Hybrid Multimodal Imaging Synthons for Chemoselective and Efficient Biomolecule Modification with Chelator and Near-Infrared Fluorescent Cyanine Dye
Abstract
1. Introduction
2. Results and Discussion
2.1. Development of a Suitable Synthetic Pathway towards NIR Cyanine Dye- and Chelator-Comprising MIS Which Can Be Introduced into Biomolecules via Click Chemistry
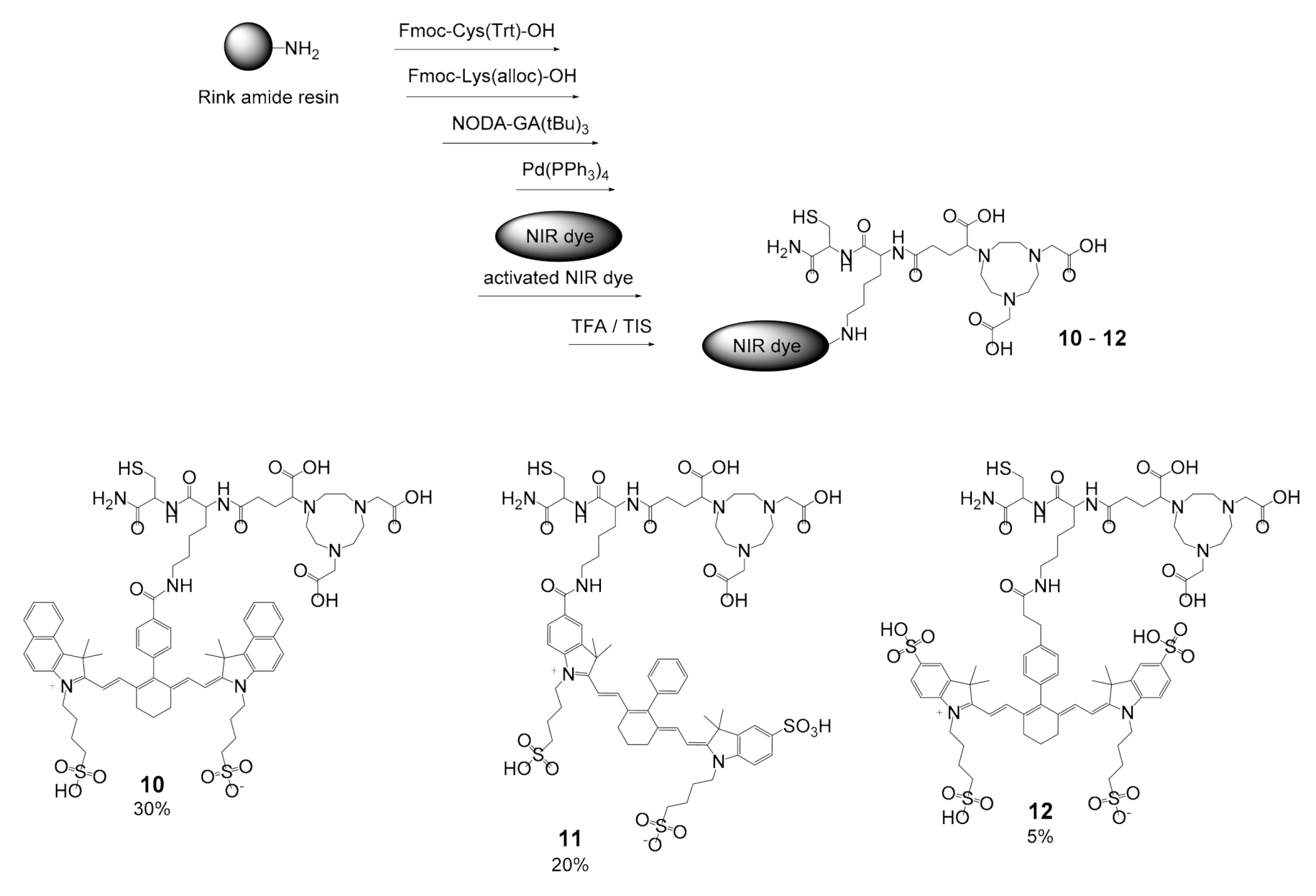
2.2. Introduction of MIS 10–12 into a Model Biomolecule
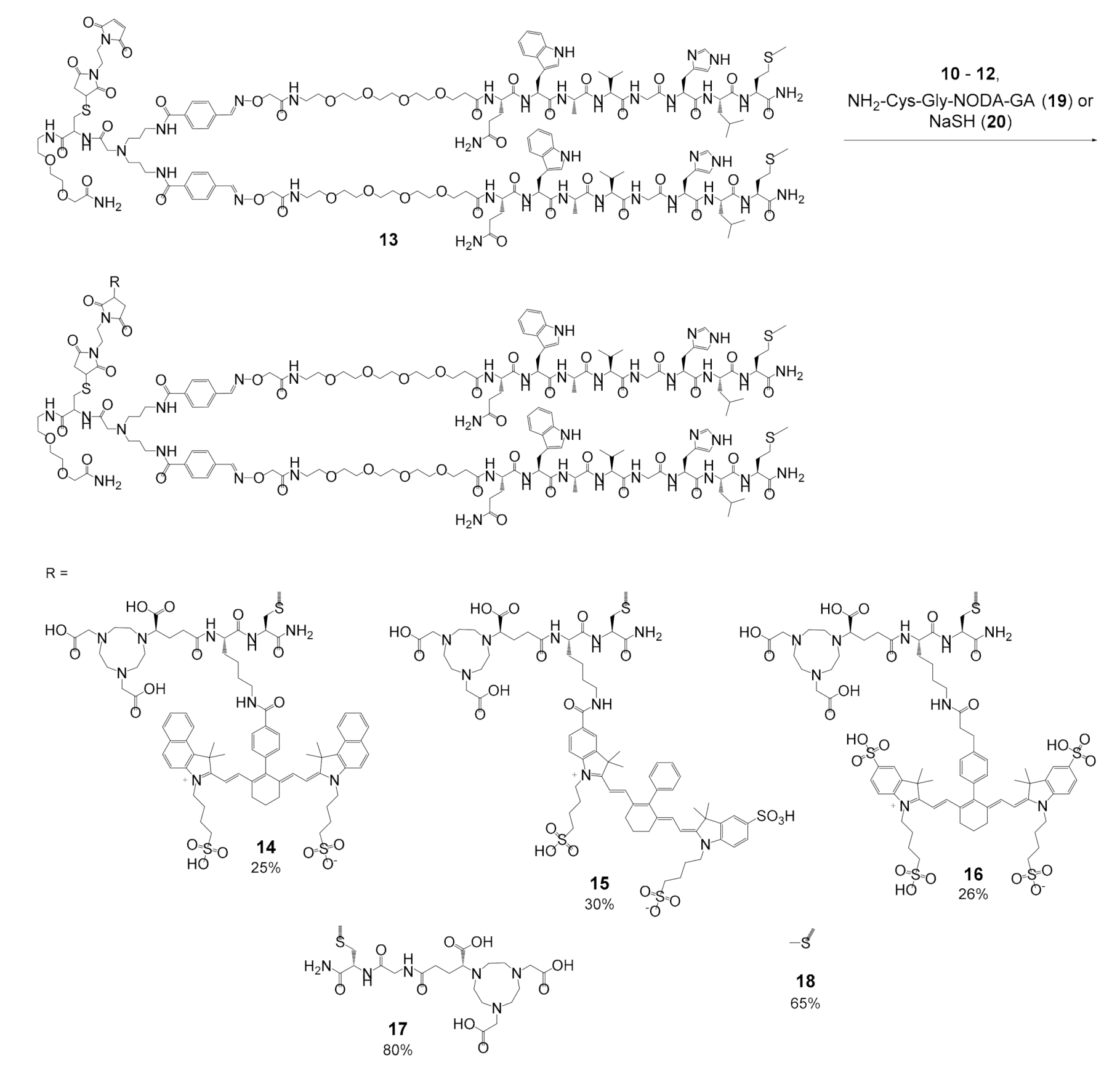
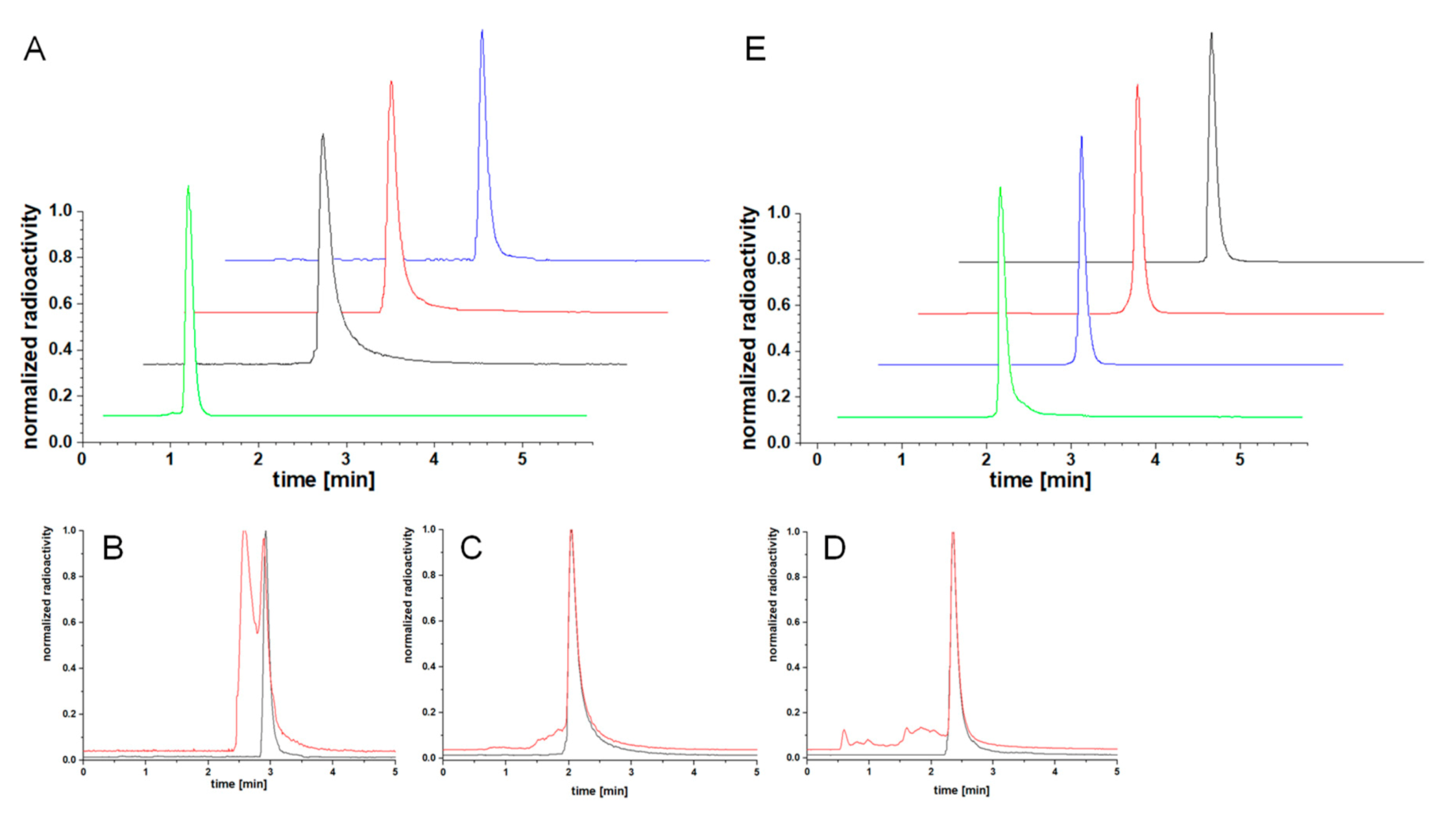
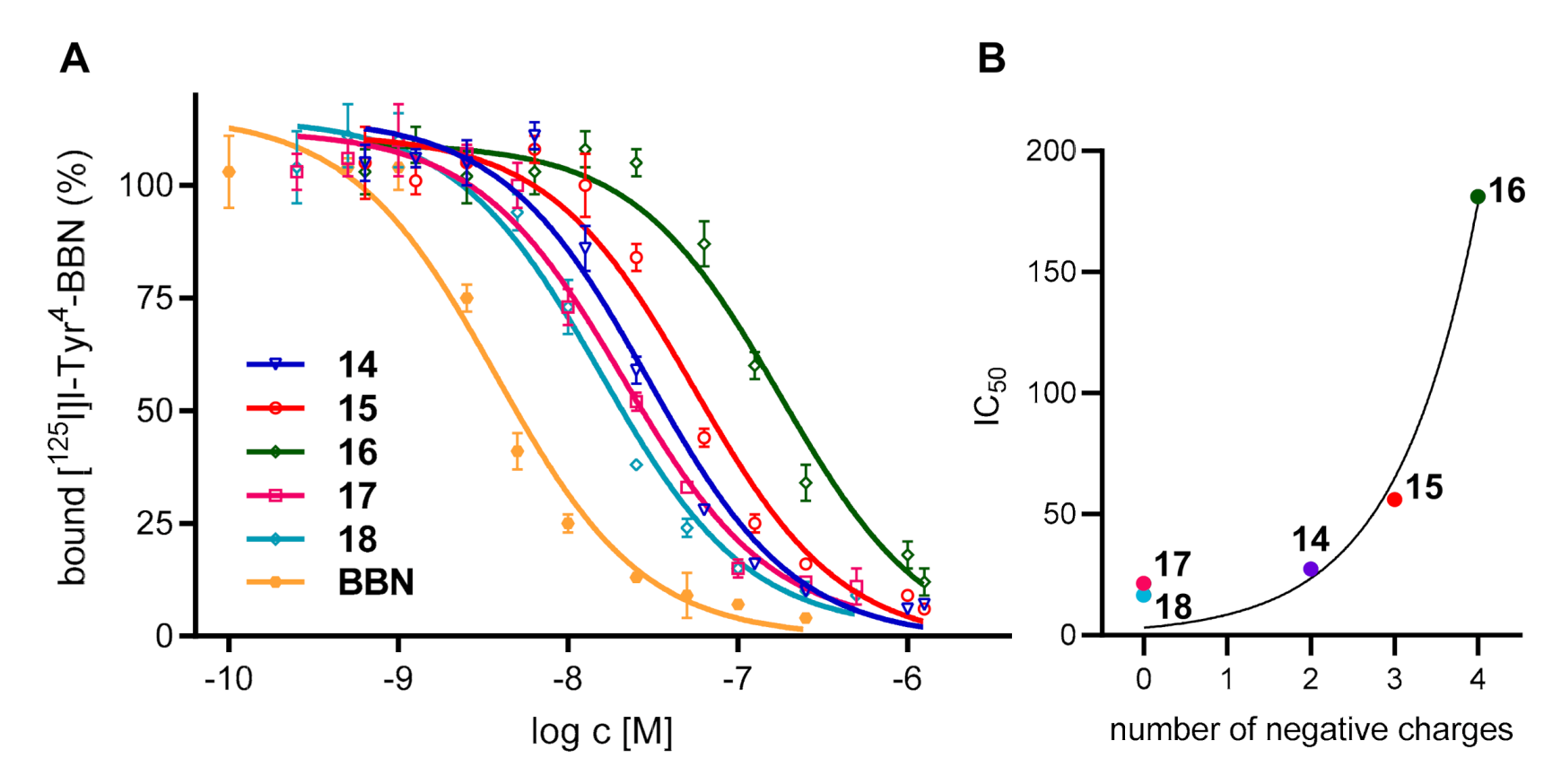
2.3. Determination of the 68Ga-Radiolabeling Efficiency of the MIS 10–12 and 19 and their Bioconjugates 14–17: Influence of the MIS on the Chemical, Biological and Photophysical Properties of the Resulting Peptide-Based Bioconjugates
3. Materials and Methods
3.1. General
3.2. General Synthesis of the Target MIS 10–12 and 19
3.3. General Synthesis of Bioconjugates 14–16 and the Reference Compounds 17 and 18
3.4. Radiochemistry
3.5. Determination of logDs
3.6. Competitive Receptor Binding Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Louie, A.Y. Multimodality imaging probes: Design and challenges. Chem. Rev. 2010, 110, 3146–3195. [Google Scholar] [CrossRef] [PubMed]
- Ni, D.L.; Ehlerding, E.B.; Cai, W.B. Multimodality imaging agents with PET as the fundamental pillar. Angew. Chem. Int. Ed. 2019, 58, 2570–2579. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Gao, Z.H.; Li, G.M.; Wang, T.D. Dual-modal in vivo fluorescence and photoacoustic imaging using a heterodimeric peptide. Chem. Commun. 2018, 54, 13196–13199. [Google Scholar] [CrossRef]
- Lee, S.; Xie, J.; Chen, X.Y. Peptides and peptide hormones for molecular imaging and disease diagnosis. Chem. Rev. 2010, 110, 3087–3111. [Google Scholar] [CrossRef] [PubMed]
- Achilefu, S. Lighting up tumors with receptor-specific optical molecular probes. Technol. Cancer Res. Treat. 2004, 3, 393–409. [Google Scholar] [CrossRef] [PubMed]
- Baranski, A.C.; Schafer, M.; Bauder-Wust, U.; Roscher, M.; Schmidt, J.; Stenau, E.; Simpfendorfer, T.; Teber, D.; Maier-Hein, L.; Hadaschik, B.; et al. PSMA-11-Derived Dual-Labeled PSMA inhibitors for preoperative PET imaging and precise fluorescence-guided surgery of prostate cancer. J. Nucl. Med. 2018, 59, 639–645. [Google Scholar] [CrossRef]
- An, F.F.; Chan, M.; Kommidi, H.; Ting, R. Dual PET and Near-Infrared fluorescence imaging probes as tools for imaging in oncology. Am. J. Roentgenol. 2016, 207, 266–273. [Google Scholar] [CrossRef]
- van Dam, G.M.; Themelis, G.; Crane, L.M.A.; Harlaar, N.J.; Pleijhuis, R.G.; Kelder, W.; Sarantopoulos, A.; de Jong, J.S.; Arts, H.J.G.; van der Zee, A.G.J.; et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-alpha targeting: First in-human results. Nat. Med. 2011, 17, 1315–1319. [Google Scholar] [CrossRef]
- Renard, E.; Dancer, P.A.; Portal, C.; Denat, F.; Prignon, A.; Goncalves, V. Design of bimodal ligands of neurotensin receptor 1 for positron emission tomography imaging and fluorescence-guided surgery of pancreatic cancer. J. Med. Chem. 2020, 63, 2426–2433. [Google Scholar] [CrossRef]
- Nguyen, Q.T.; Tsien, R.Y. Fluorescence-guided surgery with live molecular navigation—A new cutting edge. Nat. Rev. Cancer 2013, 13, 653–662. [Google Scholar] [CrossRef]
- Lim, W.; Jo, G.; Kim, E.J.; Cho, H.; Park, M.H.; Hyun, H. Zwitterionic near-infrared fluorophore for targeted photothermal cancer therapy. J. Mater. Chem. B 2020, 8, 2589–2597. [Google Scholar] [CrossRef] [PubMed]
- Seibold, U.; Wängler, B.; Schirrmacher, R.; Wängler, C. Bimodal imaging probes for combined PET and OI: Recent developments and future directions for hybrid agent development. Biomed. Res. Int. 2014, 2014, 153741. [Google Scholar] [CrossRef] [PubMed]
- Hübner, R.; Cheng, X.; Wängler, B.; Wängler, C. PESIN-Homodimers Combined with Multimodal Molecular Imaging Probes for Positron Emission Tomography (PET) and Optical Imaging (OI): Suited for Tracking of GRPR-Positive Malignant Tissue. Chem. Eur. J. 2020. [Google Scholar] [CrossRef] [PubMed]
- Paulus, A.; Desai, P.; Carney, B.; Carlucci, G.; Reiner, T.; Brand, C.; Weber, W.A. Development of a clickable bimodal fluorescent/PET probe for in vivo imaging. EJNMMI Res. 2015, 5, 43. [Google Scholar] [CrossRef]
- Singh, G.; Gott, M.D.; Pietzsch, H.J.; Stephan, H. Nuclear and optical dual-labelled imaging agents design and challenges. Nuklearmedizin 2016, 55, 41–50. [Google Scholar] [CrossRef]
- Fani, M.; Del Pozzo, L.; Abiraj, K.; Mansi, R.; Tamma, M.L.; Cescato, R.; Waser, B.; Weber, W.A.; Reubi, J.C.; Maecke, H.R. PET of Somatostatin Receptor-Positive Tumors Using Cu-64- and Ga-68-Somatostatin Antagonists: The Chelate Makes the Difference. J. Nucl. Med. 2011, 52, 1110–1118. [Google Scholar] [CrossRef]
- Litau, S.; Seibold, U.; Vall-Sagarra, A.; Fricker, G.; Wangler, B.; Wangler, C. Comparative assessment of complex stabilities of radiocopper chelating agents by a combination of complex challenge and in vivo experiments. ChemMedChem 2015, 10, 1200–1208. [Google Scholar] [CrossRef]
- Ebert, B.; Riefke, B.; Sukowski, U.; Kai, L.C. Cyanine dyes as contrast agents for near-infrared imaging in vivo: Acute tolerance, pharmacokinetics, and fluorescence imaging. J. Biomed. Opt. 2011, 16, 066003. [Google Scholar] [CrossRef]
- Lee, H.; Mason, J.C.; Achilefu, S. Heptamethine cyanine dyes with a robust C-C bond at the central position of the chromophore. J. Org. Chem. 2006, 71, 7862–7865. [Google Scholar] [CrossRef]
- König, S.G.; Krämer, R. Accessing structurally diverse near-infrared cyanine dyes for folate receptor-targeted cancer cell staining. Chem. Eur. J. 2017, 23, 9306–9312. [Google Scholar] [CrossRef]
- Nair, D.P.; Podgorski, M.; Chatani, S.; Gong, T.; Xi, W.; Fenoli, C.R.; Bowman, C.N. The Thiol-Michael Addition Click Reaction: A powerful and widely used tool in materials chemistry. Chem. Mater. 2013, 26, 724–744. [Google Scholar] [CrossRef]
- Chen, H.Y.; Wan, S.N.; Zhu, F.X.; Wang, C.; Cui, S.S.; Du, C.L.; Ma, Y.X.; Gu, Y.Q. A fast tumor-targeting near-infrared fluorescent probe based on bombesin analog for in vivo tumor imaging. Contrast Media Mol. Imaging 2014, 9, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Lindner, S.; Michler, C.; Wängler, B.; Bartenstein, P.; Fischer, G.; Schirrmacher, R.; Wängler, C. PESIN Multimerization Improves Receptor Avidities and in Vivo Tumor Targeting Properties to GRPR-Overexpressing Tumors. Bioconjug. Chem. 2014, 25, 489–500. [Google Scholar] [CrossRef]
- Fischer, G.; Lindner, S.; Litau, S.; Schirrmacher, R.; Wängler, B.; Wängler, C. Next Step toward Optimization of GRP Receptor Avidities: Determination of the Minimal Distance between BBN(7-14) Units in Peptide Homodimers. Bioconjug. Chem. 2015, 26, 1479–1483. [Google Scholar] [CrossRef] [PubMed]
- West, W.; Pearce, S. Dimeric state of cyanine dyes. J. Phys. Chem. 1965, 69, 1894–1903. [Google Scholar] [CrossRef]
- Zhegalova, N.G.; He, S.; Zhou, H.Y.; Kim, D.M.; Berezin, M.Y. Minimization of self-quenching fluorescence on dyes conjugated to biomolecules with multiple labeling sites via asymmetrically charged NIR fluorophores. Contrast Media Mol. Imaging 2014, 9, 355–362. [Google Scholar] [CrossRef]
- Hübner, R.; Benkert, V.; Cheng, X.; Wängler, B.; Krämer, R.; Wängler, C. Probing two PESIN-indocyanine-dye-conjugates: Significance of the used fluorophore. J. Mater. Chem. B 2020, 8, 1302–1309. [Google Scholar] [CrossRef]
- Wetzl, B.K.; Yarmoluk, S.M.; Craig, D.B.; Wolfbeis, O.S. Chameleon labels for staining and quantifying proteins. Angew. Chem. Int. Ed. 2004, 43, 5400–5402. [Google Scholar] [CrossRef]
- Adamczyk, M.; Fishpaugh, J.R.; Heuser, K.J. Preparation of succinimidyl and pentafluorophenyl active esters of 5- and 6-carboxyfluorescein. Bioconjug. Chem. 1997, 8, 253–255. [Google Scholar] [CrossRef]
- Wängler, C.; Maschauer, S.; Prante, O.; Schafer, M.; Schirrmacher, R.; Bartenstein, P.; Eisenhut, M.; Wängler, B. Multimerization of cRGD peptides by click chemistry: Synthetic strategies, chemical limitations, and influence on biological properties. ChemBioChem 2010, 11, 2168–2181. [Google Scholar] [CrossRef]
- Thieriet, N.; Alsina, J.; Giralt, E.; Guibe, F.; Albericio, F. Use of Alloc-amino acids in solid-phase peptide synthesis. Tandem deprotection-coupling reactions using neutral conditions. Tetrahedron Lett. 1997, 38, 7275–7278. [Google Scholar] [CrossRef]

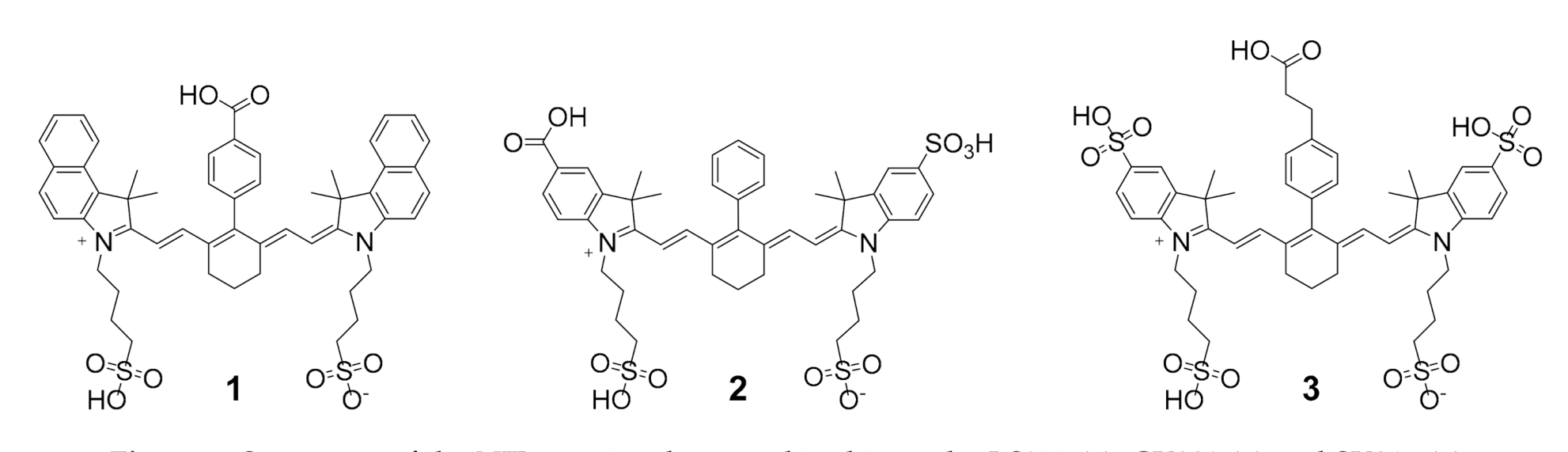
| Compound | RCY ± SD (%) | logD | IC50 (nM) | λmax(abs) (nm) | log ε (M−1 cm−1) | λmax(em) (nm) | Stokes Shift (nm) |
|---|---|---|---|---|---|---|---|
| 1 | - | −1.03 ± 0.11 | - | 804 | 5.00 | 815 | 11 |
| 10 | 98.0 ± 2.1 | −1.29 ± 0.11 | - | 720/790 | 4.80/4.61 | 800 | 10 |
| 14 | 98.3 ± 0.6 | −1.33 ± 0.04 | 27.39 ± 2.01 | 735/810 | 4.57/4.61 | 820 | 10 |
| 2 | - | −4.00 ± 0.11 | - | 761 | 5.44 | 781 | 20 |
| 11 | 98.3 ± 0.8 | −3.05 ± 0.08 | - | 690/760 | 4.88/5.11 | 790 | 30 |
| 15 | 95.8 ± 0.2 | −2.48 ± 0.14 | 56.07 ± 1.47 | 705/770 | 4.58/5.06 | 800 | 30 |
| 3 | - | −3.95 ± 0.15 | - | 756 | 5.32 | 775 | 19 |
| 12 | 96.2 ± 0.6 | −3.47 ± 0.17 | - | 690/760 | 4.83/5.30 | 790 | 30 |
| 16 | 96.8 ± 1.3 | −2.55 ± 0.13 | 181.23 ± 2.45 | 700/770 | 4.50/5.07 | 790 | 20 |
| 19 | 99.0 ± 0.4 | −3.35 ± 0.15 | - | - | - | - | - |
| 17 | 99.1 ± 0.8 | −2.38 ± 0.03 | 21.48 ± 1.22 | - | - | - | - |
| 18 | - | −0.99 ± 0.03 | 16.64 ± 1.06 | - | - | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hübner, R.; von Kiedrowski, V.; Benkert, V.; Wängler, B.; Schirrmacher, R.; Krämer, R.; Wängler, C. Hybrid Multimodal Imaging Synthons for Chemoselective and Efficient Biomolecule Modification with Chelator and Near-Infrared Fluorescent Cyanine Dye. Pharmaceuticals 2020, 13, 250. https://doi.org/10.3390/ph13090250
Hübner R, von Kiedrowski V, Benkert V, Wängler B, Schirrmacher R, Krämer R, Wängler C. Hybrid Multimodal Imaging Synthons for Chemoselective and Efficient Biomolecule Modification with Chelator and Near-Infrared Fluorescent Cyanine Dye. Pharmaceuticals. 2020; 13(9):250. https://doi.org/10.3390/ph13090250
Chicago/Turabian StyleHübner, Ralph, Valeska von Kiedrowski, Vanessa Benkert, Björn Wängler, Ralf Schirrmacher, Roland Krämer, and Carmen Wängler. 2020. "Hybrid Multimodal Imaging Synthons for Chemoselective and Efficient Biomolecule Modification with Chelator and Near-Infrared Fluorescent Cyanine Dye" Pharmaceuticals 13, no. 9: 250. https://doi.org/10.3390/ph13090250
APA StyleHübner, R., von Kiedrowski, V., Benkert, V., Wängler, B., Schirrmacher, R., Krämer, R., & Wängler, C. (2020). Hybrid Multimodal Imaging Synthons for Chemoselective and Efficient Biomolecule Modification with Chelator and Near-Infrared Fluorescent Cyanine Dye. Pharmaceuticals, 13(9), 250. https://doi.org/10.3390/ph13090250







