The New Pharmaceutical Compositions of Zinc Oxide Nanoparticles and Triterpenoids for the Burn Treatment
Abstract
1. Introduction
2. Results and Discussion
2.1. Properties of Zinc Oxide Nanoparticles Modified by Triterpenoid
2.2. Biomedical Studies
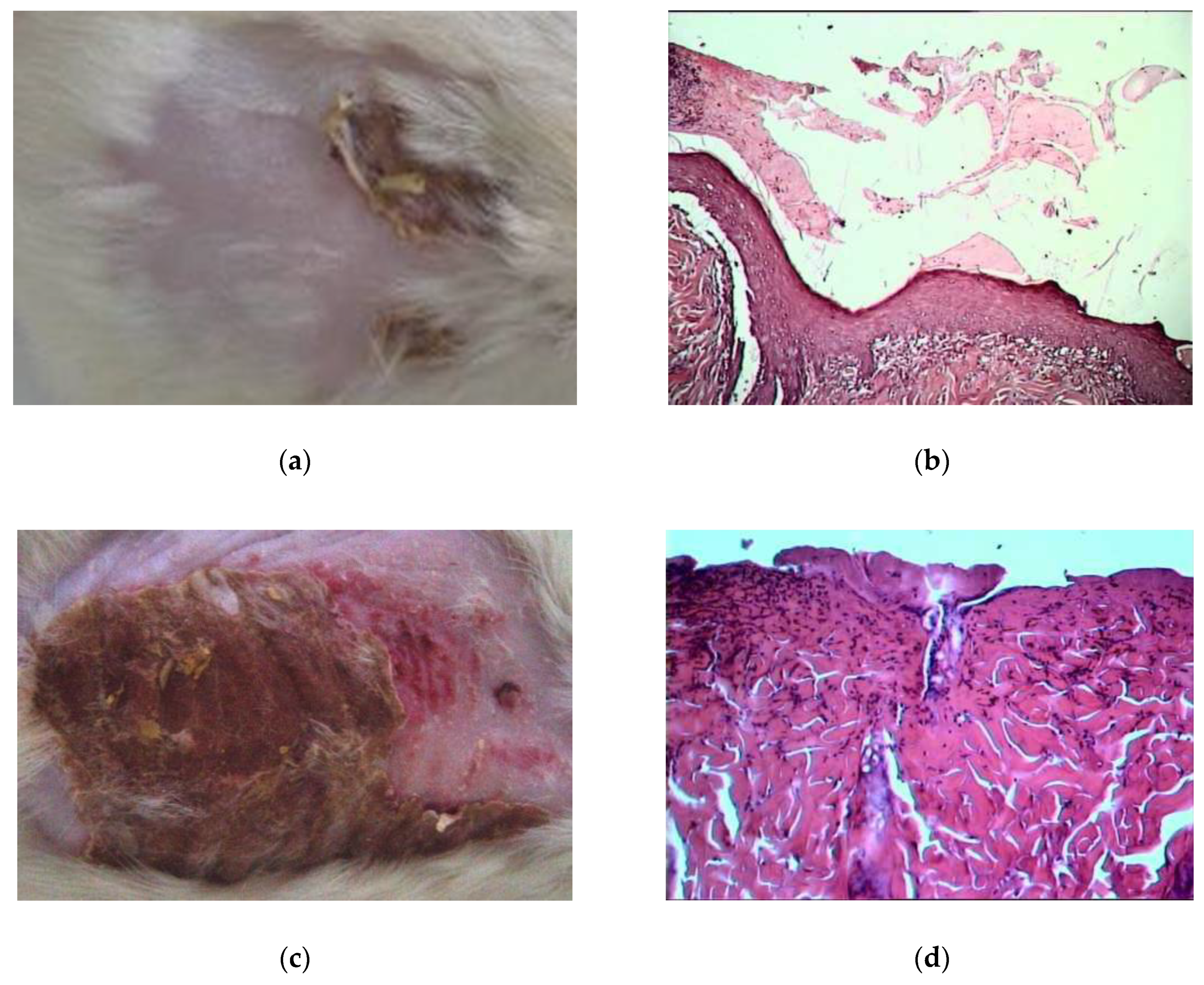
2.2.1. Morpho-histological Studies
2.2.2. Vasodilatation Effect of Pharmaceutical Compositions of ZnO NPs Modified by Triterpenoids
2.2.3. The Enzyme Activity
3. Materials and Methods
3.1. Materials
3.2. Obtaining of Zinc Oxide Nanoparticles [18,19]
3.3. FTIR Analysis
3.4. UV Analysis
3.5. RP-HPLC Analysis
3.6. Powder X-ray Diffraction Analysis
3.7. Photoluminescence Analysis
3.8. Pharmaceutical Composition
3.9. Biological Activity
3.9.1. Modeling of Thermal Burns in Animals
3.9.2. Wound Area Measurement
3.9.3. Biological Activity In Vitro
3.9.4. Morpho-histology Research
3.9.5. Microcirculation Research
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Negut, I.; Grumezescu, V.; Grumezescu, A.M. Treatment Strategies for Infected Wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef]
- Udy, A.A.; Roberts, J.A.; Lipman, J.; Blot, S. The effects of major burn related pathophysiological changes on the pharmacokinetics and pharmacodynamics of drug use: An appraisal utilizing antibiotics. Adv. Drug Deliv. Rev. 2018, 123, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Vinaik, R.; Barayan, D.; Shahrokhi, S.; Jeschke, M.G. Management and prevention of drug resistant infections in burn patients. Expert Rev. Anti Infect. Ther. 2019, 17, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Günel, E.; Çağlayan, F.; Çağlayan, O.; Canbilen, A.; Tosun, M. Effect of antioxidant therapy on collagen synthesis in corrosive esophageal burns. Pediatr. Surg. Int. 2002, 18, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Wessels, I.; Maywald, M.; Rink, L. Zinc as a Gatekeeper of Immune Function. Nutrients 2017, 9, 1286. [Google Scholar] [CrossRef]
- Gupta, M.; Mahajan, V.K.; Mehta, K.S.; Chauhan, P.S. Zinc Therapy in Dermatology: A Review. Dermatol. Res. Pract. 2014, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Pi, J.; Cai, J. The Advancing of Zinc Oxide Nanoparticles for Biomedical Applications. Bioinorg. Chem. Appl. 2018, 1–18. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Ur Rahman, A.; Tajuddin; Husen, A. Properties of Zinc Oxide Nanoparticles and Their Activity AGAINST Microbes. Nanoscale Res. Lett. 2018, 13, 141. [Google Scholar] [CrossRef]
- McClain, P.E.; Wiley, E.R.; Beecher, G.R.; Anthony, W.L.; Hsu, J.M. Influence of zinc deficiency on synthesis and cross-linking of rat skin collagen. BBA Gen. Subj. 1973, 304, 457–465. [Google Scholar] [CrossRef]
- Caldis-Coutris, N.; Gawaziuk, J.P.; Logsetty, S. Zinc supplementation in burn patients. J. Burn Care Res. 2012, 33, 678–682. [Google Scholar] [CrossRef]
- Mishra, P.K.; Mishra, H.; Ekielski, A.; Talegaonkar, S.; Vaidya, B. Zinc oxide nanoparticles: A promising nanomaterial for biomedical applications. Drug Discov. Today 2017, 22, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Drąg-Zalesińska, M.; Rembiałkowska, N.; Borska, S.; Saczko, J.; Drąg, M.; Poręba, M.; Kulbacka, J. A New Betulin Derivative Stimulates the Synthesis of Collagen in Human Fibroblasts Stronger than its Precursor. In Vivo 2019, 33, 1087–1093. [Google Scholar] [CrossRef]
- Bonte, F.; Dumas, M.; Chaudagne, C.; Meybeck, A. Influence of asiatic acid, madecassic acid, and asiaticoside on human collagen I synthesis. Planta Med. 1994, 60, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Maquart, F.X.; Bellon, G.; Gillery, P.; Wegrowski, Y.; Borel, J.P. Stimulation of collagen synthesis in fibroblast cultures by a triterpene extracted from Centella asiatica. Connect Tissue Res. 1990, 24, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Barret, J.P.; Podmelle, F.; Lipový, B.; Rennekampff, H.O.; Schumann, H.; Schwieger-Briel, A.; Zahn, T.R.; Metelmann, H.R. Accelerated re-epithelialization of partial-thickness skin wounds by a topical betulin gel: Results of a randomized phase III clinical trials program. Burns 2017, 43, 1284–1294. [Google Scholar] [CrossRef]
- Frew, Q.; Rennekampff, H.O.; Dziewulski, P.; Moiemen, N.; Moiemen, N.; BBW-11 Study Group; Zahn, T.; Hartmann, B. Betulin wound gel accelerated healing of superficial partial thickness burns: Results of a randomized, intra-individually controlled, phase III trial with 12-months follow-up. Burns 2019, 45, 876–890. [Google Scholar] [CrossRef]
- Suguna, L.; Sivakumar, P.; Chandrakasan, G. Effects of Centella asiatica extract on dermal wound healing in rats. Indian J. Exp. Biol. 1996, 34, 1208–1211. [Google Scholar]
- Bera, D.; Qian, L.; Sabui, S.; Santra, A.; Holloway, P.H. Photoluminescence of ZnO quantum dots produced by a sol-gel process. Opt. Mater. 2008, 30, 1233–1239. [Google Scholar] [CrossRef]
- Kim, J.-S.; Kang, B.-H.; Jeong, H.-M.; Kim, S.-W.; Xu, B. Quantum dot light emitting diodes using size-controlled ZnO NPs. Curr. Appl. Phys. 2018, 18, 681–685. [Google Scholar] [CrossRef]
- Efafi, B.; Majles Ara, M.H.; Mousavi, S.S. Strong blue emission from ZnO nanocrystals synthesized in acetone-based solvent. J. Lumin. 2016, 178, 384–387. [Google Scholar] [CrossRef]
- Vafaee, M.; Sasani Ghamsari, M.; Radiman, S. Highly concentrated zinc oxide nanocrystals sol with strong blueemission. J. Lumin. 2011, 131, 155–158. [Google Scholar] [CrossRef]
- Jangir, L.K.; Kumari, Y.; Kumar, A.; Kumara, M.; Awasthi, K. Investigation of luminescence and structural properties of ZnO nanoparticles, synthesized with different precursors. Mater. Chem. Front. 2017, 1, 1413–1421. [Google Scholar] [CrossRef]
- Oliva, J.; Diaz-Torres, L.; Torres-Castro, A.; Salas, P.; Perez-Mayen, L.; De la Rosa, E. Effect of TEA on the blue emission of ZnO quantum dots with high quantum yield. Opt. Mater. Express 2015, 5, 1109–1121. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Davar, F.; Khansari, A. Nanosphericals and nanobundles of ZnO: Synthesis and characterization. J. Alloy. Compd. 2011, 509, 61–65. [Google Scholar] [CrossRef]
- Chen, Z.; Li, X.X.; Du, G.; Chen, N.; Suen, A.Y.M. A sol-gel method for preparing ZnO quantum dots with strong blue emission. J. Lumin. 2011, 131, 2072–2077. [Google Scholar] [CrossRef]
- Abdullahi, A.; Amini-Nik, S.; Jeschke, M.G. Animal models in burn research. Cell. Mol. Life Sci. 2014, 71, 3241–3255. [Google Scholar] [CrossRef]
- Kang, M.; Zhao, L.; Ren, M.; Deng, M.; Li, C. Zinc mediated hepatic stellate cell collagen synthesis reduction through TGF-β signaling pathway inhibition. Int. J. Clin. Exp. Med. 2015, 8, 20463–20471. [Google Scholar]
- Seo, H.-J.; Cho, Y.-E.; Kim, T.; Shin, H.-I.; Kwun, I.-S. Zinc may increase bone formation through stimulating cell proliferation, alkaline phosphatase activity and collagen synthesis in osteoblastic MC3T3-E1 cells. Nutr. Res. Pract. 2010, 4, 356–361. [Google Scholar] [CrossRef]
- Cho, S.H.; Gottlieb, K.; Santhanam, U. Cosmetic Compositions Containing Betulinic Acid. Patent No. EP 0,717,983 B1, 31 January 2001. [Google Scholar]
- Pakhomova, A.E.; Pakhomova, E.E.; Pakhomova, J.V.; Yavorsky, E.M. Method Experimental Modeling of Thermal Combustion at Laboratory Animals. Patent No. RU 2,582,458 C1, 24 December 2014. [Google Scholar]
- Pakhomova, A.E.; Pakhomova, J.V.; Ovsyanko, E.V.; Zhurakovsky, I.P.; Karabintseva, N.O.; Pakhomova, E.E. Preclinical research of repalen ointment at treatment of thermal combustions in experiment. J. Sib. Med. Sci. 2015, 3, 98. [Google Scholar]
- Wang, H.J.; Chen, T.M.; Yang, T.S.; Wang, D.S.; Lin, S.Z. Regional skin blood flow in deep burn wounds: A preliminary report. Burns 1995, 21, 340–343. [Google Scholar] [CrossRef]
- OʼReilly, T.J.; Spence, R.J.; Taylor, R.M.; Scheulen, J.J. Laser Doppler Flowmetry Evaluation of Burn Wound Depth. J. Burn Care Rehabil. 1989, 10, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.R.; Ordaz, J.; Lo, C.-L.; Damayanti, N.P.; Zhou, F.; Irudayaraj, J. From the Cover: Zinc oxide nanoparticles-induced reactive oxygen species promotes multimodal cyto- and epigenetic toxicity. Toxicol. Sci. 2017, 156, 261–274. [Google Scholar] [CrossRef]
- Kumar, A.; Pandey, A.K.; Singh, S.S.; Shanker, R.; Dhawan, A. Engineered ZnO and TiO2 nanoparticles induce oxidative stress and DNA damage leading to reduced viability of Escherichia coli. Free Radic. Biol. Med. 2011, 51, 1872–1881. [Google Scholar] [CrossRef] [PubMed]
- Ali, D.; Alarifi, S.; Kumar, S.; Ahamed, M.; Siddiqui, M.A. Oxidative stress and genotoxic effect of zinc oxide nanoparticles in freshwater snail Lymnaea luteola L. Aqua. Toxicol. 2012, 124–125, 83–90. [Google Scholar] [CrossRef]
- Klotz, L.O.; Kröncke, K.D.; Buchczyk, D.P.; Sies, H. Role of copper, zinc, selenium and tellurium in the cellular defense against oxidative and nitrosative stress. J. Nutr. 2003, 133 (Suppl. 1), 1448S–1451S. [Google Scholar] [CrossRef] [PubMed]
- Melnikova, N.B.; Malygina, D.S.; Klabukova, I.N.; Belov, D.V.; Vasin, V.A.; Petrov, P.S.; Knyazev, A.V.; Markin, A.V. Betulin-3,28-diphosphate. Physico-Chemical Properties and In Vitro Biological Activity Experiments. Molecules 2018, 23, 1175. [Google Scholar] [CrossRef]
- Melnikova, N.; Burlova, I.; Kiseleva, T.; Klabukova, I.; Gulenova, M.; Kislitsin, C.; Vasin, V.; Tanaseichuk, B. A Practical Synthesis of Betulonic Acid Using Selective Oxidation of Betulin on Aluminium Solid Support. Molecules 2012, 17, 11849–11863. [Google Scholar] [CrossRef]
- Mihara, M.; Uchiyama, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [CrossRef]
- Melnikova, N.B.; Vorobyova, O.A.; Solovieva, A.G.; Peretyagin, P.V.; Belyaeva, K.L.; Malygina, D.S.; Klabukova, I.N. Composition for Skin Diseases Treatment and Cosmetic Use. Patent No. RU 2,724,342 C1, 25 June 2019. [Google Scholar]
- Kochetygov, N.I. Burn Disease; Medicine: Leningrad, Russia, 1973; p. 247. [Google Scholar]
- Hosseinimehr, S.J.; Khorasani, G.; Azadbakht, M.; Zamani, P.; Ghasemi, M.; Ahmadi, A. Effect of aloe cream versus silver sulfadiazine for healing burn wounds in rats. Acta Dermatovenerol. Croat. 2010, 18, 2–7. [Google Scholar]
- Levdansky, V.; Kondrasenko, A.; Levdansky, A.; Kuznetsov, B. Synthesis of Betulin Diacetate and Betulin Dipropionate. J. Sib. Fed. Univ. Chem. 2016, 9, 337–344. [Google Scholar] [CrossRef]
- Sirota, T.V. A new approach to studying the autoxidation of adrenaline: Possibility of the determination of superoxide dismutase activity and the antioxidant properties of various preparations by polarography. Biomed. Khi. 2012, 58, 77–87. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Packer, L. Catalase in Vitro. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. ISBN 012182005X. [Google Scholar]
- Sibgatullina, G.V.; Khartendinova, L.R.; Gumerova, E.A.; Akulov, A.N.; Kostyukova, Y.A.; Nikonorova, N.A.; Rumyantseva, N.I. Methods for Determining the Redox Status of Cultured Plant Cells; Kazan (Privolzhsky) Federal University: Kazan, Russia, 2011; pp. 18–20. [Google Scholar]
- Kochetov, G.A. Practical Guide to Enzymology, 2nd ed.; Severin, S.E., Ed.; High School: Moscow, Russia, 1980; p. 272. [Google Scholar]
- Solov’eva, A.G.; Zimin, Y.V. A new way to assess the dynamics of blood metabolism in patients with thermal trauma. Mod. Technol. Med. 2012, 2, 116–117. [Google Scholar]
- Dawson, J.M.; Heatlic, P.L. Lowry method of protein quantification evidence for photosensitivity. Anal. Biochem. 1984, 140, 391–393. [Google Scholar] [CrossRef]
- Martusevich, A.K.; Larionova, K.D.; Peretyagin, S.P.; Peretyagin, P.V.; Davyduk, A.V. Experimental estimation of pharmacological compositions effect on microcirculation state at early postburn period. Fundam. Res. 2013, 3, 332–336. [Google Scholar]
- Dahmus, J.D.; Bruning, R.S.; Kenney, W.L.; Alexander, L.M. Oral clopidogrel improves cutaneuos microvascular function through EDHF-dependent mechanisms in middle-aged humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R452–R458. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.; Arora, S.; Singh, B.; Manamalli, A.; Dolia, P.B. Association of macrovascular complications of type 2 diabetes mellitus with serum magnesium levels. Diabetes Metab. Syndr. Clin. Res. Rev. 2011, 5, 41–44. [Google Scholar] [CrossRef] [PubMed]


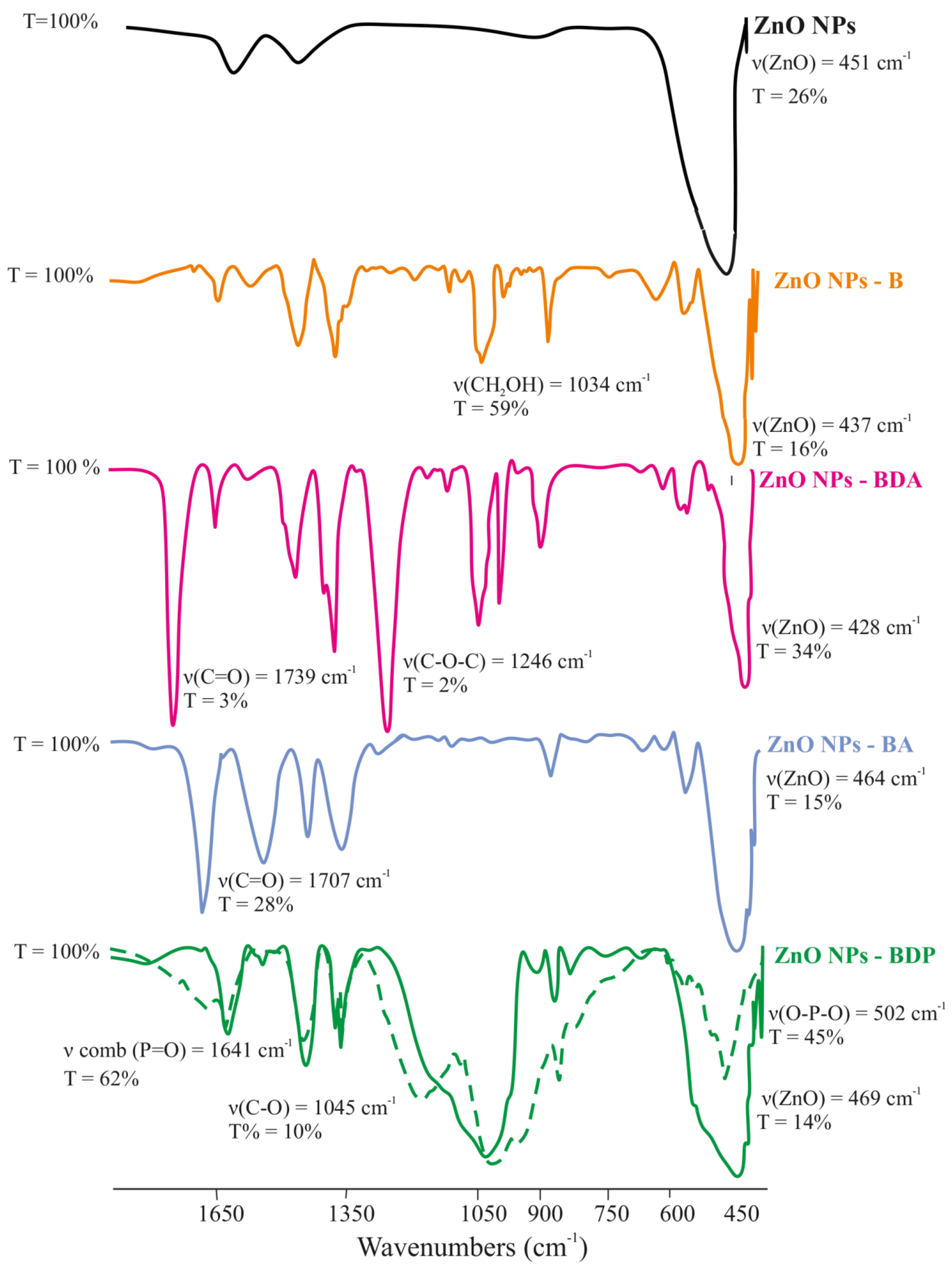
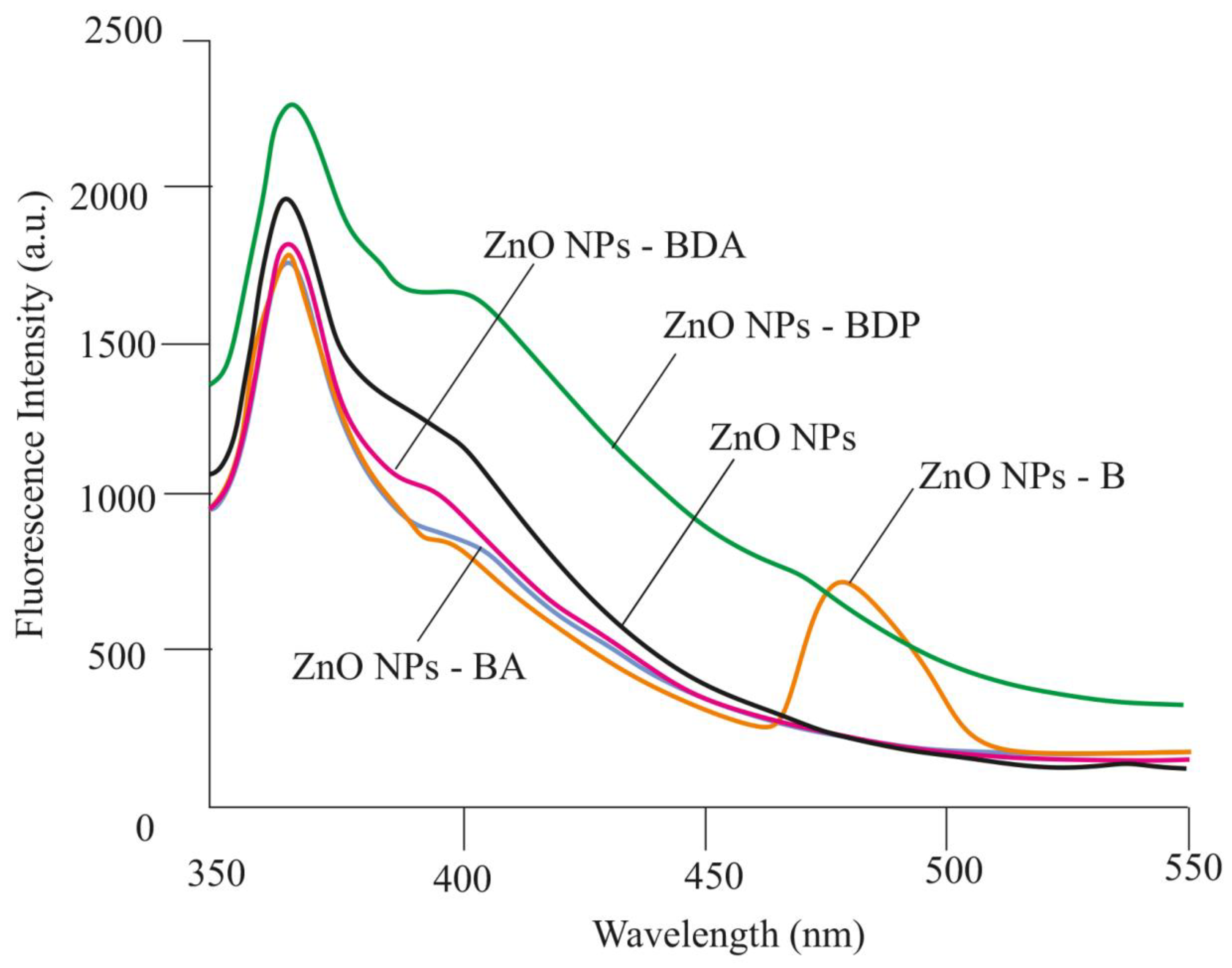
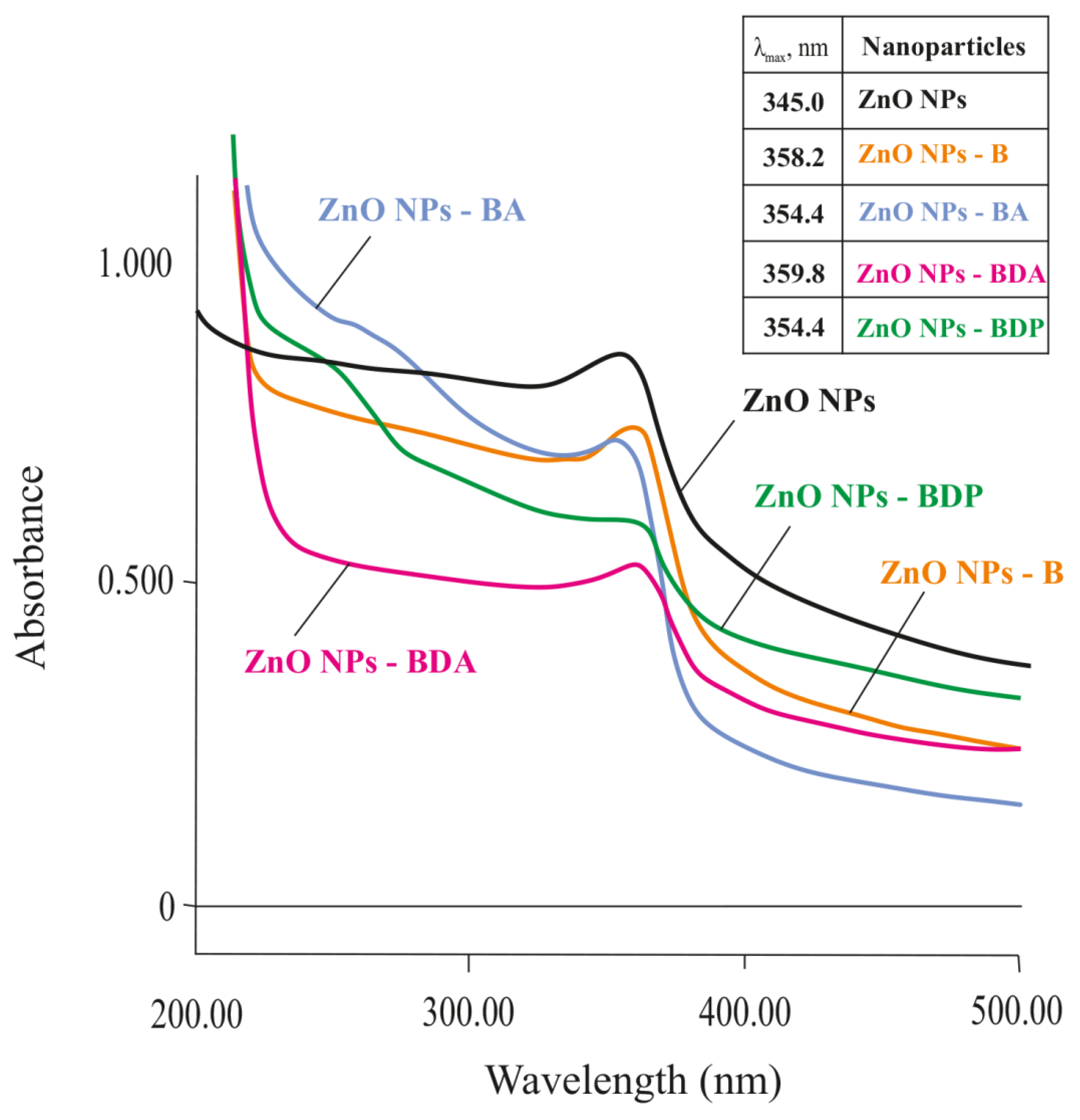
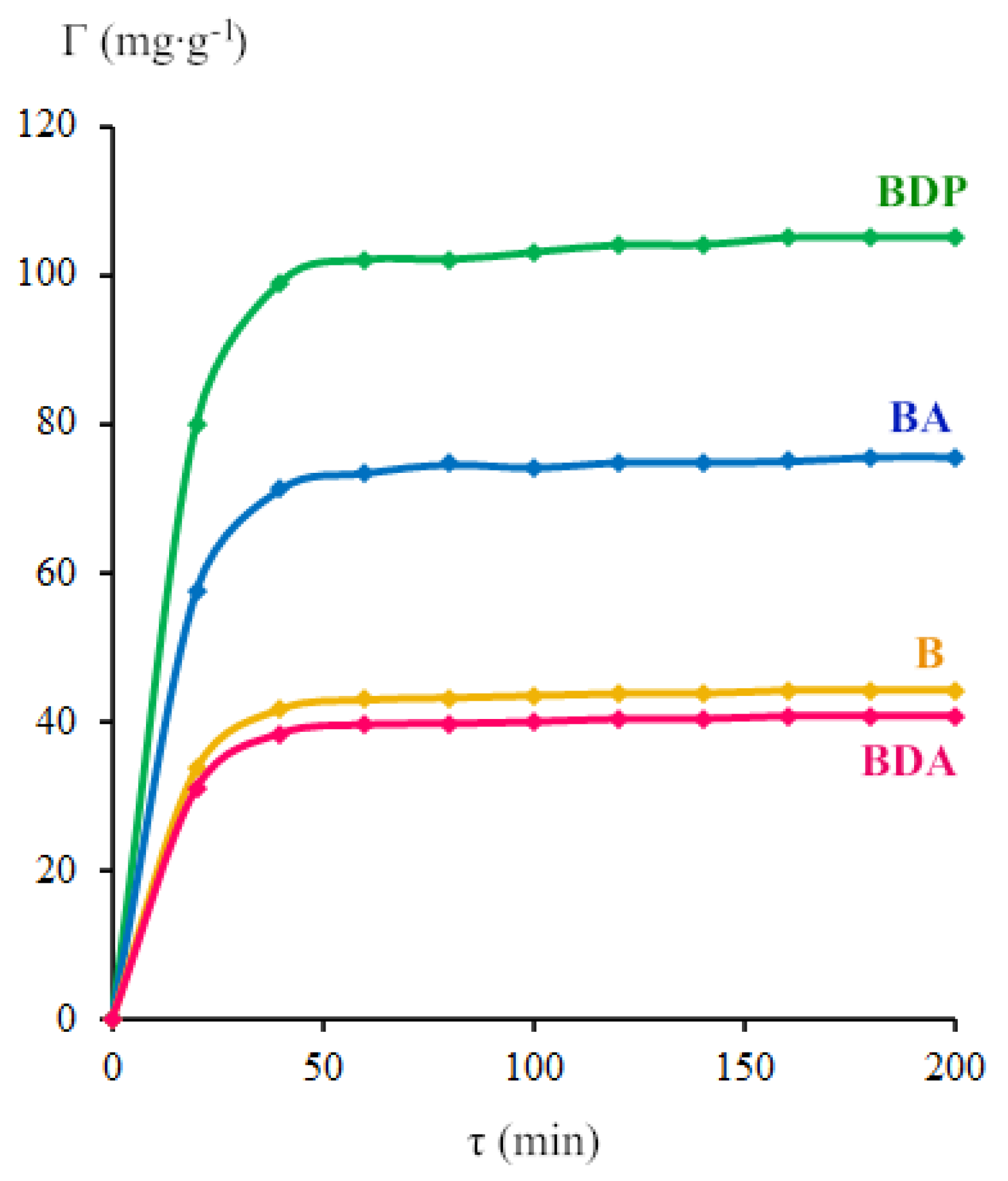

| Nanoparticles 2 | Peaks | β‘, 2θ | β, rad | 2θ, ° | Cosθ | D, nm |
|---|---|---|---|---|---|---|
| ZnO NPs | 100 | 0.468 | 0.0082 | 36.22 | 0.950 | 17.8 |
| 002 | 0.545 | 0.0082 | 47.54 | 0.911 | 16.0 | |
| 110 | 0.625 | 0.0109 | 56.62 | 0.878 | 14.5 | |
| ZnO NPs-B | 101 | 0.595 | 0.0103 | 31.68 | 0.959 | 17.3 |
| 002 | 0.476 | 0.0083 | 34.34 | 0.955 | 17.5 | |
| 100 | 0.595 | 0.0103 | 36.42 | 0.950 | 14.0 | |
| ZnO NPs-BDA | 101 | 0.487 | 0.0085 | 31.70 | 0.959 | 16.9 |
| 002 | 0.365 | 0.0064 | 34.38 | 0.956 | 22.6 | |
| 100 | 0.512 | 0.0089 | 36.2 | 0.950 | 16.4 | |
| ZnO NPs-BA | 101 | 0.730 | 0.0127 | 31.64 | 0.959 | 11.5 |
| 002 | 0.487 | 0.0085 | 34.38 | 0.954 | 17.0 | |
| 100 | 0.714 | 0.0125 | 36.16 | 0.950 | 11.7 | |
| ZnO NPs-BDP | 101 | 0.714 | 0.0125 | 31.76 | 0.959 | 11.6 |
| 002 | 0.476 | 0.0083 | 34.36 | 0.954 | 17.5 | |
| 100 | 0.714 | 0.0125 | 36.22 | 0.950 | 11.7 |
| Substance | FTIR Spectrum, ν, cm−1 | |||
|---|---|---|---|---|
| ZnO | C-O st | C=O st | PO-H comb; PO-H st P=O st; -O-PO(OH)2 | |
| ZnO | 451 | - | - | - |
| B | - | 1080, 1028 | - | - |
| ZnO NPs-B | 440–480 | 1032 | - | - |
| BDA | - | 1032, 980 | 1738 | - |
| ZnO NPs-BDA | 471 | 1080, 1033, 980 | 1739 | - |
| BA | - | 1221 | 1705 | - |
| ZnO NPs-BA | 490–450 | 1385 | 1706 | - |
| BDP | - | 1200–900 (int. comb. bands 1 of C-O and P-O) | - | 1641 (PO-H comb.); 1193 (P=O st); 983, 973 (PO-H st); 501 (-O-PO(OH)2) |
| ZnO NPs-BDP | 500–450 | 1200–950 (int. comb. bands of C-O and P-O) | - | 1640 (PO-H comb.)—more int. |
| Oleogel (Group, n = 5) | Betulin, m, g | ZnO NPs Triterpenoid | Stabilizer | Sunflower Oil | |
|---|---|---|---|---|---|
| Type | m, g | ||||
| ZnO NPs | 10.0 g in all compositions | ZnO NPs | 5.0 | Ascorbic acid + α-tokoferol acetate 0.001–0.010 g | up to 100.0 g |
| ZnO NPs-BDA | ZnO NPs-BDA | 5.3 | |||
| ZnO NPs-BA | ZnO NPs-BA | 5.4 | |||
| ZnO NPs-BDP | ZnO NPs-BDP | 5.5 | |||
| Group | Burn Wound Area, cm2 (% of 1st day) |
|---|---|
| Intact | - |
| Burned | 15.75 ± 0.35 (112.5 ± 2.5%) |
| ZnO NPs | 7.7 ± 0.74 (55 ± 5.3%) |
| ZnO NPs-BDA | 6.6 ± 0.19 (47 ± 1.4%) |
| ZnO NPs-BA | 6.3 ± 0.26 (45 ± 1.9%) |
| ZnO NPs-BDP | 7.3 ± 0.11 (52 ± 0.8%) |
| Group | Day | MI 1 | % of Intact | |
|---|---|---|---|---|
| Mean ± SD, perf. un. | RSD % | |||
| Intact | 0 | 13.54 ± 0.96 | 7.09 | 100 |
| Burned 2 | 0 | 6.18 ± 0.02 | 0.32 | 45.64 |
| 10 | 8.25 ± 0.05 | 0.61 | 60.93 | |
| ZnO NPs | 10 | 12.66 ± 0.98 | 7.78 | 93.50 |
| ZnO NPs-BDA | 10 | 13.07 ± 0.50 | 3.85 | 96.53 |
| ZnO NPs-BA | 10 | 12.42 ± 0.87 | 7.04 | 91.73 |
| ZnO NPs-BDP | 10 | 10.74 ± 0.47 | 4.34 | 79.32 |
| Value is Taken as 100% (Control Group) | MDA Level, % of Control | ||||
|---|---|---|---|---|---|
| ZnO NPs | ZnO NPs-BDA | ZnO NPs-BA | ZnO NPs-BDP | ||
| Plasma | 0.90 µmol/L (intact) | 50.66 ± 1.46 | 69.03 ± 3.01 | 85.88 ± 6.90 | 87.86 ± 5.70 |
| 1.08 µmol/L (burned) | 42.33 ± 0.58 | 57.67 ± l.96 | 71.75 ± 8.42 | 73.41 ± 6.64 | |
| Erythrocytes | 8.94 µmol/L (intact) | 60.14 ± 4.74 | 66.33 ± 5.53 | 59.35 ± 4.09 | 57.44 ± 5.06 |
| 11.25 µmol/L (burned) | 47.80 ± 3.47 | 52.72 ± 3.71 | 47.17 ± 3.56 | 45.65 ± 3.67 | |
| Enzyme | Biochemical Indexes 1 | ||||
|---|---|---|---|---|---|
| Value is Taken as 100% (Control group) | % of Control | ||||
| ZnO NPs | ZnO NPs-BDA | ZnO NPs-BA | ZnO NPs-BDP | ||
| SOD | 900.76 Ru/mg protein (intact) | 71.40 ± 2.12 | 81.29 ± 3.36 | 84.57 ± 9.66 | 77.47 ± 5.40 |
| 597.82 Ru/mg protein (burned) | 107.59 ± 5.35 | 122.48 ± 0.64 | 127.43 ± 12.87 | 116.72 ± 10.45 | |
| Catalase | 30.51 Ru/mg protein (intact) | 57.85 ± 2.67 | 62.81 ± 4.29 | 79.65 ± 4.37 | 82.61 ± 5.03 |
| 17.80 Ru/mg protein (burned) | 99.18 ± 4.55 | 107.68 ± 6.56 | 136.57 ± 10.33 | 141.63 ± 9.12 | |
| GR | 90.83 nmol NADH/min/mg protein (intact) | 104.81 ± 7.23 | 78.61 ± 3.09 | 152.58 ± 9.76 | 112.75 ± 12.44 |
| 57.44 nmol NADH/min/mg protein (burned) | 165.74 ± 6.70 | 124.30 ± 5.49 | 241.27 ± 13.45 | 178.28 ± 15.62 | |
| G6PD | 40.57 nmol NADPH/min/mg protein (intact) | 95.95 ± 8.39 | 101.90 ± 8.94 | 107.95 ± 8.56 | 120.79 ± 8.43 |
| 25.78 nmol NADPH/min/mg protein (burned) | 151.01 ± 4.95 | 160.36 ± 5.23 | 169.90 ± 4.69 | 190.11 ± 9.99 | |
| LDHdirect | 42.77 nmol NADH/min/mg protein (intact) | 75.10 ± 6.74 | 77.77 ± 4.82 | 83.61 ± 4.95 | 73.78 ± 3.78 |
| 25.87 nmol NADH/min/mg protein (burned) | 124.18 ± 8.05 | 128.59 ± 7.33 | 138.25 ± 8.14 | 121.99 ± 5.76 | |
| LDHreverse | 174.18 nmol NADH/min/mg protein (intact) | 107.02 ± 7.72 | 87.22 ± 1.98 | 90.58 ± 3.94 | 98.06 ± 4.85 |
| 146.49 nmol NADH/min/mg protein (burned) | 127.25 ± 9.42 | 103.71 ± 9.55 | 107.70 ± 5.62 | 116.60 ± 9.53 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melnikova, N.; Vorobyova, O.; Balakireva, A.; Malygina, D.; Solovyeva, A.; Belyaeva, K.; Orekhov, D.; Knyazev, A. The New Pharmaceutical Compositions of Zinc Oxide Nanoparticles and Triterpenoids for the Burn Treatment. Pharmaceuticals 2020, 13, 207. https://doi.org/10.3390/ph13090207
Melnikova N, Vorobyova O, Balakireva A, Malygina D, Solovyeva A, Belyaeva K, Orekhov D, Knyazev A. The New Pharmaceutical Compositions of Zinc Oxide Nanoparticles and Triterpenoids for the Burn Treatment. Pharmaceuticals. 2020; 13(9):207. https://doi.org/10.3390/ph13090207
Chicago/Turabian StyleMelnikova, Nina, Olga Vorobyova, Alyona Balakireva, Darina Malygina, Anna Solovyeva, Kseniya Belyaeva, Dmitry Orekhov, and Alexander Knyazev. 2020. "The New Pharmaceutical Compositions of Zinc Oxide Nanoparticles and Triterpenoids for the Burn Treatment" Pharmaceuticals 13, no. 9: 207. https://doi.org/10.3390/ph13090207
APA StyleMelnikova, N., Vorobyova, O., Balakireva, A., Malygina, D., Solovyeva, A., Belyaeva, K., Orekhov, D., & Knyazev, A. (2020). The New Pharmaceutical Compositions of Zinc Oxide Nanoparticles and Triterpenoids for the Burn Treatment. Pharmaceuticals, 13(9), 207. https://doi.org/10.3390/ph13090207






