia and Perioperative Care
4.2.2. I.c.v. Cannula Implantation
4.2.3. I.p. Catheter Implantation
4.3. Experimental Setups
4.3.1. Thermocouple Thermometry
4.3.2. Respirometry Thermometry
4.3.3. Laser Speckle Contrast Imaging
4.3.4. Drugs and Drug Administration
4.4. Measurement of Trpa1 mRNA Expression
4.4.1. RT-qPCR
4.4.2. RNAscope In Situ Hybridization
4.5. Data Processing and Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Szabo, C. A timeline of hydrogen sulfide (H2S) research: From environmental toxin to biological mediator. Biochem. Pharmacol. 2018, 149, 5–19. [Google Scholar] [CrossRef]
- Murphy, B.; Bhattacharya, R.; Mukherjee, P. Hydrogen sulfide signaling in mitochondria and disease. FASEB J. 2019, 33, 13098–13125. [Google Scholar] [CrossRef] [Green Version]
- Kimura, H. Hydrogen sulfide (H2S) and polysulfide (H2Sn) signaling: The first 25 years. Biomolecules 2021, 11, 896. [Google Scholar] [CrossRef]
- Szabo, C.; Papapetropoulos, A. International Union of Basic and Clinical Pharmacology. CII: Pharmacological modulation of H2S levels: H2S donors and H2S biosynthesis inhibitors. Pharmacol. Rev. 2017, 69, 497–564. [Google Scholar] [CrossRef] [Green Version]
- Stein, A.; Bailey, S.M. Redox biology of hydrogen sulfide: Implications for physiology, pathophysiology, and pharmacology. Redox Biol. 2013, 1, 32–39. [Google Scholar] [CrossRef] [Green Version]
- Branco, L.G.; Soriano, R.N.; Steiner, A.A. Gaseous mediators in temperature regulation. Compr. Physiol. 2014, 4, 1301–1338. [Google Scholar] [CrossRef]
- Blackstone, E.; Morrison, M.; Roth, M.B. H2S induces a suspended animation-like state in mice. Science 2005, 308, 518. [Google Scholar] [CrossRef] [Green Version]
- Volpato, G.P.; Searles, R.; Yu, B.; Scherrer-Crosbie, M.; Bloch, K.D.; Ichinose, F.; Zapol, W.M. Inhaled hydrogen sulfide: A rapidly reversible inhibitor of cardiac and metabolic function in the mouse. Anesthesiology 2008, 108, 659–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aslami, H.; Heinen, A.; Roelofs, J.J.; Zuurbier, C.J.; Schultz, M.J.; Juffermans, N.P. Suspended animation inducer hydrogen sulfide is protective in an in vivo model of ventilator-induced lung injury. Intensive Care Med. 2010, 36, 1946–1952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pozsgai, G.; Payrits, M.; Saghy, E.; Sebestyen-Batai, R.; Steen, E.; Szoke, E.; Sandor, Z.; Solymar, M.; Garami, A.; Orvos, P.; et al. Analgesic effect of dimethyl trisulfide in mice is mediated by TRPA1 and sst4 receptors. Nitric Oxide 2017, 65, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Hemelrijk, S.D.; Dirkes, M.C.; van Velzen, M.H.N.; Bezemer, R.; van Gulik, T.M.; Heger, M. Exogenous hydrogen sulfide gas does not induce hypothermia in normoxic mice. Sci. Rep. 2018, 8, 3855. [Google Scholar] [CrossRef]
- Kwiatkoski, M.; Soriano, R.N.; Francescato, H.D.; Batalhao, M.E.; Coimbra, T.M.; Carnio, E.C.; Branco, L.G. Hydrogen sulfide as a cryogenic mediator of hypoxia-induced anapyrexia. Neuroscience 2012, 201, 146–156. [Google Scholar] [CrossRef]
- Simon, F.; Giudici, R.; Duy, C.N.; Schelzig, H.; Oter, S.; Groger, M.; Wachter, U.; Vogt, J.; Speit, G.; Szabo, C.; et al. Hemodynamic and metabolic effects of hydrogen sulfide during porcine ischemia/reperfusion injury. Shock 2008, 30, 359–364. [Google Scholar] [CrossRef]
- Bracht, H.; Scheuerle, A.; Groger, M.; Hauser, B.; Matallo, J.; McCook, O.; Seifritz, A.; Wachter, U.; Vogt, J.A.; Asfar, P.; et al. Effects of intravenous sulfide during resuscitated porcine hemorrhagic shock*. Crit. Care Med. 2012, 40, 2157–2167. [Google Scholar] [CrossRef]
- Dirkes, M.C.; Milstein, D.M.; Heger, M.; van Gulik, T.M. Absence of hydrogen sulfide-induced hypometabolism in pigs: A mechanistic explanation in relation to small nonhibernating mammals. Eur. Surg. Res. 2015, 54, 178–191. [Google Scholar] [CrossRef]
- Drabek, T.; Kochanek, P.M.; Stezoski, J.; Wu, X.; Bayir, H.; Morhard, R.C.; Stezoski, S.W.; Tisherman, S.A. Intravenous hydrogen sulfide does not induce hypothermia or improve survival from hemorrhagic shock in pigs. Shock 2011, 35, 67–73. [Google Scholar] [CrossRef]
- Haouzi, P.; Notet, V.; Chenuel, B.; Chalon, B.; Sponne, I.; Ogier, V.; Bihain, B. H2S induced hypometabolism in mice is missing in sedated sheep. Respir. Physiol. Neurobiol. 2008, 160, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Soriano, R.N.; Braga, S.P.; Breder, J.S.C.; Batalhao, M.E.; Oliveira-Pelegrin, G.R.; Ferreira, L.F.R.; Rocha, M.J.A.; Carnio, E.C.; Branco, L.G.S. Endogenous peripheral hydrogen sulfide is propyretic: Its permissive role in brown adipose tissue thermogenesis in rats. Exp. Physiol. 2018, 103, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkoski, M.; Soriano, R.N.; Araujo, R.M.; Azevedo, L.U.; Batalhao, M.E.; Francescato, H.D.; Coimbra, T.M.; Carnio, E.C.; Branco, L.G. Hydrogen sulfide inhibits preoptic prostaglandin E2 production during endotoxemia. Exp. Neurol. 2013, 240, 88–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez, R.A.; Soriano, R.N.; Francescato, H.D.; Sabino, J.P.; Coimbra, T.M.; Branco, L.G. Cryogenic role of central endogenous hydrogen sulfide in the rat model of endotoxic shock. Brain Res. 2016, 1650, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Sabino, J.P.J.; Oliveira, L.V.C.; Soriano, R.N.; Kwiatkoski, M.; Branco, L.G.S.; da Silva, G.S.F. Role of hydrogen sulfide in ventilatory responses to hypercapnia in the medullary raphe of adult rats. Exp. Physiol. 2021. [Google Scholar] [CrossRef]
- Romanovsky, A.A. The thermoregulation system and how it works. Handb. Clin. Neurol. 2018, 156, 3–43. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Li, G.; Yang, L. Role of H2S in pain: Growing evidences of mystification. Eur. J. Pharmacol. 2020, 883, 173322. [Google Scholar] [CrossRef] [PubMed]
- Pozsgai, G.; Batai, I.Z.; Pinter, E. Effects of sulfide and polysulfides transmitted by direct or signal transduction-mediated activation of TRPA1 channels. Br. J. Pharmacol. 2019, 176, 628–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aubdool, A.A.; Graepel, R.; Kodji, X.; Alawi, K.M.; Bodkin, J.V.; Srivastava, S.; Gentry, C.; Heads, R.; Grant, A.D.; Fernandes, E.S.; et al. TRPA1 is essential for the vascular response to environmental cold exposure. Nat. Commun. 2014, 5, 5732. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, C.; Garami, A.; Lehto, S.G.; Pakai, E.; Tekus, V.; Pohoczky, K.; Youngblood, B.D.; Wang, W.; Kort, M.E.; Kym, P.R.; et al. Transient receptor potential channel ankyrin-1 is not a cold sensor for autonomic thermoregulation in rodents. J. Neurosci. 2014, 34, 4445–4452. [Google Scholar] [CrossRef] [PubMed]
- Garami, A.; Pakai, E.; McDonald, H.A.; Reilly, R.M.; Gomtsyan, A.; Corrigan, J.J.; Pinter, E.; Zhu, D.X.D.; Lehto, S.G.; Gavva, N.R.; et al. TRPV1 antagonists that cause hypothermia, instead of hyperthermia, in rodents: Compounds’ pharmacological profiles, in vivo targets, thermoeffectors recruited and implications for drug development. Acta Physiol. 2018, 223, e13038. [Google Scholar] [CrossRef] [Green Version]
- Papapetropoulos, A.; Whiteman, M.; Cirino, G. Pharmacological tools for hydrogen sulphide research: A brief, introductory guide for beginners. Br. J. Pharmacol. 2015, 172, 1633–1637. [Google Scholar] [CrossRef] [Green Version]
- Banki, E.; Pakai, E.; Gaszner, B.; Zsiboras, C.; Czett, A.; Bhuddi, P.R.; Hashimoto, H.; Toth, G.; Tamas, A.; Reglodi, D.; et al. Characterization of the thermoregulatory response to pituitary adenylate cyclase-activating polypeptide in rodents. J. Mol. Neurosci. 2014, 54, 543–554. [Google Scholar] [CrossRef] [Green Version]
- Garami, A.; Pakai, E.; Oliveira, D.L.; Steiner, A.A.; Wanner, S.P.; Almeida, M.C.; Lesnikov, V.A.; Gavva, N.R.; Romanovsky, A.A. Thermoregulatory phenotype of the Trpv1 knockout mouse: Thermoeffector dysbalance with hyperkinesis. J. Neurosci. 2011, 31, 1721–1733. [Google Scholar] [CrossRef] [Green Version]
- Romanovsky, A.A.; Ivanov, A.I.; Shimansky, Y.P. Selected contribution: Ambient temperature for experiments in rats: A new method for determining the zone of thermal neutrality. J. Appl. Physiol. 2002, 92, 2667–2679. [Google Scholar] [CrossRef] [Green Version]
- Guinamard, R.; Hof, T. TRPA1 and TRPV1, do we hold you in our heart? Acta Physiol. 2021, 232, e13695. [Google Scholar] [CrossRef] [PubMed]
- Virk, H.S.; Rekas, M.Z.; Biddle, M.S.; Wright, A.K.A.; Sousa, J.; Weston, C.A.; Chachi, L.; Roach, K.M.; Bradding, P. Validation of antibodies for the specific detection of human TRPA1. Sci. Rep. 2019, 9, 18500. [Google Scholar] [CrossRef] [Green Version]
- Bell, J.T.; Loomis, A.K.; Butcher, L.M.; Gao, F.; Zhang, B.; Hyde, C.L.; Sun, J.; Wu, H.; Ward, K.; Harris, J.; et al. Differential methylation of the TRPA1 promoter in pain sensitivity. Nat. Commun. 2014, 5, 2978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gombert, S.; Rhein, M.; Winterpacht, A.; Munster, T.; Hillemacher, T.; Leffler, A.; Frieling, H. Transient receptor potential ankyrin 1 promoter methylation and peripheral pain sensitivity in Crohn’s disease. Clin. Epigenetics 2019, 12, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sukenaga, N.; Ikeda-Miyagawa, Y.; Tanada, D.; Tunetoh, T.; Nakano, S.; Inui, T.; Satoh, K.; Okutani, H.; Noguchi, K.; Hirose, M. Correlation between DNA methylation of TRPA1 and chronic pain states in human whole blood cells. Pain Med. 2016, 17, 1906–1910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Femino, A.M.; Fay, F.S.; Fogarty, K.; Singer, R.H. Visualization of single RNA transcripts in situ. Science 1998, 280, 585–590. [Google Scholar] [CrossRef] [Green Version]
- Romanovsky, A.A. Skin temperature: Its role in thermoregulation. Acta Physiol. 2014, 210, 498–507. [Google Scholar] [CrossRef]
- Skop, V.; Liu, N.; Guo, J.; Gavrilova, O.; Reitman, M.L. The contribution of the mouse tail to thermoregulation is modest. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E438–E446. [Google Scholar] [CrossRef]
- Whiteman, M.; Winyard, P.G. Hydrogen sulfide and inflammation: The good, the bad, the ugly and the promising. Expert Rev. Clin. Pharmacol. 2011, 4, 13–32. [Google Scholar] [CrossRef]
- Li, L.; Whiteman, M.; Guan, Y.Y.; Neo, K.L.; Cheng, Y.; Lee, S.W.; Zhao, Y.; Baskar, R.; Tan, C.H.; Moore, P.K. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): New insights into the biology of hydrogen sulfide. Circulation 2008, 117, 2351–2360. [Google Scholar] [CrossRef] [Green Version]
- Whiteman, M.; Perry, A.; Zhou, Z.; Bucci, M.; Papapetropoulos, A.; Cirino, G.; Wood, M.E. Phosphinodithioate and phosphoramidodithioate hydrogen sulfide donors. Handb. Exp. Pharmacol. 2015, 230, 337–363. [Google Scholar] [CrossRef]
- Garami, A.; Shimansky, Y.P.; Rumbus, Z.; Vizin, R.C.L.; Farkas, N.; Hegyi, J.; Szakacs, Z.; Solymar, M.; Csenkey, A.; Chiche, D.A.; et al. Hyperthermia induced by transient receptor potential vanilloid-1 (TRPV1) antagonists in human clinical trials: Insights from mathematical modeling and meta-analysis. Pharmacol. Ther. 2020, 208, 107474. [Google Scholar] [CrossRef]
- Romanovsky, A.A.; Almeida, M.C.; Garami, A.; Steiner, A.A.; Norman, M.H.; Morrison, S.F.; Nakamura, K.; Burmeister, J.J.; Nucci, T.B. The transient receptor potential vanilloid-1 channel in thermoregulation: A thermosensor it is not. Pharmacol. Rev. 2009, 61, 228–261. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Mikami, Y.; Osumi, K.; Tsugane, M.; Oka, J.; Kimura, H. Polysulfides are possible H2S-derived signaling molecules in rat brain. FASEB J. 2013, 27, 2451–2457. [Google Scholar] [CrossRef] [PubMed]
- Poole, D.P.; Pelayo, J.C.; Cattaruzza, F.; Kuo, Y.M.; Gai, G.; Chiu, J.V.; Bron, R.; Furness, J.B.; Grady, E.F.; Bunnett, N.W. Transient receptor potential ankyrin 1 is expressed by inhibitory motoneurons of the mouse intestine. Gastroenterology 2011, 141, 565–575.e4. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.F.; Nakamura, K. Central neural pathways for thermoregulation. Front. Biosci. 2011, 16, 74–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Story, G.M.; Peier, A.M.; Reeve, A.J.; Eid, S.R.; Mosbacher, J.; Hricik, T.R.; Earley, T.J.; Hergarden, A.C.; Andersson, D.A.; Hwang, S.W.; et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 2003, 112, 819–829. [Google Scholar] [CrossRef] [Green Version]
- Kozyreva, T.V.; Evtushenko, A.A.; Voronova, I.P.; Khramova, G.M.; Kozaruk, V.P. Effect of activation of peripheral ion channel TRPM8 on gene expression of thermosensitive TRP ion channels in the hypothalamus. Comparison with the effect of cooling. Bull. Exp. Biol. Med. 2018, 166, 188–191. [Google Scholar] [CrossRef]
- Kozyreva, T.V.; Evtushenko, A.A.; Voronova, I.P.; Khramova, G.M.; Kozaruk, V.P. Effects of acute cooling on expression of genes for thermosensitive TRP ion channels in the hypothalamus. Neurosci. Behav. Physiol. 2019, 49, 804–808. [Google Scholar] [CrossRef]
- Kunert-Keil, C.; Bisping, F.; Kruger, J.; Brinkmeier, H. Tissue-specific expression of TRP channel genes in the mouse and its variation in three different mouse strains. BMC Genom. 2006, 7, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.P.; Yu, X.; Yan, X.J.; Lei, F.; Chai, Y.S.; Jiang, J.F.; Yuan, Z.Y.; Xing, D.M.; Du, L.J. TRPM8 in the negative regulation of TNFalpha expression during cold stress. Sci. Rep. 2017, 7, 45155. [Google Scholar] [CrossRef] [PubMed]
- Pereira, I.; Mendes, S.J.; Pereira, D.M.; Muniz, T.F.; Colares, V.L.; Monteiro, C.R.; Martins, M.M.; Grisotto, M.A.; Monteiro-Neto, V.; Monteiro, S.G.; et al. Transient receptor potential ankyrin 1 channel expression on peripheral blood leukocytes from rheumatoid arthritic patients and correlation with pain and disability. Front. Pharmacol. 2017, 8, 53. [Google Scholar] [CrossRef] [Green Version]
- Billeter, A.T.; Galbraith, N.; Walker, S.; Lawson, C.; Gardner, S.A.; Sarojini, H.; Galandiuk, S.; Polk, H.C., Jr. TRPA1 mediates the effects of hypothermia on the monocyte inflammatory response. Surgery 2015, 158, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Bertin, S.; Aoki-Nonaka, Y.; Lee, J.; de Jong, P.R.; Kim, P.; Han, T.; Yu, T.; To, K.; Takahashi, N.; Boland, B.S.; et al. The TRPA1 ion channel is expressed in CD4+ T cells and restrains T-cell-mediated colitis through inhibition of TRPV1. Gut 2017, 66, 1584–1596. [Google Scholar] [CrossRef]
- Sahoo, S.S.; Majhi, R.K.; Tiwari, A.; Acharya, T.; Kumar, P.S.; Saha, S.; Kumar, A.; Goswami, C.; Chattopadhyay, S. Transient receptor potential ankyrin1 channel is endogenously expressed in T cells and is involved in immune functions. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef] [Green Version]
- Stokes, A.; Wakano, C.; Koblan-Huberson, M.; Adra, C.N.; Fleig, A.; Turner, H. TRPA1 is a substrate for de-ubiquitination by the tumor suppressor CYLD. Cell Signal. 2006, 18, 1584–1594. [Google Scholar] [CrossRef]
- Nagata, K.; Duggan, A.; Kumar, G.; Garcia-Anoveros, J. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J. Neurosci. 2005, 25, 4052–4061. [Google Scholar] [CrossRef] [Green Version]
- Sawada, Y.; Hosokawa, H.; Hori, A.; Matsumura, K.; Kobayashi, S. Cold sensitivity of recombinant TRPA1 channels. Brain Res. 2007, 1160, 39–46. [Google Scholar] [CrossRef]
- Koppelkamm, A.; Vennemann, B.; Lutz-Bonengel, S.; Fracasso, T.; Vennemann, M. RNA integrity in post-mortem samples: Influencing parameters and implications on RT-qPCR assays. Int. J. Legal. Med. 2011, 125, 573–580. [Google Scholar] [CrossRef]
- Osterloh, M.; Bohm, M.; Kalbe, B.; Osterloh, S.; Hatt, H. Identification and functional characterization of TRPA1 in human myoblasts. Pflugers Arch. 2016, 468, 321–333. [Google Scholar] [CrossRef]
- Chung, S.; Baumlin, N.; Dennis, J.S.; Moore, R.; Salathe, S.F.; Whitney, P.L.; Sabater, J.; Abraham, W.M.; Kim, M.D.; Salathe, M. Electronic cigarette vapor with nicotine causes airway mucociliary dysfunction preferentially via TRPA1 receptors. Am. J. Respir. Crit. Care Med. 2019, 200, 1134–1145. [Google Scholar] [CrossRef] [Green Version]
- Shigetomi, E.; Tong, X.; Kwan, K.Y.; Corey, D.P.; Khakh, B.S. TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat. Neurosci. 2011, 15, 70–80. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.W.; Lindberg, J.E.; Peters, J.H. Genetic and pharmacological evidence for low-abundance TRPV3 expression in primary vagal afferent neurons. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R794–R805. [Google Scholar] [CrossRef] [Green Version]
- Jin, C.; Kim, S.; Kang, H.; Yun, K.N.; Lee, Y.; Zhang, Y.; Kim, Y.; Kim, J.Y.; Han, K. Shank3 regulates striatal synaptic abundance of Cyld, a deubiquitinase specific for Lys63-linked polyubiquitin chains. J. Neurochem. 2019, 150, 776–786. [Google Scholar] [CrossRef]
- Kheradpezhouh, E.; Choy, J.M.C.; Daria, V.R.; Arabzadeh, E. TRPA1 expression and its functional activation in rodent cortex. Open Biol. 2017, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, B.; Bang, S.I.; Jin, Y.H. Transient receptor potential A1 increase glutamate release on brain stem neurons. Neuroreport 2009, 20, 1002–1006. [Google Scholar] [CrossRef]
- Yokoyama, T.; Ohbuchi, T.; Saito, T.; Sudo, Y.; Fujihara, H.; Minami, K.; Nagatomo, T.; Uezono, Y.; Ueta, Y. Allyl isothiocyanates and cinnamaldehyde potentiate miniature excitatory postsynaptic inputs in the supraoptic nucleus in rats. Eur. J. Pharmacol. 2011, 655, 31–37. [Google Scholar] [CrossRef]
- Bud Craig, A.D. Central neural substrates involved in temperature discrimination, thermal pain, thermal comfort, and thermoregulatory behavior. Handb. Clin. Neurol. 2018, 156, 317–338. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, K. Central mechanisms for thermoregulation in a hot environment. Ind. Health 2006, 44, 359–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gentry, C.; Andersson, D.A.; Bevan, S. TRPA1 mediates the hypothermic action of acetaminophen. Sci. Rep. 2015, 5, 12771. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, K.; Arkwright, P.D.; Mori, Y.; Oikawa, M.A.; Muko, R.; Tanaka, A.; Matsuda, H. A rapid shift from chronic hyperoxia to normoxia induces systemic anaphylaxis via transient receptor potential ankyrin 1 channels on mast cells. J. Immunol. 2020, 205, 2959–2967. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, T.; Isosaka, T.; Hayashi, Y.; Tang, L.; Doi, A.; Yasuda, A.; Hayashi, M.; Lee, C.Y.; Cao, L.; Kutsuna, N.; et al. Thiazoline-related innate fear stimuli orchestrate hypothermia and anti-hypoxia via sensory TRPA1 activation. Nat. Commun. 2021, 12, 2074. [Google Scholar] [CrossRef]
- Hayes, M.R.; Leichner, T.M.; Zhao, S.; Lee, G.S.; Chowansky, A.; Zimmer, D.; De Jonghe, B.C.; Kanoski, S.E.; Grill, H.J.; Bence, K.K. Intracellular signals mediating the food intake-suppressive effects of hindbrain glucagon-like peptide-1 receptor activation. Cell Metab. 2011, 13, 320–330. [Google Scholar] [CrossRef] [Green Version]
- Robson, M.J.; Quinlan, M.A.; Margolis, K.G.; Gajewski-Kurdziel, P.A.; Veenstra-VanderWeele, J.; Gershon, M.D.; Watterson, D.M.; Blakely, R.D. p38alpha MAPK signaling drives pharmacologically reversible brain and gastrointestinal phenotypes in the SERT Ala56 mouse. Proc. Natl. Acad. Sci. USA 2018, 115, E10245–E10254. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; DiVittorio, J.R.; Joseph, A.M.; Correa, S.M. The effects of estrogens on neural circuits that control temperature. Endocrinology 2021, 162. [Google Scholar] [CrossRef]
- Pakai, E.; Tekus, V.; Zsiboras, C.; Rumbus, Z.; Olah, E.; Keringer, P.; Khidhir, N.; Matics, R.; Deres, L.; Ordog, K.; et al. The neurokinin-1 receptor contributes to the early phase of lipopolysaccharide-induced fever via stimulation of peripheral cyclooxygenase-2 protein expression in mice. Front. Immunol. 2018, 9, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linkous, C.A.; Huang, C.; Fowler, J.R. UV photochemical oxidation of aqueous sodium sulfide to produce hydrogen and sulfur. J. Photochem. Photobiol. A Chem. 2004, 168, 153–160. [Google Scholar] [CrossRef]
- Palkovits, M.; Graf, L.; Hermann, I.; Borvendeg, J.; Acs, Z.; Lang, T. Regional distribution of enkephalins, endorphins and ACTH in the central nervous system of rats determined by radioimmunoassay. In Endorphins’78; Graf, L., Palkovits, M., Ronai, A.Z., Eds.; Akademiai Kiado: Budapest, Hungary, 1978; pp. 187–195. [Google Scholar]
- Paxinos, G.; Franklin, K.B.J. The Mouse Brain in Stereotaxic Coordinates; Academic Press: San Diego, CA, USA, 2001. [Google Scholar]
- Ali, A.; Syed, S.M.; Jamaluddin, M.F.B.; Colino-Sanguino, Y.; Gallego-Ortega, D.; Tanwar, P.S. Cell lineage tracing identifies hormone-regulated and Wnt-responsive vaginal epithelial stem cells. Cell Rep. 2020, 30, 1463–1477.e1467. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, M.; Mitani, Y.; Holmborn, K.; Kato, T.; Koinuma, D.; Maruyama, J.; Vasilaki, E.; Sawada, H.; Kobayashi, M.; Ozawa, T.; et al. The ALK-1/SMAD/ATOH8 axis attenuates hypoxic responses and protects against the development of pulmonary arterial hypertension. Sci. Signal. 2019, 12, eaay4430. [Google Scholar] [CrossRef] [PubMed]
- Garami, A.; Steiner, A.A.; Romanovsky, A.A. Fever and hypothermia in systemic inflammation. Handb. Clin. Neurol. 2018, 157, 565–597. [Google Scholar] [CrossRef]
- Bhatia, M.; Gaddam, R.R. Hydrogen sulfide in inflammation: A novel mediator and therapeutic target. Antioxid. Redox Signal. 2021, 34, 1368–1377. [Google Scholar] [CrossRef] [PubMed]

| Gene Amplified (Mus Musculus) | Nucleotide Sequence of Primer | Primer Type | Product Length in bp | NCBI RefSeq |
|---|---|---|---|---|
| Trpa1 | ATCCAAATAGACCCAGGCACG | sense | 101 | NM_177781.5 |
| CAAGCATGTGTCAATGTTTGGTACT | antisense | |||
| Gapdh | TTCACCACCATGGAGAAGGC | sense | 237 | NM_001289726.1 |
| GGCATGGACTGTGGTCATGA | antisense | |||
| Actb | CTGTATGCCTCTGGTCGTAC | sense | 214 | NM_007393.5 |
| TGATGTCACGCACGATTTCC | antisense |
| Probes (Mus Musculus) | Catalog Number | Fluorophores | Dilution |
|---|---|---|---|
| Trpa1 | 400211 | TSA Plus Cyanine 3 | 1:750 |
| 3-plex Negative Control Probe | 320871 | TSA Plus Fluorescein, Cyanine 3 and 5 | 1:750 |
| 3-plex Positive Control Probe | 320881 | TSA Plus Fluorescein, Cyanine 3 and 5 | 1:750 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olah, E.; Rumbus, Z.; Kormos, V.; Tekus, V.; Pakai, E.; Wilson, H.V.; Fekete, K.; Solymar, M.; Kelava, L.; Keringer, P.; et al. The Hypothermic Effect of Hydrogen Sulfide Is Mediated by the Transient Receptor Potential Ankyrin-1 Channel in Mice. Pharmaceuticals 2021, 14, 992. https://doi.org/10.3390/ph14100992
Olah E, Rumbus Z, Kormos V, Tekus V, Pakai E, Wilson HV, Fekete K, Solymar M, Kelava L, Keringer P, et al. The Hypothermic Effect of Hydrogen Sulfide Is Mediated by the Transient Receptor Potential Ankyrin-1 Channel in Mice. Pharmaceuticals. 2021; 14(10):992. https://doi.org/10.3390/ph14100992
Chicago/Turabian StyleOlah, Emoke, Zoltan Rumbus, Viktoria Kormos, Valeria Tekus, Eszter Pakai, Hannah V. Wilson, Kata Fekete, Margit Solymar, Leonardo Kelava, Patrik Keringer, and et al. 2021. "The Hypothermic Effect of Hydrogen Sulfide Is Mediated by the Transient Receptor Potential Ankyrin-1 Channel in Mice" Pharmaceuticals 14, no. 10: 992. https://doi.org/10.3390/ph14100992
APA StyleOlah, E., Rumbus, Z., Kormos, V., Tekus, V., Pakai, E., Wilson, H. V., Fekete, K., Solymar, M., Kelava, L., Keringer, P., Gaszner, B., Whiteman, M., Keeble, J., Pinter, E., & Garami, A. (2021). The Hypothermic Effect of Hydrogen Sulfide Is Mediated by the Transient Receptor Potential Ankyrin-1 Channel in Mice. Pharmaceuticals, 14(10), 992. https://doi.org/10.3390/ph14100992








