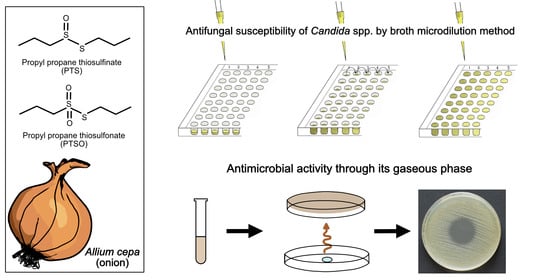
Antibacterial and Antifungal Activity of Propyl-Propane-Thiosulfinate and Propyl-Propane-Thiosulfonate, Two Organosulfur Compounds from Allium cepa: In Vitro Antimicrobial Effect via the Gas Phase
Abstract
1. Introduction
2. Results
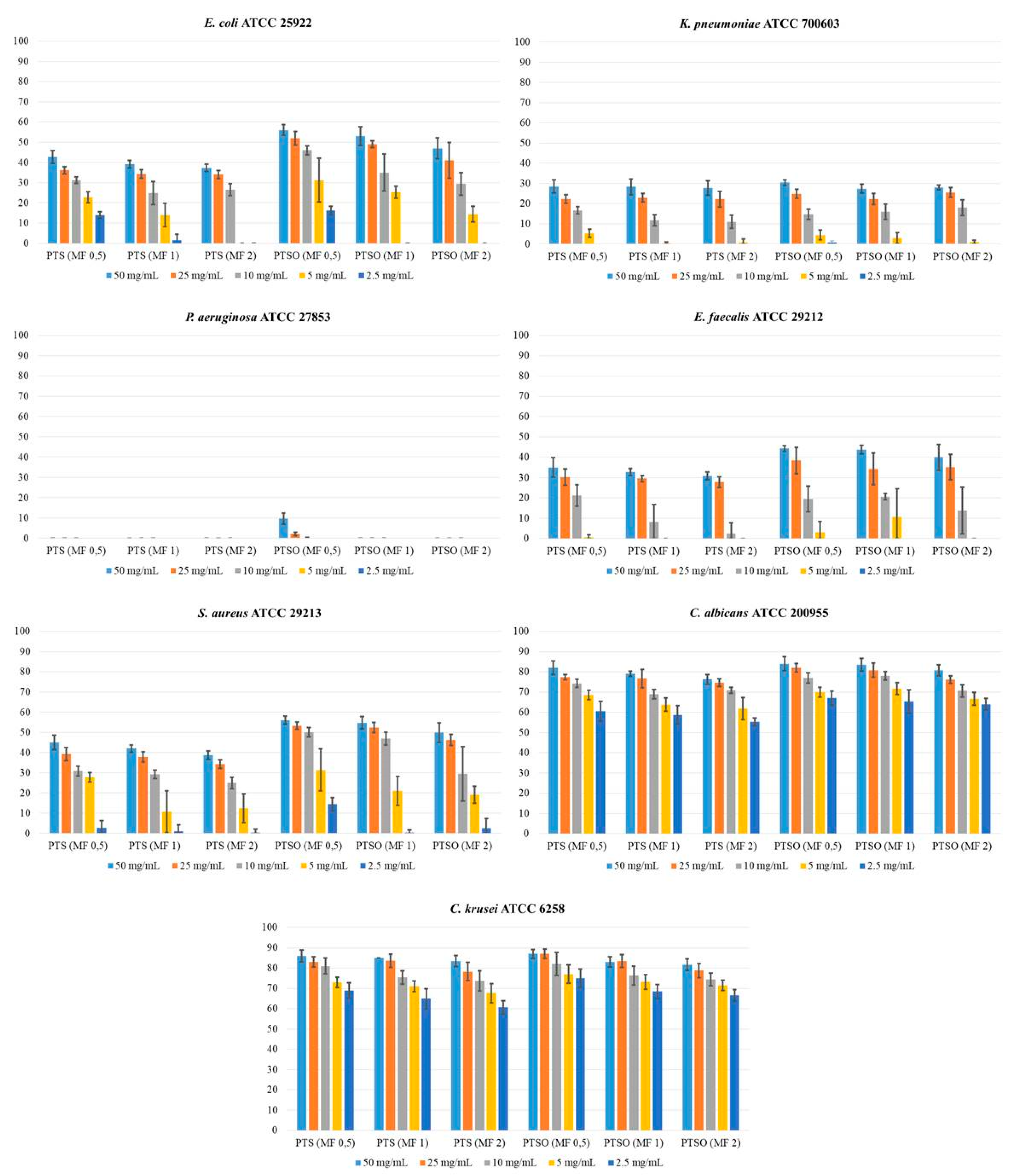
2.1. Antifungal Susceptibility
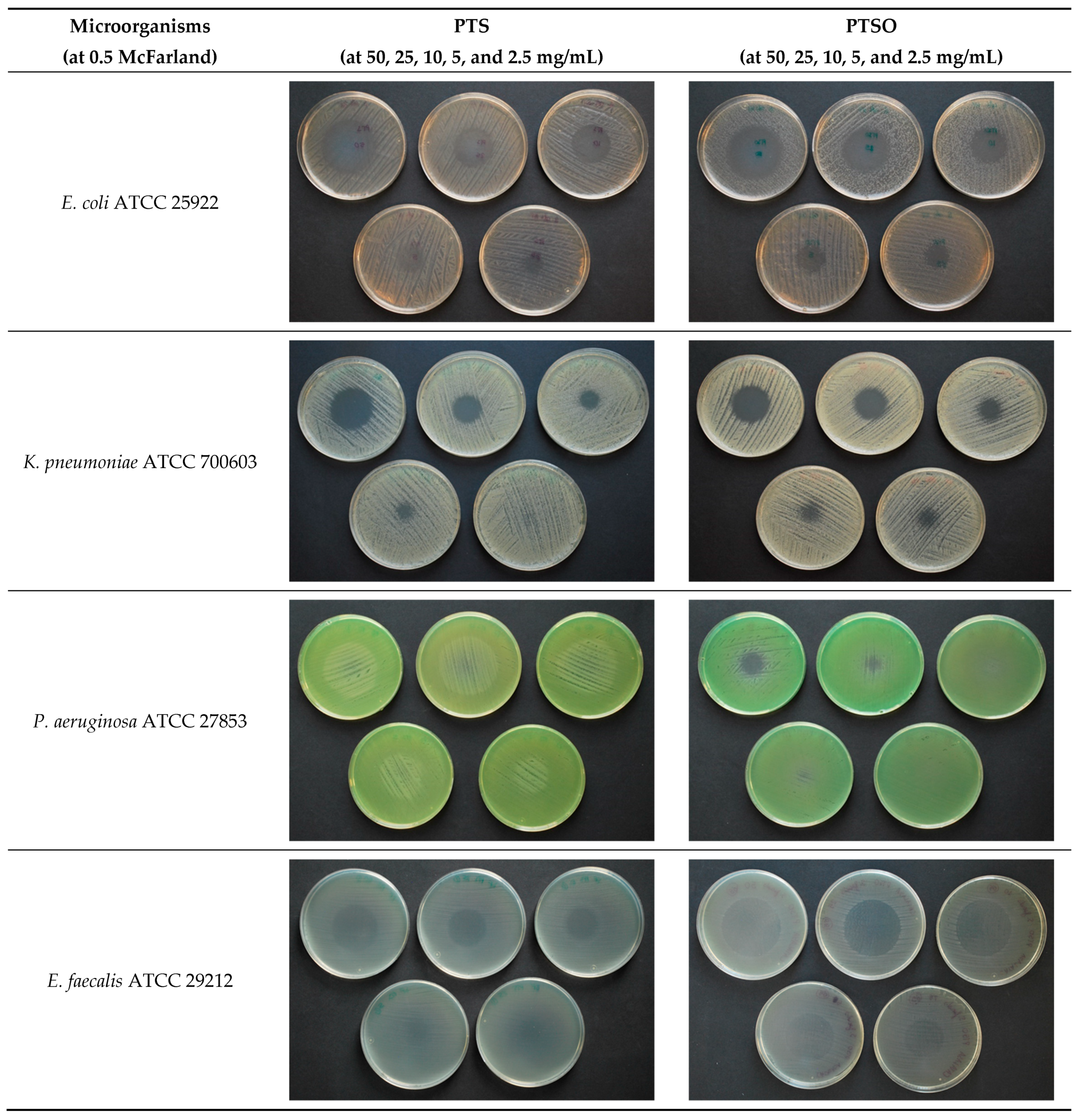
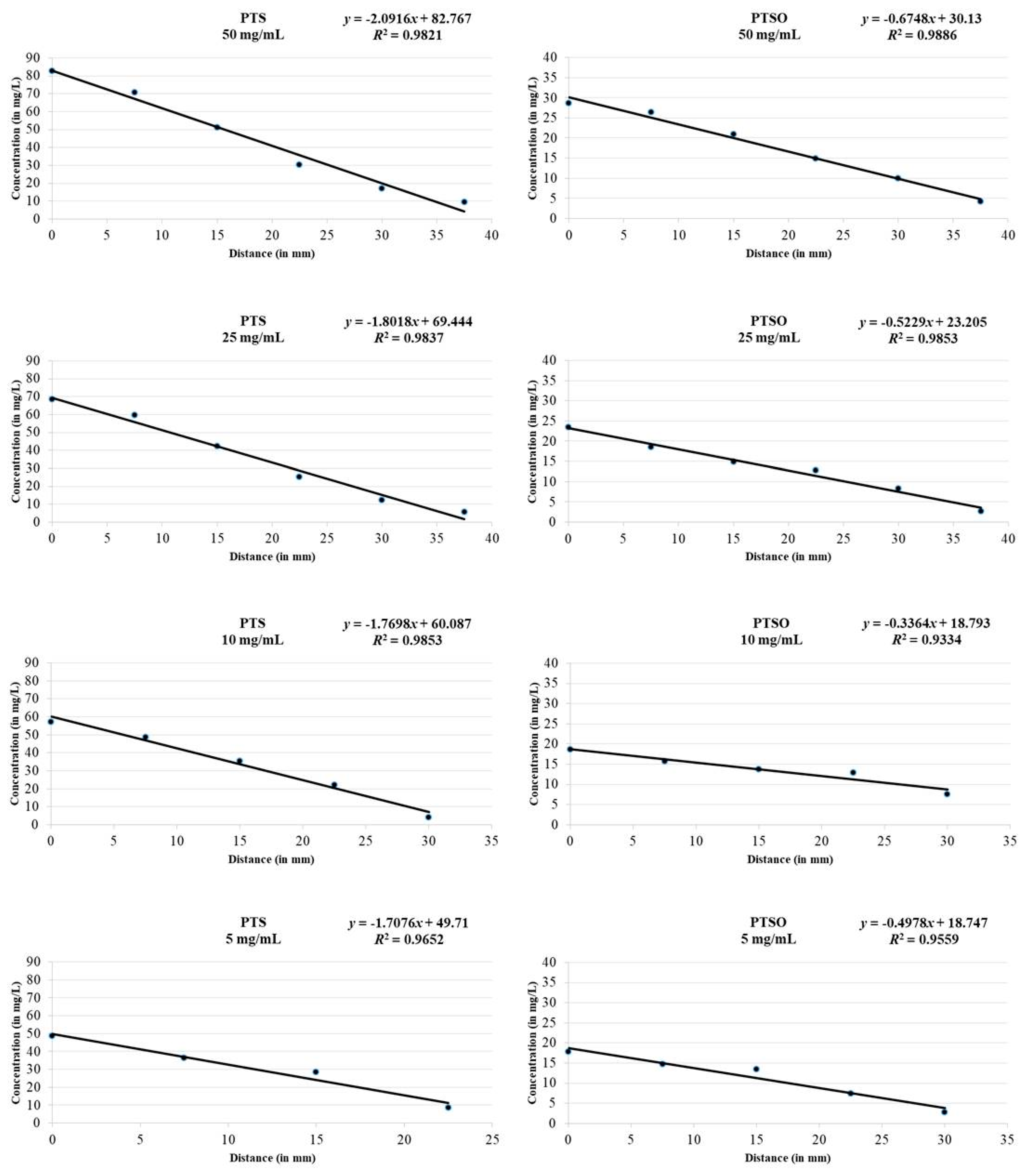
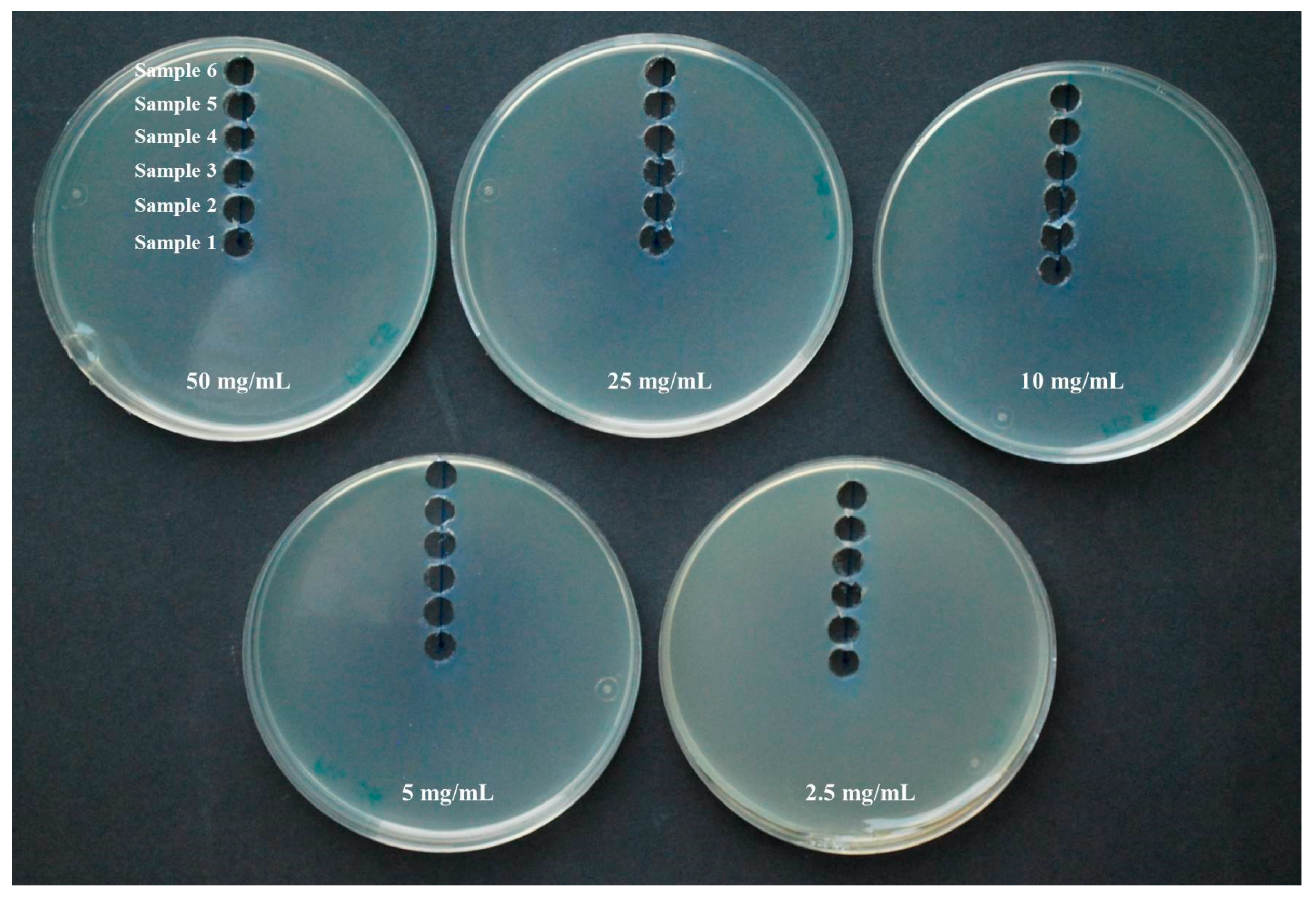
2.2. Antimicrobial Activity of Vapor
3. Discussion
3.1. Antifungal Susceptibility
3.2. Antimicrobial Activity of Vapor
4. Materials and Methods
4.1. Antifungal Susceptibility Testing
4.1.1. Antifungals, PTS, and PTSO
4.1.2. Candida Isolates and Identification
4.1.3. In Vitro Antifungal Assay
4.2. Antimicrobial Activity of Gaseous PTS and PTSO
4.3. HPLC-UV Analysis
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borlinghaus, J.; Albrecht, F.; Gruhlke, M.C.; Nwachukwu, I.D.; Slusarenko, A.J. Allicin: Chemistry and biological properties. Molecules 2014, 19, 12591–12618. [Google Scholar] [CrossRef]
- Chan, J.Y.; Yuen, A.C.; Chan, R.Y.; Chan, S.W. A review of the cardiovascular benefits and antioxidant properties of allicin. Phytother. Res. 2013, 27, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Reiter, J.; Levina, N.; van der Linden, M.; Gruhlke, M.; Martin, C.; Slusarenko, A.J. Diallylthiosulfinate (Allicin), a volatile antimicrobial from garlic (Allium sativum), kills human lung pathogenic bacteria, including MDR strains, as a vapor. Molecules 2017, 22, 1711. [Google Scholar] [CrossRef] [PubMed]
- Ankri, S.; Mirelman, D. Antimicrobial properties of allicin from garlic. Microbes Infect. 1999, 1, 125–129. [Google Scholar] [CrossRef]
- Focke, M.; Feld, A.; Lichtenthaler, K. Allicin, a naturally occurring antibiotic from garlic, specifically inhibits acetyl-CoA synthetase. FEBS Lett. 1990, 261, 106–108. [Google Scholar] [CrossRef]
- Rabinkov, A.; Miron, T.; Konstantinovski, L.; Wilchek, M.; Mirelman, D.; Weiner, L. The mode of action of allicin: Trapping of radicals and interaction with thiol containing proteins. Biochim. Biophys. Acta 1998, 1379, 233–244. [Google Scholar] [CrossRef]
- Gruhlke, M.C.; Portz, D.; Stitz, M.; Anwar, A.; Schneider, T.; Jacob, C.; Schlaich, N.L.; Slusarenko, A.J. Allicin disrupts the cell’s electrochemical potential and induces apoptosis in yeast. Free Radic. Biol. Med. 2010, 49, 1916–1924. [Google Scholar] [CrossRef]
- Feldberg, R.S.; Chang, S.C.; Kotik, A.N.; Nadler, M.; Neuwirth, Z.; Sundstrom, D.C.; Thompson, N.H. In vitro mechanism of inhibition of bacterial cell growth by allicin. Antimicrob. Agents Chemother. 1988, 32, 1763–1768. [Google Scholar] [CrossRef]
- Keusgen, M.; Schulz, H.; Glodek, J.; Krest, I.; Krüger, H.; Herchert, N.; Keller, J. Characterization of some Allium hybrids by aroma precursors, aroma profiles, and alliinase activity. J. Agric. Food Chem. 2002, 50, 2884–2890. [Google Scholar] [CrossRef]
- Guillamón, E. Effect of phytochemical compounds of the genus Allium on the immune system and the inflammatory response. Ars Pharm. 2018, 59, 185–196. [Google Scholar] [CrossRef]
- Sorlozano-Puerto, A.; Albertuz-Crespo, M.; Lopez-Machado, I.; Ariza-Romero, J.J.; Baños-Arjona, A.; Exposito-Ruiz, M.; Gutierrez-Fernandez, J. In vitro antibacterial activity of propyl-propane-thiosulfinate and propyl-propane-thiosulfonate derived from Allium spp. against gram-negative and gram-positive multidrug-resistant bacteria isolated from human samples. Biomed Res. Int. 2018, 2018, 7861207. [Google Scholar] [CrossRef] [PubMed]
- Singla, N.; Gulati, N.; Kaistha, N.; Chander, J. Candida colonization in urine samples of ICU patients: Determination of etiology, antifungal susceptibility testing and evaluation of associated risk factors. Mycopathologia 2012, 174, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Sobel, J.D.; Fisher, J.F.; Kauffman, C.A.; Newman, C.A. Candida urinary tract infections—Epidemiology. Clin. Infect. Dis. 2011, 52, S433–S436. [Google Scholar] [CrossRef] [PubMed]
- Leontiev, R.; Hohaus, N.; Jacob, C.; Gruhlke, M.; Slusarenko, A.J. A comparison of the antibacterial and antifungal activities of thiosulfinate analogues of Allicin. Sci. Rep. 2018, 8, 6763. [Google Scholar] [CrossRef]
- Miceli, M.H.; Díaz, J.A.; Lee, S.A. Emerging opportunistic yeast infections. Lancet Infect. Dis. 2011, 11, 142–151. [Google Scholar] [CrossRef]
- Kucukates, E.; Gultekin, N.; Alisan, Z.; Hondur, N.; Ozturk, R. Identification of Candida species and susceptibility testing with Sensititre YeastOne microdilution panel to 9 antifungal agents. Saudi Med. J. 2016, 37, 750–757. [Google Scholar] [CrossRef]
- Alexander, B.D.; Johnson, M.D.; Pfeiffer, C.D.; Jiménez-Ortigosa, C.; Catania, J.; Booker, R.; Castanheira, M.; Messer, S.A.; Perlin, D.S.; Pfaller, M.A. Increasing echinocandin resistance in Candida glabrata: Clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin. Infect. Dis. 2013, 56, 1724–1732. [Google Scholar] [CrossRef]
- Castanheira, M.; Messer, S.A.; Jones, R.N.; Farrell, D.J.; Pfaller, M.A. Activity of echinocandins and triazoles against a contemporary (2012) worldwide collection of yeast and moulds collected from invasive infections. Int. J. Antimicrob. Agents 2014, 44, 320–326. [Google Scholar] [CrossRef]
- Cretella, D.; Barber, K.E.; King, S.T.; Stover, K.R. Comparison of susceptibility patterns using commercially available susceptibility testing methods performed on prevalent Candida spp. J. Med. Microbiol. 2016, 65, 1445–1451. [Google Scholar] [CrossRef]
- Wang, E.; Farmakiotis, D.; Yang, D.; McCue, D.A.; Kantarjian, H.M.; Kontoyiannis, D.P.; Mathisen, M.S. The ever-evolving landscape of candidaemia in patients with acute leukaemia: Non-susceptibility to caspofungin and multidrug resistance are associated with increased mortality. J. Antimicrob. Chemother. 2015, 70, 2362–2368. [Google Scholar] [CrossRef]
- An, M.; Shen, H.; Cao, Y.; Zhang, J.; Cai, Y.; Wang, R.; Jiang, Y. Allicin enhances the oxidative damage effect of amphotericin B against Candida albicans. Int. J. Antimicrob. Agents 2009, 33, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Khodavandi, A.; Alizadeh, F.; Aala, F.; Sekawi, Z.; Chong, P.P. In vitro investigation of antifungal activity of allicin alone and in combination with azoles against Candida species. Mycopathologia 2010, 169, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Khodavandi, A.; Alizadeh, F.; Harmal, N.S.; Sidik, S.M.; Othman, F.; Sekawi, Z.; Jahromi, M.A.; Ng, K.P.; Chong, P.P. Comparison between efficacy of allicin and fluconazole against Candida albicans in vitro and in a systemic candidiasis mouse model. FEMS Microbiol. Lett. 2011, 315, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Heras-Cañas, V.; Ros, L.; Sorlózano, A.; Gutiérrez-Soto, B.; Navarro-Marí, J.M.; Gutiérrez-Fernández, J. Isolated yeast species in urine samples in a Spanish regional hospital. Rev. Argent. Microbiol. 2015, 47, 331–334. [Google Scholar] [CrossRef]
- Jiménez-Guerra, G.; Casanovas Moreno-Torres, I.; Gutiérrez-Soto, M.; Vazquez-Alonso, F.; Sorlózano-Puerto, A.; Navarro-Marí, J.M.; Gutiérrez-Fernández, J. Inpatient candiduria: Etiology, susceptibility to antifungal drugs and risk factors. Rev. Esp. Quimioter. 2018, 31, 323–328. [Google Scholar]
- Aigner, M.; Erbeznik, T.; Gschwentner, M.; Lass-Flörl, C. Etest and Sensititre YeastOne susceptibility testing of echinocandins against Candida species from a single center in Austria. Antimicrob. Agents Chemother. 2017, 61, e00512-17. [Google Scholar] [CrossRef]
- Alfouzan, W.; Al-Enezi, T.; AlRoomi, E.; Sandhya, V.; Chandy, R.; Khan, Z.U. Comparison of the VITEK 2 antifungal susceptibility system with Etest using clinical isolates of Candida species. Rev. Iberoam. Micol. 2017, 34, 171–174. [Google Scholar] [CrossRef]
- Oz, Y.; Gokbolat, E. Evaluation of direct antifungal susceptibility testing methods of Candida spp. from positive blood culture bottles. J. Clin. Lab. Anal. 2018, 32, e22297. [Google Scholar] [CrossRef]
- Siqueira, R.A.; Doi, A.M.; de Petrus Crossara, P.P.; Koga, P.; Marques, A.G.; Nunes, F.G.; Pasternak, J.; Martino, M. Evaluation of two commercial methods for the susceptibility testing of Candida species: Vitek 2® and Sensititre YeastOne®. Rev. Iberoam. Micol. 2018, 35, 83–87. [Google Scholar] [CrossRef]
- Diba, A.; Alizadeh, F. In vitro and in vivo antifungal activity of Allium hirtifolium and Allium sativum. Avicenna J. Phytomed. 2018, 8, 465–474. [Google Scholar]
- Shams-Ghahfarokhi, M.; Shokoohamiri, M.R.; Amirrajab, N.; Moghadasi, B.; Ghajari, A.; Zeini, F.; Sadeghi, G.; Razzaghi-Abyaneh, M. In vitro antifungal activities of Allium cepa, Allium sativum and ketoconazole against some pathogenic yeasts and dermatophytes. Fitoterapia 2006, 77, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Gruhlke, M.; Schlembach, I.; Leontiev, R.; Uebachs, A.; Gollwitzer, P.; Weiss, A.; Delaunay, A.; Toledano, M.; Slusarenko, A.J. Yap1p, the central regulator of the S. cerevisiae oxidative stress response, is activated by allicin, a natural oxidant and defence substance of garlic. Free Radic. Biol. Med. 2017, 108, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Li, W.R.; Shi, Q.S.; Dai, H.Q.; Liang, Q.; Xie, X.B.; Huang, X.M.; Zhao, G.Z.; Zhang, L.X. Antifungal activity, kinetics and molecular mechanism of action of garlic oil against Candida albicans. Sci. Rep. 2016, 6, 22805. [Google Scholar] [CrossRef] [PubMed]
- Khodavandi, A.; Alizadeh, F.; Harmal, N.S.; Sidik, S.M.; Othman, F.; Sekawi, Z.; Chong, P.P. Expression analysis of SIR2 and SAPs1-4 gene expression in Candida albicans treated with allicin compared to fluconazole. Trop. Biomed. 2011, 28, 589–598. [Google Scholar]
- Ebrahimy, F.; Dolatian, M.; Moatar, F.; Majd, H.A. Comparison of the therapeutic effects of Garcin® and fluconazole on Candida vaginitis. Singap. Med. J. 2015, 56, 567–572. [Google Scholar] [CrossRef]
- Curtis, H.; Noll, U.; Störmann, J.; Slusarenko, A.J. Broad-spectrum activity of the volatile phytoanticipin allicin in extracts of garlic (Allium sativum L.) against plant pathogenic bacteria, fungi and Oomycetes. Physiol. Mol. Plant Pathol. 2004, 65, 79–89. [Google Scholar] [CrossRef]
- Abubakar, E.-m.M. Efficacy of crude extracts of garlic (Allium sativum Linn.) against nosocomial Escherichia coli, Staphylococcus aureus, Streptococcus pneumoniae and Pseudomonas aeruginosa. J. Med. Plant Res. 2009, 3, 179–185. [Google Scholar]
- Müller, A.; Eller, J.; Albrecht, F.; Prochnow, P.; Kuhlmann, K.; Bandow, J.E.; Slusarenko, A.J.; Leichert, L.I. Allicin induces thiol stress in bacteria through S-allylmercapto modification of protein cysteines. J. Biol. Chem. 2016, 291, 11477–11490. [Google Scholar] [CrossRef]
- Lemar, K.M.; Turner, M.P.; Lloyd, D. Garlic (Allium sativum) as an anti-Candida agent: A comparison of the efficacy of fresh garlic and freeze-dried extracts. J. Appl. Microbiol. 2002, 93, 398–405. [Google Scholar] [CrossRef]
- Shadkchan, Y.; Shemesh, E.; Mirelman, D.; Miron, T.; Rabinkov, A.; Wilchek, M.; Osherov, N. Efficacy of allicin, the reactive molecule of garlic, in inhibiting Aspergillus spp. in vitro, and in a murine model of disseminated aspergillosis. J. Antimicrob. Chemother. 2004, 53, 832–836. [Google Scholar] [CrossRef]
- Abouelfetouh, A.Y.; Moussa, N.K. Enhancement of antimicrobial activity of four classes of antibiotics combined with garlic. Asian J. Plant Sci. 2012, 11, 148–152. [Google Scholar] [CrossRef][Green Version]
- Jonkers, D.; Sluimer, J.; Stobberingh, E. Effect of garlic on vancomycin-resistant enterococci. Antimicrob. Agents Chemother. 1999, 43, 3045. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ogita, A.; Nagao, Y.; Fujita, K.; Tanaka, T. Amplification of vacuole-targeting fungicidal activity of antibacterial antibiotic polymyxin B by allicin, an allyl sulfur compound from garlic. J. Antibiot. 2007, 60, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Pillai, R.; Trivedi, N.A.; Bhatt, J.D. Studies on in vitro interaction of ampicillin and fresh garlic extract against Staphylococcus aureus by checkerboard method. Anc. Sci. Life 2013, 33, 114–118. [Google Scholar] [CrossRef]
- Yalindag-Ozturk, N.; Ozdamar, M.; Cengiz, P. Trial of garlic as an adjunct therapy for multidrug resistant Pseudomonas aeruginosa pneumonia in a critically ill infant. J. Altern. Complement. Med. 2011, 17, 379–380. [Google Scholar] [CrossRef]
- Guo, N.; Wu, X.; Yu, L.; Liu, J.; Meng, R.; Jin, J.; Lu, H.; Wang, X.; Yan, S.; Deng, X. In vitro and in vivo interactions between fluconazole and allicin against clinical isolates of fluconazole-resistant Candida albicans determined by alternative methods. FEMS Immunol. Med. Microbiol. 2010, 58, 193–201. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Fourth Informational Supplement M27-S4; CLSI: Wayne, PA, USA, 2012. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antifungal Susceptibility Testing of Yeasts, 1st ed.; Supplement M60; CLSI: Wayne, PA, USA, 2017. [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). Antifungal Agents-Breakpoint Tables for Interpretation of MICs. Version 8.1. 2017. Available online: http://www.eucast.org/ (accessed on 24 October 2017).
- Abad, P.; Lara, F.J.; Arroyo-Manzanares, N.; Baños, A.; Guillamón, E.; García-Campaña, A.M. High-performance liquid chromatography method for the monitoring of the Allium derivative propyl propane thiosulfonate used as natural additive in animal feed. Food Anal. Methods 2015, 8, 916–921. [Google Scholar] [CrossRef]
- Abad, P.; Arroyo-Manzanares, N.; Gil, L.; García-Campaña, A.M. Use of onion extract as a dairy cattle feed supplement: Monitoring propyl propane thiosulfonate as a marker of its effect on milk attributes. J. Agric. Food Chem. 2017, 65, 793–799. [Google Scholar] [CrossRef]

| Species of Yeasts (Number of Isolates) | Range of MIC (in mg/L) | MIC50 (in mg/L) | MIC90 (in mg/L) | Range of MFC (in mg/L) | MFC50 (in mg/L) | MFC90 (in mg/L) | Number of Resistant Isolates |
|---|---|---|---|---|---|---|---|
| Candida albicans (n = 83) | |||||||
| Amphotericin B | 0.125–0.25 | 0.25 | 0.25 | 1–16 | 4 | 8 | 0 |
| Anidulafungin | ≤0.008–0.25 | 0.015 | 0.06 | 0.015–>4 | 0.25 | 1 | 0 |
| Micafungin | ≤0.008–0.25 | 0.03 | 0.125 | 0.03–4 | 0.5 | 2 | 0 |
| Caspofungin | ≤0.008–0.25 | 0.06 | 0.06 | 0.06–4 | 0.5 | 2 | 0 |
| Fluconazole | ≤0.125–2 | 0.25 | 2 | 8–>64 | >64 | >64 | 0 |
| Voriconazole | ≤0.008–0.06 | ≤0.008 | ≤0.008 | 0.03–0.5 | 0.5 | 0.5 | 0 |
| PTS | 16–64 | 32 | 32 | 16–>128 | 32 | 128 | - |
| PTSO | 8–64 | 16 | 32 | 8–128 | 32 | 64 | - |
| Candida glabrata (n = 73) | |||||||
| Amphotericin B | 0.06–1 | 0.25 | 0.5 | 1–>16 | 4 | 8 | 0 |
| Anidulafungin | ≤0.008–0.125 | 0.015 | 0.03 | 0.06–>4 | 0.25 | 0.5 | 0 |
| Micafungin | ≤0.008–0.06 | ≤0.008 | 0.03 | 0.008–4 | 0.06 | 0.25 | 0 |
| Caspofungin | ≤0.008–0.125 | 0.03 | 0.03 | 0.06–4 | 1 | 2 | 0 |
| Fluconazole | 0.25–≥64 | 4 | ≥64 | 16–>64 | >64 | >64 | 73 |
| Voriconazole | 0.03–≥4 | 0.5 | ≥4 | 2–>4 | >4 | >4 | 73 |
| PTS | 8–64 | 32 | 32 | 16–>128 | 64 | 128 | - |
| PTSO | 16–32 | 16 | 32 | 8–>128 | 32 | 64 | - |
| Candida krusei (n = 12) | |||||||
| Amphotericin B | 0.25–1 | 0.5 | 1 | 4–16 | 8 | 16 | 0 |
| Anidulafungin | ≤0.008–0.06 | 0.015 | 0.03 | 0.125–0.5 | 0.25 | 0.5 | 0 |
| Micafungin | 0.06–0.25 | 0.125 | 0.125 | 0.5–4 | 0.5 | 1 | 0 |
| Caspofungin | 0.06–0.25 | 0.125 | 0.25 | 1–4 | 1 | 2 | 0 |
| Fluconazole | 8–≥64 | 16 | ≥64 | >64 | >64 | >64 | 12 |
| Voriconazole | 0.03–0.5 | 0.125 | 0.5 | 2–>4 | >4 | >4 | 0 |
| PTS | 4–32 | 16 | 32 | 32–>128 | 32 | 128 | - |
| PTSO | 4–16 | 4 | 8 | 64–>128 | 128 | 128 | - |
| Candida tropicalis (n = 35) | |||||||
| Amphotericin B | ≤0.03–1 | 0.125 | 0.25 | 2–16 | 2 | 8 | 0 |
| Anidulafungin | ≤0.008–0.25 | 0.06 | 0.25 | 0.015–2 | 1 | 2 | 0 |
| Micafungin | ≤0.008–0.25 | 0.06 | 0.25 | 0.008–2 | 0.5 | 2 | 0 |
| Caspofungin | ≤0.008–0.25 | 0.06 | 0.25 | 0.06–4 | 1 | 4 | 0 |
| Fluconazole | ≤0.125–2 | ≤0.125 | 1 | 8–>64 | >64 | >64 | 0 |
| Voriconazole | ≤0.008–0.06 | ≤0.008 | 0.015 | 0.03–0.5 | 0.5 | 0.5 | 0 |
| PTS | 32–64 | 64 | 64 | 64–>128 | 128 | 128 | - |
| PTSO | 4–32 | 16 | 32 | 4–128 | 32 | 64 | - |
| Microorganisms | McFarland | PTS Concentration (Growth Inhibition in mm) | PTSO Concentration (Growth Inhibition in mm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 mg/mL | 25 mg/mL | 10 mg/m L | 5 mg/mL | 2.5 mg/mL | 50 mg/mL | 25 mg/mL | 10 mg/mL | 5 mg/mL | 2.5 mg/mL | ||
| E. coli ATCC 25922 | 0.5 | 43 ± 3.2 | 36 ± 1.9 | 31 ± 1.6 | 23 ± 2.7 | 14 ± 1.6 | 56 ± 2.7 | 52 ± 3.3 | 46 ± 2.2 | 31 ± 10.8 | 16 ± 2.1 |
| 1 | 39 ± 2.0 | 34 ± 2.1 | 25 ± 5.6 | 14 ± 5.7 | 1 ± 3.0 | 53 ± 4.5 | 49 ± 1.8 | 35 ± 9.2 | 25 ± 3.0 | 0 | |
| 2 | 37 ± 1.8 | 34 ± 2.0 | 27 ± 3.0 | 0 | 0 | 47 ± 5.1 | 42 ± 8.8 | 29 ± 5.5 | 14 ± 3.9 | 0 | |
| K. pneumoniae ATCC 700603 | 0.5 | 28 ± 3.3 | 22 ± 2.2 | 17 ± 1.8 | 5 ± 2.0 | 0 | 30 ± 1.3 | 25 ± 2.2 | 15 ± 2.5 | 5 ± 2.5 | 1 ± 0.7 |
| 1 | 28 ± 3.9 | 23 ± 2.2 | 12 ± 2.8 | 0 | 0 | 27 ± 2.3 | 22 ± 2.7 | 16 ± 3.8 | 3 ± 2.7 | 0 | |
| 2 | 28 ± 3.6 | 22 ± 3.8 | 11 ± 3.3 | 1 ± 1.8 | 0 | 28 ± 1.2 | 26 ± 2.4 | 18 ± 3.9 | 1 ± 0.9 | 0 | |
| P. aeruginosa ATCC 27853 | 0.5 | 0 | 0 | 0 | 0 | 0 | 10 ± 2.7 | 2 ± 0.9 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| E. faecalis ATCC 29212 | 0.5 | 35 ± 4.6 | 30 ± 4.0 | 21 ± 5.2 | 1 ± 1.2 | 0 | 44 ± 1.3 | 38 ± 6.5 | 20 ± 6.3 | 3 ± 5.3 | 0 |
| 1 | 33 ± 1.8 | 30 ± 1.6 | 8 ± 8.7 | 0 | 0 | 44 ± 2.1 | 34 ± 7.8 | 21 ± 1.6 | 11 ± 14.0 | 0 | |
| 2 | 31 ± 1.9 | 28 ± 2.7 | 3 ± 5.3 | 0 | 0 | 40 ± 6.3 | 35 ± 6.2 | 14 ± 11.6 | 0 | 0 | |
| S. aureus ATCC 29213 | 0.5 | 45 ± 3.6 | 39 ± 3.3 | 31 ± 2.4 | 28 ± 2.4 | 3 ± 3.5 | 56 ± 2.0 | 53 ± 1.8 | 50 ± 2.3 | 31 ± 10.4 | 14 ± 3.3 |
| 1 | 42 ± 1.8 | 38 ± 2.5 | 29 ± 2.0 | 11 ± 10.3 | 1 ± 3.2 | 55 ± 3.1 | 52 ± 2.5 | 47 ± 3.2 | 21 ± 7.1 | 0 | |
| 2 | 39 ± 2.1 | 34 ± 2.1 | 25 ± 2.8 | 12 ± 7.1 | 1 ± 1.6 | 50 ± 4.9 | 46 ± 2.7 | 29 ± 13.5 | 19 ± 4.3 | 3 ± 4.8 | |
| C. albicans ATCC 200955 | 0.5 | 82 ± 3.4 | 77 ± 1.2 | 74 ± 1.9 | 69 ± 2.3 | 61 ± 4.9 | 84 ± 3.4 | 82 ± 2.2 | 77 ± 2.5 | 70 ± 2.4 | 67 ± 3.5 |
| 1 | 79 ± 1.3 | 77 ± 4.5 | 69 ± 2.3 | 64 ± 3.3 | 59 ± 4.5 | 84 ± 3.2 | 81 ± 3.4 | 78 ± 2.1 | 72 ± 3.0 | 65 ± 5.8 | |
| 2 | 76 ± 2.5 | 75 ± 1.9 | 71 ± 1.5 | 62 ± 5.5 | 55 ± 1.9 | 81 ± 2.8 | 76 ± 1.9 | 71 ± 3.0 | 67 ± 3.2 | 64 ± 2.9 | |
| C. krusei ATCC 6258 | 0.5 | 86 ± 3.0 | 83 ± 2.5 | 81 ± 3.9 | 73 ± 2.5 | 69 ± 3.9 | 87 ± 2.1 | 87 ± 2.3 | 82 ± 5.6 | 77 ± 4.5 | 75 ± 4.5 |
| 1 | 85 ± 0.0 | 84 ± 3.3 | 75 ± 3.2 | 71 ± 2.6 | 65 ± 5.0 | 83 ± 2.5 | 84 ± 3.2 | 76 ± 4.7 | 73 ± 3.6 | 69 ± 3.4 | |
| 2 | 84 ± 2.7 | 78 ± 4.5 | 74 ± 4.9 | 68 ± 4.7 | 61 ± 3.2 | 82 ± 2.8 | 79 ± 3.5 | 75 ± 3.2 | 72 ± 2.5 | 67 ± 2.8 | |
| Organosulfur Compound | Initial Concentration | Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 | Sample 6 |
|---|---|---|---|---|---|---|---|
| PTS | 50 mg/mL | 82.7 ± 0.1 | 70.8 ± 0.3 | 51.2 ± 0.1 | 30.2 ± 0.2 | 17.0 ± 0.3 | 9.4 ± 0.1 |
| 25 mg/mL | 68.6 ± 0.2 | 59.6 ± 0.1 | 42.4 ± 0.3 | 25.1 ± 0.2 | 12.4 ± 0.1 | 5.8 ± 0.2 | |
| 10 mg/mL | 57.3 ± 0.2 | 48.6 ± 0.1 | 35.5 ± 0.1 | 22.1 ± 0.2 | 4.2 ± 0.1 | <LD | |
| 5 mg/mL | 48.5 ± 0.2 | 36.4 ± 0.2 | 28.6 ± 0.3 | 8.5 ± 0.2 | <LD | <LD | |
| 2.5 mg/mL | <LD | <LD | <LD | <LD | <LD | <LD | |
| PTSO | 50 mg/mL | 28.6 ± 0.2 | 26.3 ± 0.2 | 20.9 ± 0.2 | 14.8 ± 0.1 | 9.9 ± 0.4 | 4.2 ± 0.2 |
| 25 mg/mL | 23.4 ± 0.2 | 18.6 ± 0.2 | 14.8 ± 0.2 | 12.7 ± 0.2 | 8.2 ± 0.2 | 2.6 ± 0.1 | |
| 10 mg/mL | 18.7 ± 0.1 | 15.8 ± 0.1 | 13.8 ± 0.1 | 12.9 ± 0.3 | 7.5 ± 0.1 | <LD | |
| 5 mg/mL | 17.9 ± 0.1 | 14.8 ± 0.3 | 13.4 ± 0.2 | 7.5 ± 0.1 | 2.8 ± 0.3 | <LD | |
| 2.5 mg/mL | <LD | <LD | <LD | <LD | <LD | <LD |
| Microorganism | McFarland | Limit of the Microbial Growth | PTS Concentration | PTSO Concentration | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 50 mg/mL | 25 mg/mL | 10 mg/mL | 5 mg/mL | 50 mg/mL | 25 mg/mL | 10 mg/mL | 5 mg/mL | |||
| E. coli ATCC 25922 | 0.5 | Radius | 21.5 | 18 | 15.5 | 11.5 | 28 | 26 | 23 | 15.5 |
| Concentration | 38.1 ± 3.3 | 36.9 ± 1.7 | 32.5 ± 1.4 | 30.3 ± 2.3 | 11.3 ± 0.9 | 9.5 ± 0.9 | 11.1 ± 0.4 | 11.0 ± 2.7 | ||
| 1 | Radius | 19.5 | 17 | 12.5 | 7 | 26.5 | 24.5 | 17.5 | 12.5 | |
| Concentration | 41.9 ± 2.1 | 38.5 ± 1.9 | 38.1 ± 5.0 | 37.8 ± 4.9 | 12.2 ± 1.5 | 10.4 ± 0.5 | 12.9 ± 1.5 | 12.4 ± 0.7 | ||
| 2 | Radius | 18.5 | 17 | 13.5 | 0 | 23.5 | 21 | 14.5 | 7 | |
| Concentration | 43.8 ± 1.8 | 38.8 ± 1.8 | 36.5 ± 2.6 | ≥48.5 | 14.2 ± 1.7 | 12.2 ± 2.3 | 13.8 ± 0.9 | 15.2 ± 1.0 | ||
| K. pneumoniae ATCC 700603 | 0.5 | Radius | 14 | 11 | 8.5 | 2.5 | 15 | 12.5 | 7.5 | 2.5 |
| Concentration | 53.1 ± 3.4 | 49.4 ± 1.9 | 45.3 ± 1.6 | 45.2 ± 1.7 | 19.9 ± 0.5 | 16.7 ± 0.6 | 16.3 ± 0.4 | 17.6 ± 0.6 | ||
| 1 | Radius | 14 | 11.5 | 6 | 0 | 13.5 | 11 | 8 | 1.5 | |
| Concentration | 53.2 ± 4.0 | 48.8 ± 2.0 | 49.6 ± 2.5 | ≥48.5 | 20.9 ± 0.8 | 17.4 ± 0.7 | 16.1 ± 0.6 | 18.0 ± 0.7 | ||
| 2 | Radius | 14 | 11 | 5.5 | 0.5 | 14 | 13 | 9 | 0.5 | |
| Concentration | 53.7 ± 3.7 | 49.4 ± 3.4 | 50.4 ± 2.9 | ≥48,5 | 20.7 ± 0.4 | 16.5 ± 0.6 | 15.8 ± 0.6 | ≥17.9 | ||
| E. faecalis ATCC 29212 | 0.5 | Radius | 17.5 | 15 | 10.5 | 0.5 | 22 | 19 | 10 | 1.5 |
| Concentration | 46.2 ± 4.9 | 42.2 ± 3.6 | 41.3 ± 4.6 | ≥48.5 | 15.2 ± 0.5 | 13.2 ± 1.7 | 15.5 ± 1.1 | 18.0 ± 1.3 | ||
| 1 | Radius | 16.5 | 15 | 4 | 0 | 22 | 17 | 10.5 | 5.5 | |
| Concentration | 48.5 ± 1.8 | 42.8 ± 1.4 | 52.9 ± 7.7 | ≥48.5 | 15.4 ± 0.7 | 14.3 ± 2.0 | 15.3 ± 0.3 | 16.1 ± 3.5 | ||
| 2 | Radius | 15.5 | 14 | 1.5 | 0 | 20 | 17.5 | 7 | 0 | |
| Concentration | 50.5 ± 2.0 | 44.3 ± 2.4 | 57.9 ± 4.7 | ≥48.5 | 16.7 ± 2.1 | 14.0 ± 1.6 | 16.5 ± 2.0 | ≥17.9 | ||
| S. aureus ATCC 29213 | 0.5 | Radius | 22.5 | 19.5 | 15.5 | 14 | 28 | 26.5 | 25 | 15.5 |
| Concentration | 36.1 ± 3.7 | 34.0 ± 2.9 | 32.8 ± 2.1 | 26.1 ± 2.0 | 11.3 ± 0.7 | 9.3 ± 0.5 | 10.4 ± 0.4 | 10.9 ± 2.6 | ||
| 1 | Radius | 21 | 19 | 14.5 | 5.5 | 27.5 | 26 | 23.5 | 10.5 | |
| Concentration | 39.4 ± 1.9 | 35.4 ± 2.2 | 34.2 ± 1.8 | 40.6 ± 8.8 | 11.7 ± 1.0 | 9.5 ± 0.7 | 10.9 ± 0.5 | 13.5 ± 1.8 | ||
| 2 | Radio | 19.5 | 17 | 12.5 | 6 | 25 | 26 | 14.5 | 9.5 | |
| Concentration | 42.3 ± 2.2 | 38.6 ± 1.9 | 38.0 ± 2.5 | 39.1 ± 6.1 | 13.3 ± 1.7 | 11.1 ± 0.7 | 13.8 ± 2.3 | 14.0 ± 1.1 | ||
| C. albicans ATCC 200955 | 0.5 | Radius | 41 | 38.5 | 37 | 34.5 | 42 | 41 | 38.5 | 35 |
| Concentration | ≤9.4 | ≤5.8 | ≤ 4.2 | ≤8.5 | ≤4.2 | ≤2.6 | ≤7.5 | ≤2.8 | ||
| 1 | Radius | 39.5 | 38.5 | 34.5 | 32 | 42 | 40.5 | 39 | 36 | |
| Concentration | ≤9.4 | ≤5.8 | ≤4.2 | ≤8.5 | ≤4.2 | ≤2.6 | ≤7.5 | ≤2.8 | ||
| 2 | Radius | 38 | 37.5 | 35.5 | 31 | 40.5 | 38 | 35.5 | 33.5 | |
| Concentration | ≤9.4 | ≤5.8 | ≤4.2 | ≤8.5 | ≤4.2 | ≤2.6 | ≤7.5 | ≤2.8 | ||
| C. krusei ATCC 6258 | 0.5 | Radius | 43 | 41.5 | 40.5 | 36.5 | 43.5 | 43.5 | 41 | 38.5 |
| Concentration | ≤9.4 | ≤5.8 | ≤4.2 | ≤8.5 | ≤4.2 | ≤2.6 | ≤7.5 | ≤2.8 | ||
| 1 | Radius | 42.5 | 42 | 37.5 | 35.5 | 41.5 | 42 | 38 | 36.5 | |
| Concentration | ≤9.4 | ≤5.8 | ≤4.2 | ≤8.5 | ≤4.2 | ≤2.6 | ≤7.5 | ≤2.8 | ||
| 2 | Radius | 42 | 36 | 37 | 34 | 41 | 39.5 | 37.5 | 36 | |
| Concentration | ≤9.4 | ≤5.8 | ≤4.2 | ≤8.5 | ≤4.2 | ≤2.6 | ≤7.5 | ≤2.8 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorlozano-Puerto, A.; Albertuz-Crespo, M.; Lopez-Machado, I.; Gil-Martinez, L.; Ariza-Romero, J.J.; Maroto-Tello, A.; Baños-Arjona, A.; Gutierrez-Fernandez, J. Antibacterial and Antifungal Activity of Propyl-Propane-Thiosulfinate and Propyl-Propane-Thiosulfonate, Two Organosulfur Compounds from Allium cepa: In Vitro Antimicrobial Effect via the Gas Phase. Pharmaceuticals 2021, 14, 21. https://doi.org/10.3390/ph14010021
Sorlozano-Puerto A, Albertuz-Crespo M, Lopez-Machado I, Gil-Martinez L, Ariza-Romero JJ, Maroto-Tello A, Baños-Arjona A, Gutierrez-Fernandez J. Antibacterial and Antifungal Activity of Propyl-Propane-Thiosulfinate and Propyl-Propane-Thiosulfonate, Two Organosulfur Compounds from Allium cepa: In Vitro Antimicrobial Effect via the Gas Phase. Pharmaceuticals. 2021; 14(1):21. https://doi.org/10.3390/ph14010021
Chicago/Turabian StyleSorlozano-Puerto, Antonio, Maria Albertuz-Crespo, Isaac Lopez-Machado, Lidia Gil-Martinez, Juan Jose Ariza-Romero, Alba Maroto-Tello, Alberto Baños-Arjona, and Jose Gutierrez-Fernandez. 2021. "Antibacterial and Antifungal Activity of Propyl-Propane-Thiosulfinate and Propyl-Propane-Thiosulfonate, Two Organosulfur Compounds from Allium cepa: In Vitro Antimicrobial Effect via the Gas Phase" Pharmaceuticals 14, no. 1: 21. https://doi.org/10.3390/ph14010021
APA StyleSorlozano-Puerto, A., Albertuz-Crespo, M., Lopez-Machado, I., Gil-Martinez, L., Ariza-Romero, J. J., Maroto-Tello, A., Baños-Arjona, A., & Gutierrez-Fernandez, J. (2021). Antibacterial and Antifungal Activity of Propyl-Propane-Thiosulfinate and Propyl-Propane-Thiosulfonate, Two Organosulfur Compounds from Allium cepa: In Vitro Antimicrobial Effect via the Gas Phase. Pharmaceuticals, 14(1), 21. https://doi.org/10.3390/ph14010021