Prophylactic Peripheral Blood Stem Cell Collection in Patients with Extensive Bone-Marrow Infiltration of Neuroendocrine Tumours Prior to Peptide Receptor Radionuclide Therapy with 177Lu-DOTATATE
Abstract
1. Introduction
2. Methods
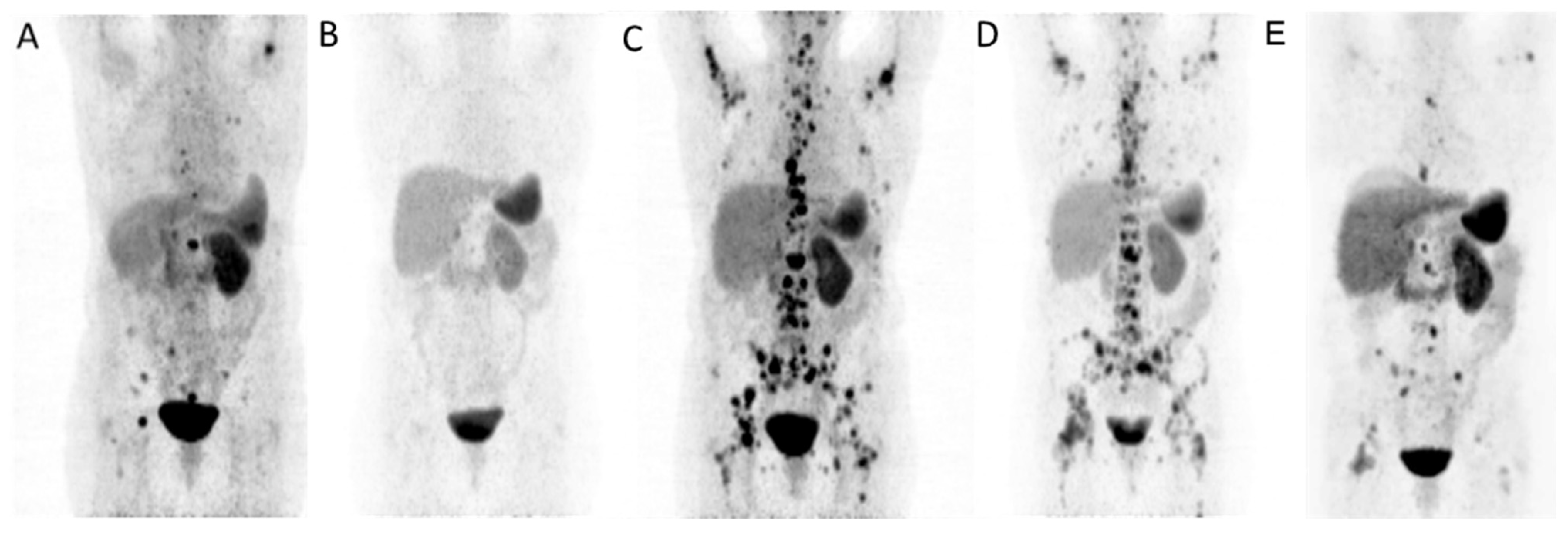
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 177Lu | Lutetium-177 |
| CTCAE | Common Terminology Criteria for Adverse Events |
| G-CSF | Granulocyte-colony stimulating factor |
| NET | Neuroendocrine tumor |
| PBSC | Peripheral blood stem-cells collection |
| PRRT | Peptide receptor radionuclide therapy |
References
- Kwekkeboom, D.J.; de Herder, W.W.; Kam, B.L.; van Eijck, C.H.; van Essen, M.; Kooij, P.P.; Feelders, R.A.; van Aken, M.O.; Krenning, E.P. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: Toxicity, efficacy, and survival. J. Clin. Oncol. 2008, 26, 2124–2130. [Google Scholar] [CrossRef]
- Bodei, L.; Kidd, M.; Paganelli, G.; Grana, C.M.; Drozdov, I.; Cremonesi, M.; Lepensky, C.; Kwekkeboom, D.J.; Baum, R.P.; Krenning, E.P.; et al. Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: The value and limitations of clinical factors. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 5–19. [Google Scholar] [CrossRef]
- Hamiditabar, M.; Ali, M.; Roys, J.; Wolin, E.M.; O’Dorisio, T.M.; Ranganathan, D.; Tworowska, I.; Strosberg, J.R.; Delpassand, E.S. Peptide Receptor Radionuclide Therapy With 177Lu-Octreotate in Patients With Somatostatin Receptor Expressing Neuroendocrine Tumors: Six Years’ Assessment. Clin. Nucl. Med. 2017, 42, 436–443. [Google Scholar] [CrossRef]
- Yordanova, A.; Wicharz, M.M.; Mayer, K.; Brossart, P.; Gonzalez-Carmona, M.A.; Strassburg, C.P.; Fimmers, R.; Essler, M.; Ahmadzadehfar, H. The Role of Adding Somatostatin Analogues to Peptide Receptor Radionuclide Therapy as a Combination and Maintenance Therapy. Clin. Cancer Res. 2018, 24, 4672–4679. [Google Scholar] [CrossRef]
- Yordanova, A.; Ahrens, H.; Feldmann, G.; Brossart, P.; Gaertner, F.C.; Fottner, C.; Weber, M.M.; Ahmadzadehfar, H.; Schreckenberger, M.; Miederer, M.; et al. Peptide Receptor Radionuclide Therapy Combined With Chemotherapy in Patients With Neuroendocrine Tumors. Clin. Nucl. Med. 2019, 44, e329–e335. [Google Scholar] [CrossRef]
- Paganelli, G.; Sansovini, M.; Nicolini, S.; Grassi, I.; Ibrahim, T.; Amadori, E.; Di Iorio, V.; Monti, M.; Scarpi, E.; Bongiovanni, A.; et al. (177)Lu-PRRT in advanced gastrointestinal neuroendocrine tumors: 10-year follow-up of the IRST phase II prospective study. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 152–160. [Google Scholar] [CrossRef]
- Sabet, A.; Dautzenberg, K.; Haslerud, T.; Aouf, A.; Sabet, A.; Simon, B.; Mayer, K.; Biersack, H.J.; Ezziddin, S. Specific efficacy of peptide receptor radionuclide therapy with (177)Lu-octreotate in advanced neuroendocrine tumours of the small intestine. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1238–1246. [Google Scholar] [CrossRef]
- Ezziddin, S.; Opitz, M.; Attassi, M.; Biermann, K.; Sabet, A.; Guhlke, S.; Brockmann, H.; Willinek, W.; Wardelmann, E.; Biersack, H.J.; et al. Impact of the Ki-67 proliferation index on response to peptide receptor radionuclide therapy. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 459–466. [Google Scholar] [CrossRef]
- Delpassand, E.S.; Samarghandi, A.; Zamanian, S.; Wolin, E.M.; Hamiditabar, M.; Espenan, G.D.; Erion, J.L.; O’Dorisio, T.M.; Kvols, L.K.; Simon, J.; et al. Peptide receptor radionuclide therapy with 177Lu-DOTATATE for patients with somatostatin receptor-expressing neuroendocrine tumors: The first US phase 2 experience. Pancreas 2014, 43, 518–525. [Google Scholar] [CrossRef]
- Hamiditabar, M.; Ali, M.; Bolek, L.; Vahdati, G.; Tworowska, I.; Delpassand, E.S. Safety and Effectiveness of 177Lu-DOTATATE Peptide Receptor Radionuclide Therapy After Regional Hepatic Embolization in Patients With Somatostatin-Expressing Neuroendocrine Tumors. Clin. Nucl. Med. 2017, 42, 822–828. [Google Scholar] [CrossRef]
- Foster, J.H.; Sher, A.; Seghers, V.; Poston, J.; Wells, D.; Delpassand, E.S.; Potter, S.; Mahajan, P.; Venkatramani, R. Peptide receptor radionuclide therapy for treatment of metastatic neuroendocrine tumors in children. Pediatr. Blood Cancer 2021, 68, e29056. [Google Scholar] [CrossRef]
- Braat, A.; Kwekkeboom, D.J.; Kam, B.L.R.; Teunissen, J.J.M.; de Herder, W.W.; Dreijerink, K.M.A.; van Rooij, R.; Krijger, G.C.; de Jong, H.; van den Bosch, M.; et al. Additional hepatic (166)Ho-radioembolization in patients with neuroendocrine tumours treated with (177)Lu-DOTATATE; a single center, interventional, non-randomized, non-comparative, open label, phase II study (HEPAR PLUS trial). BMC Gastroenterol. 2018, 18, 84. [Google Scholar] [CrossRef]
- Brabander, T.; van der Zwan, W.A.; Teunissen, J.J.M.; Kam, B.L.R.; Feelders, R.A.; de Herder, W.W.; van Eijck, C.H.J.; Franssen, G.J.H.; Krenning, E.P.; Kwekkeboom, D.J. Long-Term Efficacy, Survival, and Safety of [(177)Lu-DOTA(0),Tyr(3)]octreotate in Patients with Gastroenteropancreatic and Bronchial Neuroendocrine Tumors. Clin. Cancer Res. 2017, 23, 4617–4624. [Google Scholar] [CrossRef]
- van Vliet, E.I.; van Eijck, C.H.; de Krijger, R.R.; Nieveen van Dijkum, E.J.; Teunissen, J.J.; Kam, B.L.; de Herder, W.W.; Feelders, R.A.; Bonsing, B.A.; Brabander, T.; et al. Neoadjuvant Treatment of Nonfunctioning Pancreatic Neuroendocrine Tumors with [177Lu-DOTA0,Tyr3]Octreotate. J. Nucl. Med. 2015, 56, 1647–1653. [Google Scholar] [CrossRef]
- Strosberg, J.; Krenning, E. 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 1391–1392. [Google Scholar] [CrossRef]
- van Essen, M.; Krenning, E.P.; Kam, B.L.; de Herder, W.W.; Feelders, R.A.; Kwekkeboom, D.J. Salvage therapy with (177)Lu-octreotate in patients with bronchial and gastroenteropancreatic neuroendocrine tumors. J. Nucl. Med. 2010, 51, 383–390. [Google Scholar] [CrossRef]
- Sabet, A.; Haug, A.R.; Eiden, C.; Auernhammer, C.J.; Simon, B.; Bartenstein, P.; Biersack, H.J.; Ezziddin, S. Efficacy of peptide receptor radionuclide therapy with (177)Lu-octreotate in metastatic pulmonary neuroendocrine tumors: A dual-centre analysis. Am. J. Nucl. Med. Mol. Imaging 2017, 7, 74–83. [Google Scholar]
- Ezziddin, S.; Khalaf, F.; Vanezi, M.; Haslerud, T.; Mayer, K.; Al Zreiqat, A.; Willinek, W.; Biersack, H.J.; Sabet, A. Outcome of peptide receptor radionuclide therapy with 177Lu-octreotate in advanced grade 1/2 pancreatic neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 925–933. [Google Scholar] [CrossRef]
- Bodei, L.; Cwikla, J.B.; Kidd, M.; Modlin, I.M. The role of peptide receptor radionuclide therapy in advanced/metastatic thoracic neuroendocrine tumors. J. Thorac. Dis. 2017, 9, S1511–S1523. [Google Scholar] [CrossRef]
- Hicks, R.J.; Kwekkeboom, D.J.; Krenning, E.; Bodei, L.; Grozinsky-Glasberg, S.; Arnold, R.; Borbath, I.; Cwikla, J.; Toumpanakis, C.; Kaltsas, G.; et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Neoplasia: Peptide Receptor Radionuclide Therapy with Radiolabeled Somatostatin Analogues. Neuroendocrinology 2017, 105, 295–309. [Google Scholar] [CrossRef]
- Albertelli, M.; Dotto, A.; Di Dato, C.; Malandrino, P.; Modica, R.; Versari, A.; Colao, A.; Ferone, D.; Faggiano, A.; Nike. PRRT: Identikit of the perfect patient. Rev. Endocr. Metab. Disord. 2021, 22, 563–579. [Google Scholar] [CrossRef]
- Ezziddin, S.; Attassi, M.; Yong-Hing, C.J.; Ahmadzadehfar, H.; Willinek, W.; Grunwald, F.; Guhlke, S.; Biersack, H.J.; Sabet, A. Predictors of long-term outcome in patients with well-differentiated gastroenteropancreatic neuroendocrine tumors after peptide receptor radionuclide therapy with 177Lu-octreotate. J. Nucl. Med. 2014, 55, 183–190. [Google Scholar] [CrossRef]
- Kwekkeboom, D.J.; Kam, B.L.; van Essen, M.; Teunissen, J.J.; van Eijck, C.H.; Valkema, R.; de Jong, M.; de Herder, W.W.; Krenning, E.P. Somatostatin-receptor-based imaging and therapy of gastroenteropancreatic neuroendocrine tumors. Endocr. Relat. Cancer 2010, 17, R53–R73. [Google Scholar] [CrossRef]
- Sabet, A.; Khalaf, F.; Yong-Hing, C.J.; Sabet, A.; Haslerud, T.; Ahmadzadehfar, H.; Guhlke, S.; Grunwald, F.; Biersack, H.J.; Ezziddin, S. Can peptide receptor radionuclide therapy be safely applied in florid bone metastases? A pilot analysis of late stage osseous involvement. Nuklearmedizin 2014, 53, 54–59. [Google Scholar] [CrossRef]
- Bergsma, H.; van Lom, K.; Raaijmakers, M.; Konijnenberg, M.; Kam, B.; Teunissen, J.J.M.; de Herder, W.W.; Krenning, E.P.; Kwekkeboom, D.J. Persistent Hematologic Dysfunction after Peptide Receptor Radionuclide Therapy with (177)Lu-DOTATATE: Incidence, Course, and Predicting Factors in Patients with Gastroenteropancreatic Neuroendocrine Tumors. J. Nucl. Med. 2018, 59, 452–458. [Google Scholar] [CrossRef]
- Valkema, R.; Pauwels, S.A.; Kvols, L.K.; Kwekkeboom, D.J.; Jamar, F.; de Jong, M.; Barone, R.; Walrand, S.; Kooij, P.P.; Bakker, W.H.; et al. Long-term follow-up of renal function after peptide receptor radiation therapy with (90)Y-DOTA(0),Tyr(3)-octreotide and (177)Lu-DOTA(0), Tyr(3)-octreotate. J. Nucl. Med. 2005, 46 (Suppl. S1), 83–91. [Google Scholar]
- Sabet, A.; Ezziddin, K.; Pape, U.F.; Reichman, K.; Haslerud, T.; Ahmadzadehfar, H.; Biersack, H.J.; Nagarajah, J.; Ezziddin, S. Accurate assessment of long-term nephrotoxicity after peptide receptor radionuclide therapy with (177)Lu-octreotate. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 505–510. [Google Scholar] [CrossRef]
- Bergsma, H.; Konijnenberg, M.W.; van der Zwan, W.A.; Kam, B.L.; Teunissen, J.J.; Kooij, P.P.; Mauff, K.A.; Krenning, E.P.; Kwekkeboom, D.J. Nephrotoxicity after PRRT with (177)Lu-DOTA-octreotate. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1802–1811. [Google Scholar] [CrossRef]
- Mangan, K.F. Peripheral blood stem cell transplantation: From laboratory to clinical practice. Semin. Oncol. 1995, 22, 202–209. [Google Scholar]
- Talmadge, J.E.; Reed, E.; Ino, K.; Kessinger, A.; Kuszynski, C.; Heimann, D.; Varney, M.; Jackson, J.; Vose, J.M.; Bierman, P.J. Rapid immunologic reconstitution following transplantation with mobilized peripheral blood stem cells as compared to bone marrow. Bone Marrow Transplant. 1997, 19, 161–172. [Google Scholar] [CrossRef]
- Siena, S.; Schiavo, R.; Pedrazzoli, P.; Carlo-Stella, C. Therapeutic relevance of CD34 cell dose in blood cell transplantation for cancer therapy. J. Clin. Oncol. 2000, 18, 1360–1377. [Google Scholar] [CrossRef]
- Wuchter, P.; Ran, D.; Bruckner, T.; Schmitt, T.; Witzens-Harig, M.; Neben, K.; Goldschmidt, H.; Ho, A.D. Poor mobilization of hematopoietic stem cells-definitions, incidence, risk factors, and impact on outcome of autologous transplantation. Biol. Blood Marrow Transplant. 2010, 16, 490–499. [Google Scholar] [CrossRef]
- Lemoli, R.M.; D’Addio, A. Hematopoietic stem cell mobilization. Haematologica 2008, 93, 321–324. [Google Scholar] [CrossRef][Green Version]
- Kwekkeboom, D.J.; de Herder, W.W.; van Eijck, C.H.; Kam, B.L.; van Essen, M.; Teunissen, J.J.; Krenning, E.P. Peptide receptor radionuclide therapy in patients with gastroenteropancreatic neuroendocrine tumors. Semin. Nucl. Med. 2010, 40, 78–88. [Google Scholar] [CrossRef]
- Forrer, F.; Uusijarvi, H.; Storch, D.; Maecke, H.R.; Mueller-Brand, J. Treatment with 177Lu-DOTATOC of patients with relapse of neuroendocrine tumors after treatment with 90Y-DOTATOC. J. Nucl. Med. 2005, 46, 1310–1316. [Google Scholar] [PubMed]
- Sabet, A.; Haslerud, T.; Pape, U.F.; Sabet, A.; Ahmadzadehfar, H.; Grunwald, F.; Guhlke, S.; Biersack, H.J.; Ezziddin, S. Outcome and toxicity of salvage therapy with 177Lu-octreotate in patients with metastatic gastroenteropancreatic neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 205–210. [Google Scholar] [CrossRef] [PubMed]
- van der Zwan, W.A.; Brabander, T.; Kam, B.L.R.; Teunissen, J.J.M.; Feelders, R.A.; Hofland, J.; Krenning, E.P.; de Herder, W.W. Salvage peptide receptor radionuclide therapy with [(177)Lu-DOTA,Tyr(3)]octreotate in patients with bronchial and gastroenteropancreatic neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 704–717. [Google Scholar] [CrossRef] [PubMed]
- Ezziddin, S.; Sabet, A.; Heinemann, F.; Yong-Hing, C.J.; Ahmadzadehfar, H.; Guhlke, S.; Holler, T.; Willinek, W.; Boy, C.; Biersack, H.J. Response and long-term control of bone metastases after peptide receptor radionuclide therapy with (177)Lu-octreotate. J. Nucl. Med. 2011, 52, 1197–1203. [Google Scholar] [CrossRef]
- Bergsma, H.; Konijnenberg, M.W.; Kam, B.L.; Teunissen, J.J.; Kooij, P.P.; de Herder, W.W.; Franssen, G.J.; van Eijck, C.H.; Krenning, E.P.; Kwekkeboom, D.J. Subacute haematotoxicity after PRRT with (177)Lu-DOTA-octreotate: Prognostic factors, incidence and course. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Sabet, A.; Ezziddin, K.; Pape, U.F.; Ahmadzadehfar, H.; Mayer, K.; Poppel, T.; Guhlke, S.; Biersack, H.J.; Ezziddin, S. Long-term hematotoxicity after peptide receptor radionuclide therapy with 177Lu-octreotate. J. Nucl. Med. 2013, 54, 1857–1861. [Google Scholar] [CrossRef]
- Fitzgerald, P.A.; Goldsby, R.E.; Huberty, J.P.; Price, D.C.; Hawkins, R.A.; Veatch, J.J.; Dela Cruz, F.; Jahan, T.M.; Linker, C.A.; Damon, L.; et al. Malignant pheochromocytomas and paragangliomas: A phase II study of therapy with high-dose 131I-metaiodobenzylguanidine (131I-MIBG). Ann. N. Y. Acad. Sci. 2006, 1073, 465–490. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, L.O.; Skerlj, R.T.; Bridger, G.J.; Schwartz, T.W. Molecular interactions of cyclam and bicyclam non-peptide antagonists with the CXCR4 chemokine receptor. J. Biol. Chem. 2001, 276, 14153–14160. [Google Scholar] [CrossRef] [PubMed]
- Debnath, B.; Xu, S.; Grande, F.; Garofalo, A.; Neamati, N. Small molecule inhibitors of CXCR4. Theranostics 2013, 3, 47–75. [Google Scholar] [CrossRef] [PubMed]
| Age/Sex | Prmary/Grade | Previous Therapies | Metastatic Site | Mobilizing Agent | PBSC Results | Activity (Cycles) | Response | |
|---|---|---|---|---|---|---|---|---|
| Before PBSC | After PBSC | |||||||
| 60y/w | Ileum/G2 | CTx (Dox/5FU), SSA | liver, bone, LN | G-CSF | Successful | 0 GBq (0) | 43.0 GBq (6) | SD |
| 54y/w | Kidney/G2 | Surgery, SSA, PRRT | bone | G-CSF | Successful | 29.0 GBq (4) | 33.4 GBq (5) | PR |
| 70y/m | Jejunum/G1 | Surgery, SSA, PRRT | liver, bone | G-CSF, Plerixafor | Successful | 15.7 GBq (2) | 5.6 GBq (1) | PD |
| 53y/m | CUP/G2 | CTx (Cis/5FU), Radiation | liver, bone, LN | G-CSF | Successful | 0 GBq (0) | 39.0 GBq (6) | PR |
| 58y/w | Pancreas/G2 | CTx (STZ/5FU), SSA Surgery, PRRT | liver, bone | G-CSF, Plerixafor | Successful | 37.3 GBq (5) | 7.5 GBq (1) | PR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabet, A.; Mader, N.; Bittenbring, J.T.; Khreish, F.; Grünwald, F.; Biersack, H.J.; Ezziddin, S. Prophylactic Peripheral Blood Stem Cell Collection in Patients with Extensive Bone-Marrow Infiltration of Neuroendocrine Tumours Prior to Peptide Receptor Radionuclide Therapy with 177Lu-DOTATATE. Pharmaceuticals 2021, 14, 1022. https://doi.org/10.3390/ph14101022
Sabet A, Mader N, Bittenbring JT, Khreish F, Grünwald F, Biersack HJ, Ezziddin S. Prophylactic Peripheral Blood Stem Cell Collection in Patients with Extensive Bone-Marrow Infiltration of Neuroendocrine Tumours Prior to Peptide Receptor Radionuclide Therapy with 177Lu-DOTATATE. Pharmaceuticals. 2021; 14(10):1022. https://doi.org/10.3390/ph14101022
Chicago/Turabian StyleSabet, Amir, Nicolai Mader, Jörg Thomas Bittenbring, Fadi Khreish, Frank Grünwald, Hans Jürgen Biersack, and Samer Ezziddin. 2021. "Prophylactic Peripheral Blood Stem Cell Collection in Patients with Extensive Bone-Marrow Infiltration of Neuroendocrine Tumours Prior to Peptide Receptor Radionuclide Therapy with 177Lu-DOTATATE" Pharmaceuticals 14, no. 10: 1022. https://doi.org/10.3390/ph14101022
APA StyleSabet, A., Mader, N., Bittenbring, J. T., Khreish, F., Grünwald, F., Biersack, H. J., & Ezziddin, S. (2021). Prophylactic Peripheral Blood Stem Cell Collection in Patients with Extensive Bone-Marrow Infiltration of Neuroendocrine Tumours Prior to Peptide Receptor Radionuclide Therapy with 177Lu-DOTATATE. Pharmaceuticals, 14(10), 1022. https://doi.org/10.3390/ph14101022







