Current Insights on Antifungal Therapy: Novel Nanotechnology Approaches for Drug Delivery Systems and New Drugs from Natural Sources
Abstract
1. Introduction
- There are 20–40% mortality rates with invasive mycoses, therefore these figures need to be improved;
- The increase in patients undergoing prolonged antifungal therapies reflects the need to develop better fungicidal drugs and thus reduce the length of the treatments and the costs associated;
- There is still space for improvement in pharmacokinetics and pharmacodynamics, in order to reduce the frequency of drug use;
- More attention needs to be given to the host toxicities and drug–drug interactions of current therapy so that their effects can be eliminated or, at least, minimized;
- New therapy groups with different mechanisms of action are needed; this way, these new drugs may synergize with present ones and allow better responses;
- There is an alarming growth in antifungal resistance in all therapeutic groups [8].
- The resistance mechanisms can be prevented by packaging multiple antimicrobial drugs within the same nanoparticle, because the likelihood of multiple simultaneous gene mutations in the same cell is low. The most striking examples are the encapsulation of antifungal drugs in chitosan or silver nanoparticles, combining the antifungal properties of both and decreasing the possibility of drug resistance [104,106];
- Some nanoparticles, such as liposomes and dendrimers, are able to overcome the resistance mechanisms of decreased uptake and increased efflux of drug from the microbial cell. Liposomes are able to quickly fuse with the plasma membrane of the microbial cell and release a high concentration of drug into its plasma membrane or cytoplasm, thereby circumventing the decreased uptake mechanism of resistance. This means a faster delivery and avoidance of the transmembrane pumps that catalyze increased efflux of drugs. Dendrimers, on the other hand, are extensively branched molecules, whose surface can be filled with positively charged quaternary ammonium compounds, which bind to negatively charged microbial cell envelopes and increase membrane permeability. This allows the entrance of more dendrimers to the microbial cell, the flow of its cytoplasmic contents to the exterior, and the ultimate destruction of the microbial cell membrane. This goes to show that dendrimers are also able to surpass the resistance mechanism of decreased uptake of drug [107]. Other nanoparticles, specifically nitric oxide nanoparticles made of silica and zinc oxide nanoparticles are able to overcome biofilm formation by killing the microbes present in already formed biofilms or by inhibiting biofilm formation through the generation of reactive oxygen species, respectively [108,109];
- Nanoparticles have been used to target antifungal drugs to the specific site of infection, allowing the local release of high concentrations of drug, while keeping the total dose of drug administered low. This high local dose is able to destroy the infecting fungi before they can develop resistance, thereby overcoming this worrisome issue and translating into fewer side effects upon the patient [104].
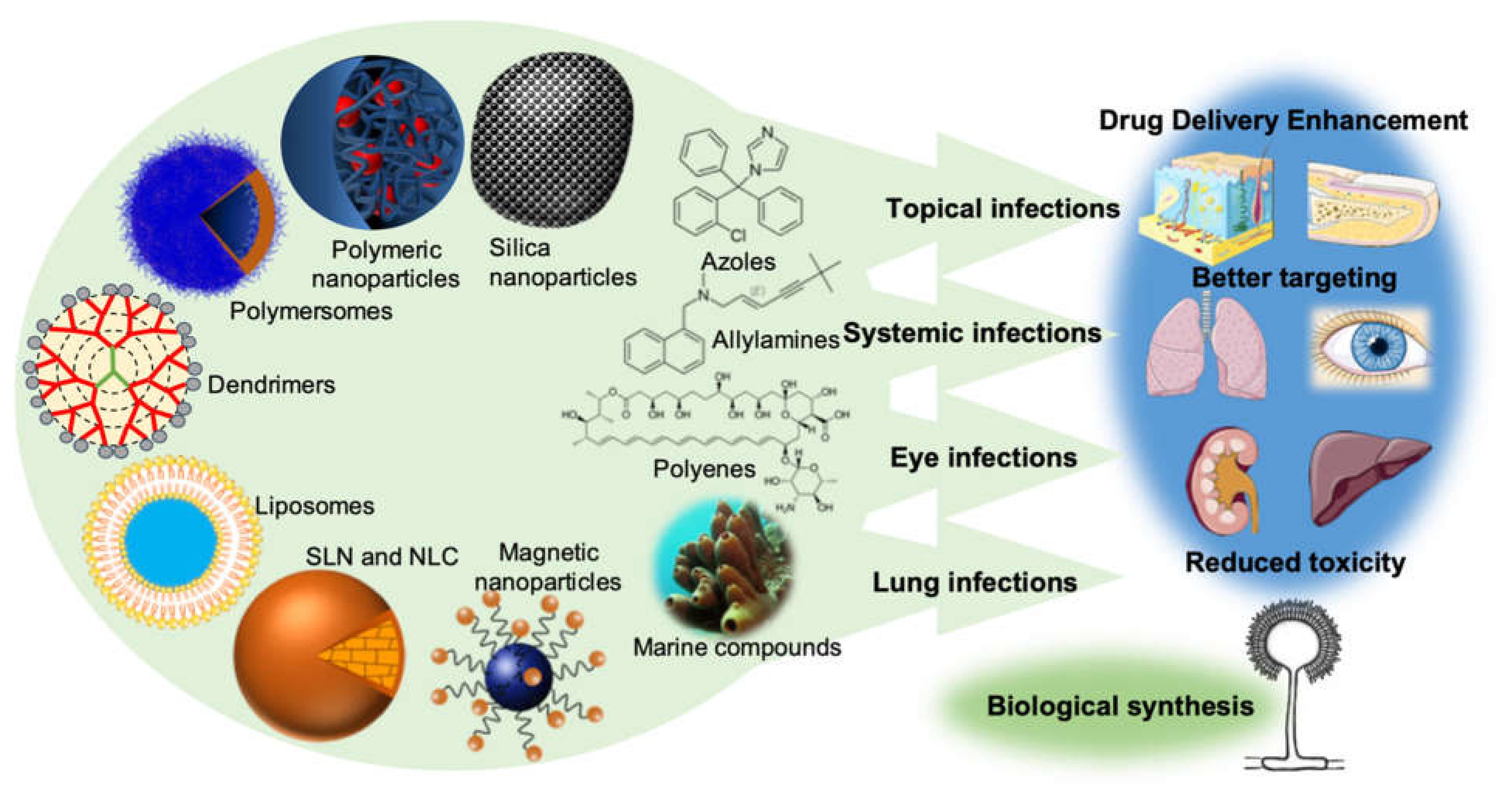
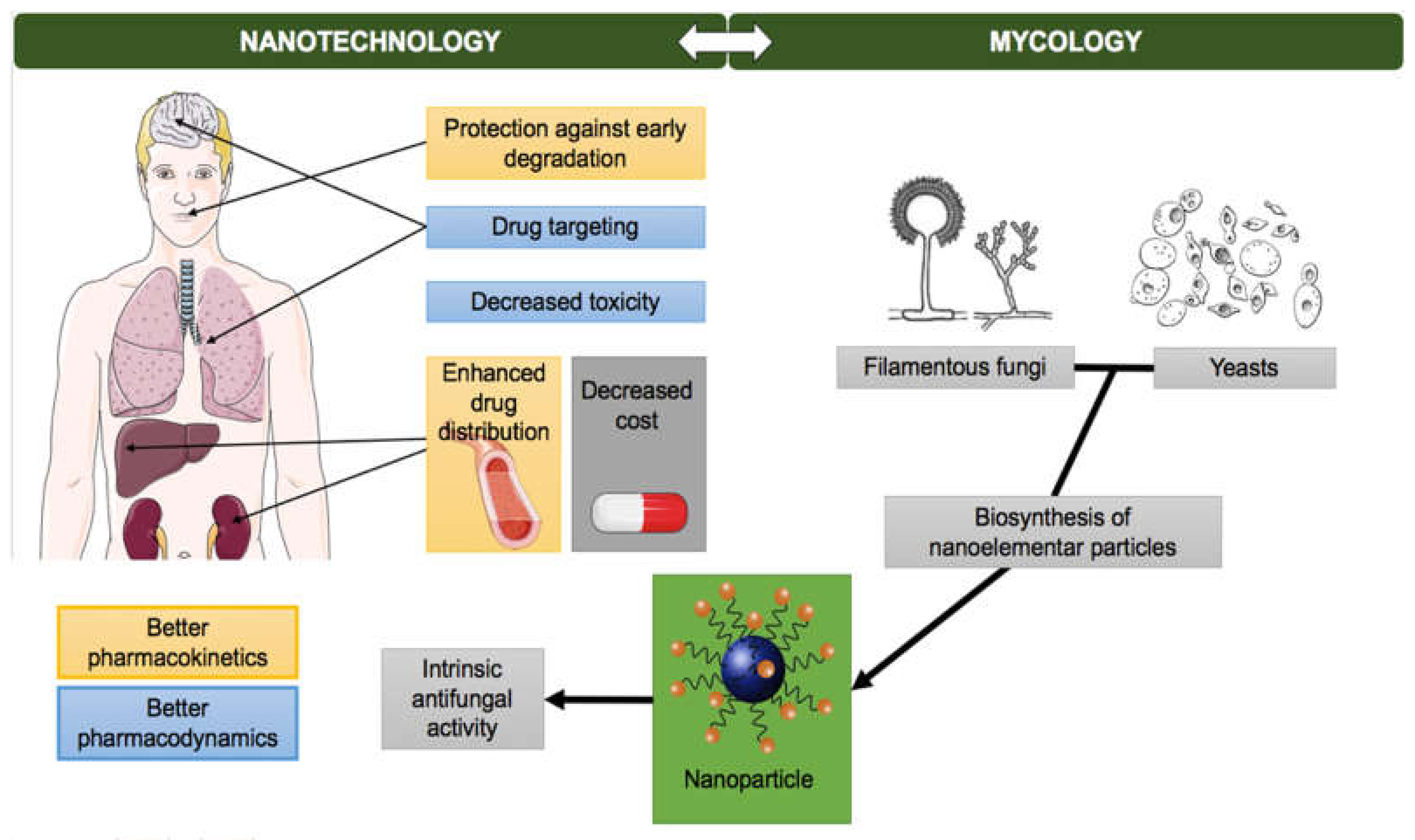
2. Nanotechnology and Mycology
2.1. Antifungal Potential of Nanoparticles
2.2. Synthesis of Nanoparticles by Fungi
2.3. Antifungal Drug Administration
2.3.1. The Transungual Route
2.3.2. Pulmonary Delivery
2.3.3. The Ocular Route
2.4. An Overview of Nanoparticle Types and Their Applicability on Antifungal Therapy
2.4.1. Lipid Nanoparticles
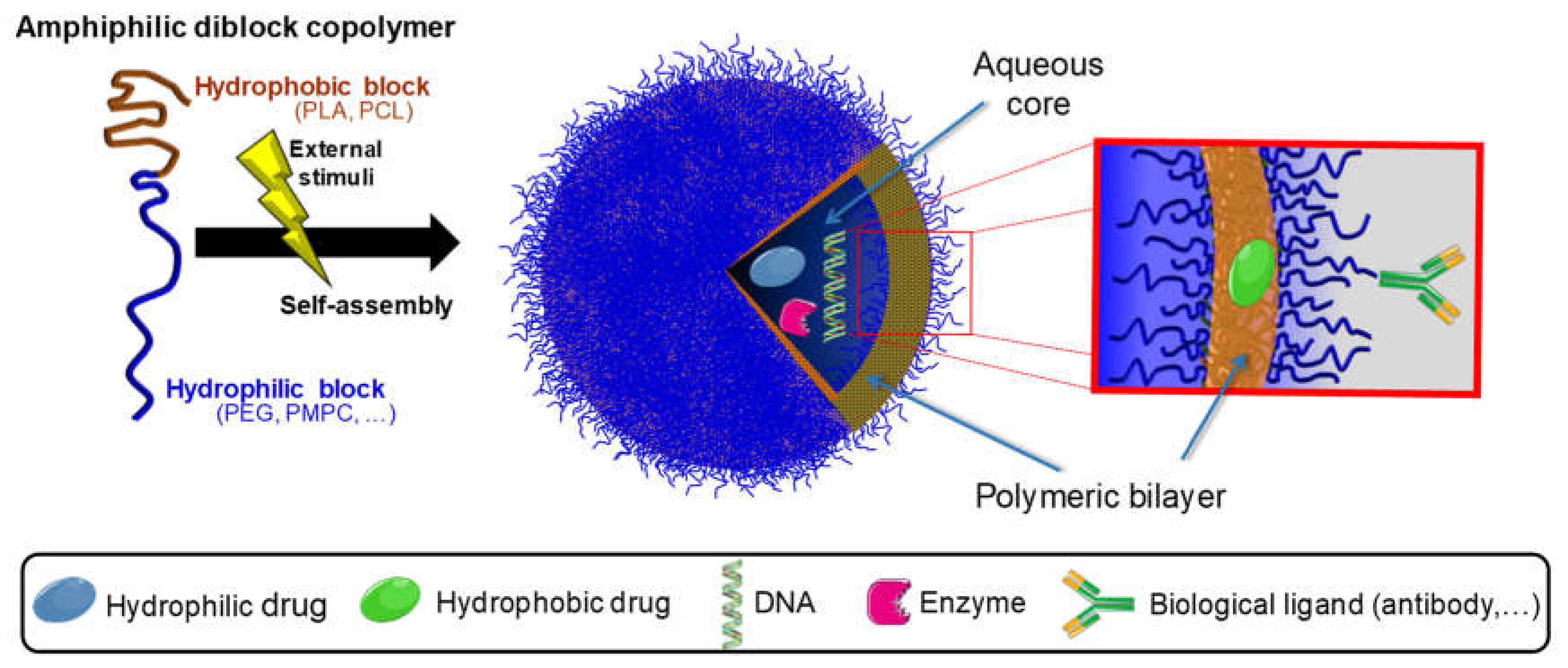
2.4.2. Polymeric Nanoparticles
2.4.3. Metallic Nanoparticles
2.4.4. Other Drug Delivery Systems
- Nitric oxide-silica nanoparticles with proven anti-biofilm activity [108];
- Metal modified silica nanoparticles, which can include silver or copper, metals that have a very well documented antimicrobial effect, derived from the cell membrane and DNA damages, interaction with enzymes from thiol groups or are associated with generating hydrogen peroxide [176];
- Bioglasses and bioceramics [179].
3. Hidden Potential and Challenges of Natural Antifungal Compounds
4. Ongoing Clinical Trials on Myconanotechnology
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rai, M.; Ingle, A.P.; Pandit, R.; Paralikar, P.; Gupta, I.; Anasane, N.; Dolenc-Voljč, M. Nanotechnology for the Treatment of Fungal Infections on Human Skin. In The Microbiology of Skin, Soft Tissue, Bone and Joint Infections; Academic Press: Cambridge, MA, USA, 2017; pp. 169–184. [Google Scholar]
- Pianalto, K.M.; Alspaugh, J.A. New Horizons in Antifungal Therapy. J. Fungi 2016, 2, 26. [Google Scholar] [CrossRef]
- Chang, Y.L.; Yu, S.J.; Heitman, J.; Wellington, M.; Chen, Y.L. New facets of antifungal therapy. Virulence 2017, 8, 222–236. [Google Scholar] [CrossRef] [PubMed]
- Nami, S.; Aghebati-Maleki, A.; Morovati, H.; Aghebati-Maleki, L. Current antifungal drugs and immunotherapeutic approaches as promising strategies to treatment of fungal diseases. Biomed. Pharm. 2019, 110, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.C.; Amaral, A.C. Antifungal Therapy for Systemic Mycosis and the Nanobiotechnology Era: Improving Efficacy, Biodistribution and Toxicity. Front. Microbiol. 2017, 8, 336. [Google Scholar] [CrossRef] [PubMed]
- Scorzoni, L.; de Paula e Silva, A.C.A.; Marcos, C.M.; Assato, P.A.; de Melo, W.C.M.A.; de Oliveira, H.C.; Costa-Orlandi, C.B.; Mendes-Giannini, M.J.S.; Fusco-Almeida, A.M. Antifungal Therapy: New Advances in the Understanding and Treatment of Mycosis. Front. Microbiol. 2017, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Sirish Sadhna Khatry, S.N.; Sadanandan, M. Novel Drug Delivery Systems for Antifungal Therapy. Int. J. Pharm. Pharm. Sci. 2010, 2, 6–9. [Google Scholar]
- Perfect, J.R. Is there an emerging need for new antifungals? Expert Opin Emerg Drugs 2016, 21, 129–131. [Google Scholar] [CrossRef] [PubMed]
- Roli Jain, A.P. A Review of Kinetics of Nanoparticulated Delayed Release Formulations. J. Nanomed. Nanotechnol. 2015, 6, 2. [Google Scholar]
- Hasan, S. A review on Nanoparticles: Their Synthesis and Types. Res. J. Recent Sci. 2015, 4, 1–3. [Google Scholar]
- Nagavarma, B.V.N.; Ayaz, A.H.K.S.Y.; Vasudha, L.S.; Shivahumar, H.G. Different Techniques for Preparation of Polymeric Nanoparticles—A review. Asian J. Pharm. Clin. Res. 2012, 5, 16–23. [Google Scholar]
- Bhatt, P.; Lalani, R.; Vhora, I.; Patil, S.; Amrutiya, J.; Misra, A.; Mashru, R. Liposomes encapsulating native and cyclodextrin enclosed Paclitaxel: Enhanced loading efficiency and its pharmacokinetic evaluation. Int. J. Pharm. 2017, 536, 95–107. [Google Scholar] [CrossRef]
- Jinhyun Hannah Lee, Y.Y. Controlled drug release from pharmaceutical nanocarriers. Chem. Eng. Sci. 2015, 125, 75–84. [Google Scholar]
- D’Souza, S. A Review of In Vitro Drug Release Test Methods for Nano-Sized Dosage Forms. Adv. Pharm. 2014, 2014, 12. [Google Scholar]
- Goyal, R.; Macri, L.K.; Kaplan, H.M.; Kohn, J. Nanoparticles and nanofibers for topical drug delivery. J. Control Release 2016, 240, 77–92. [Google Scholar] [CrossRef]
- Rangari, A.T. Polymeric Nanoparticles Based Topical Drug Delivery: An Overview. Asian J. Biomed. Pharm. Sci. 2015, 5, 5–12. [Google Scholar] [CrossRef]
- Siegel, R.A.; Rathbone, M.J. Chapter 2—Overview of Controlled Release Mechanisms. In Fundamentals and Applications of Controlled Release Drug Delivery, Advances in Delivery Science and Technology; Society, C.R., Ed.; Springer: New York, NY, USA, 2012. [Google Scholar]
- Firthouse, P.U.M.; Halith, S.M.; Wahab, S.U.; Sirajudeen, M.; Mohideen, S.K. Formulation and Evaluation of Miconazole Niosomes. Int. J. Pharmtech Res. 2011, 3, 1019–1022. [Google Scholar]
- Aljaeid, B.M.; Hosny, K.M. Miconazole-loaded solid lipid nanoparticles: Formulation and evaluation of a novel formula with high bioavailability and antifungal activity. Int. J. Nanomed. 2016, 11, 441–447. [Google Scholar] [CrossRef]
- Bhalekar, M.R.; Pokharkar, V.; Madgulkar, A.; Patil, N.; Patil, N. Preparation and evaluation of miconazole nitrate-loaded solid lipid nanoparticles for topical delivery. AAPS Pharmscitech 2009, 10, 289–296. [Google Scholar] [CrossRef]
- Shahzadi, I.; Masood, M.I.; Chowdhary, F.; Anjum, A.A.; Nawaz, M.A.; Maqsood, I.; Zaman, M.Q. Microemulsion Formulation for Topical Delivery of Miconazole Nitrate. Int. J. Pharm. Sci. Rev. Res. 2014, 24, 30–36. [Google Scholar]
- Elmoslemany, R.M.; Abdallah, O.Y.; El-Khordagui, L.K.; Khalafallah, N.M. Propylene glycol liposomes as a topical delivery system for miconazole nitrate: Comparison with conventional liposomes. AAPS Pharmscitech 2012, 13, 723–731. [Google Scholar] [CrossRef]
- Maha, H.L.; Sinaga, K.R.; Masfria, M. Formulation and evaluation of miconazole nitrate nanoemulsion and cream. Asian J. Pharm. Clin. Res. 2018, 11, 319–321. [Google Scholar] [CrossRef]
- Kumar, P.S.; Hematheerthani, N.; Ratna, J.V.; Saikishore, V. Design and characterization of miconazole nitrate loaded nanosponges containing vaginal gels. Int. J. Pharm. Anal. Res. 2016, 5, 410–417. [Google Scholar]
- Qushawy, M.; Nasr, A.; Abd-Alhaseeb, M.; Swidan, S. Design, Optimization and Characterization of a Transfersomal Gel Using Miconazole Nitrate for the Treatment of Candida Skin Infections. Pharmaceutics 2018, 10, 26. [Google Scholar] [CrossRef]
- Ge, S.; Lin, Y.; Lu, H.; Li, Q.; He, J.; Chen, B.; Wu, C.; Xu, Y. Percutaneous delivery of econazole using microemulsion as vehicle: Formulation, evaluation and vesicle-skin interaction. Int. J. Pharm. 2014, 465, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Evelyn, D.; Wooi, C.C.; Kumar, J.R.; Muralidharan, S.; Dhanaraj, S.A. Development and evaluation of microemulsion based gel (MBGs) containing econazole nitrate for nail fungal infection. J. Pharm. Res. 2012, 5, 2385–2390. [Google Scholar]
- Sanna, V.; Gavini, E.; Cossu, M.; Rassu, G.; Giunchedi, P. Solid lipid nanoparticles (SLN) as carriers for the topical delivery of econazole nitrate: In-vitro characterization, ex-vivo and in-vivo studies. J. Pharm. Pharm. 2007, 59, 1057–1064. [Google Scholar] [CrossRef]
- Keshri, L.; Pathak, K. Development of thermodynamically stable nanostructured lipid carrier system using central composite design for zero order permeation of econazole nitrate through epidermis. Pharm. Dev. Technol. 2013, 18, 634–644. [Google Scholar] [CrossRef]
- Xianrong, Q.; Liu, M.H.; Liu, H.Y.; Maitani, Y.; Nagai, T. Topical econazole delivery using liposomal gel. S.T.P. Pharma Sci. 2003, 13, 241–245. [Google Scholar]
- Verma, P.; Pathak, K. Nanosized ethanolic vesicles loaded with econazole nitrate for the treatment of deep fungal infections through topical gel formulation. Nanomedicine 2012, 8, 489–496. [Google Scholar] [CrossRef]
- Verma, S.; Utreja, P. Transethosomes of Econazole Nitrate for Transdermal Delivery: Development, In-vitro Characterization, and Ex-vivo Assessment. Pharm. Nanotechnol. 2018, 6, 171–179. [Google Scholar] [CrossRef]
- Sharma, R.; Walker, R.B.; Pathak, K. Evaluation of the Kinetics and Mechanism of Drug Release from Econazole nitrate Nanosponge Loaded Carbapol Hydrogel. Indian J. Pharm. Educ. Res. 2011, 45, 25–31. [Google Scholar]
- Kumar, Y.P.; Kumar, K.V.; Shekar, R.R.; Ravi, M.; Kishore, V.S. Formulation and Evaluation of Econazole Niosomes. Sch. Acad. J. Pharm. 2013, 2, 315–318. [Google Scholar]
- Bachhav, Y.G.; Mondon, K.; Kalia, Y.N.; Gurny, R.; Möller, M. Novel micelle formulations to increase cutaneous bioavailability of azole antifungals. J. Control. Release 2011, 153, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Youenang Piemi, M.P.; Korner, D.; Benita, S.; Jean-Paul, M. Positively and negatively charged submicron emulsions for enhanced topical delivery of antifungal drugs. J. Control. Release 1999, 58, 177–187. [Google Scholar] [CrossRef]
- Souto, E.B.; Muller, R.H. SLN and NLC for topical delivery of ketoconazole. J. Microencapsul. 2005, 22, 501–510. [Google Scholar] [CrossRef]
- Shirsand, S.; Para, M.; Nagendrakumar, D.; Kanani, K.; Keerthy, D. Formulation and evaluation of Ketoconazole niosomal gel drug delivery system. Int. J. Pharm. Investig. 2012, 2, 201–207. [Google Scholar] [CrossRef]
- Tiwari, N.; Sivakumar, A.; Mukherjee, A.; Chandrasekaran, N. Enhanced antifungal activity of Ketoconazole using rose oil based novel microemulsion formulation. J. Drug Deliv. Sci. Technol. 2018, 47, 434–444. [Google Scholar] [CrossRef]
- Kakkar, S.; Kaur, I.P. Spanlastics—A novel nanovesicular carrier system for ocular delivery. Int. J. Pharm. 2011, 413, 202–210. [Google Scholar] [CrossRef]
- Winnicka, K.; Wroblewska, M.; Wieczorek, P.; Sacha, P.T.; Tryniszewska, E. Hydrogel of ketoconazole and PAMAM dendrimers: Formulation and antifungal activity. Molecules 2012, 17, 4612–4624. [Google Scholar] [CrossRef]
- Ashe, S.; Nayak, D.; Tiwari, G.; Rauta, P.R.; Nayak, B. Development of liposome-encapsulated ketoconazole: Formulation, characterisation and evaluation of pharmacological therapeutic efficacy. Micro. Nano Lett. 2015, 10, 126–129. [Google Scholar] [CrossRef]
- Ning, M.; Guo, Y.; Pan, H.; Chen, X.; Gu, Z. Preparation, in vitro and in vivo evaluation of liposomal/niosomal gel delivery systems for clotrimazole. Drug Dev. Ind. Pharm. 2005, 31, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.S.; Sheri, P.S.; Kuriachan, M.A. Formulation and Evaluation of Antifungal Nanosponge Loaded Hydrogel for Topical Delivery. Int. J. Pharm. Pharm. Res. 2018, 13, 362–379. [Google Scholar]
- Akhtar, N.; Pathak, K. Cavamax W7 Composite Ethosomal Gel of Clotrimazole for Improved Topical Delivery: Development and Comparison with Ethosomal Gel. Am. Assoc. Pharm. Sci. 2012, 13, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Shirsand, S.; Kumar, R.; Keshavshetti, G.; Bushetti, S.S.; Padivala, V.S. Formulation and Evaluation of Clotrimazole Niosomal Gel for Topical Application. Rajiv Gandhi Univ. Health Sci. J. Pharm. Sci. 2015, 5, 32–38. [Google Scholar] [CrossRef]
- Yassin, G.E. Formulation and Evaluation of Optimized Clotrimazole Emulgel Formulations. Br. J. Pharm. Res. 2014, 4, 1014–1030. [Google Scholar] [CrossRef]
- Souto, E.B.; Wissing, S.A.; Barbosa, C.M.; Müller, R.H. Development of a controlled release formulation based on SLN and NLC for topical clotrimazole delivery. Int. J. Pharm. 2004, 278, 71–77. [Google Scholar] [CrossRef]
- Kaewbanjong, J.; Heng, P.W.S.; Boonme, P. Clotrimazole microemulsion and microemulsion-based gel: Evaluation of buccal drug delivery and irritancy using chick chorioallantoic membrane as the model. J. Pharm. Pharm. 2017, 69, 1716–1723. [Google Scholar] [CrossRef]
- Bachhav, Y.G.; Patravale, V.B. Microemulsion-based vaginal gel of clotrimazole: Formulation, in vitro evaluation, and stability studies. AAPS Pharmscitech 2009, 10, 476–481. [Google Scholar] [CrossRef]
- Maheshwari, R.G.S.; Tekade, R.K.; Sharma, P.A.; Darwhekar, G.; Tyagi, A.; Patel, R.P.; Jain, D.K. Ethosomes and ultradeformable liposomes for transdermal delivery of clotrimazole: A comparative assessment. Saudi Pharm. J. Spj. Off. Publ. Saudi Pharm. Soc. 2012, 20, 161–170. [Google Scholar] [CrossRef]
- Zheng, W.S.; Fang, X.Q.; Wang, L.L.; Zhang, Y.J. Preparation and quality assessment of itraconazole transfersomes. Int. J. Pharm. 2012, 436, 291–298. [Google Scholar] [CrossRef]
- Mohanty, B.; Majumdar, D.K.; Mishra, S.K.; Panda, A.K.; Patnaik, S. Development and characterization of itraconazole-loaded solid lipid nanoparticles for ocular delivery. Pharm. Dev. Technol. 2015, 20, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Pardeike, J.; Weber, S.; Haber, T.; Wagner, J.; Zarfl, H.P.; Plank, H.; Zimmer, A. Development of an itraconazole-loaded nanostructured lipid carrier (NLC) formulation for pulmonary application. Int. J. Pharm. 2011, 419, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Wagh, V.D.; Deshmukh, O.J. Itraconazole Niosomes Drug Delivery System and Its Antimycotic Activity against Candida albicans. Isrn. Pharm. 2012, 2012, 653465. [Google Scholar] [CrossRef] [PubMed]
- Chudasama, A.; Patel, V.; Nivsarkar, M.; Vasu, K.; Shishoo, C. Investigation of microemulsion system for transdermal delivery of itraconazole. J. Adv. Pharm. Technol. Res. 2011, 2, 30–38. [Google Scholar]
- Leal, A.F.; Leite, M.C.; Medeiros, C.S.; Cavalcanti, I.M.; Wanderley, A.G.; Magalhaes, N.S.; Neves, R.P. Antifungal activity of a liposomal itraconazole formulation in experimental Aspergillus flavus keratitis with endophthalmitis. Mycopathologia 2015, 179, 225–229. [Google Scholar] [CrossRef]
- Leal, A.F.; Leite, M.C.; Medeiros, C.S.; Cavalcanti, I.M.; Wanderley, A.G.; Magalhaes, N.S.; Neves, R.P. Development of an itraconazole encapsulated polymeric nanoparticle platform for effective antifungal therapy. J. Mater. Chem. B 2016, 4, 1787–1796. [Google Scholar]
- ElMeshad, A.N.; Mohsen, A.M. Enhanced corneal permeation and antimycotic activity of itraconazole against Candida albicans via a novel nanosystem vesicle. Drug Deliv. 2016, 23, 2115–2123. [Google Scholar] [CrossRef]
- Mellaerts, R.; Mols, R.; Jammaer, J.A.G.; Aerts, C.A.; Annaert, P.; Van Humbeeck, J.; Van den Mooter, G.; Augustijns, P.; Martens, J.A. Increasing the oral bioavailability of the poorly water soluble drug itraconazole with ordered mesoporous silica. Eur. J. Pharm. Biopharm. 2008, 69, 223–230. [Google Scholar] [CrossRef]
- Bachhav, Y.G.; Patravale, V.B. Microemulsion based vaginal gel of fluconazole: Formulation, in vitro and in vivo evaluation. Int. J. Pharm. 2009, 365, 175–179. [Google Scholar] [CrossRef]
- Soliman, O.A.E.; Mohamed, E.A.; Khatera, N.A.A. Enhanced ocular bioavailability of fluconazole from niosomal gels and microemulsions: Formulation, optimization, and in vitro-in vivo evaluation. Pharm. Dev. Technol. 2017, 24, 1–52. [Google Scholar] [CrossRef]
- Gupta, S.K.; Dhingra, N.; Velpandian, T.; Jaiswal, J. Efficacy of fluconazole and liposome entrapped fluconazole for C. albicans induced experimental mycotic endophthalmitis in rabbit eyes. Acta Ophthalmol. Scand 2000, 78, 448–450. [Google Scholar] [CrossRef] [PubMed]
- El-Housiny, S.; Shams Eldeen, M.A.; El-Attar, Y.A.; Salem, H.A.; Attia, D.; Bendas, E.R.; El-Nabarawi, M.A. Fluconazole-loaded solid lipid nanoparticles topical gel for treatment of pityriasis versicolor: Formulation and clinical study. Drug Deliv. 2017, 25, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Kelidari, H.R.; Moazeni, M.; Babaei, R.; Saeedi, M.; Akbari, J.; Parkoohi, P.I.; Nabili, M.; Morteza-Semnani, A.A.G.K.; Nokhodchi, A. Improved Yeast Delivery of Fluconazole with a Nanostructured Lipid Carrier System. Biomed. Pharmacother. 2017, 89, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Moin, A.; Deb, T.K.; Osmani, R.A.M.; Bhosale, R.R.; Hani, U. Fabrication, characterization, and evaluation of microsponge delivery system for facilitated fungal therapy. J. Basic Clin. Pharm. 2016, 7, 39–48. [Google Scholar] [PubMed]
- Indora, N.; Kaushik, D. Design, development and evaluation of ethosomal gel of fluconazole for topical fungal infection. Int. J. Eng. Sci. Invent. Res. Dev. 2015, 1, 280–306. [Google Scholar]
- Kaur, I.P.; Rana, C.; Singh, M.; Bhushan, S.; Singh, H.; Kakkar, S. Development and evaluation of novel surfactant-based elastic vesicular system for ocular delivery of fluconazole. J. Ocul. Pharm. 2012, 28, 484–496. [Google Scholar] [CrossRef]
- Lalit, S.K.; Panwar, A.S.; Darwhekar, G.; Jain, D.K. Formulation and Evaluation of Fluconazole Amphiphilogel. Der. Pharm. Lett. 2011, 3, 125–131. [Google Scholar]
- Kumar, R.; Sinha, V.R. Preparation and optimization of voriconazole microemulsion for ocular delivery. Colloids Surf. B Biointerfaces 2014, 117, 82–88. [Google Scholar] [CrossRef]
- Basaran, E.; Karaca, H.; Yenilmez, E.; Guven, U. Voriconazole incorporated polymeric nanoparticles for ocular application. Lat. Am. J. Pharm. 2017, 36, 1983–1994. [Google Scholar]
- Das, P.J.; Paul, P.; Mukherjee, B.; Mazumder, B.; Mondal, L.; Baishya, R.; Debnath, M.C.; Dey, K.S. Pulmonary Delivery of Voriconazole Loaded Nanoparticles Providing a Prolonged Drug Level in Lungs: A Promise for Treating Fungal Infection. Mol. Pharm. 2015, 12, 2651–2664. [Google Scholar] [CrossRef]
- Pandurangan, D.K.; Bodagala, P.; Palanirajan, V.K.; Govindaraj, S. Formulation and evaluation of voriconazole ophthalmic solid lipid nanoparticles in situ gel. Int. J. Pharm. Investig. 2016, 6, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Song, C.K.; Balakrishnan, P.; Shim, C.K.; Chung, S.-J.; Chong, S.; Kim, D.-D. A novel vesicular carrier, transethosome, for enhanced skin delivery of voriconazole: Characterization and in vitro/in vivo evaluation. Colloids Surf. B Biointerfaces 2011, 92, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Faisal, W.; Soliman, G.M.; Hamdan, A.M. Enhanced skin deposition and delivery of voriconazole using ethosomal preparations. J. Liposome. Res. 2018, 28, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Tanriverdi, S.T.; Hilmioglu Polat, S.; Yesim Metin, D.; Kandiloglu, G.; Ozer, O. Terbinafine hydrochloride loaded liposome film formulation for treatment of onychomycosis: In vitro and in vivo evaluation. J. Liposome Res. 2016, 26, 163–173. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Liu, D.-Z.; Liu, J.-J.; Chang, T.-W.; Ho, H.-O.; Sheu, M.-T. Development of terbinafine solid lipid nanoparticles as a topical delivery system. Int. J. Nanomed. 2012, 7, 4409–4418. [Google Scholar]
- Ghannoum, M.; Isham, N.; Henry, W.; Kroon, H.A.; Yurdakul, S. Evaluation of the morphological effects of TDT 067 (terbinafine in Transfersome) and conventional terbinafine on dermatophyte hyphae in vitro and in vivo. Antimicrob. Agents Chemother. 2012, 56, 2530–2534. [Google Scholar] [CrossRef]
- Elsherif, N.I.; Shamma, R.N.; Abdelbary, G. Terbinafine Hydrochloride Trans-ungual Delivery via Nanovesicular Systems: In Vitro Characterization and Ex Vivo Evaluation. Aaps Pharmscitech 2017, 18, 551–562. [Google Scholar] [CrossRef]
- Ozcan, I.; Abaci, O.; Uztan, A.H.; Aksu, B.; Boyacioglu, H.; Guneri, T.; Ozer, O. Enhanced topical delivery of terbinafine hydrochloride with chitosan hydrogels. Aaps Pharmscitech 2009, 10, 1024–1031. [Google Scholar] [CrossRef]
- Erdal, M.S.; Ozhan, G.; Mat, M.C.; Ozsoy, Y.; Gungor, S. Colloidal nanocarriers for the enhanced cutaneous delivery of naftifine: Characterization studies and in vitro and in vivo evaluations. Int. J. Nanomed. 2016, 11, 1027–1037. [Google Scholar] [CrossRef]
- Barakat, H.S.; Darwish, I.A.; El-Khordagui, L.K.; Khalafallah, N.M. Development of naftifine hydrochloride alcohol-free niosome gel. Drug Dev. Ind. Pharm. 2009, 35, 631–637. [Google Scholar] [CrossRef]
- Pillai, A.B.; Nair, J.V.; Gupta, N.K.; Gupta, S. Microemulsion-loaded hydrogel formulation of butenafine hydrochloride for improved topical delivery. Arch Derm. Res. 2015, 307, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Hay, R.J. Liposomal amphotericin B, AmBisome. J. Infect. 1994, 28 (Suppl. 1), 35–43. [Google Scholar] [CrossRef]
- Jansook, P.; Pichayakorn, W.; Ritthidej, G.C. Amphotericin B-loaded solid lipid nanoparticles (SLNs) and nanostructured lipid carrier (NLCs): Effect of drug loading and biopharmaceutical characterizations. Drug Dev. Ind. Pharm. 2018, 44, 1693–1700. [Google Scholar] [CrossRef] [PubMed]
- Butani, D.; Yewale, C.; Misra, A. Topical Amphotericin B solid lipid nanoparticles: Design and development. Colloids Surf. B Biointerfaces 2016, 139, 17–24. [Google Scholar] [CrossRef]
- Saldanha, C.A.; Garcia, M.P.; Iocca, D.C.; Rebelo, L.G.; Souza, A.C.; Bocca, A.L.; Almeida Santos Mde, F.; Morais, P.C.; Azevedo, R.B. Antifungal Activity of Amphotericin B Conjugated to Nanosized Magnetite in the Treatment of Paracoccidioidomycosis. PLoS Negl. Trop Dis. 2016, 10, e0004754. [Google Scholar] [CrossRef]
- Sosa, L.; Clares, B.; Alvarado, H.L.; Bozal, N.; Domenech, O.; Calpena, A.C. Amphotericin B releasing topical nanoemulsion for the treatment of candidiasis and aspergillosis. Nanomedicine 2017, 13, 2303–2312. [Google Scholar] [CrossRef]
- Souza, A.C.; Nascimento, A.L.; de Vasconcelos, N.M.; Jeronimo, M.S.; Siqueira, I.M.; R-Santos, L.; Cintra, D.O.; Fuscaldi, L.L.; Pires Junior, O.R.; Titze-de-Almeida, R.; et al. Activity and in vivo tracking of Amphotericin B loaded PLGA nanoparticles. Eur. J. Med. Chem. 2015, 95, 267–276. [Google Scholar] [CrossRef]
- Italia, J.L.; Kumar, M.N.; Carter, K.C. Evaluating the potential of polyester nanoparticles for per oral delivery of amphotericin B in treating visceral leishmaniasis. J. Biomed. Nanotechnol. 2012, 8, 695–702. [Google Scholar] [CrossRef]
- Tang, X.; Zhu, H.; Sun, L.; Hou, W.; Cai, S.; Zhang, R.; Liu, F. Enhanced antifungal effects of amphotericin B-TPGS-b-(PCL-ran-PGA) nanoparticles in vitro and in vivo. Int. J. Nanomed. 2014, 9, 5403–5413. [Google Scholar]
- Jay Prakash Jain, N.K. Development of amphotericin B loaded polymersomes based on (PEG)3-PLA co-polymers: Factors affecting size and in vitro evaluation. Eur. J. Pharm. Sci. 2010, 40, 456–465. [Google Scholar] [CrossRef]
- Perez, A.P.; Altube, M.J.; Schilrreff, P.; Apezteguia, G.; Celes, F.S.; Zacchino, S.; de Oliveira, C.I.; Romero, E.L.; Morilla, M.J. Topical amphotericin B in ultradeformable liposomes: Formulation, skin penetration study, antifungal and antileishmanial activity in vitro. Colloids Surf. B Biointerfaces 2016, 139, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Rodriguez, A.C.; Torrado-Duran, S.; Molero, G.; Garcia-Rodriguez, J.J.; Torrado-Santiago, S. Efficacy and toxicity evaluation of new amphotericin B micelle systems for brain fungal infections. Int. J. Pharm. 2015, 494, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Lykov, A.; Gaidul, K.; Goldina, I.; Konenkov, V.; Kozlov, V.; Lyakhov, N.; Dushkin, A. Silica Nanoparticles as a Basis for Efficacy of Antimicrobial Drugs. In Nanostructures for Antimicrobial Therapy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 551–575. [Google Scholar]
- Khalil, R.M.; Rahman, A.A.A.E.; Kassem, M.A.; Ridi, M.S.E.; Samra, M.M.A.; Awad, G.E.A.; Mansy, S.S. Preparation and in vivo Assessment of Nystatin-Loaded Solid Lipid Nanoparticles for Topical Delivery against Cutaneous Candidiasis. Int. J. Pharmacol. Pharm. Sci. 2014, 8, 401–409. [Google Scholar]
- Fernandez-Campos, F.; Clares Naveros, B.; Lopez Serrano, O.; Alonso Merino, C.; Calpena Campmany, A.C. Evaluation of novel nystatin nanoemulsion for skin candidosis infections. Mycoses 2013, 56, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Offner, F.; Krcmery, V.; Boogaerts, M.; Doyen, C.; Engelhard, D.; Ribaud, P.; Cordonnier, C.; de Pauw, B.; Durrant, S.; Marie, J.P.; et al. Liposomal nystatin in patients with invasive aspergillosis refractory to or intolerant of amphotericin B. Antimicrob. Agents Chemother. 2004, 48, 4808–4812. [Google Scholar] [CrossRef]
- El-Ridy, M.S.; Abdelbary, A.; Essam, T.; Abd El-Salam, R.M.; Aly Kassem, A.A. Niosomes as a potential drug delivery system for increasing the efficacy and safety of nystatin. Drug Dev. Ind. Pharm. 2011, 37, 1491–1508. [Google Scholar] [CrossRef]
- Jadon, P.S.; Gajbhiye, V.; Jadon, R.S.; Gajbhiye, K.R.; Ganesh, N. Enhanced oral bioavailability of griseofulvin via niosomes. Aaps Pharmscitech 2009, 10, 1186–1192. [Google Scholar] [CrossRef]
- Shirsand, S.B.; Keshavshetti, G.G. Formulaiton and characterization of drug loaded niosomes for antifungal activity. Sper. J. Adv. Nov. Drug Deliv. 2016, 1, 12–17. [Google Scholar]
- Jaya raja Kumar, S.M.; Subramani, P. Antifugal Agents: New Approach for Novel Delivery Systems. J. Pharm. Sci. Res. 2014, 6, 229–235. [Google Scholar]
- Srinivasan, A.; Lopez-Ribot, J.L.; Ramasubramanian, A.K. Overcoming antifungal resistance. Drug Discov. Today Technol. 2014, 11, 65–71. [Google Scholar] [CrossRef]
- Pelgrift, R.Y.; Friedman, A.J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 2013, 65, 1803–1815. [Google Scholar] [CrossRef] [PubMed]
- Schairer, D.O.; Chouake, J.S.; Nosanchuk, J.D.; Friedman, A.J. The potential of nitric oxide releasing therapies as antimicrobial agents. Virulence 2012, 3, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Blecher, K.; Nasir, A.; Friedman, A. The growing role of nanotechnology in combating infectious disease. Virulence 2011, 2, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.D.P.; Hu, C.-M.J.; Huang, C.-M. Development of Nanoparticles for Antimicrobial Drug Delivery. Curr. Med. Chem. 2010, 17, 585–594. [Google Scholar] [CrossRef]
- Hetrick, E.M.; Shin, J.H.; Paul, H.S.; Schoenfisch, M.H. Anti-biofilm efficacy of nitric oxide-releasing silica nanoparticles. Biomaterials 2009, 30, 2782–2789. [Google Scholar] [CrossRef]
- Hajipour, M.J.; Fromm, K.M.; Akbar Ashkarran, A.; Jimenez de Aberasturi, D.; Larramendi, I.R.d.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef]
- Mashitah, M.D.; Chan, Y.S.; Jason, J. Antifungal nanomaterials: Syntesis, properties and applications. In Nanobiomaterials in Antimicrobial Therapy; William Andrew: San Diego, CA, USA, 2016; pp. 343–383. [Google Scholar]
- Mashitah, M.D.; Chan, Y.S.; Jason, J. Antimicrobial properties of nanobiomaterials and the mechanism. In Nanobiomaterials in Antimicrobial Therapy; William Andrew: San Diego, CA, USA, 2016; pp. 261–312. [Google Scholar]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Qidway, A.; Pandey, A.; Kumar, R.; Shukla, S.K.; Dikshit, A. Advances in Biogenic Nanoparticles and the Mechanisms of Antimicrobial Effects. Indian J. Pharm. Sci. 2018, 80, 592–603. [Google Scholar] [CrossRef]
- Zhang, X.F.; Liu, Z.G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Bocate, K.P.; Reis, G.F.; de Souza, P.C.; Oliveira Junior, A.G.; Duran, N.; Nakazato, G.; Furlaneto, M.C.; de Almeida, R.S.; Panagio, L.A. Antifungal activity of silver nanoparticles and simvastatin against toxigenic species of Aspergillus. Int. J. Food Microbiol. 2018, 291, 79–86. [Google Scholar] [CrossRef]
- Calvo, N.L.; Sreekumar, S.; Svetaz, L.A.; Lamas, M.C.; Moerschbacher, B.M.; Leonardi, D. Design and Characterization of Chitosan Nanoformulations for the Delivery of Antifungal Agents. Int. J. Mol. Sci. 2019, 20, 3686. [Google Scholar] [CrossRef] [PubMed]
- Kucharska, M.S.; Sikora, M.; Brzoza-Malczewska, K.; Owczarek, M. Antimicrobial Properties of Chitin and Chitosan. In Chitin and Chitosan: Properties and Applications; John Wiley & Sons, Ltd.: West Sussex, UK, 2020. [Google Scholar]
- Sun, Q.; Li, J.; Le, T. Zinc Oxide Nanoparticle as a Novel Class of Antifungal Agents: Current Advances and Future Perspectives. J. Agric. Food Chem. 2018, 66, 11209–11220. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.N.; Nanda, A. Antimicrobial and antifungal potential of zinc oxide nanoparticles in comparison to conventional zinc oxide particles. J. Chem. Pharm. Res. 2013, 5, 457–463. [Google Scholar]
- Rai, M.; Yadav, A.; Bridge, P.; Gade, A. Myconanotechnology: A New and Emerging Science. In Applied Mycology, 14th ed.; York, C.I.N., Ed.; CABI: Wallingford, UK, 2009. [Google Scholar]
- Ingle, A.; Rai, M.; Gade, A.; Bawaskar, M. Fusarium solani: A novel biological agent for the extracellular synthesis of silver nanoparticles. J. Nanoparticle Res. 2009, 11, 2079–2085. [Google Scholar] [CrossRef]
- Korbekandi, H.; Ashari, Z.; Iravani, S.; Abbasi, S. Optimization of Biological Synthesis of Silver Nanoparticles using Fusarium oxysporum. Iran. J. Pharm. Res. IJPR 2013, 12, 289–298. [Google Scholar]
- Kamil, D.; Prameeladevi, T.; Ganesh, S.; Prabhakaran, N.; Nareshkumar, R.; Thomas, S.P. Green synthesis of silver nanoparticles by entomopathogenic fungus Beauveria bassiana and their bioefficacy against mustard aphid (Lipaphis erysimi Kalt.). Indian J. Exp. Biol. 2017, 55, 555–561. [Google Scholar]
- Huang, W.; Fang, X.; Wang, H.; Chen, F.; Duan, H.; Bi, Y.; Yu, H. Biosynthesis of AgNPs by B. maydis and its antifungal effect against Exserohilum turcicum. IET Nanobiotechnol. 2018, 12, 585–590. [Google Scholar] [CrossRef]
- Ogar, A.; Tylko, G.; Turnau, K. Antifungal properties of silver nanoparticles against indoor mould growth. Sci. Total Environ. 2015, 521–522, 305–314. [Google Scholar] [CrossRef]
- Hariharan, H.; Al-Harbi, N.A.; Karuppiah, P.; Rajaram, S.K. Microbial synthesis of Selenium Nanocomposite using Saccharomyces cerevisiae and its antimicrobial activity against pathogens causing nosocomial infection. Chalcogenide Lett. 2012, 9, 509–515. [Google Scholar]
- Muthupandian Saravanan, T.A.; Letemichael, N.; Araya, G.; Arokiyaraj, S.; Vinoth, R.; Karthik, D. Extracellular Biosynthesis and Biomedical Application of Silver Nanoparticles Synthesized from Baker’s Yeast. Int. J. Res. Pharm. Biomed. Sci. 2013, 4, 822–828. [Google Scholar]
- Shanbhaga, P.P.; Janib, U. Drug delivery through nails: Present and future. New Horiz. Transl. Med. 2017, 3, 252–263. [Google Scholar]
- Rajendra, V.B.; Baro, A.; Kumari, A.; Dhamecha, D.L.; Lahoti, S.R.; Shelke, S.D. Transungual Drug Delivery: An Overview. J. Appl. Pharm. Sci. 2012, 2, 203–209. [Google Scholar]
- Tiwary, A.K.; Sapra, B. High failure rate of transungal drug delivery: Need for new strategies. Ther. Deliv. 2017, 8, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Beloqui, A.; Solinis, M.A.; Rodriguez-Gascon, A.; Almeida, A.J.; Preat, V. Nanostructured lipid carriers: Promising drug delivery systems for future clinics. Nanomedicine 2016, 12, 143–161. [Google Scholar] [CrossRef]
- Puglia, C.; Blasi, P.; Ostacolo, C.; Sommella, E.; Bucolo, C.; Platania, C.B.M.; Romano, G.L.; Geraci, F.; Drago, F.; Santonocito, D.; et al. Innovative Nanoparticles Enhance N-Palmitoylethanolamide Intraocular Delivery. Front Pharm. 2018, 9, 285. [Google Scholar] [CrossRef]
- Kumar, L.; Verma, S.; Bhardwaj, A.; Vaidya, S.; Vaidya, B. Eradication of superficial fungal infections by conventional and novel approaches: A comprehensive review. Artif. Cells Nanomed. Biotechnol. 2014, 42, 32–46. [Google Scholar] [CrossRef]
- Soliman, G.M. Nanoparticles as safe and effective delivery systems of antifungal agents: Achievements and challenges. Int. J. Pharm. 2017, 523, 15–32. [Google Scholar] [CrossRef]
- Mishra, G.P.; Bagui, M.; Tamboli, V.; Mitra, A.K. Recent applications of liposomes in ophthalmic drug delivery. J. Drug Deliv. 2011, 2011, 863734. [Google Scholar] [CrossRef]
- Habib, F.S.; Fouad, E.A.; Abdel-Rhaman, M.S.; Fathalla, D. Liposomes as an ocular delivery system of fluconazole: In-vitro studies. Acta Ophthalmol. 2010, 88, 901–904. [Google Scholar] [CrossRef]
- Fetih, G. Fluconazole-loaded niosomal gels as a topical ocular drug delivery system for corneal fungal infections. J. Drug Deliv. Sci. Technol. 2016, 35, 8–15. [Google Scholar] [CrossRef]
- Mohanta, P.; Pandey, N.; Kapoor, D.N.; Singh, S.; Sarvi, Y.; Sharma, P. Development of surfactant-based nanocarrier system for delivery of an antifungal drug. J. Pharm. Res. 2017, 11, 1153–1158. [Google Scholar]
- Baishakhi Bhowmik, A.R.A.S.; Gowda, D.V.; Singh, A.; Srivastava, A.; Ali, R.; Osmani, M. Recent trends and advances in fungal drug delivery. J. Chem. Pharm. Res. 2016, 8, 169–178. [Google Scholar]
- Jensen, G.M. The care and feeding of a commercial liposomal product: Liposomal amphotericin B (AmBisome((R))). J. Liposome Res. 2017, 27, 173–179. [Google Scholar] [CrossRef]
- Jung, S.H.; Lim, D.H.; Jung, S.H.; Lee, J.E.; Jeong, K.S.; Seong, H.; Shin, B.C. Amphotericin B-entrapping lipid nanoparticles and their in vitro and in vivo characteristics. Eur. J. Pharm Sci. 2009, 37, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Korting, H.C.; Klövekorn, W.; Klövekorn, G. Comparative Efficacy and Tolerability of Econazole Liposomal Gel 1%, Branded Econazole Conventional Cream 1% and Generic Clotrimazole Cream 1% in Tinea Pedis. Clin. Drug Investig. 1997, 14, 286–293. [Google Scholar] [CrossRef]
- Dhawade Nakusha, K.P.; Pande, V. Emerging Trends in Topical Antifungal Therapy: A Review. Inventi J. 2015, 2015, 1–5. [Google Scholar]
- Ascenso, A.; Raposo, S.; Batista, C.; Cardoso, P.; Mendes, T.; Praca, F.G.; Bentley, M.V.; Simoes, S. Development, characterization, and skin delivery studies of related ultradeformable vesicles: Transfersomes, ethosomes, and transethosomes. Int. J. Nanomed. 2015, 10, 5837–5851. [Google Scholar] [CrossRef]
- Verma, S.; Utreja, P. Vesicular nanocarrier based treatment of skin fungal infections: Potential and emerging trends in nanoscale pharmacotherapy. Asian J. Pharm. Sci. 2018, 14, 117–129. [Google Scholar] [CrossRef]
- Shaji, J.; Bajaj, R. Transethosomes: A new prospect for enhanced transdermal delivery. Int. J. Pharm. Sci. Res. 2018, 9, 2681–2685. [Google Scholar]
- Ramasamy, T.; Khandasami, U.S.; Ruttala, H.; Shanmugam, S. Development of solid lipid nanoparticles enriched hydrogels for topical delivery of anti-fungal agent. Macromol. Res. 2012, 20, 682–692. [Google Scholar] [CrossRef]
- Pardeike, J.; Hommoss, A.; Muller, R.H. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int. J. Pharm. 2009, 366, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Jain, S.; Khare, P.; Gulbake, A.; Bansal, D.; Jain, S.K. Design and development of solid lipid nanoparticles for topical delivery of an anti-fungal agent. Drug Deliv. 2010, 17, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Crucho, C.I.C.; Barros, M.T. Polymeric nanoparticles: A study on the preparation variables and characterization methods. Mater Sci. Eng. C Mater Biol. Appl. 2017, 80, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Patel, E.K.; Oswal, R. Nanosponge and Micro Sponges: A Novel Drug Delivery System. Int. J. Res. Pharm. Chem. 2012, 2, 2281–2781. [Google Scholar]
- Amaral, A.C.; Bocca, A.L.; Ribeiro, A.M.; Nunes, J.; Peixoto, D.L.; Simioni, A.R.; Primo, F.L.; Lacava, Z.G.; Bentes, R.; Titze-de-Almeida, R.; et al. Amphotericin B in poly(lactic-co-glycolic acid) (PLGA) and dimercaptosuccinic acid (DMSA) nanoparticles against paracoccidioidomycosis. J. Antimicrob. Chemother. 2009, 63, 526–533. [Google Scholar] [CrossRef]
- Nazila Kamaly, B.Y.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef]
- Caon, T.; Porto, L.C.; Granada, A.; Tagliari, M.P.; Silva, M.A.; Simoes, C.M.; Borsali, R.; Soldi, V. Chitosan-decorated polystyrene-b-poly(acrylic acid) polymersomes as novel carriers for topical delivery of finasteride. Eur. J. Pharm. Sci. 2014, 52, 165–172. [Google Scholar] [CrossRef]
- Ing, L.Y.; Zin, N.M.; Sarwar, A.; Katas, H. Antifungal activity of chitosan nanoparticles and correlation with their physical properties. Int. J. Biomater. 2012, 2012, 632698. [Google Scholar] [CrossRef]
- Serrano, D.R.; Lalatsa, A.; Dea-Ayuela, M.A.; Bilbao-Ramos, P.E.; Garrett, N.L.; Moger, J.; Guarro, J.; Capilla, J.; Ballesteros, M.P.; Schätzlein, A.G.; et al. Oral Particle Uptake and Organ Targeting Drives the Activity of Amphotericin B Nanoparticles. Mol. Pharm. 2015, 12, 420–431. [Google Scholar] [CrossRef]
- Makhmalzade, B.S.; Chavoshy, F. Polymeric micelles as cutaneous drug delivery system in normal skin and dermatological disorders. J. Adv. Pharm. Technol. Res. 2018, 9, 2–8. [Google Scholar]
- Thambi, T.; Deepagan, V.G.; Ko, H.; Lee, D.S.; Park, J.H. Bioreducible polymersomes for intracellular dual-drug delivery. J. Mater. Chem. 2012, 22, 22028–22036. [Google Scholar] [CrossRef]
- Lee, J.S.; Feijen, J. Polymersomes for drug delivery: Design, formation and characterization. J. Control. Release 2012, 161, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Meng, F.; Cheng, R.; Deng, C.; Zhong, Z. Reduction and pH dual-bioresponsive crosslinked polymersomes for efficient intracellular delivery of proteins and potent induction of cancer cell apoptosis. Acta Biomater. 2014, 10, 2159–2168. [Google Scholar] [CrossRef] [PubMed]
- Sherje, A.P.; Jadhav, M.; Dravyakar, B.R.; Kadam, D. Dendrimers: A versatile nanocarrier for drug delivery and targeting. Int. J. Pharm. 2018, 548, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Caminade, A.M.; Laurent, R.; Majoral, J.P. Characterization of dendrimers. Adv. Drug Deliv. Rev. 2005, 57, 2130–2146. [Google Scholar] [CrossRef] [PubMed]
- Gupta, U.; Perumal, O. Dendrimers and Its Biomedical Applications. In Natural and Synthetic Biomedical Polymers; Elsevier: Amsterdam, The Netherlands, 2014; pp. 243–257. [Google Scholar]
- Nimesh, S. Dendrimers. In Gene therapy: Potential Applications of Nanotechnology; Woodhead Publishing Limited: Cambridge, UK, 2013; pp. 259–285. [Google Scholar]
- Nanjwade, B.K.; Bechra, H.M.; Derkar, G.K.; Manvi, F.V.; Nanjwade, V.K. Dendrimers: Emerging polymers for drug-delivery systems. Eur. J. Pharm. Sci. 2009, 38, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, E.; Milani, M.; Fekri Aval, S.; Kouhi, M.; Akbarzadeh, A.; Tayefi Nasrabadi, H.; Nikasa, P.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; et al. Silver nanoparticles: Synthesis methods, bio-applications and properties. Crit. Rev. Microbiol. 2016, 42, 173–180. [Google Scholar] [CrossRef]
- Vallabani, N.V.S.; Singh, S. Recent advances and future prospects of iron oxide nanoparticles in biomedicine and diagnostics. 3 Biotech 2018, 8, 279. [Google Scholar] [CrossRef]
- Seabra, A.B.; Pasquoto, T.; Ferrarini, A.C.; Santos Mda, C.; Haddad, P.S.; de Lima, R. Preparation, characterization, cytotoxicity, and genotoxicity evaluations of thiolated- and s-nitrosated superparamagnetic iron oxide nanoparticles: Implications for cancer treatment. Chem. Res. Toxicol. 2014, 27, 1207–1218. [Google Scholar] [CrossRef]
- Sankhyan, A.; Pawar, P. Recent Trends in Niosome as Vesicular Drug Delivery System. J. Appl. Pharm. Sci. 2012, 2, 20–32. [Google Scholar]
- Boonme, P.; Kaewbanjong, J.; Amnuaikit, T.; Andreani, T.; Silva, A.M.; Souto, E.B. Microemulsion and Microemulsion-Based Gels for Topical Antifungal Therapy with Phytochemicals. Curr. Pharm. Des. 2016, 22, 4257–4263. [Google Scholar] [CrossRef] [PubMed]
- Mehta, K.; Bhatt, D. Preparation, Optimization and in vitro Microbiological Efficacy of Antifungal Microemulsion. Int. J. Pharm. Sci. Res. 2011, 2, 2424–2429. [Google Scholar]
- Güngör, S.; Erdal, M.S.; Aksu, B. New Formulation Strategies in Topical Antifungal Therapy. J. Cosmet. Dermatol. Sci. Appl. 2013, 3, 56–65. [Google Scholar] [CrossRef]
- El-Hadidy, G.N.; Ibrahim, H.K.; Mohamed, M.I.; El-Milligi, M.F. Microemulsions as vehicles for topical administration of voriconazole: Formulation and in vitro evaluation. Drug Dev. Ind. Pharm. 2012, 38, 64–72. [Google Scholar] [CrossRef]
- De Souza, M.L.; Oliveira, D.D.; Pereira, N.P.; Soares, D.M. Nanoemulsions and dermatological diseases: Contributions and therapeutic advances. Int. J. Derm. 2018, 57, 894–900. [Google Scholar] [CrossRef]
- Zazo, H.; Colino, C.I.; Lanao, J.M. Current applications of nanoparticles in infectious diseases. J. Control Release 2016, 224, 86–102. [Google Scholar] [CrossRef]
- Peszke, J.; Dulski, M.; Nowak, A.; Balin, K.; Zubko, M.; Sułowicz, S.; Nowak, B.; Piotrowska-Seget, Z.; Talik, E.; Wojtyniak, M.; et al. Unique properties of silver and copper silica-based nanocomposites as antimicrobial agents. RSC Adv. 2017, 7, 28092–28104. [Google Scholar] [CrossRef]
- Botequim, D.; Maia, J.; Lino, M.M.; Lopes, L.M.; Simoes, P.N.; Ilharco, L.M.; Ferreira, L. Nanoparticles and surfaces presenting antifungal, antibacterial and antiviral properties. Langmuir 2012, 28, 7646–7656. [Google Scholar] [CrossRef]
- Cristiana, S.; Vidal, M.; Ferreira, L.S. Antifungal Nanoparticles and Surfaces. Biomacromolecules 2010, 11, 2810–2817. [Google Scholar]
- Camporotondi, D.E.; Foglia, M.L.; Alvarez, G.S.; Mebert, A.; Diaz, L.E.; Coradin, T. Antimicrobial properties of silica modified nanoparticles. In Microbial Pathogens and Strategies for Combating Them: Science, Technology and Education; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2013. [Google Scholar]
- Derbalah, A.; Shenashen, M.; Hamza, A.; Mohamed, A.; El Safty, S. Antifungal activity of fabricated mesoporous silica nanoparticles against early blight of tomato. Egypt. J. Basic Appl. Sci. 2018, 5, 145–150. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Kelkawi, A.H.A.; Kajani, A.A.; Bordbar, A.K. Green synthesis of silver nanoparticles using Mentha pulegium and investigation of their antibacterial, antifungal and anticancer activity. IET Nanobiotechnol. 2017, 11, 370–376. [Google Scholar] [CrossRef]
- El-Hossary, E.M.; Cheng, C.; Hamed, M.M.; El-Sayed Hamed, A.N.; Ohlsen, K.; Hentschel, U.; Abdelmohsen, U.R. Antifungal potential of marine natural products. Eur. J. Med. Chem. 2017, 126, 631–651. [Google Scholar] [CrossRef] [PubMed]
- Vitali, A. Antimicrobial Peptides Derived from Marine Sponges. Am. J. Clin. Microbiol. Antimicrob. 2018, 1, 1006. [Google Scholar]
- Ratnayake, A.S.; Bugni, T.S.; Feng, X.; Harper, M.K.; Skalicky, J.J.; Mohammed, K.A.; Andjelic, C.D.; Barrows, L.R.; Ireland, C.M. Theopapuamide, a Cyclic Depsipeptide from a Papua New Guinea Lithistid Sponge Theonella swinhoei. J. Nat. Prod. 2006, 69, 1582–1586. [Google Scholar] [CrossRef] [PubMed]
- Alberto Plaza, G.B.; Jessica, L.K.; John, R.L.; Heather, L.B.; Carole, A.B. Celebesides A-C and Theopapuamides B-D, Depsipeptides from an Indonesian Sponge that Inhibit HIV-1 Entry. J. Org. Chem. 2010, 74, 504–512. [Google Scholar] [CrossRef]
- Negri, M.; Salci, T.P.; Shinobu-Mesquita, C.S.; Capoci, I.R.G.; Svidzinski, T.I.E.; Kioshima, E.S. Early state research on antifungal natural products. Molecules 2014, 19, 2925–2956. [Google Scholar] [CrossRef] [PubMed]
- Krause, J.; Tobi, G. Discovery, Development, and Regulation of Natural Products. In Using Old Solutions to New Problems—Natural Drug Discovery in the 21st Century; BoD–Books on Demand: Norderstedt, Germany, 2013. [Google Scholar]
- Roemer, T.; Xu, D.; Singh, S.B.; Parish, C.A.; Harris, G.; Wang, H.; Davies, J.E.; Bills, G.F. Confronting the Challenges of Natural Product-Based Antifungal Discovery. Chem. Biol. 2011, 18, 148–164. [Google Scholar] [CrossRef]
- Wright, G.D. Unlocking the potential of natural products in drug discovery. Microb. Biotechnol. 2018, 12, 55–57. [Google Scholar] [CrossRef]
- Gupta, N.; Rai, D.B.; Jangid, A.K.; Kulhari, H. Use of nanotechnology in antimicrobial therapy. In Nanotechnology; Academic Press: Cambridge, MA, USA, 2019; pp. 143–172. [Google Scholar]
- Pooja, D.; Kadari, A.; Kulhari, H.; Sistla, R. Lipid-based nanomedicines. In Lipid Nanocarriers for Drug Targeting; William Andrew: San Diego, CA, USA, 2018; pp. 509–528. [Google Scholar]
- Koppa Raghu, P.; Bansal, K.K.; Thakor, P.; Bhavana, V.; Madan, J.; Rosenholm, J.M.; Mehra, N.K. Evolution of Nanotechnology in Delivering Drugs to Eyes, Skin and Wounds via Topical Route. Pharmaceuticals 2020, 13, 167. [Google Scholar] [CrossRef]
- NIH. ClinicalTrials. Available online: https://clinicaltrials.gov/ct2/home (accessed on 10 September 2020).
| Class | Target (Mechanism of Action) | Antifungal | |
|---|---|---|---|
| Azoles | Ergosterol (inhibition of lanosterol 14-α-demethylase) | Imidazoles | Miconazole |
| Econazole | |||
| Ketoconazole | |||
| Clotrimazole | |||
| Triazoles | Itraconazole | ||
| Fluconazole | |||
| Voriconazole | |||
| Allylamines | Ergosterol (inhibition of squalene epoxidase) | Terbinafine | |
| Naftifine | |||
| Butenafine | |||
| Polyenes | Cell membrane (production of ROS) | Amphotericin B | |
| Ergosterol (inhibition of lanosterol 14-α-demethylase) | Nystatin | ||
| Echinocandines | Cell wall (block of β-1,3 glucan synthesis) | Caspofungin, Micafungin, Anidulafungin | |
| Other antifungals | Chelation of polyvalent metal cations | Ciclopirox | |
| Microtubules (prevention of the formation of the mitotic spindle) | Griseofulvin | ||
| Ergosterol (inhibition of D14 reductase and D7-D8 isomerase) | Amorolfine | ||
| Antifungal Drugs | Novel Drug Delivery Systems | Routes of Administration | Dosage Forms | References |
|---|---|---|---|---|
| Miconazole | Niosomes | Transdermal | Gel | [18] |
| SLN | Oral | N.A. | [19] | |
| Topical | Gel | [20] | ||
| Microemulsion | Topical | N.A. | [21] | |
| Liposomes | Topical | Gel | [22] | |
| Nanoemulsion | Topical | N.A. | [23] | |
| Nanosponges | Vaginal | Gel | [24] | |
| Transfersomes | Topical | Gel | [25] | |
| Econazole | Microemulsion | Percutaneous | N.A. | [26] |
| Topical | Gel | [27] | ||
| SLN | Topical | Gel | [28] | |
| NLC | Topical | Gel | [29] | |
| Liposomes | Topical | Gel | [30] | |
| Ethosomes | Topical | Gel | [31] | |
| Transethosomes | Transdermal | Gel | [32] | |
| Nanosponges | Topical | Hydrogel | [33] | |
| Niosomes | Transdermal | Gel | [34] | |
| Polymeric micelles | Topical | N.A. | [35] | |
| Nanoemulsion | Topical | N.A. | [36] | |
| Ketoconazole | SLN/NLC | Topical | Gel | [37] |
| Niosomes | Topical | Gel | [38] | |
| Microemulsion | Oral | N.A. | [39] | |
| Spanlastics | Ocular | N.A. | [40] | |
| Dendrimers | Topical | Hydrogel | [41] | |
| Liposomes | Topical | N.A. | [42] | |
| Clotrimazole | Liposomes | Topical | Gel | [43] |
| Nanosponges | Topical | Hydrogel | [44] | |
| Ethosomes | Topical | Gel | [45] | |
| Niosomes | Topical | Gel | [46] | |
| Polymeric emulgel | Topical | Gel | [47] | |
| Polymeric micelles | Topical | N.A. | [35] | |
| SLN/NLC | Topical | N.A. | [48] | |
| Microemulsion | Buccal | Gel | [49] | |
| Vaginal | Gel | [50] | ||
| Transfersomes | Transdermal/Topical | N.A. | [51] | |
| Itraconazole | Transfersomes | Transdermal | N.A. | [52] |
| SLN | Ocular | N.A. | [53] | |
| NLC | Inhalation | N.A. | [54] | |
| Niosomes | Topical | N.A. | [55] | |
| Microemulsion | Transdermal | N.A. | [56] | |
| Liposomes | Topical | N.A. | [57] | |
| Polymeric nanoparticles | Oral | N.A. | [58] | |
| Polymersome | Intravenous | N.A. | [54] | |
| Spanlastics | Ocular | N.A. | [59] | |
| Silica nanoparticles | Oral | N.A. | [60] | |
| Fluconazole | Microemulsion | Vaginal | Gel | [61] |
| Niosomes | Ocular | Gel | [62] | |
| Liposomes | Intravitral | N.A. | [63] | |
| SLN | Topical | Gel | [64] | |
| NLC | Oral | N.A. | [65] | |
| Microsponges | Topical | Gel | [66] | |
| Ethosomes | Topical | Gel | [67] | |
| Spanlastics | Ocular | N.A. | [68] | |
| Polymeric amphiphilogel | Topical | Gel | [69] | |
| Polymeric micelles | Topical | N.A. | [35] | |
| Voriconazole | Microemulsion | Ocular | N.A. | [70] |
| Polymeric nanoparticles | Ocular | N.A. | [71] | |
| Pulmonar | N.A. | [72] | ||
| SLN | Topical | Gel | [73] | |
| Transethosome | Topical | N.A. | [74] | |
| Ethosome | Topical | N.A. | [75] | |
| Terbinafine | Liposomes | Topical | Gel | [76] |
| SLN | Topical | N.A. | [77] | |
| Transfersomes | Topical | N.A. | [78] | |
| Spanlastics | Transungual | N.A. | [79] | |
| Polymeric chitosan nanoparticles | Topical | Hydrogel | [80] | |
| Naftifine | Microemulsion | Topical | N.A. | [81] |
| Niosomes | Topical | Gel | [82] | |
| Butenafine | Microemulsion | Topical | Hydrogel | [83] |
| Amphotericin B | Liposomes | Intravenous | N.A. | [84] |
| SLN/NLC | Oral | N.A. | [85] | |
| Topical | N.A. | [86] | ||
| Magnetic nanoparticles | Nasal instilation | N.A. | [87] | |
| Nanoemulsion | Topical | N.A. | [88] | |
| Polymeric nanoparticles | Intravenous | N.A. | [89] | |
| Oral | N.A. | [90] | ||
| Polymersomes | Oral | N.A. | [91,92] | |
| Transfersomes | Topical | N.A. | [93] | |
| Micelles | Intravenous | N.A. | [94] | |
| Silica nanoparticles | Intravenous | N.A. | [95] | |
| Nystatin | SLN | Topical | N.A. | [96] |
| Nanoemulsion | Topical | N.A. | [97] | |
| Liposomes | Intravenous | N.A. | [98] | |
| Niosomes | Parenteral | N.A. | [99] | |
| Griseofulvin | Niosomes | Oral | N.A. | [100] |
| Ciclopirox | Niosomes | Topical | Gel | [101] |
| Caspofungin, Micafungin, Anidulafungin, Amorolfine | No nano-tech studies yet released | |||
| Fungal Species | Nanoparticles Type | Method of Synthesis |
|---|---|---|
| Phoma sp. | Silver | Extracellular |
| Fusarium oxysporum | Gold; Magnetite | Extracellular |
| Verticillium sp. | Silver | Intracellular |
| Aspergillus fumigatus | Silver | Extracellular |
| Aspergillus niger | Silver | Extracellular |
| Fusarium semitectum | Silver | Extracellular |
| Trichoderma asperellum | Silver | Extracellular |
| Phaenerochaete chrysosporium | Silver | Extracellular |
| Marine Organism | Source Organism | Type of Compound | Compound Name | Spectrum of Activity |
|---|---|---|---|---|
| Bacteria (30% of total) | Bacillus licheniformis | Glycolipid | Ledoglucomide C, Iedoglycolipid | Aspergillus niger, Rhizoctonia solani, Botrytis cinerea, and Colletotrichum acutatum, Candida albicans |
| Bacillus subtilis | Lipopeptide | Gageopeptides A-D | R. solani, P. capsici, B. cinerea, C. acutatum | |
| Actinoalloteichus sp. NPS702 | Macrolide | Neomaclafungins A-I | Trichophyton mentagrophytes | |
| Streptomyces sp. | Peptide | Mohangamide A | C. albicans | |
| Bacillus marinus | Macrolide | Macrolactins T and B | Pyricularia oryzae, A. solani | |
| Tolypothrix | Lipopeptide | Hassallidin A | A. fumigatus and C. albicans | |
| Chondromyces pediculatus | Peptide | Pedein A | Rhodotorula glutinis | |
| Fungi (15% of total) | Stagonosporopsis cucurbitacearum | Alkaloid | Didymellamide A | C. neoformans, C. albicans, C. glabrata |
| Aspergillus sclerotiorum | Peptide | Sclerotide B | C. albicans | |
| Penicillium bilaiae MA-267 | Sesquiterpene | Penicibilaenes A and B | C. gloeosporioides | |
| Sponge (35%) | Theonella swinhoei | Peptide | Theonegramide, Theonellamide G, Cyclolithistide A | C. albicans |
| Halichondria cylindrata | Peptide | Halicylindramide D and E | Mortierella ramanniana | |
| Siliquariaspongia mirabilis, Theonella swinhoei | Peptide | Theopapuamide A; B and C | C. albicans | |
| Jaspis johnstoni | Peptide | Jasplakinolide | C. albicans, C. pseidrotropicalis, C. parapsilosis | |
| Monanchora arbuscular | Alkaloid | Batzelladine L | A. flavus | |
| Xestospongia muta | Furan | Mutafuran D | Cryptococcus neoformans var.grubii | |
| Corals (5%) | Clavelina oblonga | Alkanol | (2S,3R)-2-aminododecan-3-ol | C. albicans ATCC 10231, C. glabrata |
| Sea cucumbers (6%) | Stichopus variegates | Triterpene glycoside | Variegatuside D | C. albicans, C. pseudo- tropicalis, C. parapsilosis, and M. gypseum |
| Algae (9%) | Caulerpa racemos | Xylene | Caulerprenylol B | T. rubrum |
| Trade Name/Sponsor | ClinicalTrials.gov Identifier | Antifungal | Nanoformulation | Clinical Phase | Disease |
|---|---|---|---|---|---|
| Sara Botros, Minia University | NCT04110834 | Itraconazole | Nanoemulsion gel | II | Tinea versicolor |
| Sara Botros, Minia University | NCT04110860 | Voriconazol | Nanoemulsion gel | II | Tinea versicolor |
| Matinas BioPharma | NCT02971007 | Amphotericin B | Cochleate lipid-crystal nanoparticle | II | Vulvovaginal candidiasis |
| Matinas BioPharma | NCT02629419 | Amphotericin B | Cochleate lipid-crystal nanoparticle | II | Mucocutaneous candidiasis |
| Ahmed Abdellatif, Al-Azhar University | NCT03752424 | - | Silver nanoparticle gel | I | Mycosis |
| Mona Badran, Cairo University | NCT03666195 | - | Titanium dioxide nanoparticles | Recruiting | Candidiasis |
| Rasha Hamed, Assiut University | NCT04431804 | - | Silver nanoparticle | Recruiting | Invasive aspergillosis |
| Celtic Pharma Development Services | NCT01145807 | Terbinafine (TDT067) | Transfersome | III | Onychomycosis |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, F.; Ferreira, D.; Reis, S.; Costa, P. Current Insights on Antifungal Therapy: Novel Nanotechnology Approaches for Drug Delivery Systems and New Drugs from Natural Sources. Pharmaceuticals 2020, 13, 248. https://doi.org/10.3390/ph13090248
Sousa F, Ferreira D, Reis S, Costa P. Current Insights on Antifungal Therapy: Novel Nanotechnology Approaches for Drug Delivery Systems and New Drugs from Natural Sources. Pharmaceuticals. 2020; 13(9):248. https://doi.org/10.3390/ph13090248
Chicago/Turabian StyleSousa, Filipa, Domingos Ferreira, Salette Reis, and Paulo Costa. 2020. "Current Insights on Antifungal Therapy: Novel Nanotechnology Approaches for Drug Delivery Systems and New Drugs from Natural Sources" Pharmaceuticals 13, no. 9: 248. https://doi.org/10.3390/ph13090248
APA StyleSousa, F., Ferreira, D., Reis, S., & Costa, P. (2020). Current Insights on Antifungal Therapy: Novel Nanotechnology Approaches for Drug Delivery Systems and New Drugs from Natural Sources. Pharmaceuticals, 13(9), 248. https://doi.org/10.3390/ph13090248







