3. Materials and Methods
3.1. General Experimental Procedures
IR spectra were obtained using a Fourier transform infrared spectrometer. NMR spectra were recorded in CDCl
3 or DMSO-d
6 at 500 or 600 MHz for
1H NMR and 125 or 150 MHz for
13C NMR. Chemical shifts are given in (δ) parts per million and coupling constants (
J) in hertz (Hz).
1H and
13C spectra were referenced using the solvent signal as internal standard. Melting points were taken on a capillary melting point apparatus and are uncorrected. HREIMS were recorded using a high-resolution magnetic trisector (EBE) mass analyzer. Analytical thin-layer chromatography plates Polygram-Sil G/UV254 were used. Preparative thin-layer chromatography was carried out with Analtech silica gel GF plates (20 × 20 cm, 1000 Microns) using appropriate mixtures of ethyl acetate and hexanes. Microwave reactions were conducted in sealed glass vessels (capacity 5 mL) using a Biotage initiator microwave reactor. All solvents and reagents were purified by standard techniques reported [
28] or used as supplied from commercial sources. All compounds were named using the ACD40 Name-Pro program, which is based on IUPAC rules. The embelin (
1) used in the reactions was obtained from
Oxalis erythrorhiza Gillies ex Hook and Arn following the procedure described in reference [
29].
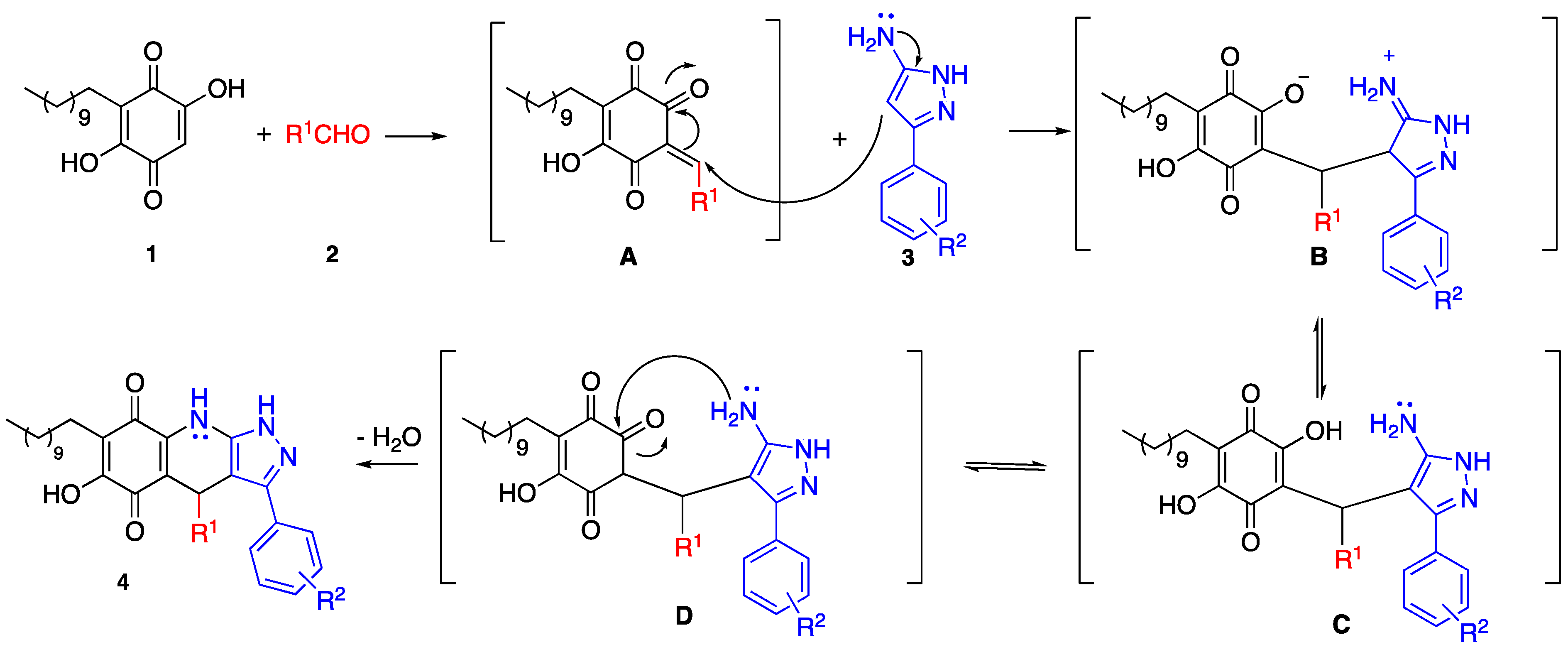
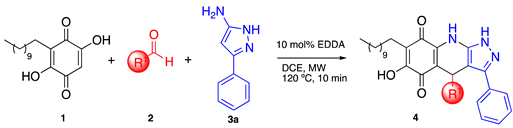
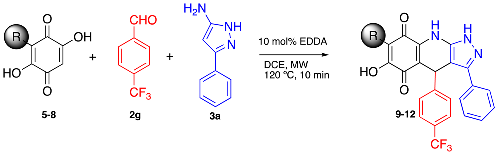
3.2. General Procedure for the Synthesis of Pyrazolo[3,4-b] Quinolin-5,8(4H,9H)-Dione Derivatives
To a MW tube equipped with a magnetic stir bar, embelin, 1.5 equiv of aldehyde, 1.5 equiv of 3-amino-5-phenylpyrazole and 10 mol % EDDA in 2 mL of DCE were added. The MW tube was sealed, and the reaction mixture was irradiated at 150 °C for 10 min. The products were isolated by filtration or purified by Shepadex LH-20.
3.3. 6-Hydroxy-4-(4-Nitrophenyl)-3-Phenyl-7-Undecyl-1H-Pyrazolo[3,4-b]Quinoline-5,8(4H,9H)-Dione (4a)
Following the general procedure described above, in a 5 mL MW tube, 30 mg of embelin (0.1 mmol), 23.4 mg of 4-nitrobenzaldehyde (0.15 mmol) and 24.4 mg of 3-amino-5-phenylpyrazole (0.15 mmol) were dissolved in 2 mL DCE and treated with 1.8 mg of EDDA (10 mol %). The tube was sealed, and the reaction mixture was irradiated at 150 °C for 10 min. Then, the reaction mixture was filtered and the obtained solid was washed with n-hex to yield 54.5 mg (94%) of 4a as an amorphous violet solid. Mp: 234.0–234.7 °C; 1H-NMR (500 MHz, CDCl3) δ 0.86 (t, J = 7.1 Hz, 3H, H-11′’’), 1.22 (bs, 16H, H, H-3′’’-H-10′’’), 1.43 (m, 2H, H-2′’’), 2.41 (t, J = 7.6 Hz, 2H, H-1′’’), 5.64 (s, 1H, H-4), 7.31 (m, 2H, H-2′’ + H-6′’), 7.39 (m, 5H, H-2′-H-6′), 8.00 (d, J = 8.5 Hz, 2H, H-3′’ + H-5′’); 13C-NMR (125 MHz, CDCl3) δ 14.1 (CH3), 22.6 (CH2), 22.7 (CH2), 28.0 (CH2), 28.1 (CH2), 29.3 (CH2), 29.4 (CH2), 29.5 (CH2), 29.5 (CH2), 29.6 (CH2), 29.7 (CH2 X 2), 31.9 (CH2), 36.3 (CH), 102.9 (C), 106.6 (C), 116.6 (C), 123.6 (CH x 2), 124.0 (CH) 125.7 (CH), 126.7 (CH), 128.1 (C), 128.7 (C), 129.0 (CH X 2), 129.2 (CH x 2), 129.6 (C), 141.3 (C), 146.6 (C), 152.0 (C), 154.1 (C), 178.6 (C), 182.2 (C); EIMS m/z (%): 568 ([M+], 100), 446 (89), 427 (24), 306 (8); HREIMS m/z 568.2682 (calcd for C33H36N4O5 [M+] 568.2686); IR vmax 3430 (N-H), 3315 (O-H), 2922 (C-H aliph), 2851 (C-H aliph), 2322 (C-C arom), 1640 (C=O), 1586, 1518, 1482, (C=N, C=C), 1347 (N-N), 1313, 1272, 1237, 1187, 1139, 1119, 1009, 981, 864 cm−1.
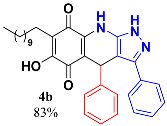
3.4. 6-Hydroxy-3,4-Diphenyl-7-Undecyl-1H-Pyrazolo[3,4-b]Quinoline-5,8 (4H,9H)-Dione (4b)
Following the general procedure described above, in a 5 mL MW tube, 30 mg of embelin (0.1 mmol), 16.2 mg of benzaldehyde (0.15 mmol, 15.6 µL) and 24.4 mg of 3-amino-5-phenylpyrazole (0.15 mmol) were dissolved in 2 mL DCE and treated with 1.8 mg of EDDA (10 mol %). The tube was sealed, and the reaction mixture was irradiated at 150 °C for 10 min. Then, the reaction mixture was filtered and the obtained solid was washed with n-hex to yield 44.3 mg (83%) of 4b as an amorphous violet solid. Mp: 216.4–217.4 °C; 1H-NMR (500 MHz, CDCl3) δ 0.86 (t, J = 6.9 Hz, 3H, H-11′’’), 1.23 (bs, 16H, H-3′’’-H-10′’’), 1.44 (m, 2H, H-2′’’), 2.38 (t, J = 7.8 Hz, 2H, H-1′’’); 5.48 (s, 1H, H-4); 7.10 (t, J = 7.3 Hz, 1H); 7.18 (t, J = 7.6 Hz, 2H); 7.27 (m, 2H); 7.33 (m, 5H); 13C-NMR (125 MHz, CDCl3) δ 14.1 (CH3), 22.6 (CH2), 22.7 (CH2), 28.2 (CH2), 29.3 (CH2), 29.5 (CH2), 29.6 (CH2 x 2), 29.7 (CH2 x 2), 31.9 (CH2), 36.2 (CH), 104.1 (C), 108.1 (C), 116.0 (C), 126.6 (CH), 126.8 (CH x 2), 128.3 (CH x 4), 128.8 (C), 128.9 (CH x 2), 129.0 (CH), 140.2 (C), 141.1 (C), 145.6 (C), 147.5 (C), 154.6 (C), 178.8 (C), 182.6 (C); EIMS m/z (%) 523 ([M+], 76), 446 (100), 382 (13), 276 (5); HREIMS 523.2852 (calcd for C33H37N3O3 [M+] 523.2835); IR vmax 3433 (N-H), 3259 (O-H), 2921 (C-H aliph), 2851 (C-H aliph), 1641 (C=O), 1571, 1528, 1506, 1483, 1437 (C=N, C=C), 1352 (N-N), 1271, 1235, 1182, 1138, 1084, 1029, 981, 878, 842 cm−1.
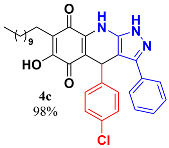
3.5. 4-(4-Chlorophenyl)-6-Hydroxy-3-Phenyl-7-Undecyl-1H-Pyrazolo[3,4-b]Quinoline-5,8 (4H,9H)-Dione (4c)
Following the general procedure described above, in a 5 mL MW tube, 30 mg of embelin (0.1 mmol), 21.5 mg of 4-chlorobenzaldehyde (0.15 mmol) and 24.4 mg of 3-amino-5-phenylpyrazole (0.15 mmol) were dissolved in 2 mL DCE and treated with 1.8 mg of EDDA (10 mol %). The tube was sealed, and the reaction mixture was irradiated at 150 °C for 10 min. Then, the reaction mixture was filtered and the obtained solid was washed with n-hex to yield 56.5 mg (98%) of 4c as an amorphous blue solid. Mp: 220.3–221.1 °C; 1H-NMR (500 MHz, CDCl3) δ 0.86 (t, J = 7.0 Hz, 3H, H-11′’’), 1.23 (bs, 16H, H-3′’’-H-10′’’), 1.43 (m, 2H, H-2′’’), 2.39 (t, J = 7.6 Hz, 2H, H-1′’’), 5.48 (s, 1H, H-4), 7.14 (d, J = 8.4 Hz, 2H, H-3′’ + H-5′’), 7.20 (d, J = 8.4 Hz, 2H, H-2′’ + H-6′’), 7.39 (m, 5H, H-2′-H-5′); 13C-NMR (125 MHz, CDCl3) 14.1 (CH3), 22.6 (CH2), 22.7 (CH2), 28.2 (CH2), 29.3 (CH2), 29.5 (CH2), 29.6 (CH2 x 2), 29.7 (CH2 x 2), 31.9 (CH2), 35.7 (CH), 103.7 (C), 107.7 (C), 116.2 (C), 126.8 (CH x 2), 128.4 (CH x 2), 128.6 (C), 129.1 (CH x 2), 129.2 (CH), 129.5 (CH2 x 2), 132.4 (C), 149.1 (C), 141.0 (C), 143.9 (C), 147.4 (C), 154.4 (C), 178.8 (C), 182.35 (C); EIMS m/z (%) 541 ([M+], 100), 446 (8), 413 (19), 400 (68); HREIMS m/z 559.2476 (calc for C33H36N3O337Cl [M+] 559.2416); 557.2471 (calcd for C33H36N3O335Cl [M+] 557.2445); IR vmax 3428 (N-H), 3240 (O-H), 2924 (C-H aliph), 2853 (C-H aliph), 2322 (C-C arom), 1642 (C=O), 1587, 1528, 1484, 1437 (C=N, C=C), 1352 (N-N), 1272, 1205, 1182, 1138, 1089 (C-Cl), 1014, 982, 826 cm−1.
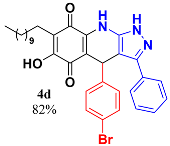
3.6. 4-(4-Bromophenyl)-6-Hydroxy-3-Phenyl-7-Undecyl-1H-Pyrazolo[3,4-b]Quinoline-5,8 (4H,9H)-Dione (4d)
Following the general procedure described above, in a 5 mL MW tube, 30 mg of embelin (0.1 mmol), 28.3 mg of 4-bromobenzaldehyde (0.15 mmol) and 24.4 mg of 3-amino-5-phenylpyrazole (0.15 mmol) were dissolved in 2 mL DCE and treated with 1.8 mg of EDDA (10 mol %). The tube was sealed, and the reaction mixture was irradiated at 150 °C for 10 min. Then, the reaction mixture was filtered and the obtained solid was washed with n-hex to yield 50.5 mg (82%) of 4d as an amorphous violet solid. Mp: 231.8–232.5 °C; 1H-NMR (500 MHz, CDCl3) δ 0.86 (t, J = 7.1 Hz, 3H, H-11′’’), 1.23 (bs, 16H, H-3′’’-H-10′’’), 1.44 (m, 2H, H-2′’’), 2.39 (t, J = 7.9 Hz, 2H, H-1′’’), 5.47 (s, 1H, H-4), 7.13 (d, J = 8.3 Hz, 2H, H-3′’ + H-5′’), 7.30 (d, J = 8.3 Hz, 2H, H-2′’ + H-6′’), 7.32 (m, 2H), 7.38 (m, 3H); 13C-NMR (125 MHz, CDCl3) δ 14.1 (CH3), 22.6 (CH2), 22.7 (CH2), 28.1 (CH2), 29.3 (CH2), 29.5 (CH2), 29.6 (CH2 x 2), 29.6 (CH2), 29.7 (CH2), 31.9 (CH2), 35.7 (CH), 103.5 (C), 107.6 (C), 116.3 (C), 120.6 (C), 126.8 (CH x 2), 128.5 (C), 129.0 (C), 129.1 (CH2 x 2), 129.2 (CH), 129.9 (CH x 2), 131.4 (CH x 2), 140.1 (C), 140.9 (C), 144.4 (C), 147.4 (C), 178.7 (C), 182.3 (C); EIMS m/z (%) 601 ([M+], 47), 460 (7), 446 (100), 306 (7); HREIMS m/z 601.1951 (calc for C33H36N3O379Br [M+] 601.1940); 603.1866 (calcd for C33H36N3O381Br [M+] 603.1920); IR vmax 3389 (N-H), 3256 (O-H), 2923, 2852 (C-H aliph), 1640 (C=O), 1573, 1526, 1503, 1482 (C=N, C=C), 1351 (N-N), 1315, 1270, 1237, 1181, 1136, 1071 (C-Br), 1009, 984, 827 cm−1.
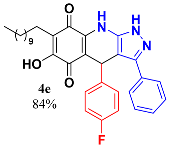
3.7. 4-(4-Fluorophenyl)-6-Hydroxy-3-Phenyl-7-Undecyl-1H-Pyrazolo[3,4-b]Quinoline-5,8 (4H,9H)-Dione (4e)
Following the general procedure described above, in a 5 mL MW tube, 30 mg of embelin (0.1 mmol), 19 mg of 4-fluorobenzaldehyde (0.15 mmol, 16.4 µL) and 24.4 mg of 3-amino-5-phenylpyrazole (0.15 mmol) were dissolved in 2 mL DCE and treated with 1.8 mg of EDDA (10 mol %). The tube was sealed, and the reaction mixture was irradiated at 150 °C for 10 min. Then, the reaction mixture was filtered and the obtained solid was washed with n-hex to yield 46.5 mg (84%) of 4e as an amorphous violet solid. Mp: 234.5–235.4 °C; 1H-NMR (500 MHz, CDCl3) δ 0.86 (t, J = 7.1 Hz, 3H, H-11′’’), 1.23 (bs, 16H, H-3′’’-H-10′’’), 1.43 (m, 2H, H-2′’’), 2.40 (t, J = 7.5 Hz, 2H, H-11′), 5.50 (s, 1H, H-4), 6.86 (t, J = 8.5 Hz, 2H, H-3′’ + H-5′’), 7.22 (m, 2H, H-2′’ + H-6′’), 7.35 (m, 5H, H-2′-H-5′); 13C-NMR (125 MHz, CDCl3) 14.1 (CH3), 22.6 (CH2), 22.7 (CH2), 28.2 (CH2), 29.3 (CH2), 29.5 (CH2), 29.6 (CH2 x 2), 29.7 (CH2), 31.9 (CH2), 35.5 (CH), 104.0 (C), 107.8 (C), 115.1 (CH x 2, J = 21.4 Hz), 116.2 (C), 126.8 (CH x 2), 128.6 (C), 129.0 (CH x 2), 129.2 (CH), 129.7 (CH x 2, J = 7.9 Hz), 140.0 (C), 141.1 (C), 141.3 (C), 141.4 (C), 147.4 (C), 154.3 (C), 161.5 (C-F, J = 248.5 Hz), 178.7 (C), 182.6 (C); EIMS m/z (%) 541 ([M+], 96); 446 (100); 401 (13); 304 (8); HREIMS m/z 541.2744 (calcd for C33H36N3O3F [M+] 541.2741); IR vmax 3426 (N-H), 3234 (O-H), 2924, 2851 (C-H aliph), 1641 (C=O), 1571, 1528, 1483 (C=C, C=N), 1353 (N-N), 1295, 1272, 1223, 1205, 1155, 1137 (C-F), 1014, 983, 835 cm−1.
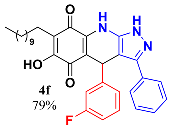
3.8. 4-(3-Fluorophenyl)-6-Hydroxy-3-Phenyl-7-Undecyl-1H-Pyrazolo[3,4-b]Quinoline-5,8 (4H,9H)-Dione (4f)
Following the general procedure described above, in a 5 mL MW tube, 30 mg of embelin (0.1 mmol), 19 mg of 3-fluorobenzaldehyde (0.15 mmol, 16.1 µL) and 24.4 mg of 3-amino-5-phenylpyrazole (0.15 mmol) were dissolved in 2 mL of DCE and treated with 1.8 mg of EDDA (10 mol %). The tube was sealed, and the reaction mixture was irradiated at 150 °C for 10 min. Then, the reaction mixture was filtered and the obtained solid was washed with n-Hex to yield 43 mg (79%) of 4f as an amorphous violet solid. Mp: 224.7–225.5 °C; 1H-NMR (500 MHz, CDCl3) δ 0.86 (t, J = 7.0 Hz, 3H, H-11′’’), 1.23 (bs, 16H, H-3′’’-H-10′’’), 1.46 (m, 2H, H-2′’’), 2.40 (t, J = 7.8 Hz, 2H, H-11′), 5.51 (s, 1H, H-4), 6.80 (td, J = 1.8, 8.3 Hz, 1H, H-2′’), 6.96 (dt, J = 2.5, 9.6 Hz, 1H, H-4′’), 7.05 (d, J = 7.7 Hz, 1H, H-1′’), 7.14 (m, 1H), 7.32 (m, 2H), 7.37 (m, 3H); 13C-NMR (125 MHz, CDCl3) 14.2 (CH3), 22.6 (CH2), 22.7 (CH2), 28.2 (CH2), 29.3 (CH2), 29.5 (CH2), 29.6 (CH2 x 2), 29.7 (CH2 x 2), 31.9 (CH2), 35.8 (CH), 103.5 (C), 107.4 (C), 113.5 (C, J = 20.8 Hz), 115.2 (CH, J = 21.6 Hz), 116.2 (C), 123.8 (CH), 126.7 (CH x 4), 128.5 (C), 129.0 (CH), 129.2 (CH), 129.6 (CH, J = 7.8 Hz), 140.1 (C), 141.1 (C), 147.3 (C), 147.8 (C), 154.3 (C), 162.8 (C, J = 247.8 Hz), 178.6 (C), 182.4 (C); EIMS m/z (%) 541 ([M+], 79); 446 (100); 400 (18); 305 (9); HREIMS 541.2756 (calcd for C33H36N3O3F [M+] 541.2741); IR vmax 3429 (N-H), 3256 (O-H), 2924, 2851 (C-H aliph), 1639 (C=O), 1585, 1527, 1481,1439 (C=C, C=N), 1350 (N-N), 1296, 1199, 1145 (C-F), 983, 929, 841, 729, 690 cm−1.
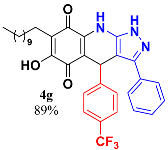
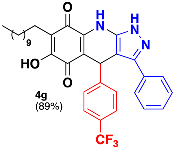
3.9. 6-Hydroxy-3-Phenyl-4-(4-(Trifluoromethyl)Phenyl)-7-Undecyl-1H-Pyrazolo [3,4-b] Quinoline-5,8 (4H,9H)-Dione (4g)
Following the general procedure described above, in a 5 mL MW tube, 30 mg of embelin (0.1 mmol), 26.6 mg of 4-(trifluromethyl)-benzaldehyde (0.15 mmol, 21 µL) and 24.4 mg of 3-amino-5-phenylpyrazole (0.15 mmol) were dissolved in 2 mL of DCE and treated with 1.8 mg of EDDA (10 mol %). The tube was sealed, and the reaction mixture was irradiated at 150 °C for 10 min. Then, the reaction mixture was filtered and the obtained solid was washed with n-hex to yield 53.7 mg (89%) of 4g as an amorphous violet solid. Mp: 213.9–214.9 °C; 1H-NMR (500 MHz, CDCl3) δ 0.87 (t, J = 7.1 Hz, 3H, H-11′’’), 1.22 (bs, 16H, H-3′’’-H-10′’’), 1.43 (m, 2H, H-2′’’), 2.37 (t, J = 7.1 Hz, 2H, H-1′’’), 5.55 (s, 1H, H-4), 7.31 (m, 2H), 7.35 (m, 5H), 7.40 (d, J = 8.0 Hz, 2H, H-3′’ + H-5′’); 13C-NMR (125 MHz, CDCl3) 14.1 (CH3), 22.7 (CH2 x 2), 28.2 (CH2), 29.3 (CH2), 29.5 (CH2), 29.6 (CH2 x 2), 29.7 (CH2 x 2), 31.9 (CH2), 36.2 (CH), 103.4 (C), 107.2 (C), 116.3 (C), 123.1 (C), 125.2 (CH x 2), 126.8 (CH x 2), 128.5 (CH x 2), 128.5 (C), 128.7 (C), 129.1 (CH x 2), 129.3 (CH), 140.2 (C), 141.5 (C), 147.3 (C), 149.2 (C), 179.2 (C), 182.1 (C); EIMS m/z (%) 591 ([M+], 100); 446 (95); 306 (8); 276 (6); HREIMS m/z 591.2720 (calcd for C34H36N3O3F3 [M+] 591.2709); IR vmax 3431 (N-H), 3253 (O-H), 2924, 2853 (C-H aliph), 1642 (C=O), 1586, 1569, 1529, 1484 (C=C, C=N), 1322, (N-N), 1272, 1236, 1161, 1120 (C-F), 1066, 1017, 986, 834 cm−1.
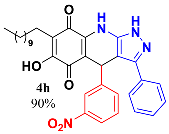
3.10. 6-Hydroxy-4-(3-Nitrophenyl)-3-Phenyl-7-Undecyl-1H-Pyrazolo[3,4-b]Quinoline-5,8 (4H,9H)-Dione (4h)
Following the general procedure described above, in a 5 mL MW tube, 30 mg of embelin (0.1 mmol), 23.4 mg of 3-nitrobenzaldehyde (0.15 mmol) and 24.4 mg of 3-amino-5-phenylpyrazole (0.15 mmol) were dissolved in 2 mL DCE and treated with 1.8 mg of EDDA (10 mol %). The tube was sealed, and the reaction mixture was irradiated at 150 °C for 10 min. Then, the reaction mixture was filtered and the obtained solid was washed with n-hex to yield 52 mg (90%) of 4h as an amorphous violet solid. Mp: 210.2–211.8 °C; 1H-NMR (500 MHz, DMSO-d6) δ 0.85 (t, J = 7.1 Hz, 3H, H-11′’’), 1.22 (bs, 16H, H-3′’’-H-10′’’), 1.33 (m, 2H, H-2′’’), 2.25 (t, J = 7.5 Hz, 2H, H-1′’’), 3.03 (bs, 1H), 5.71 (s, 1H), 7.29 (t, J = 7.5 Hz, 1H), 7.37 (t, J = 7.5 Hz, 2H), 7.41 (t, J = 8.1 Hz,1H), 7.51 (d, J = 7.2 Hz, 2H), 7.58 (d, J = 7.8 Hz, 1H), 7.88 (dd, J = 1.3, 8.1 Hz, 1H), 8.00 (t, J = 2.1 Hz, 1H); 13C-NMR (125 MHz, DMSO-d6) 13.9 (CH3), 22.0 (CH2), 22.1(CH2), 27.7 (CH2), 28.7 (CH2), 28.8 (CH2), 28.9 (CH2), 29.0 (CH2 x 2), 29.1 (CH2), 31.3 (CH2), 35.9 (CH), 101.8 (C), 106.5 (C), 115.5 (C), 121.0 (CH), 122.3 (CH), 126.4 (CH x 2), 128.2 (CH), 128.6 (CH x 2), 129.4 (CH), 137.6 (CH), 138.7 (C), 140.5 (C), 146.2 (C), 147.1 (C), 148.1 (C), 157.6(C), 179.0 (C), 180.4 (C); EIMS m/z (%) 568 ([M+], 92); 538 (7); 446 (100); 427(20); 306 (8). HREIMS 568.2669 (calcd for C33H36N4O5 [M+] 568.2686); IR 3415 (N-H), 3267 (O-H), 2924, 2851 (C-H aliph), 1639 (C=O), 1578, 1527, 1485 (C=C, C=N), 1346 (N-N), 1315 (NO2), 1269, 1192, 1123, 1088, 814, 690 cm−1.
3.11. 4-(6-Hydroxy-5,8-Dioxo-3-Phenyl-7-Undecyl-4,5,8,9-Tetrahydro-1H-Pyrazolo[3,4-b]Quinolin-4-yl)Benzoic Acid (4i)
Following the general procedure described above, in a 5 mL MW tube, 30 mg of embelin (0.1 mmol), 23 mg of 4-formylbenzoic acid (0.15 mmol, 14.4 μL) and 24.4 mg of 3-amino-5-phenylpyrazole (0.154 mmol) were dissolved in 2 mL of DCE and treated with 1.8 mg of EDDA (10 mol %). The tube was sealed, and the reaction mixture was irradiated at 150 °C for 10 min. Then, the reaction mixture was filtered and the obtained solid was washed with n-hex to yield 54.7 mg (93%) of 4i as an amorphous violet solid. Mp: 240.1–242.0 °C; 1H-NMR (500 MHz, DMSO-d6) δ 0.77 (t, J = 7.1 Hz, 3H, H-11′’’), 1.13 (bs, 16H, H-3′’’-H-10′’’), 1.30 (m, 2H, H-2′’’), 2.23 (t, J = 7.6 Hz, 2H, H-1′’’), 5.54 (s, 1H, H-4), 7.23 (d, J = 8.3 Hz, 2H, H-2′’ + H-6′’), 7.30 (m, 1H, H-4′), 7.37 (t, J = 7.5 Hz, 2H, H-3′ + H-5′), 7.46 (d, J = 7.5 Hz, 2H, H-2′ + H-6′), 7.66 (d, J = 8.2 Hz, 2H, H-3′’ + H-5′’); 13C-NMR (125 MHz, DMSO-d6) 14.1 (CH3), 22.0 (CH2 x 2), 27.7 (CH2), 28.7 (CH2), 28.8 (CH2), 28.9 (CH2), 29.0 (CH2 x 2), 29.1 (CH2), 31.3 (CH2), 36.0 (CH), 102.2 (C), 107.1 (C), 115.3 (C), 124.6 (C), 124.9 (C), 126.2 (CH x 2), 128.0 (CH x 2), 128.1 (CH), 128.5 (C), 128.7 (CH x 2), 129.0 (CH x 2), 129.8 (C), 138.5 (C), 140.3 (C), 150.9 (C), 167.0 (C), 177.3 (C), 179.0 (C); EIMS m/z (%) 567 ([M+], 38), 566 (100), 551 (62), 446 (96), 410 (31); HREIMS 567.2757 (calcd for C34H37N3O5 [M+] 567.2733); IR vmax 3413 (OH), 2375 (C-H arom), 2924, 2854 (C-H aliph), 1685 (C=O), 1574, 1527, 1427 (C=C, C=N), 1366 (N-N), 1258, 1184, 1141, 976, 860, 694 cm−1.
3.12. Methyl-4-(6-Hydroxy-5,8-Dioxo-3-Phenyl-7-Undecyl-4,5,8,9-Tetrahydro-1H-Pyrazolo[3,4-b]Quinolin-4-yl)Benzoate (4j)
Following the general procedure described above, in a 5 mL MW tube, 33 mg of embelin (0.112 mmol), 24.4 mg of methyl 4-formylbenzoate (0.17 mmol) and 27.1 mg of 3-amino-5-phenylpyrazole (0.17 mmol) were dissolved in 2 mL of DCE and treated with 2 mg of EDDA (10 mol %). The tube was sealed, and the reaction mixture was irradiated at 150 °C for 10 min. Then, the reaction mixture was filtered and the obtained solid was washed with n-hex to yield 62.2 mg (95%) of 4j as an amorphous violet solid. Mp: 219.1–220.4 °C. 1H-NMR (500 MHz, CDCl3) δ 0.86 (t, J = 7.1 Hz, 3H, H-11′’’), 1.23 (bs, 16H, H-3′’’-H-10′’’), 1.45 (m, 2H, H-2′’’), 2.40 (t, J = 8.2 Hz, 2H, H-1′), 3.85 (s, 3H, -OCH3), 5.57 (s, 1H, H-4), 7.30 (m, 2H), 7.34 (d, J = 8.3 Hz, 2H, H-2′’ + H-6′’), 7.37 (m, 3H), 7.86 (d, J = 8.3 Hz, 2H, H-3′’ + H-5′’); 13C-NMR (125 MHz, CDCl3) δ 14.1 (CH3), 22.6 (CH2), 22.7 (CH2), 28.1 (CH2), 29.3 (CH2), 29.5 (CH2), 29.6 (CH2 x 2), 29.7 (CH2 x 2), 31.9 (CH2), 36.2 (CH), 52.0 (CH3), 103.4 (C), 107.2 (C), 116.3 (C), 126.8 (CH x 2), 128.2 (CH x 2), 128.4 (C), 128.5 (C), 129.1 (CH x 2), 129.3 (CH), 129.7 (CH x 2), 140.2 (C), 141.1 (C), 147.3 (C), 150.2 (C), 154.0 (C), 166.9 (C), 178.5 (C), 182.4 (C); EIMS m/z (%) 581 ([M+], 78); 446 (100); 305 (59); 159 (47); HREIMS 581.2916 (calcd for C35H39N3O5 [M+] 581.2890); IR vmax 3433 (OH), 2923, 2854 (C-H aliph), 2360 (C-H arom), 1639 (C=O), 1589, 1569, 1523, 1485 (C=C, C=N), 1353 (N-N), 1273, 1218, 1138, 1068, 995, 891, 690 cm−1.
3.13. 4-(6-Hydroxy-5,8-Dioxo-3-Phenyl-7-Undecyl-4,5,8,9-Tetrahydro-1H-Pyrazolo[3,4-b]Quinolin-4-yl)Benzonitrile (4k)
Following the general procedure described above, in a 5 mL MW tube, 30 mg of embelin (0.1 mmol), 20.1 mg of 4-cyanobenzaldehyde (0.15 mmol, 14.4 μL) and 24.4 mg of 3-amino-5-phenylpyrazole (0.154 mmol) were dissolved in 2 mL of DCE and treated with 1.8 mg of EDDA (10 mol %). The tube was sealed, and the reaction mixture was irradiated at 150 °C for 10 min. Then, the reaction mixture was filtered and the obtained solid was washed with n-hex to yield 45.3mg (81%) of 4k as an amorphous violet solid. Mp: 206.2–207.8 °C; 1H-NMR (500 MHz, CDCl3) δ 0.86 (t, J = 7.1 Hz, 3H, H-11′’’), 1.23 (bs, 16H, H-3′’’-H-10′’’), 1.45 (m, 2H, H-2′’’), 2.40 (t, J = 7.7 Hz, 2H, H-1′’’), 5.56 (s, 1H, H-4), 7.29 (m, 2H), 7.34 (d, J = 8.1 Hz, 2H, H-2′’ + H-6′’), 7.38 (m, 3H), 7.45 (d, J = 8.1 Hz, 2H, H-3′’ + H-5′’); 13C-NMR (125 MHz, CDCl3) 14.1 (CH3), 22.6 (CH2), 22.7 (CH2), 28.1 (CH2), 29.3 (CH2), 29.5 (CH2), 29.6 (CH2 x 2), 29.7 (CH2 x 2), 31.9 (CH2), 36.5 (CH), 103.0 (C), 106.6 (C), 110.4 (C), 116.4 (C), 118.8 (C), 125.3 (C), 126.8 (CH x 2), 128.2 (C), 128.9 (CH x 2), 129.1 (CH x 2), 129.4 (CH), 132.1 (CH x 2), 140.3 (C), 141.4 (C), 147.1 (C), 150.2(C), 178.6 (C), 182.2 (C); EIMS m/z (%) 548 ([M+], 69), 532 (37), 395 (37), 274 (100), 159 (73); HREIMS 548.2787 (calcd for C34H36N4O3 [M+] 548.2787); IR vmax 3256 (OH), 2920, 2854 (CH-arom), 2804, 2677, 2229 (CN), 2052, 1643 (C=O), 1578, 1519, 1504, 1438 (C=C, C=N), 1350 (N-N), 1196, 1138, 1084, 986, 833, 694 cm−1.
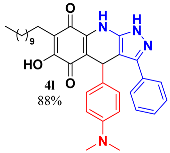
3.14. 4-(4-(Dimethylamino)Phenyl)-6-Hydroxy-3-Phenyl-7-Undecyl-1H-Pyrazolo[3,4-b]Quinoline-5,8 (4H,9H)-Dione (4l)
Following the general procedure described above, in a 5 mL MW tube, 35 mg of embelin (0.12 mmol), 27 mg of 4-dimethylaminobenzaldehyde (0.18 mmol) and 28.7 mg of 3-amino-5-phenylpyrazole (0.18 mmol) were dissolved in 2 mL of DCE and treated with 3.4 mg of EDDA (10 mol %). The tube was sealed, and the reaction mixture was irradiated at 150 °C for 10 min. Then, the reaction mixture was filtered and the obtained solid was washed with n-hex to yield 59.9 mg (88%) of 4l as an amorphous violet solid. Mp: 195.8–197.2 °C; 1H-NMR (500 MHz, CDCl3) δ 0.87 (t, J = 7.1 Hz, 3H, H-11′’’), 1.24 (bs, 16H, H-3′’’-H-10′’’), 1.45 (m, 2H, H-2′’’), 2.39 (t, J = 7.7 Hz, 2H, H-1′’’), 2.87 (s, 6H, -N(CH3)2), 3.07 (bs, 1H, -NH), 5.41 (s, 1H, H-4), 7.31 (m, 2H), 6.57 (d, J = 8.7 Hz, 2H, H-3′’ + H-5′’), 7.14 (d, J = 8.5 Hz, 2H, H-2′’ + H-6′’), 7.36 (m, 5H, H-2′-H-5′); 13C-NMR (125 MHz, CDCl3) δ 14.1 (CH3), 22.5 (CH2), 22.7 (CH2), 28.1 (CH2), 29.3 (CH2), 29.5 (CH2), 29.6 (CH2 x 2), 29.7 (CH2), 31.9 (CH2), 34.9 (CH2), 40.5 (CH), 104.4 (C), 108.8 (C), 112.3 (CH x 2), 115.8 (C), 125.3 (CH), 125.5 (C), 126.7 (CH x 2), 128.9 (CH x 2), 129.0 (CH x 2), 129.1 (C), 130.8 (C), 133.9 (C), 139.6 (C), 140.3 (C), 147.7 (C), 149.1 (C), 154.0 (C), 178.9 (C), 182.8 (C); EIMS m/z (%) 566 ([M+], 100), 550 (19), 447 (16), 426 (14), 290 (12); HREIMS 566.3282 (calcd for C35H42N4O3 [M+] 566.3257); IR vmax 3433 (N-H), 3251 (O-H), 2920, 2851, 2804 (C-H arom), 1666 (C=O), 1520, 1481, 1438 (C=C, C=N), 1354 (N-N), 1315, 1273, 1199, 1126, 1061, 1034, 964, 814, 690 cm−1.
3.15. 4-(3-Fluoro-4-Methoxyphenyl)-6-Hydroxy-3-Phenyl-7-Undecyl-1H-Pyrazolo[3,4-b]Quinoline-5,8 (4H,9H)-Dione (4m)
Following the general procedure described above, in a 5 mL MW tube, 30 mg of embelin (0.1 mmol), 23.6 mg of 3-fluoro-4-methoxybenzaldehyde (0.15mmol) and 24.4 mg of 3-amino-5-phenylpyrazole (0.15 mmol) were dissolved in 2 mL of DCE and treated with 1.8 mg of EDDA (10 mol %). The tube was sealed, and the reaction mixture was irradiated at 150 °C for 10 min. Then, the reaction mixture was filtered and the obtained solid was washed with n-hex to yield 57.6 mg (98%) of 4m as an amorphous violet solid. Mp: 189.7–190.1 °C; 1H-NMR (500 MHz, CDCl3) δ 0.86 (t, J = 7.1 Hz, 3H, H-11′’’), 1.22 (bs, 16H, H-3′’’-H-10′’’), 1.43 (m, 2H, H-2′’’), 2.38 (t, J = 7.6 Hz, 2H, H-1′’’), 3.77 (s, 3H, -OCH3), 5.44 (s, 1H, H-4), 6.73 (t, J = 8.8 Hz, 1H), 6.98 (m, 2H), 7.51 (m, 5H); 13C-NMR (125 MHz, CDCl3) δ 14.1 (CH3), 22.6 (CH2), 22.7 (CH2), 28.2 (CH2), 29.3 (CH2), 29.5 (CH2), 29.6 (CH2 x 2), 29.7 (CH2), 29.7 (CH2), 31.9 (CH2), 35.3 (CH), 56.2 (CH3), 103.7 (C), 107.7 (C), 113.0 (C), 116.0 (CH, J = 19.2 Hz), 123.7 (CH, J = 2.6 Hz), 126.7 (CH x 2), 128.7 (C), 129.0 (CH x 2), 129.0 (CH), 138.7 (C, J = 4.8 Hz), 139.9 (C), 141.1 (C) 146.2 (C, J = 10.6 Hz), 147.4 (C), 151.2 (C), 154.0 (C-F, J = 239.4 Hz), 154.9 (C), 178.9 (C), 182.43 (C); EIMS m/z (%) 571 ([M+], 99); 446 (100); 295 (82); 159 (94); HREIMS 571.2845 (calcd for C34H38N3O4F [M+] 571.2846); IR vmax 3425 (N-H), 3251 (O-H), 2924, 2852 (C-H arom), 1641 (C=O), 1586, 1571, 1506, 1436 (C=N, C=C), 1352 (N-N), 1314, 1269, 1201, 1148, 1116, 1028, 981, 872, 803 cm−1.
3.16. 4-(3,4-Dimethoxyphenyl)-6-Hydroxy-3-Phenyl-7-Undecyl-1H-Pyrazolo[3,4-b]Quinoline-5,8 (4H,9H)-Dione (4n)
Following the general procedure described above, in a 5 mL MW tube, 30 mg of embelin (0.1 mmol), 25.4 mg of 3,4-dimethoxybenzaldehyde (0.15 mmol) and 24.4 mg of 3-amino-5-phenylpyrazole (0.15 mmol) were dissolved in 2 mL of DCE and treated with 1.8 mg of EDDA (10 mol %). The tube was sealed, and the reaction mixture was irradiated at 150 °C for 10 min. Then, the reaction mixture was filtered and the obtained solid was washed with n-hex to yield 54.2 mg (91%) of 4n as an amorphous violet solid. Mp: 183.8–184.5 °C; 1H-NMR (500 MHz, CDCl3) δ 0.86 (t, J = 7.1 Hz, 3H, H-11′’’), 1.23 (bs, 16H, H-3′’’-H-10′’’), 1.44 (m, 2H), 2.40 (t, J = 7.8 Hz, 2H, H-1′’’), 3.72 (s, 3H, -OCH3), 3.78 (s, 3H, -OCH3), 5.47 (s, 1H, H-4), 6.68 (d, J = 8.4 Hz, 1H, H-5′’), 6.75 (dd, J = 8.3, 1.8 Hz, 1H, H-6′’), 6.82 (d, J = 1.8 Hz, 1H, H-2′’), 7.37 (m, 5H, H-2′-H-6′); 13C-NMR (125 MHz, CDCl3) 14.1 (CH3), 22.6 (CH2), 22.7 (CH2), 28.2 (CH2), 29.3 (CH2), 29.5 (CH2), 29.6 (CH2 x 2), 29.7 (CH2 x 2), 31.9 (CH2), 35.6 (CH), 55.8 (CH3), 55.9 (CH3), 104.3 (C), 108.1 (C), 111.1 (CH), 111.7 (CH), 116.1 (C), 120.1 (CH), 127.0 (CH x 2), 128.9 (C), 129.0 (CH x 2), 129.1 (CH), 138.4 (C), 139.9 (C), 141.1 (C), 147.5 (C), 147.7 (C), 148.7 (C), 154.3 (C), 178.9 (C), 182.8 (C); EIMS m/z (%) 583 ([M+], 66); 446 (100); 304 (26); HREIMS m/z 583.3026 (calc for C35H41N3O5 [M+] 583.3046); IR vmax 3431 (N-H), 3253 (O-H), 2922, 2852 (C-H arom), 1641 (C=O), 1586, 1506 (C=C, C=N), 1352 (N-N), 1263 (O-CH3), 1233, 1203, 1136, 1029, 982, 927, 852 cm−1.
3.17. 4-(Benzo[d][1,3]Dioxol-5-yl)-6-Hydroxy-3-Phenyl-7-Undecyl-1H-Pyrazolo[3,4-b]Quinoline-5,8 (4H, 9H)-Dione (4o)
Following the general procedure described above, in a 5 mL MW tube, 30 mg of embelin (0.1 mmol), 23 mg of piperonal (0.15 mmol) and 24.4 mg of 3-amino-5-phenylpyrazole (0.15 mmol) were dissolved in 2 mL of DCE and treated with 1.8 mg of EDDA (10 mol %). The tube was sealed, and the reaction mixture was irradiated at 150 °C for 10 min. Then, the reaction mixture was filtered and the obtained solid was washed with n-hex to yield 42.3 mg (73%) of 4o as an amorphous violet solid. Mp: 227.5–228.3 °C; 1H-NMR (500 MHz, CDCl3) δ 0.86 (t, J = 7.1 Hz, 3H, H-11′’’), 1.23 (bs, 16H, H-3′’’-H-10′’’), 1.44 (m, 2H, H-2′’’), 2.39 (t, J = 7.7 Hz, 2H, H-1′’’), 5.43 (s, 1H, H-4), 5.86 (d, J = 4.2 Hz, 2H, -OCH2O-), 6.62 (d, J = 7.8 Hz, 1H, H-6′’), 6.75 (m, 2H), 7.37 (m, 5H); 13C-NMR (125 MHz, CDCl3) 14.1 (CH3), 22.6 (CH2), 22.7 (CH2), 28.2 (CH2), 29.3 (CH2), 29.5 (CH2), 29.6 (CH2 x 2), 29.7 (CH2 x 2), 31.9 (CH2), 35.7 (CH), 100.9 (CH2), 104.1 (C), 107.9 (C), 108.2 (C), 108.8 (C), 116.1 (C), 121.4 (C), 126.8 (CH x 2), 128.7 (CH), 129.0 (CH x 2), 129.1 (CH), 139.6 (CH), 139.9 (CH), 140.7 (C), 146.2 (C), 147.5 (C), 147.7 (C), 154.4 (C), 178.8 (C), 182.5 (C); EIMS m/z (%) 567 ([M+], 67), 446 (100), 427 (7), 304 (7); HREIMS m/z 567.2746 (calcd for C34H37N3O5 [M+] 567.2733); IR vmax 3427 (N-H), 3237 (O-H), 2923, 2853 (CH-aliph), 1641, (C=O), 1571, 1528, 1483, 1438 (C=C, C=N), 1315 (N-N), 1271, 1201, 1142, 1090, 1038, 981, 939, 922, 866 cm−1.
3.18. 6-Hydroxy-4-(1H-Imidazol-4-yl)-3-Phenyl-7-Undecyl-1H-Pyrazolo[3,4-b]Quinoline-5,8 (4H,9H)-Dione (4p)
Following the general procedure described above, in a 5 mL MW tube, 30 mg of embelin (0.1 mmol), 24.7 mg of 4-(5)-imidazolecarboxaldehyde (0.15 mmol) and 24.4 mg of 3-amino-5-phenylpyrazole (0.15 mmol) were dissolved in 2 mL of DCE and treated with 1.8 mg of EDDA (10 mol %). The tube was sealed, and the reaction mixture was irradiated at 150 °C for 10 min. Then, the reaction mixture was filtered and the obtained solid was washed with n-hex to yield 30 mg (57%) of 4p as an amorphous violet solid. Mp: 265.4–266.1 °C; 1H-NMR (500 MHz, DMSO-d6) δ 0.88 (t, J = 7.1 Hz, 3H, H-11′’’), 1.26 (bs, 16H, H-3′’’-H-10′’’), 1.39 (m, 2H, H-2′’’), 2.30 (t, J = 7.7 Hz, 2H, H-1′’’), 5.58 (s, 1H, H-4), 6.75 (s, 1H, H-2′’), 7.38 (t, J = 7.4 Hz, 1H), 7.46 (m, 3H), 7.68 (d, J = 7.5 Hz, 2H); 13C-NMR (125 MHz, DMSO-d6) δ 13.9 (CH3), 22.0 (CH2 x 2), 27.8 (CH2), 28.4 (CH2), 28.6 (CH2), 28.9 (CH2 x 3), 29.0 (CH2) 29.1 (CH2), 31.2 (CH), 101.6 (C), 104.9 (C), 106.1 (C), 115.1 (C), 126.1 (CH x 2), 127.9 (CH), 128.7 (CH x 2), 133.9 (CH), 137.8 (CH), 140.5 (C), 146.9 (C), 157.6 (C), 178.9 (C), 181.0 (C); EIMS m/z (%) 513 ([M+], 50), 497 (100), 357 (29), 342 (7); HREIMS 513.2764 (calcd for C30H35N5O3 [M+] 513.2740); IR vmax 3420 (N-H), 3147 (O-H), 2921, 2850 (C-H aliph), 1637 (C=O), 1566, 1522, 1505, 1435 (C=C, C=N), 1360 (N-N), 1310, 1200, 1141, 1096, 980, 955, 843 cm−1.
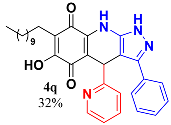
3.19. 6-Hydroxy-3-Phenyl-4-(Pyridin-3-yl)-7-Undecyl-1H-Pyrazolo[3,4-b]Quinoline-5,8 (4H,9H)-Dione (4r)
Following the general procedure described above, in a 5 mL MW tube, 30 mg of embelin (0.1 mmol), 16.4 mg of 3-pyridinecarboxaldehyde (0.15 mmol, 14.4 μL) and 24.4 mg of 3-amino-5-phenylpyrazole (0.15 mmol) were dissolved in 2 mL of DCE and treated with 1.8 mg of EDDA (10 mol %). The tube was sealed, and the reaction mixture was irradiated at 150 °C for 10 min. Then, the reaction mixture was filtered and the obtained solid was washed with n-hex to yield 30.4 mg (57%) of 4r as an amorphous violet solid. Mp: 273.6–274.6 °C; 1H-NMR (500 MHz, DMSO-d6) δ 0.81 (t, J = 7.0 Hz, 3H, H-11′’’), 1.19 (bs, 16H, H-3′’’-H-10′’’), 1.32 (m, 2H, H-2′’’), 2.25 (t, J = 7.1 Hz, 2H, H-1′’’), 5.56 (s, 1H, H-4), 7.14 (dd, J = 4.8, 7.8 Hz, 1H, H-6′’’), 7.30 (m, 1H), 7.37 (t, J = 7.4 Hz, 2H), 7.44 (dt, J = 1.9, 7.9 Hz, 1H), 7.49 (d, J = 7.6 Hz, 1H), 8.17 (dd, J = 1.4, 4.6 Hz, 1H, H-4′’), 8.39 (d, J = 1.8 Hz, 1H, H-2′’); 13C-NMR (125 MHz, DMSO-d6) δ 13.9 (CH3), 21.9 (CH2), 22.0 (CH2), 27.5 (CH2), 28.6 (CH2), 28.7 (CH2), 28.8 (CH2 x 2), 29.0 (CH2 x 2), 31.2 (CH2), 33.6 (CH), 101.9 (C), 106.9 (C), 115.9 (C), 123.5 (CH), 123.9 (C), 126.2 (CH x 2), 128.3 (CH), 128.7(CH x 2), 135.3 (CH), 137.0 (C), 140.0 (C), 141.3 (C), 147.0(CH), 148.6 (CH), 150.1 (C), 153.2(C), 178.6 (C), 181.4 (C); ESMS (-) m/z (%) 523 ([M-H]+, 30), 511 (100), 497(12), 391 (45); ESHRMS(-) 523.2708 (calcd for C32H35N4O3 [M+] 523.2709); IR vmax 3433 (OH), 2924, 2851 (C-H-alipha), 1639, (C=O), 1570, 1531, 1481, 1435 (C=C, C=N), 1357 (N-N), 1238, 1177, 1126, 1034, 976, 822, 694 cm−1.
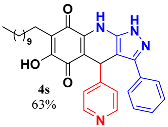
3.20. 6-Hydroxy-3-Phenyl-4-(Pyridin-4-yl)-7-Undecyl-1H-Pyrazolo[3,4-b]Quinoline-5,8 (4H,9H)-Dione (4s)
Following the general procedure described above, in a 5 mL MW tube, 30 mg of embelin (0.1 mmol), 16.4 mg of 4-pyridinecarboxaldehyde (0.15 mmol, 14.4 μL) and 24.4 mg of 3-amino-5-phenylpyrazole (0.15 mmol) were dissolved in 2 mL DCE and treated with 1.8 mg of EDDA (10 mol %). The tube was sealed, and the reaction mixture was irradiated at 150 °C for 10 min. Then, the reaction mixture was filtered and the obtained solid was washed with n-hex to yield 33.8 mg (63%) of 4s as an amorphous violet solid. Mp: 275.9–277.0 °C. 1H-NMR (500 MHz, DMSO-d6) δ 0.85 (t, J = 6.9 Hz, 3H, H-11′’’), 1.22 (bs, 16H, H-3′’’-H-10′’’), 1.34 (m, 2H, H-2′’’), 2.26 (t, J = 7.7 Hz, 2H, H-1′’’), 3.10 (bs, 1H, NH), 5.54 (s, 1H, H-4), 7.12 (d, J = 6.0 Hz, 1H, H-2′’ + H-4′’), 7.32 (m, 1H, H-4′), 7.40 (t, J = 7.8 Hz, 2H, H-3′ + H-5′), 7.52 (d, J = 7.2 Hz, 2H, H-2′ + H-6′), 8.29 (d, J = 6.0 Hz, 2H, H-3′’ + H-5′’); 13C-NMR (125 MHz, DMSO-d6) 13.9 (CH3), 21.9 (CH2), 22.0 (CH2), 27.6 (CH2), 28.7 (CH2), 28.8 (CH2), 28.9 (CH2 x 2), 29.0 (CH2 x 2), 31.2 (CH2), 35.6 (CH), 101.3 (C), 106.3 (C), 116.0 (C), 121.9 (C), 122.0 (CH x 2), 123.1 (C), 126.3 (CH x 2), 128.2 (CH), 128.7 (CH x 2), 140.3 (C), 149.1 (CH x 2), 150.2 (C), 150.4 (C), 154.0 (C), 178.5 (C), 181.4 (C); EIMS m/z (%) 524 ([M-H]+,100), 508 (100), 446 (43), 384 (33), 368 (70); HREIMS 524.2786 (calcd for C32H36N4O3 [M+] 524.2787). IR vmax 3433 (O-H), 2920, 2851 (C-H alipha), 1643 (C=O), 1589, 1531, 1481, 1439 (C=C, C=N), 1358 (N-N), 1269, 1142, 1007, 960, 791, 698, 679 cm−1.
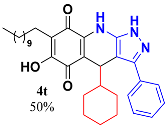
3.21. 4-Cyclohexyl-6-Hydroxy-3-Phenyl-7-Undecyl-1H-Pyrazolo[3,4-b]Quinoline-5,8 (4H,9H)-Dione (4t)
Following the general procedure described above, in a 5 mL MW tube, 30 mg of embelin (0.1 mmol), 17.2 mg of cyclohexanaldehyde (0.15 mmol, 18.5 µL) and 24.4 mg of 3-amino-5-phenylpyrazole (0.15 mmol) were dissolved in 2 mL of DCE and treated with 1.8 mg of EDDA (10 mol %). The tube was sealed, and the reaction mixture was irradiated at 150 °C for 10 min. The crude was purified by Sephadex LH-20 using hex/DCM/MeOH (2:2:1) as eluent to yield 27.2 mg (50%) of 4t as an amorphous blue solid. Mp: 184.6–185.2 °C; 1H-NMR (500 MHz, CDCl3) 0.64 (m, 2H), 0.87 (t, J = 7.1 Hz, 3H, H-11′’’), 0.98 (m, 2H), 1.25 (bs, 18H, H-3′’’-H-10′’’), 1.50 (m, 7H), 2.44 (t, J = 7.5 Hz, 2H), 4.50 (d, J = 3.3 Hz, 1H, H-4), 7.43 (t, J = 7.3 Hz, 1H), 7.49 (t, J = 7.7 Hz, 2H), 7.57 (d, J = 7.6 Hz, 2H, H-2′ + H-6′); 13C-NMR (125 MHz, CDCl3) 14.1 (CH3), 22.6 (CH2), 22.7 (CH2), 26.2 (CH2), 26.4 (CH2), 26.5 (CH2), 28.2 (CH2), 28.4 (CH2), 29.4 (CH2), 29.5 (CH2), 29.6 (CH2), 29.7 (CH2 x 3), 30.4 (CH2), 31.9 (CH2), 35.2 (CH), 46.4 (CH), 101.9 (C), 107.2 (C), 115.8 (C), 125.6 (C), 127.0 (CH x 2), 129.0 (CH), 129.2 (CH x 2), 130.2 (C), 139.9 (C); 143.1 (C), 149.1 (C), 154.0 (C), 179.1 (C), 182.5 (C); EIMS m/z (%) 529 ([M+], 2); 446 (100); 307 (10); 291 (9); HREIMS 529.3300 (calcd for C33H43N3O3 [M+] 529.3304); IR vmax 3265 (O-H), 2923, 2852 (C-H aliphat), 1637 (C=O), 1587, 1572, 1527, 1491, 1439 (C=C, C=N), 1387 (N-N), 1296, 1271, 1243, 1206, 1150, 1121, 1074, 977, 941, 893 cm−1.
3.22. 4-Ethyl-6-Hydroxy-3-Phenyl-7-Undecyl-1H-Pyrazolo[3,4-b]Quinoline-5,8 (4H,9H)-Dione (4u)
Following the general procedure described above, in a 5 mL MW tube, 30 mg of embelin (0.1 mmol), 8.9 mg of propanaldehyde (0.15 mmol, 11.1 µL) and 24.4 mg of 3-amino-5-phenylpyrazole (0.15 mmol) were dissolved in 2 mL of DCE and treated with 1.8 mg of EDDA (10 mol %). The tube was sealed, and the reaction mixture was irradiated at 150 °C for 10 min. The product was purified by Sephadex LH-20 using hex/DCM/MeOH (2:2:1) as eluent mixture to yield 18.3 mg (38%) of 4u as an amorphous blue solid. Mp: 213.4–214.4 °C; 1H-NMR (500 MHz, DMSO-d6) δ 0.46 (t, J = 7.1 Hz, 3H, -CH2CH3), 0.85 (t, J = 7.1 Hz, 3H, H-11′’’), 1.23 (bs, 17H), 1.39 (m, 2H), 1.67 (m, 1H), 2.30 (t, J = 7.7 Hz, 2H), 4.61 (t, J = 4.2 Hz, 1H, H-4), 7.40 (t, J = 7.4 Hz, 1H, H-4′), 7.52 (t, J = 7.9 Hz, 2H, H-3′ + H-5′), 7.65 (d, J = 7.4 Hz, 2H, H-2′ + H-6′); 13C-NMR (125 MHz, DMSO-d6) δ 8.7 (CH3), 13.9 (CH3), 21.9 (CH2), 22.0 (CH2), 27.1 (CH2), 27.6 (CH2), 28.6 (CH2), 28.8 (CH2), 28.9 (CH2 x 4), 30.2 (CH2), 31.2 (CH), 101.3 (C), 106.0 (C), 115.5 (C), 124.7 (C), 126.1 (CH x 2), 128.1 (CH), 128.9 (CH x 2), 137.6 (C), 141.8 (C), 147.5 (C), 155.6 (C), 179.0 (C), 182.06 (C); EIMS m/z (%) 475 ([M+], 3), 446 (100), 318 (8), 306 (7); HREIMS m/z 475.2836 (calcd for C29H37N3O3 [M+] 475.2836); IR vmax 3442 (N-H), 3318 (O-H), 2917, 2849 (C-H-aliphat), 1637, (C=O), 1562, 1526, 1483 (C=C, C=N), 1359 (N-N), 1330, 1270, 1228, 1204, 1146, 1120, 1024, 980, 909, 871, 832 cm−1.
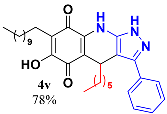
3.23. 4-Heptyl-6-Hydroxy-3-Phenyl-7-Undecyl-1H-Pyrazolo[3,4-b]Quinoline-5,8 (4H,9H)-Dione (4v)
Following the general procedure described above, in a 5 mL MW tube, 30 mg of embelin (0.1 mmol), 17.5 mg of heptanaldehyde (0.15 mmol, 21.4 µL) and 24.4 mg of 3-amino-5-phenylpyrazole (0.15 mmol) were dissolved in 2 mL of DCE and treated with 1.8 mg of EDDA (10 mol %). The tube was sealed, and the reaction mixture was irradiated at 150 °C for 10 min. The product was purified by Sephadex LH-20 using hex/DCM/MeOH (2:2:1) as eluent to yield 42.3 mg (78%) of 4v as an amorphous blue solid. Mp: 172.5–173.2 °C; 1H-NMR (500 MHz, CDCl3) δ 0.73 (t, J = 7.2 Hz, 3H, (CH2)CH3), 0.87 (t, J = 6.9 Hz, 3H, H-11′’’), 0.97 (m, 7H), 1.25 (bs, 16H), 1.50 (m, 2H), 1.63 (m, 2H), 2.44 (t, J = 7.6 Hz, 2H), 4.64 (t, J = 4.2 Hz, 1H, H-4), 7.43 (t, J = 7.1 Hz, 1H, H-4′), 7.50 (t, J = 7.4 Hz, 2H, H-3′ + H-5′), 7.58 (d, J = 7.4 Hz, 2H, H-2′ + H-6′); 13C-NMR (125 MHz, CDCl3) δ 14.0 (CH3), 14.1 (CH3), 22.5 (CH2), 22.6 (CH2), 22.7 (CH2), 24.9 (CH2), 28.2 (CH2), 29.2 (CH2), 29.4 (CH2), 29.5 (CH2), 29.6 (CH2), 29.7 (CH2 x 2), 29.7 (CH2), 30.0 (CH2), 31.6 (CH2), 31.9 (CH2), 35.5 (CH), 103.5 (C), 107.3 (C), 115.8 (C), 126.6 (CH x 2), 129.0 (CH), 129.3 (CH x 2), 129.5 (CH), 139.4 (C), 142.5 (C), 147.9 (C), 154.1 (C), 178.8 (C), 181.41 (C); EIMS m/z (%) 531 ([M+], 3), 474 (5), 446 (100), 307 (7); HREIMS 531.3461 (calcd for C33H45N3O3 [M+] 531.3461); IR 3440 (N-H), 3298 (O-H), 2922, 2853 (C-H aliph), 1639 (C=O), 1526, 1483, 1438 (C=N, C=C), 1378 (N-N), 1268, 1232, 1205, 1147, 1120, 1072, 1014, 977, 870, 823 cm−1.
3.24. 4-(Tert-Butyl)-6-Hydroxy-3-Phenyl-7-Undecyl-1H-Pyrazolo[3,4-b]Quinoline-5,8 (4H,9H)-Dione (4w)
Following the general procedure described above, in a 5 mL MW tube, 30 mg of embelin (0.1 mmol), 13.2 mg of pyvalaldehyde (0.15 mmol, 18.5 µL) and 24.4 mg of 3-amino-5-phenylpyrazole (0.15 mmol) were dissolved in 2 mL of DCE and treated with 1.8 mg of EDDA (10 mol %). The tube was sealed, and the reaction mixture was irradiated at 150 °C for 10 min. The product was purified by Sephadex LH-20 using hex/DCM/MeOH (2:2:1) as eluent to yield 24.3 mg (47%) of 4w as an amorphous blue solid. Mp: 160.1–160.9 °C; 1H-NMR (500 MHz, CDCl3) δ 0.87 (t, J = 7.1 Hz, 3H, H-11′’’), 0.93 (s, 9H, -C(CH3)3), 1.25 (bs, 16H, H-4′’’-H-10′’’), 1.49 (m, 2H, H-3′’’), 2.45 (t, J = 7.8 Hz, 2H, H-1′’’), 5.41 (s, 1H, H-4), 6.20 (bs, 1H, OH), 7.32 (t, J = 7.3 Hz, 1H, H-4′), 7.40 (t, J = 7.7 Hz, 2H, H-3′ + H-5′), 7.80 (d, J = 7.3 Hz, 2H, H-2′ + H-6′); 13C-NMR (125 MHz, CDCl3) δ 14.1 (CH3), 22.6 (CH2), 22.7 (CH2), 27.1 (CH3 x 3), 27.6 (CH2), 28.1 (CH2), 29.3 (CH2), 29.4 (CH2), 29.6 (CH2 x 2), 29.7 (CH2), 31.9 (CH2), 40.7 (CH), 61.9 (C), 87.7 (C), 104.3 (C), 116.6 (C), 125.6 (CH x 2), 128.0 (CH), 128.6 (CH x 2), 133.2 (C), 138.0 (C), 139.6 (C) 150.9 (C), 154.0 (C), 178.9 (C), 180.9 (C); EIMS m/z (%) 446 ([M+-C4H9], 100), 418 (4), 307 (15), 276 (5); HRMS: 446.2423 (calcd for C27H32N3O3 [M+-C4H9] 446.2444); IR vmax 3310 (N-H), 3223 (O-H), 2955, 2916, 2850 (C-H aliph), 1635 (C=O), 1556, 1519, 1497, 1464, 1428 (C=C, C=N), 1359 (N-N), 1305, 1264, 1223, 1184, 1119, 1073, 1026, 996, 956, 916, 882, 841 cm−1.
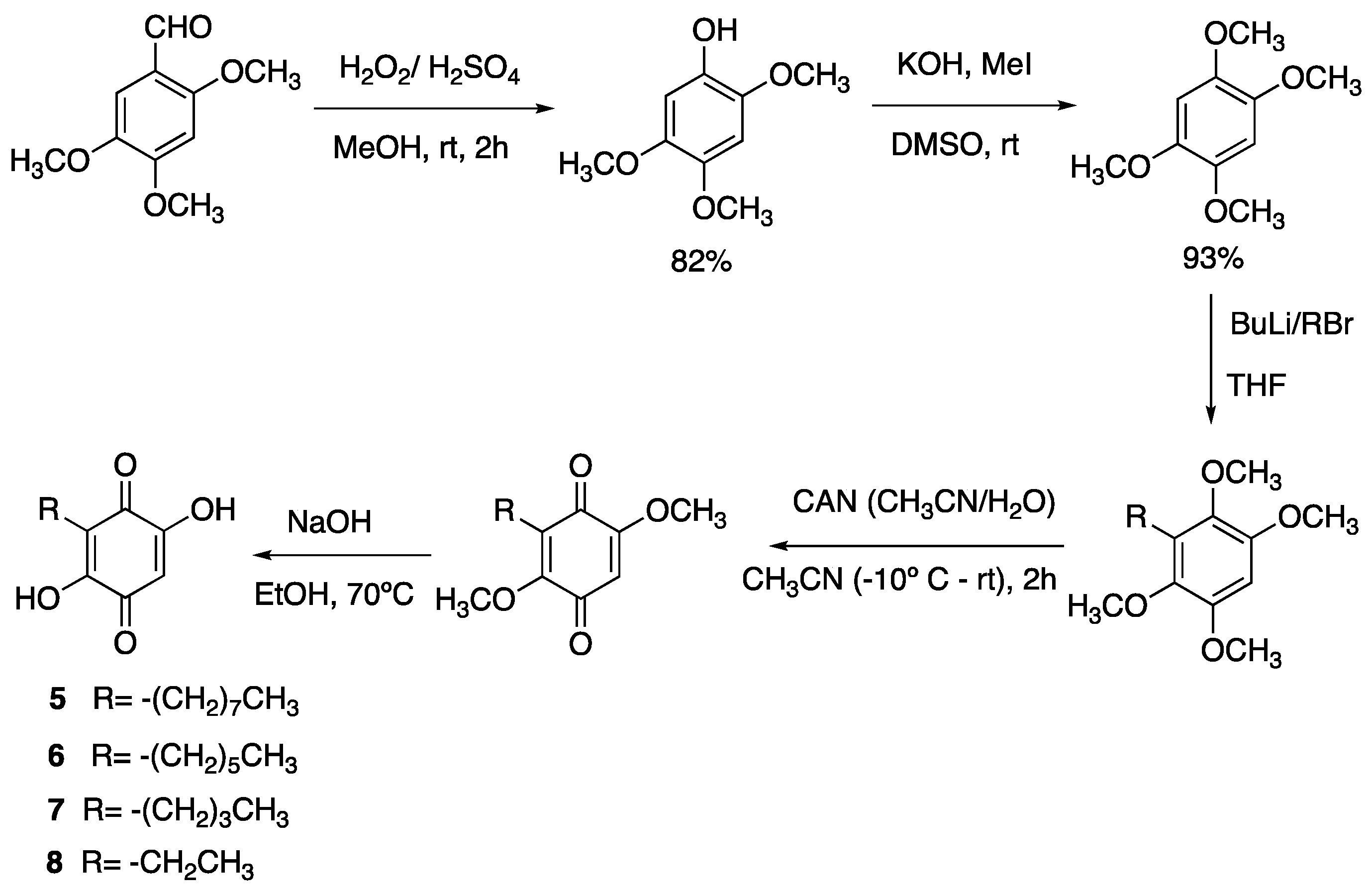
3.25. 6-Hydroxy-7-Octyl-3-Phenyl-4-(4-(Trifluoromethyl)Phenyl)-1H-Pyrazolo[3,4-b]Quinoline-5,8 (4H,9H)-Dione (9)
Following the general procedure described above, in a 5 mL MW tube, 30 mg of 2,5-dihydroxy-3-octylcyclohexa-2,5-diene-1,4-dione (0.12 mmol), 31.1 mg of 4-(trifluoromethyl)-benzaldehyde (0.18 mmol, 24.4 µL) and 28.4 mg of 3-amino-5-phenylpyrazole (0.18 mmol) were dissolved in 2 mL of DCE and treated with 2.1 mg of EDDA (10 mol % m). The tube was sealed, and the reaction mixture was irradiated at 150 °C for 10 min. The product was purified by filtration and washed with n-hex to yield 28.5 mg (43%) of 9 as an amorphous blue solid. Mp: 243.7–244.4 °C; 1H-NMR (500 MHz, CDCl3) 0.85 (t, J = 7.1 Hz, 3H, H-8′’’), 1.25 (m, 10H, H-3′’’-H-7′’’), 1.45 (m, 2H, H-2′’’), 2.41 (t, J = 7.8 Hz, 2H, H-1′’’), 5.58 (s, 1H, H-4), 7.30 (m, 2H, H-3′’ + H-5′’), 7.39 (m, 4H), 7.37 (d, J = 8.1 Hz, 2H, H-2′’ + H-6′’); 13C-NMR (125 MHz, CDCl3) δ 14.1 (CH3), 22.6 (CH2), 22.7 (CH2), 28.1 (CH2), 29.2 (CH2), 29.4 (CH2), 29.7 (CH2), 31.9 (CH2), 36.0 (CH), 103.3 (C), 107.2 (C), 116.1 (C), 116.3 (C), 123.2 (C), 125.2 (CH x 2, J = 2.8 Hz), 126.7 (CH x 2), 128.3 (C), 128.4 (CH x 2), 128.2 (C, J = 31.2 Hz), 129.1 (CH x 2), 129.3 (CH), 140.2(C), 141.0(C), 147.3(C), 149.0(C), 154.1(C), 178.6 (C), 182.3 (C); EIMS m/z (%) 549 ([M+], 90), 450 (17), 404 (100), 306 (7); HREIMS 549.2214 (calcd for C31H30N3O3F3 [M+] 549.2239); IR vmax 3433 (N-H), 3251 (O-H), 2927, 2854 (C-H aliph), 1643 (C=O), 1570, 1504 (C=N, C=C), 1354 (N-N), 1323, 1274, 1161, 1118, 1064, 1018, 833, 690 cm−1.
3.26. 7-Hexyl-6-Hydroxy-3-Phenyl-4-(4-(Trifluoromethyl)Phenyl)-1H-Pyrazolo[3,4-b]Quinoline-5,8 (4H,9H)-Dione (10)
Following the general procedure described above, in a 5 mL MW tube, 30 mg of 3-hexyl-2,5-dihydroxycyclohexa-2,5-diene-1,4-dione (0.134 mmol), 35 mg of 4-(trifluoromethyl)-benzaldehyde (0.20 mmol, 27.4 µL) and 32 mg of 3-amino-5-phenylpyrazole (0.20 mmol) were dissolved in 2 mL DCE and treated with 2.4 mg of EDDA (10 mol %). The tube was sealed, and the reaction mixture was irradiated at 150 °C for 10 min. The product was purified by filtration and washed with n-hex to yield 35.6 mg (51%) of 10 as an amorphous blue solid. Mp: 239.9–240.7 °C; 1H-NMR (500 MHz, CDCl3) δ 0.86 (t, J = 7.2 Hz, 3H, H-6′’’), 1.29 (m, 6H, H-3′’’-H-5′’’), 1.46 (m, 2H, H-2′’’), 2.41 (t, J = 7.5 Hz, 2H, H-1′’’), 5.58 (s, 1H, H-4), 7.30 (m, 2H), 7.39 (m, 4H), 7.38 (m, 5H), 7.44 (d, J = 8.3 Hz, 2H); 13C-NMR (125 MHz, CDCl3) δ 14.1 (CH3), 22.6 (CH2 x 2), 28.0 (CH2), 29.3 (CH2), 31.6 (CH2), 36.0 (CH), 103.3 (C), 107.2 (C), 116.3 (C), 123.9 (C, J = 271.6 Hz), 125.3 (CH x 2, J = 3.7 Hz), 125.9 (C), 126.7 (CH x 2), 128.3 (C), 128.4 (CH x 2), 128.8 (C, J = 31.2 Hz), 129.1 (CH x 2), 129.3 (CH), 140.2 (C), 141.0 (C), 147.2 (C), 148.9 (C), 154.0 (C), 178.6 (C), 182.3 (C); EIMS m/z (%) 521 ([M+], 1); 423 (29); 359 (100); 303 (62); 301 (69); 289 (47); HREIMS 493.1601 (calcd for C27H22N3O3F3 [M+] 493.1613); IR vmax 3435 (N-H), 3260 (O-H), 2928, 2858 (C-H aliph), 1643 (C=O), 1569, 1504 (C=C, C=N), 1385 (N-N), 1323, 1269, 1165, 1122, 1065, 979, 833, 694 cm−1.
3.27. 7-Butyl-6-Hydroxy-3-Phenyl-4-(4-(Trifluoromethyl)Phenyl)-1H-Pyrazolo[3,4-b]Quinoline-5,8 (4H,9H)-Dione (11)
Following the general procedure described above, in a 5 mL MW tube, 30 mg of 3-butyl-2,5-dihydroxycyclohexa-2,5-diene-1,4-dione (0.153 mmol), 40 mg of 4-(trifluromethyl)-benzaldehyde (0.23 mmol, 31.3 µL) and 36.5 mg of 3-amino-5-phenylpyrazole (0.23 mmol) were dissolved in 2 mL DCE and treated with 2.8 mg of EDDA (10 mol %). The tube was sealed, and the reaction mixture was irradiated at 150 °C for 10 min. The product was purified by filtration and washed with n-hex to yield 49.7 mg (67%) of compound 11 as an amorphous violet solid. Mp: 257.2–258.6 °C; 1H-NMR (500 MHz, DMSO-d6) δ 0.87 (t, J = 7.2 Hz, 3H, H-4′’’), 1.27 (m, 2H, H-3′’’), 1.34 (m, 2H, H-2′’’), 2.27 (t, J = 7.5 Hz, 2H, H-1′’’), 5.63 (s, 1H, H-4), 5.76 (s, 1H, OH), 7.31 (m, 1H), 7.39 (m, 4H), 7.49 (d, J = 8.4 Hz, 2H, H-2′’ + H-6′’), 7.53 (d, J = 7.4 Hz, 2H, H-3′’ + H-5′’); 13C-NMR (125 MHz, CDCl3) δ 14.3 (CH3), 22.2 (CH2), 22.7 (CH2), 30.4 (CH2), 36.4 (CH), 107.5 (C), 116.2 (C), 123.8 (C), 125.2 (CH x 2, J = 3.2 Hz), 125.6 (C), 126.7 (CH x 2), 127.1 (C, J = 32.3 Hz), 127.4 (C), 128.7 (CH), 129.1 (CH x 2), 129.2 (CH x 2), 179.1 (C), 181.7 (C); EIMS m/z (%) 493 ([M+], 31), 450 (12), 348 (100), 306 (9); HREIMS 493.1601 (calcd for C27H22N3O3F3 [M+] 493.1613); IR vmax 3441 (N-H), 3356 (O-H), 2967, 2932, 2870 (C-H aliph), 1636 (C=O), 1566, 1527, 1493 (C=C, C=N), 1327 (N-N), 1296, 1204, 1165, 1111, 1068, 980, 930, 833, 690, 660 cm−1.
3.28. 7-Ethyl-6-Hydroxy-3-Phenyl-4-(4-(Trifluoromethyl)Phenyl)-1H-Pyrazolo[3,4-b]Quinoline-5,8 (4H,9H)-Dione (12)
Following the general procedure described above, in a 5 mL MW tube, 30 mg of 3-ethyl-2,5-dihydroxycyclohexa-2,5-diene-1,4-dione (0.18 mmol), 46.6 mg of 4-(trifluromethyl)-benzaldehyde (0.27 mmol, 36.5 µL) and 42.6 mg of 3-amino-5-phenylpyrazole (0.27 mmol) were dissolved in 2 mL DCE and treated with 3.2 mg of EDDA (10 mol %). The tube was sealed, and the reaction mixture was irradiated at 150 °C for 10 min. The product was purified by filtration and washed with n-hex to yield 27.9 mg (34%) of compound 12 as an amorphous blue solid. Mp: 175.2–177.0 °C; 1H-NMR (500 MHz, DMSO-d6) δ 0.95 (t, J = 7.2 Hz, 3H, H-2′’’), 2.28 (q, J = 7.2 Hz, 2H, H-1′’’), 5.60 (s, 1H, H-4), 5.76 (s, 1H, OH), 7.31 (m, 1H), 7.39 (m, 4H), 7.49 (d, J = 8.2 Hz, 2H, H-2′’ + H-6′’), 7.53 (d, J = 7.6 Hz, 2H, H-3′’ + H-5′’); 13C-NMR (125 MHz, CDCl3) δ 12.6 (CH3), 15.3 (CH2), 35.9 (CH), 101.9 (C), 107.1 (C), 117.3 (C), 124.2 (C, J = 271.9 Hz), 124.7 (CH, J = 3.4 Hz), 126.2 (CH x 2), 126.6 (C, J = 31.7 Hz), 127.4 (C), 128.2 (CH), 128.6 (CH x 2), 128.7 (CH x 2), 128.8 (C), 138.5 (C), 140.0 (C), 146.5 (C), 150.4 (C), 155.1 (C), 178.5 (C), 181.5 (C); EIMS m/z (%) 465 ([M+], 34), 321 (21), 320 (100), 292 (5); HREIMS 465.1291 (calcd for C25H18N3O3F3 [M+] 465.1300); IR vmax 3433 (N-H), 3275 (O-H), 2974, 2932 (C=C, C=N), 1643 (C=O), 1589, 1531, 1504 (C=C, C=N), 1346 (N-N), 1318, 1273, 1161, 1111, 1065, 1022, 976, 841, 690 cm−1.
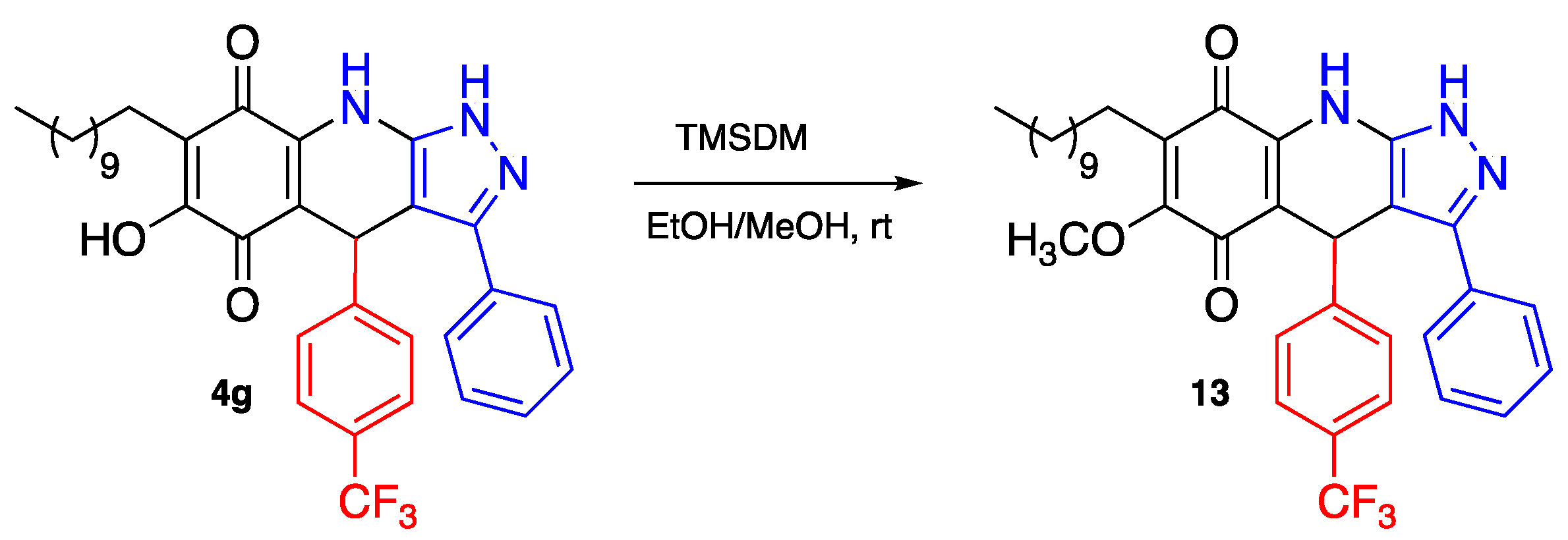
3.29. 6-Methoxy-3-Phenyl-4-(4-(Trifluoromethyl)Phenyl)-7-Undecyl-4,9-Dihydro-1H-Pyrazolo[3,4-b]Quinoline-5,8-Dione (13)
To 15 mg of 4g (0.025 mmol) dissolved in 7.5 mL of a mixture diethyl ether/MeOH (2:1) an excess of trimethylsilyldiazomethane (Me3SiCHN2, 2 equiv) was added. The reaction mixture was stirred at room temperature until disappearance of the starting material (24 h). The solvent was removed under reduced pressure and the product 13 was quantitatively obtained without further purifications (15.1 mg, 100%). Mp: 241.8–242.5 °C. 1H-NMR (500 MHz, DMSO-d6) δ 0.84 (t, J = 7.1 Hz, 3H, H-11′’’), 1.22 (bs, 16H, H-3′’’-H-10′’’), 1.34 (m, 2H, H-2′’’), 2.30 (t, J = 7.7 Hz, 2H, H-1′’’), 3.89 (s, 3H, -OCH3), 5.62 (s, 1H, H-4), 7.34 (m, 3H), 7.40 (t, J = 7.7 Hz, 2H, H-2′’-H-6′’), 7.49 (d, J = 8.1 Hz, 2H), 7.53 (d, J = 7.5 Hz, 2H, H-3′’-H-5′’); 13C-NMR (125 MHz, DMSO-d6) δ 13.9 (CH3), 22.0 (CH2), 22.3 (CH2), 28.0 (CH2), 28.6 (CH2), 28.7 (CH2), 28.8 (CH2), 28.9 (CH2 x 3), 31.2 (CH2), 35.7 (CH), 61.3 (CH3), 101.9 (C), 109.2 (C), 113.6 (C), 124.1 (C, JC-F = 273.4 Hz), 124.9 (CH x 2, JC-F = 3.5 Hz), 126.2 (CH x 2), 126.6 (C, JC-F = 31.4 Hz), 127.3 (C), 128.2 (CH), 128.3 (CH x 2), 128.8 (CH x 2), 129.3 (C), 138.9 (C), 146.9 (C), 150.5 (C), 156.9 (C), 179.3 (C), 182.7 (C); EIMS m/z (%) 605 ([M+], 56), 474 (20), 460 (100), 432 (14); HREIMS 605.2889 (calcd for C35H38N3O3F3 [M+] 605.2865).
3.30. 3-(4-Chlorophenyl)-6-Hydroxy-4-(4-(Trifluoromethyl)Phenyl)-7-Undecyl-4,9-Dihydro-1H-Pyrazolo[3,4-b]Quinoline-5,8-Dione (14a)
Following the general procedure, in a 5 mL MW tube, 30 mg of embelin (0.1 mmol), 21.3 μL of 4-(trifluoromethyl)benzaldehyde (0.15 mmol) and 27.5 mg of 3-(4-chlorophenyl)-1H-pyrazol-5-amine (0.15 mmol) were dissolved in 2 mL of DCE and treated with 1.8 mg of EDDA (10 mol %). The tube was sealed, and the reaction mixture was irradiated at 150 °C for 10 min. The product was purified by filtration and washed with n-hex to yield 51.6 mg (83%) of 14a as an amorphous violet solid. Mp: 231.6–233.0 °C; 1H-NMR (500 MHz, DMSO-d6) δ 0.84 (t, J = 6.8 Hz, 3H, H-11′’’), 1.21 (bs, 16H, H-3′’’-H-10′’’), 1.33 (m, 2H, H-2′’’), 2.25 (t, J = 7.4 Hz, 2H, H-1′’’), 5.61 (s, 1H, H-4), 7.36 (d, J = 8.0 Hz, 2H, H-2′’ + H-6′’), 7.45 (d, J = 7.1 Hz, 2H, H-2′ + H-6′), 7.48 (d, J = 8.0 Hz, 2H, H-3′’ + H-5′’), 7.56 (d, J = 8.5 Hz, 2H, H-3′ + H-5′); 13C-NMR (125 MHz, DMSO-d6) δ 14.4 (CH3), 22.5 (CH2), 22.6 (CH2), 28.2 (CH2), 29.2 (CH2), 29.4 (CH2 x 2), 29.5 (CH2 x 2), 29.6 (CH2), 31.7 (CH2), 36.3 (CH), 102.8 (C), 107.5 (C), 115.9 (C), 123.8 (C), 125.3 (CH x 2, JC-F = 3.4 Hz), 125.6 (C, JC-F = 273.4 Hz), 127.1 (C, JC-F = 31.8 Hz), 128.5 (CH x 2), 129.1 (CH x 2), 129.2 (C); 129.3 (CH x 2), 133.3 (C), 137.8 (C), 140.9 (C), 147.0 (C), 150.9 (C), 179.5 (C), 180.7 (C); EIMS m/z (%) 624 ([M+], 100), 606 (15), 590 (18), 478 (11); HREIMS 624.2239 (calcd for C34H34N3O3F335Cl [M+] 624.2241), 626.2224 (calcd for C34H34N3O3F337Cl [M+] 626.2211).
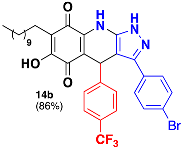
3.31. 3-(4-Bromophenyl)-6-Hydroxy-4-(4-(Trifluoromethyl)Phenyl)-7-Undecyl-4,9-Dihydro-1H–Pyrazolo[3,4-b]Quinoline-5,8-Dione (14b)
Following the general procedure, in a 5 mL MW tube, 30 mg of embelin (0.1 mmol), 21.3 μL of 4-(trifluoromethyl)benzaldehyde (0.15 mmol) and 36.4 mg of 3-(4-bromophenyl)-1H-pyrazol-5-amine (0.15 mmol) were dissolved in 2 mL of DCE and treated with 1.8 mg of EDDA (10 mol %). The tube was sealed, and the reaction mixture was irradiated at 150 °C for 10 min. The product was purified by filtration and washed with n-hex to yield 58 mg (86%) of 14b as an amorphous violet solid. Mp: 227.6–229.0 °C. 1H-NMR (500 MHz, DMSO-d6) δ 0.84 (t, J = 7.0 Hz, 3H, H-11′’’), 1.21 (bs, 16H, H-3′’’-H-10′’’), 1.34 (m, 2H, H-2′’), 2.26 (t, J = 7.7 Hz, 2H, H-1′’), 5.63 (s, 1H, H-4), 7.37 (d, J = 7.9 Hz, 2H), 7.48 (m, 4H), 7.59 (d, J = 8.6 Hz, 2H); 13C-NMR (125 MHz, DMSO-d6) δ 14.4 (CH3), 22.4 (CH2), 22.5 (CH2), 28.1 (CH2), 29.2 (CH2), 29.3 (CH2), 29.4 (CH2 x 2), 29.5 (CH2 x 2), 31.7 (CH2), 36.3 (CH), 102.9 (C), 107.6 (C), 116.5 (C), 122.8 (C, JC-F = 271.6 Hz), 125.2 (CH x 2, JC-F = 3.6 Hz), 125.5 (C), 126.7 (C), 127.2 (C, JC-F = 30.5 Hz), 128.7 (CH x 2), 129.1 (CH x 2), 132.2 (CH x 2), 137.7 (C), 140.5 (C), 147.2 (C), 150.8 (C), 156.2 (C), 179.0 (C), 182.0 (C).
3.32. 3-(4-Fluorophenyl)-6-Hydroxy-4-(4-(Trifluoromethyl)Phenyl)-7-Undecyl-4,9-Dihydro-1H-Pyrazolo[3,4-b]Quinoline-5,8-Dione (14c)
Following the general procedure, in a 5 mL MW tube, 30 mg of embelin (0.1 mmol), 21.3 μL of 4-(trifluoromethyl)benzaldehyde (0.15 mmol) and 27.1 mg of 3-(4-fluorophenyl)-1H-pyrazol-5-amine (0.15 mmol) were dissolved in 2 mL of DCE and treated with 1.8 mg of EDDA (10 mol %). The tube was sealed, and the reaction mixture was irradiated at 150 °C for 10 min. The product was purified by filtration and washed with n-Hex to yield 52.1 mg (85%) of 14c as an amorphous violet solid. Mp: 228.2–229.8 °C; 1H-NMR (500 MHz, DMSO-d6) δ 0.83 (t, J = 7.2 Hz, 3H, H-11′’’), 1.21 (bs, 16H, H-3′’’-H-10′’’), 1.34 (m, 2H, H-2′’’), 2.26 (t, J = 7.8 Hz, 2H, H-1′’’), 5.62 (s, 1H, H-4), 7.23 (t, J = 8.4 Hz, 2H, H-3′’ + H-5′’), 7.35 (d, J = 7.8 Hz, 2H, H-3′ + H-5′), 7.48 (d, J = 7.8 Hz, 2H, H-2′ + H-6′), 7.57 (m, 2H, H-2′’ + H-6′’); 13C-NMR (125 MHz, DMSO-d6) δ 14.3 (CH3), 22.4 (CH2), 22.5 (CH2), 28.1 (CH2), 29.1 (CH2), 29.2 (CH2), 29.3 (CH2), 29.4 (CH2), 29.5 (CH2 x 2), 31.7 (CH2), 36.3 (CH), 102.5 (C), 107.5 (C), 116.2 (CH x 2, JC-F = 21.1 Hz), 116.5 (C), 125.3 (CH x 2, JC-F = 2.2 Hz), 127.3 (C), 129.0 (CH x 4), 129.1 (C), 140.5 (C), 142.5 (C), 143.8 (C), 150.9 (C), 156.1 (C), 161.5 (C), 163.1 (C), 178.9 (C), 182.1 (C); EIMS m/z (%) 609 ([M+], 93), 668 (56), 464 (100), 323 (39). HREIMS 609.2632 (calcd for C34H35N3O3F4 [M+] 609.2615).
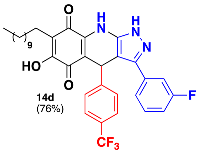
3.33. 3-(3-Fluorophenyl)-6-Hydroxy-4-(4-(Trifluoromethyl)Phenyl)-7-Undecyl-4,9-Dihydro-1H-Pyrazolo[3,4-b]Quinoline-5,8-Dione (14d)
Following the general procedure, in a 5 mL MW tube, 30 mg of embelin (0.1 mmol), 21.3 μL of 4-(trifluoromethyl)benzaldehyde (0.15 mmol) and 27.1 mg of 3-(3-fluorophenyl)-1H-pyrazol-5-amine (0.15 mmol) were dissolved in 2 mL of DCE and treated with 1.8 mg of EDDA (10 mol %). The tube was sealed and the reaction mixture was irradiated at 150 °C for 10 min. The product was purified by filtration and washed with n-hex to yield 46.6 mg (76%) of 14d as an amorphous violet solid. Mp: 213.8–215.0 °C. 1H-NMR (500 MHz, DMSO-d6) δ 0.83 (t, J = 7.1 Hz, 3H, H-11′’’), 1.21 (bs, 16H, H-3′’’-H-10′’’), 1.31 (m, 2H, H-2′’’), 2.23 (t, J = 7.7 Hz, 2H, H-1′’’), 5.61 (s, 1H, H-4), 7.14 (td, J = 2.3, 8.6 Hz, 1H), 7.33 (dt, J = 2.2, 10.7 Hz, 1H), 7.36 (m, 3H), 7.43 (m, 1H), 7.50 (d, J = 8.4 Hz, 2H, H-3′’ + H-5′’); 13C-NMR (125 MHz, DMSO-d6) δ 13.8 (CH3), 21.0 (CH2), 22.0 (CH2), 27.8 (CH2), 28.6 (CH2), 28.8 (CH2), 28.9 (CH2 x 2), 29.0 (CH2 x 2), 31.2 (CH2), 35.8 (CH), 102.4 (C), 106.9 (C), 112.8 (CH, JC-F = 22.6 Hz), 114.9 (CH, JC-F = 21.5 Hz), 118.8 (C, JC-F = 14.8 Hz), 115.1 (C), 122.4 (CH x 2, JC-F = 2.6 Hz), 124.1 (C, JC-F = 272.3 Hz), 124.8 (CH, JC-F = 4.4 Hz), 126.6 (C, JC-F = 31.3 Hz), 128.6 (CH x 2), 129.0 (C), 130.8 (CH, JC-F = 8.5 Hz), 140.5 (C), 150.4 (C), 161.1 (C), 163.0 (C), 171.9 (C), 179.3 (C), 179.7 (C); EIMS m/z (%) 609 ([M+], 100), 468 (38), 464 (96), 324 (19); HREIMS 609.2596 (calcd for C34H35N3O3F4 [M+] 609.2615).
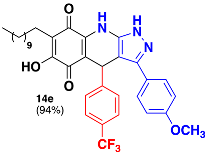
3.34. 6-Hydroxy-3-(4-Methoxyphenyl)-4-(4-(Trifluoromethyl)Phenyl)-7-Undecyl-4,9-Dihydro-1H-Pyrazolo[3,4-b]Quinoline-5,8-Dione (14e)
Following the general procedure, in a 5 mL MW tube, 30 mg of embelin (0.1 mmol), 21.3 μL of 4-(trifluoromethyl)benzaldehyde (0.15 mmol) and 28.9 mg of 3-(4-methoxyphenyl)-1H-pyrazol-5-amine (0.15 mmol) were dissolved in 2 mL of DCE and treated with 1.8 mg of EDDA (10 mol %). The tube was sealed and the reaction mixture was irradiated at 150 °C for 10 min. The product was purified by filtration and washed with n-hex to yield 58.6 mg (94%) of 14e as an amorphous violet solid. Mp: 206.6–207.5 °C; 1H-NMR (500 MHz, DMSO-d6) δ 0.84 (t, J = 7.5 Hz, 3H, H-11′’’), 1.21 (bs, 16H, H-3′’’-H-10′’’), 1.33 (m, 2H, H-2′’’), 2.25 (t, J = 7.6 Hz, 2H, H-1′’’), 3.75 (s, 3H, OCH3), 5.57 (s, 1H, H-4), 6.95 (d, J = 8.5 Hz, 2H, H-3′ + H-5′), 7.36 (d, J = 8.0 Hz, 2H, H-2′’ + H-6′’), 7.46 (d, J = 8.9 Hz, 2H, H-3′’ + H-5′’), 7.50 (d, J = 8.5 Hz, 2H, H-2′ + H-6′); 13C-NMR (125 MHz, DMSO-d6) δ 14.4 (CH3), 22.5 (CH2 x 2), 28.2 (CH2), 29.2 (CH2), 29.3 (CH2), 29.4 (CH2 x 2), 29.5 (CH2 x 2), 31.7 (CH2), 36.3 (CH), 55.6 (CH3), 101.7 (C), 107.5 (C), 114.7 (CH x 2), 116.0 (C), 121.8 (C), 124.7 (C, JC-F = 272.9 Hz), 125.3 (CH x 2, JC-F = 3.2 Hz), 126.5 (C), 127.1 (C, JC-F = 31.5 Hz), 128.1 (CH x 2), 129.0 (CH x 2), 139.1 (C); 140.8 (C), 147.0 (C), 151.1 (C), 159.6 (C), 179.2 (C), 181.3 (C); EIMS m/z (%) 621 ([M+], 65), 480 (21), 476 (100), 345 (30); HREIMS 621.2825 (calcd for C35H38N3O4F3 [M+] 621.2814).
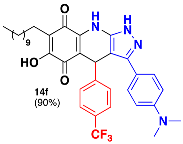
3.35. 3-(4-(Dimethylamino)Phenyl)-6-Hydroxy-4-(4-(Trifluoromethyl)Phenyl)-7-Undecyl-4,9-Dihydro-1H-Pyrazolo[3,4-b]Quinoline-5,8-Dione (14f)
Following the general procedure, in a 5 mL MW tube, 30 mg of embelin (0.1 mmol), 21.3 μL of 4-(trifluoromethyl)benzaldehyde (0.15 mmol) and 30.9 mg of 3-(4-(dimethylamino)phenyl)-1H-pyrazol-5-amine (0.15 mmol) were dissolved in 2 mL of DCE and treated with 1.8 mg of EDDA (10 mol %). The tube was sealed, and the reaction mixture was irradiated at 150 °C for 10 min. The product was purified by filtration and washed with n-hex to yield 56.9 mg (90%) of 14f as an amorphous violet solid. Mp: 242.4–244.0 °C; 1H-NMR (500 MHz, DMSO-d6) δ 0.84 (t, J = 7.5 Hz, 3H, H-11′’’), 1.21 (bs, 16H, H-3′’’-H-10′’’), 1.33 (m, 2H, H-2′’’), 2.25 (t, J = 7.6 Hz, 2H, H-1′’’), 3.75 (s, 6H, -N(CH3)2), 5.57 (s, 1H, H-4), 6.95 (d, J = 8.5 Hz, 2H, H-3′ + H-5′), 7.36 (d, J = 8.0 Hz, 2H, H-2′’ + H-6′’), 7.46 (d, J = 8.9 Hz, 2H, H-3′’ + H-5′’), 7.50 (d, J = 8.5 Hz, 2H, H-2′ + H-6′); 13C-NMR (125 MHz, DMSO-d6) δ 13.9 (CH3), 21.9 (CH2), 22.0 (CH2), 27.6 (CH2), 28.6 (CH2), 28.8 (CH2), 28.9 (CH2 x 2), 29.0 (CH2 x 2), 31.2 (CH2), 35.9 (CH), 40.2 (CH3 x 2), 100.2 (C), 107.1 (C), 118.8 (CH x 2), 115.8 (C), 116.3 (C), 124.2 (C, JC-F = 274.9 Hz), 124.8 (CH x 2, JC-F = 3.7 Hz), 126.6 (C, JC-F = 30.7 Hz), 126.9 (CH x 2), 128.6 (CH x 2), 138.9 (C), 140.0 (C), 146.6 (C), 149.9 (C), 150.7 (C), 156.1 (C), 178.3 (C), 181.5 (C); EIMS m/z (%) 634 ([M+], 83), 493 (16), 489 (100), 347 (10); HREIMS 634.3113 (calcd for C36H41N4O3F3 [M+] 634.3131).
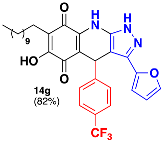
3.36. 3-(Furan-2-yl)-6-Hydroxy-4-(4-(Trifluoromethyl)Phenyl)-7-Undecyl-4,9-Dihydro-1H-Pyrazolo[3,4-b]Quinoline-5,8-Dione (14g)
Following the general procedure, in a 5 mL MW tube, 30 mg of embelin (0.1 mmol), 21.3 μL of 4-(trifluoromethyl)benzaldehyde (0.15 mmol) and 22.8 mg of 3-(furan-2-yl)-1H-pyrazol-5-amine (0.15 mmol) were dissolved in 2 mL of DCE and treated with 1.8 mg of EDDA (10 mol %). The tube was sealed, and the reaction mixture was irradiated at 150 °C for 10 min. The product was purified by filtration and washed with n-hex to yield 48.8 mg (82%) of 14g as an amorphous violet solid. Mp: 165.0–166.8 °C. 1H-NMR (500 MHz, DMSO-d6) δ 0.83 (t, J = 6.2 Hz, 3H, H-11′’’), 1.21 (bs, 16H, H-3′’’-H-10′’’), 1.33 (m, 2H, H-2′’’), 2.26 (t, J = 7.3 Hz, 2H, H-1′’’), 5.49 (s, 1H, H-4), 6.53 (bs, 1H, H-4′), 6.63 (bs, 1H, H-5′), 7.46 (d, J = 7.3 Hz, 2H, H-2′’ + H-6′’), 7.54 (d, J = 7.6 Hz, 2H, H-3′’ + H-5′’), 7.74 (s, 1H, H-3′); 13C-NMR (125 MHz, DMSO-d6) δ 13.9 (CH3), 22.0 (CH2 x 2), 27.6 (CH2), 28.6 (CH2), 28.8 (CH2), 28.9 (CH2 x 2), 29.0 (CH2 x 2), 31.2 (CH2), 35.7 (CH), 101.5 (C), 107.1 (C), 107.6 (CH), 111.7 (CH), 115.7 (C), 124.1 (C, JC-F = 272.6 Hz), 124.7 (CH x 2, JC-F = 3.6 Hz), 126.6 (C, JC-F = 31.5 Hz), 128.7 (CH x 2), 140.3 (C), 143.1 (CH), 143.8 (C), 146.2 (C), 150.0 (C), 150.8 (C); 156.9 (C), 178.9 (C), 180.6 (C); EIMS m/z (%) 581 ([M+], 89), 439 (44), 435 (100), 295 (23); HREIMS 581.2491 (calcd for C32H34N3O4F3 [M+] 581.2501).
3.37. 6-Hydroxy-3-Methyl-4-(4-(Trifluoromethyl-6-Hydroxy-3-Methyl-4-(4-(Trifluoromethyl) Phenyl)-7-Undecyl-4,9-Dihydro-1H-Pyrazolo[3,4-b]Quinoline-5,8-Dione (14h)
Following the general procedure, in a 5 mL MW tube, 30 mg of embelin (0.1 mmol), 21.3 μL of 4-(trifluoromethyl)benzaldehyde (0.15 mmol) and 14.9 mg of 3-methyl-1H-pyrazol-5-amine (0.15 mmol) were dissolved in 2 mL of DCE and treated with 1.8 mg of EDDA (10 mol %). The tube was sealed, and the reaction mixture was irradiated at 150 °C for 10 min. The product was purified by filtration and washed with n-hex to yield 41.4 mg (78%) of 14h as an amorphous violet solid. Mp: 249.8–250.9 °C. 1H-NMR (500 MHz, DMSO-d6) δ 0.83 (t, J = 6.6 Hz, 3H, H-11′’’), 1.20 (bs, 16H, H-3′’’-H-10′’’), 1.33 (m, 2H, H-2′’’), 1.88 (s, 3H, CH3), 2.25 (t, J = 6.8 Hz, 2H, H-1′’’), 5.24 (s, 1H, H-4), 7.43 (d, J = 7.6 Hz, 2H, H-2′’ + H-6′’), 7.58 (d, J = 7.6 Hz, 2H, H-3′’ + H-5′’); 13C-NMR (125 MHz, DMSO-d6) δ 9.4 (CH3), 13.9 (CH3), 21.9 (CH2), 22.0 (CH2), 27.6 (CH2), 28.7 (CH2), 28.8 (CH2), 28.9 (CH2 x 2), 29.0 (CH2 x 2), 31.2 (CH2), 35.6 (CH), 102.7 (C), 106.5 (C), 115.8 (C), 124.2 (C, JC-F = 272.9 Hz), 124.9 (CH x 2, JC-F = 3.3 Hz), 126.5 (C, JC-F = 31.8 Hz), 128.3 (CH x 2), 135.9 (C), 140.8 (C), 144.9 (C), 151.3 (C), 155.9 (C); 178.6 (C), 181.6 (C); EIMS m/z (%) 529 ([M+], 100), 388 (47), 384 (86), 244 (22); HREIMS 529.2531 (calcd for C29H34N3O3F3 [M+] 529.2552).
3.38. 6-Hydroxy-3-Phenyl-4-(4-(Trifluoromethyl)Phenyl)-7-Undecyl-1H-Pyrazolo[3,4-b]Quinoline-5,8-Dione (15)
To 15 mg of compound 4g in 3 mL of DCM 9.6 mg of DDQ (1 equiv) was added at room temperature. The reaction mixture was stirred until the disappearance of the starting material, then it was washed with a solution of saturated NaHCO3 and extracted with DCM. The organic layers were dried over anhydrous MgSO4 and filtered. The solvent was removed under reduced pressure to yield 15.4 mg (82%) of compound 15 as an amorphous orange oil. 1H-NMR (500 MHz, CDCl3) δ 0.86 (t, J = 7.1 Hz, 3H, H-11′’’), 1.23 (bs, 16H, H-3′’’-H-10′’’), 1.39 (m, 2H, H-2′’’), 2.73 (t, J = 7.8 Hz, 2H, H-1′’’), 6.97 (s, J = 7.1 Hz, 2H), 7.05 (t, J = 7.8 Hz, 2H), 7.19 (m, 3H), 7.42 (d, J = 8.1 Hz, 2H), 7.52 (s, 1H); 13C-NMR (125 MHz, CDCl3) δ 14.1 (CH3), 22.7 (CH2), 23.6 (CH2), 28.1 (CH2), 29.3 (CH2), 29.4 (CH2), 29.6 (CH2 x 2), 29.7 (CH2), 29.8 (CH2), 31.9 (CH2), 115.4 (C), 117.0 (C), 123.7 (C, JC-F = 272.3 Hz), 124.6 (CH x 2, JC-F = 3.6 Hz), 125.5 (C), 127.7 (CH x 2), 128.1 (CH), 128.7 (CH x 2), 129.1 (CH x 2), 130.7 (C, JC-F = 32.6 Hz), 131.7 (C), 138.5 (C), 148.1 (C), 149.2 (C), 149.3 (C), 152.6 (C), 154.6 (C), 180.3 (C), 183.4 (C); EIMS m/z (%) 589 ([M+], 87), 561 (32), 461 (54), 449 (88); HREIMS 589.2537 (calcd for C34H34N3O3F3 [M+] 589.2552).
3.39. Cells
Cell lines were purchased from the American Type Culture Collection (ATCC). The cell lines were growth at 37 °C under 5% CO2 under humidified atmosphere. The human hematologic cell lines K562 (derived from patients during the blast crisis phase of chronic myelogenous leukemia), HEL (erythroleukemia), HL60 (acute myeloid leukemia), and the human breast cancer cells BT-549 (triple negative breast cancer) and MCF7 (ER+) were grown in RPMI-1640 medium. The triple-negative breast cancer cells MDA-MB-231 and HS-578T were grown in DMEM medium. The HER+ breast cancer cells SKBR3 were maintained in McCoy′s 5A medium. The primate non-malignant kidney Vero cells were grown in DMEM low glucose medium. Cell culture media were supplemented with 10% FBS, L-glutamine (2 mM) and PEST (50 units/mL penicillin, 50 μg/mL streptomycin).
3.40. Cell Viability Assay
The effects of compounds on cell viability were examined in hematological and breast cancer cells and in primate non-tumor kidney Vero cells seeded at exponential growth (5000–10,000 cells per well) in 96-well plates (BD Falcon, France). Cells were treated with vehicle (0.05% DMSO) or test compounds (0.01 to 10 µM) for 48 h. Then, mitochondrial metabolization of the MTT was used as indicator of cell viability [
30]. Briefly, the tetrazolium salt 3-(4,5-methyltiazol-2yl-)-2,5diphenyl-tetrazolium bromide (MTT) (Applichen, Germany) was added to cells and incubated for 2–4 h at 37 °C, cells were lysed in 10% SDS and optical density was measured at 595 nm with the iMark Microplate Reader (BioRad).
3.41. ADME Property Predictions of Dihydro-1H-Pyrazolo[1,3-b] Pyridine Embelin Derivatives
The physicochemical parameters and ADME descriptors were predicted using QikProp program version 6.3 (Schrödinger, New York, NY, USA, 2020) [
31] in fast mode and based on the method of Jorgensen [
32,
33]. Preparation of compounds and the 2D-to-3D conversion was performed using LigPrep tool, a module of the Small-Molecule Drug Discovery Suite in the Schrödinger software package, followed by MacroModel v12.3 (Schrödinger, LLC, New York, NY, USA, 2020). A conformational search was implemented using Molecular Mechanics, followed by the minimization of the energy of each conformer. The global minimum energy conformer of each compound was used as input for the ADME studies.
3.42. Protein Preparation and Docking
The X-ray coordinates of human protein kinase CK2 alpha subunit in complex with the inhibitor CX-4945 (PDB 3PE1). The PDB structures were prepared for docking using the Protein Preparation Workflow (Schrodinger, New York, NY, USA, 2018) accessible from within the Maestro program (Maestro, version 11.6; Schrodinger, New York, NY, USA, 2018). The substrate and water molecules were removed beyond 5 Å, bond corrections were applied to the co-crystallized ligands and an exhaustive sampling of the orientations of groups was performed. Finally, the receptors were optimized in Maestro 11.6 by using OPLS3 force field before docking study. In the final stage, the optimization and minimization on the ligand–protein complexes were carried out with the OPLS3 force field and the default value for rmsd of 0.30 Å for non-hydrogen atoms were used. The receptor grids were generated using the prepared proteins, with the docking grids centered on the center of the bound ligand for each receptor. A receptor grid was generated using a 1.00 van der Waals (vdW) radius scaling factor and 0.25 partial charge cutoff. The binding sites were enclosed in a grid box of 20 Å
3 with default parameters and without constrains. The three-dimensional structures of the ligands to be docked were generated and prepared using LigPrep, as implemented in Maestro 11.6 (LigPrep, Schrodinger, New York, NY, USA, 2018), to generate the most probable ionization states at pH 7 ± 1 (retain original ionization state). These conformations were used as the initial input structures for the docking. In this stage a series of treatments are applied to the structures. Finally, the geometries are optimized using OPLS3 force field. These conformations were used as the initial input structures for the docking. The ligands were docked using the extra precision mode (XP) [
34] without using any constraints and a 0.80 van der Waals (vdW) radius scaling factor and 0.15 partial charge cutoff. The dockings were carried out with flexibility of the residues of the pocket near to the ligand. The generated ligand poses were evaluated with empirical scoring function, GlideScore a modified version of ChemScore [
35], GlideScore implemented in Glide, was used to estimate binding affinity and rank ligands [
36]. The XP Pose Rank was used to select the best-docked pose for each ligand. The best correlation with the human protein kinase CK2 alpha subunit was achieved when the PDB 3PE1 was used.