Echinacea purpurea (L.) Moench: Chemical Characterization and Bioactivity of Its Extracts and Fractions
Abstract
1. Introduction
2. Results and Discussion
2.1. HPLC–DAD–ESI/MS Analysis of Phenolic Compounds
2.2. GC/MS Analysis of Non-Polar Extracts
2.3. Biological Properties
2.3.1. Antimicrobial Activity
2.3.2. Cytotoxic Properties of Extracts and Fractions
3. Materials and Methods
3.1. Standards and Reagents
3.2. Plant Material
3.3. Preparation of Extracts
3.3.1. Organic Extracts
3.3.2. Aqueous Extracts
3.4. Fractionation of the Extracts
3.4.1. Dichloromethane Extracts
3.4.2. n-Hexane Extracts
3.5. Chemical Characterization
3.5.1. Analysis of Phenolic Compounds by HPLC–DAD–ESI/MS
3.5.2. Analysis of Non-Polar Compounds by GC–MS
3.6. Evaluation of the Bioactive Properties
3.6.1. Antimicrobial Activity
3.6.2. Cytotoxic Activity
3.6.3. Hepatotoxicity
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nadaf, M.; Joharchi, M.R.; Amiri, M.S. Ethnomedicinal uses of plants for the treatment of nervous disorders at the herbal markets of Bojnord, North Khorasan Province, Iran. Avicenna J. Phytomed. 2019, 9, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Sousa, S.G.; Oliveira, L.A.; de Aguiar Magalhaes, D.; de Brito, T.V.; Batista, J.A.; Pereira, C.M.C.; de Souza Costa, M.; Mazulo, J.C.R.; de Carvalho Filgueiras, M.; Vasconselos, D.F.P.; et al. Chemical structure and anti-inflammatory effect of polysaccharide extracted from Morinda citrifolia Linn (Noni). Carbohydr. Polym. 2018, 197, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, M.O.; Christensen, L.P.; Grevsen, K. Harvest strategies for optimization of the content of bioactive alkamides and caffeic acid derivatives in aerial parts and in roots of Echinacea purpurea. J. Agric. Food Chem. 2018, 66, 11630–11639. [Google Scholar] [CrossRef] [PubMed]
- Chiou, S.-Y.; Sung, J.-M.; Huang, P.-W.; Lin, S.-D. Antioxidant, antidiabetic, and antihypertensive properties of Echinacea purpurea flower extract and caffeic acid derivatives using in vitro models. J. Med. Food 2017, 20, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Wang, Z.; Wang, L.; Gao, M.; Wang, L.; Gan, C.; Yang, C. Simultaneous determination of seven phenolic acids in rat plasma using UHPLC-ESI-MS/MS after oral administration of Echinacea purpurea extract. Molecules 2017, 22, 1494. [Google Scholar] [CrossRef]
- Aarland, R.C.; Bañuelos-Hernández, A.E.; Fragoso-Serrano, M.; Del Carmen Sierra-Palacios, E.; Díaz de León-Sánchez, F.; Pérez-Flores, L.J.; Rivera-Cabrera, F.; Mendoza-Espinoza, J.A. Studies on phytochemical, antioxidant, anti-inflammatory, hypoglycaemic and antiproliferative activities of Echinacea purpurea and Echinacea angustifolia extracts. Pharm. Biol. 2017, 55, 649–656. [Google Scholar] [CrossRef]
- Barnes, J.; Anderson, L.A.; Gibbons, S.; Phillipson, J.D. Echinacea species (Echinacea angustifolia (DC.) Hell., Echinacea pallida (Nutt.) Nutt., Echinacea purpurea (L.) Moench): A review of their chemistry, pharmacology and clinical properties. J. Pharm. Pharmacol. 2005, 57, 929–954. [Google Scholar] [CrossRef]
- Oláh, A.; Szabó-Papp, J.; Soeberdt, M.; Knie, U.; Dähnhardt-Pfeiffer, S.; Abels, C.; Bíró, T. Echinacea purpurea-derived alkylamides exhibit potent anti-inflammatory effects and alleviate clinical symptoms of atopic eczema. J. Dermatol. Sci. 2017, 88, 67–77. [Google Scholar] [CrossRef]
- Pires, C.; Martins, N.; Carvalho, A.M.; Barros, L.; Ferreira, I.C.F.R. Phytopharmacologic preparations as predictors of plant bioactivity: A particular approach to Echinacea purpurea (L.) Moench antioxidant properties. Nutrition 2016, 32, 834–839. [Google Scholar] [CrossRef]
- Hudson, J.B. Applications of the phytomedicine Echinacea purpurea (Purple Coneflower) in infectious diseases. J. Biomed. Biotechnol. 2012, 2012, 1–16. [Google Scholar] [CrossRef]
- Senica, M.; Mlinsek, G.; Veberic, R.; Mikulic-Petkovsek, M. Which plant part of purple coneflower (Echinacea purpurea (L.) Moench) should be used for tea and which for tincture? J. Med. Food 2019, 22, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Sultan, M.T.; Buttxs, M.S.; Qayyum, M.M.N.; Suleria, H.A.R. Immunity: Plants as effective mediators. Crit. Rev. Food Sci. Nutr. 2014, 54, 1298–1308. [Google Scholar] [CrossRef] [PubMed]
- Manayi, A.; Vazirian, M.; Saeidnia, S. Echinacea purpurea: Pharmacology, phytochemistry and analysis methods. Pharmacogn. Rev. 2015, 9, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, A.A.; Hussein, S.; Tohamy, A.F.; Marouf, S.; Yassa, H.D.; Zaki, A.R.; Bishayee, A. Protective effect of Echinacea purpurea (Immulant) against cisplatin-induced immunotoxicity in rats. DARU J. Pharm. Sci. 2019, 27, 233–241. [Google Scholar] [CrossRef]
- Pillai, S.; Pillai, C.; Mitscher, L.A.; Cooper, R. Use of quantitative flow cytometry to measure ex vivo immunostimulant activity of Echinacea: The case for polysaccharides. J. Altern. Complement. Med. 2007, 13, 625–634. [Google Scholar] [CrossRef]
- Todd, D.A.; Gulledge, T.V.; Britton, E.R.; Oberhofer, M.; Leyte-Lugo, M.; Moody, A.N.; Shymanovich, T.; Grubbs, L.F.; Juzumaite, M.; Graf, T.N.; et al. Ethanolic Echinacea purpurea extracts contain a mixture of cytokine-suppressive and cytokine-inducing compounds, including some that originate from endophytic bacteria. PLoS ONE 2015, 10, e0124276. [Google Scholar] [CrossRef]
- Yang, G.; Li, K.; Liu, C.; Peng, P.; Bai, M.; Sun, J.; Li, Q.; Yang, Z.; Yang, Y.; Wu, H. A comparison of the immunostimulatory effects of polysaccharides from tetraploid and diploid Echinacea purpurea. Biomed. Res. Int. 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Yao, L.; Bai, L.; Tan, Y.; Sun, J.; Qu, Q.; Shi, D.; Guo, S.; Liu, C. The immunoregulatory effect of sulfated Echinacea purpurea polysaccharide on chicken bone marrow-derived dendritic cells. Int. J. Biol. Macromol. 2019, 139, 1123–1132. [Google Scholar] [CrossRef]
- Sloley, B.D.; Urichuk, L.J.; Tywin, C.; Coutts, R.T.; Pang, P.K.T.; Shan, J.J. Comparison of chemical components and antioxidant capacity of different Echinacea species. J. Pharm. Pharmacol. 2001, 53, 849–857. [Google Scholar] [CrossRef]
- Ma, Y.; Kosińska-Cagnazzo, A.; Kerr, W.L.; Amarowicz, R.; Swanson, R.B.; Pegg, R.B. Separation and characterization of phenolic compounds from dry-blanched peanut skins by liquid chromatography-electrospray ionization mass spectrometry. J. Chromatogr. A 2014, 1356, 64–81. [Google Scholar] [CrossRef]
- Waidyanatha, S.; Pierfelice, J.; Cristy, T.; Mutlu, E.; Burback, B.; Rider, C.V.; Ryan, K. A strategy for test article selection and phytochemical characterization of Echinacea purpurea extract for safety testing. Food Chem. Toxicol. 2020, 137, 111125. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.N.; Chan, M.; Paley, L.; Betz, J.M. Determination of major phenolic compounds in Echinacea spp. raw materials and finished products by high-performance liquid chromatography with ultraviolet detection: Single-laboratory validation matrix extension. J. AOAC Int. 2011, 94, 1400–1410. [Google Scholar] [CrossRef] [PubMed]
- Vandyshev, V.V.; Babaeva, E.Y.; Drozdovskaya, D.D. Triacylglycerols of the lipid fraction from fruits of two Echinacea species. Pharm. Chem. J. 2009, 43, 154–156. [Google Scholar] [CrossRef]
- Sharma, M.; Vohra, S.; Arnason, J.T.; Hudson, J.B. Echinacea extracts contain significant and selective activities against human pathogenic bacteria. Pharm. Biol. 2008, 46, 111–116. [Google Scholar] [CrossRef]
- Tsai, Y.-L.; Chiu, C.-C.; Yi-Fu Chen, J.; Chan, K.-C.; Lin, S.-D. Cytotoxic effects of Echinacea purpurea flower extracts and cichoric acid on human colon cancer cells through induction of apoptosis. J. Ethnopharmacol. 2012, 143, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Chicca, A.; Adinolfi, B.; Pellati, F.; Orlandini, G.; Benvenuti, S.; Nieri, P. Cytotoxic activity and G1 cell cycle arrest of a Dienynone from Echinacea pallida. Planta Med. 2010, 76, 444–446. [Google Scholar] [CrossRef]
- Graça, V.C.; Barros, L.; Calhelha, R.C.; Dias, M.I.; Carvalho, A.M.; Santos-Buelga, C.; Santos, P.F.; Ferreira, I.C.F.R. Chemical characterization and bioactive properties of aqueous and organic extracts of Geranium robertianum L. Food Funct. 2016, 7. [Google Scholar] [CrossRef]
- Graça, V.C.; Barros, L.; Calhelha, R.C.; Dias, M.I.; Ferreira, I.C.F.R.; Santos, P.F. Bio-guided fractionation of extracts of Geranium robertianum L.: Relationship between phenolic profile and biological activity. Ind. Crops Prod. 2017, 108, 543–552. [Google Scholar] [CrossRef]
- Bessada, S.M.F.; Barreira, J.C.M.; Barros, L.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P. Phenolic profile and antioxidant activity of Coleostephus myconis (L.) Rchb.f.: An underexploited and highly disseminated species. Ind. Crops Prod. 2016, 89, 45–51. [Google Scholar] [CrossRef]
- Mascoloti Spréa, R.; Fernandes, Â.; Calhelha, R.C.; Pereira, C.; Pires, T.C.S.P.; Alves, M.J.; Canan, C.; Barros, L.; Amaral, J.S.; Ferreira, I.C.F.R. Chemical and bioactive characterization of the aromatic plant Levisticum officinale W.D.J. Koch: A comprehensive study. Food Funct. 2020, 11, 1292–1303. [Google Scholar] [CrossRef]
- Alves, M.J.; Ferreira, I.C.F.R.; Martins, A.; Pintado, M. Antimicrobial activity of wild mushroom extracts against clinical isolates resistant to different antibiotics. J. Appl. Microbiol. 2012, 113, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Pereira, E.; Calhelha, R.C.; Dueñas, M.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Bioactivity and chemical characterization in hydrophilic and lipophilic compounds of Chenopodium ambrosioides L. J. Funct. Foods 2013, 5, 1732–1740. [Google Scholar] [CrossRef]
- Abreu, R.M.V.; Ferreira, I.C.F.R.; Calhelha, R.C.; Lima, R.T.; Vasconcelos, M.H.; Adega, F.; Chaves, R.; Queiroz, M.-J.R.P. Anti-hepatocellular carcinoma activity using human HepG2 cells and hepatotoxicity of 6-substituted methyl 3-aminothieno[3,2-b]pyridine-2-carboxylate derivatives: In vitro evaluation, cell cycle analysis and QSAR studies. Eur. J. Med. Chem. 2011, 46, 5800–5806. [Google Scholar] [CrossRef] [PubMed]
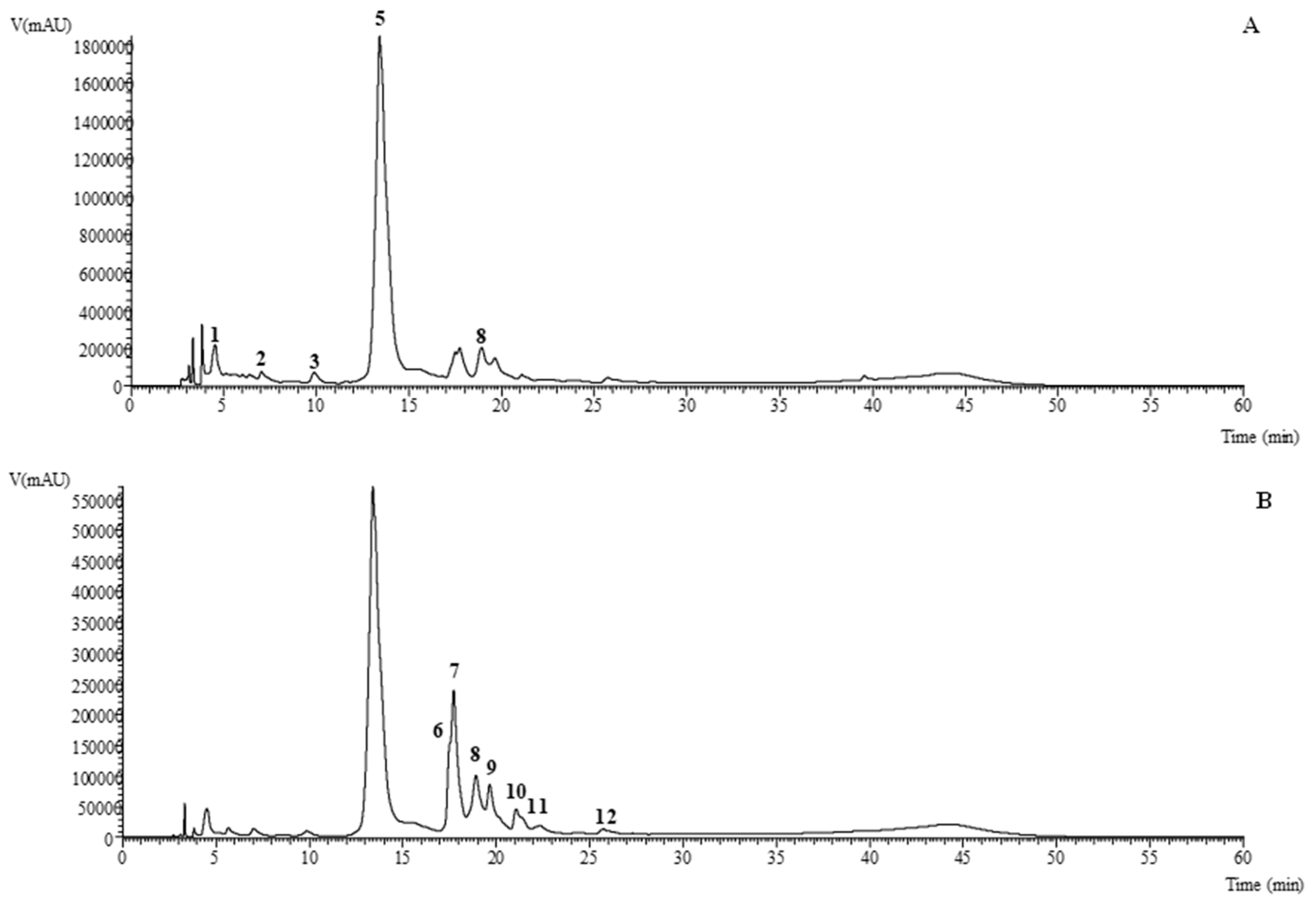
| Peak | Rt (min) | λmax (nm) | [M-H]− (m/z) | MS2 (m/z) | Tentative Identification | Organic | Aqueous | |||
|---|---|---|---|---|---|---|---|---|---|---|
| EtOAc | Acetone | MeOH | Infusion | Decoction | ||||||
| 1 | 4.54 | 327 | 311 | 179(6),149(100),135(5),113(3) | Caftaric acid | nd | nd | 1.19 ± 0.02 a | 0.16 ± 0.01 c | 0.22 ± 0.01 b |
| 2 | 7.04 | 325 | 353 | 191(100),179(8),173(3),135(3) | 5-O-caffeoylquinic acid | nd | nd | 0.286 ± 0.003 a | 0.024 ± 0.001 b | nd |
| 3 | 9.87 | 324 | 179 | 135(100) | Caffeic acid | 0.20 ± 0.01 b | 0.066 ± 0.001 c | 0.63 ± 0.02 a | nd | nd |
| 4 | 11.84 | 328 | 473 | 311(100),293(87),179(5),149(5) | cis Chicoric acid | nd | nd | nd | 12.0 ± 0.1 * | 10.5 ± 0.1 * |
| 5 | 13.40 | 328 | 473 | 311(100),293(87),179(5),149(5) | trans Chicoric acid | nd | nd | 41.0 ± 0.3 a | 4.0 ± 0.1 b | 4.5 ± 0.1 b |
| 6 | 17.50 | 342 | 609 | 301(100) | Quercetin-O-deoxyhexosyl-hexoside | nd | tr | 1.8836 ± 0.0005 | nd | nd |
| 7 | 17.73 | 342 | 609 | 301(100) | Quercetin-3-O-rutinoside | nd | tr | 5.6 ± 0.2 a | 1.4 ± 0.1 b | 0.26 ± 0.01 c |
| 8 | 18.93 | 328 | 487 | 325(85),307(51),293(100),193(10),179(15) | Feruloylcaffeoyltartaric acid | nd | 0.030 ± 0.001 d | 1.36 ± 0.04 a | 0.10 ± 0.01 b | 0.040 ± 0.001 c |
| 9 | 19.66 | 329 | 593 | 285(100) | Kaempferol-O-deoxyhexosyl-hexoside | nd | tr | 0.60 ± 0.01 | nd | tr |
| 10 | 21.10 | 334 | 593 | 285(100) | Kaempferol-3-O-rutinoside | nd | tr | 0.536 ± 0.001 | nd | tr |
| 11 | 21.42 | 334 | 593 | 285(100) | Kaempferol-O-deoxyhexosyl-hexoside | nd | tr | 0.058 ± 0.003 | nd | nd |
| 12 | 22.35 | 327 | 549 | 505(5),463(13),301(30) | Quercetin-O-malonylhexoside | nd | nd | nd | nd | nd |
| 13 | 24.10 | 340 | 607 | 285(100) | Kaempferol-O-deoxyhexosyl-glucuronide | nd | tr | nd | nd | nd |
| 14 | 25.73 | 327 | 501 | 337(100),307(50),277(15),233(61),203(5) | p-Coumaroylsinapoyltartaric acid | nd | nd | 0.050 ± 0.001 a | 0.010 ± 0.001 b | 0.010 ± 0.001 b |
| 15 | 39.56 | 308 | 577 | 299(100) | Diosmetin-O-p-coumaroyl-pentoside | nd | 5.29 ± 0.04 | nd | nd | nd |
| Total phenolic acids | 0.20 ± 0.01 b | 0.066 ± 0.001 a | 44.5 ± 0.4 b | 16.4 ± 0.2 b | 15.2 ± 0.2 b | |||||
| Total flavonoids | nd | 5.29 ± 0.04 b | 8.6 ± 0.2 a | 1.4 ± 0.1 c | 0.26 ± 0.01 d | |||||
| Total phenolic compounds | 0.20 ± 0.01 e | 5.36 ± 0.04 d | 53 ± 1 a | 17.8 ± 0.1 b | 15.5 ± 0.2 c | |||||
| Number | Compound | RT (min) | LRI a | Relative % b | t-Students Test p-Value | |
|---|---|---|---|---|---|---|
| n-Hexane | DCM | |||||
| 1 | 2,3-Butanediol | 14.97 | 1044 * | - | 0.055 ± 0.003 | - |
| 2 | Hexanoic acid | 16.56 | 1079 * | 0.017 ± 0.004 | 0.061 ± 0.001 | <0.001 |
| 3 | 2-Methyl-4-pentenoic acid | 16.83 | 1085 * | - | 0.027 ± 0.002 | - |
| 4 | (E)-2-Hexenoic acid | 18.83 | 1127 * | - | 0.018 ± 0.002 | - |
| 5 | Verbenone | 22.92 | 1215 | 0.009 ± 0.001 | - | - |
| 6 | (+)-cis-Verbenol | 24.11 | 1241 * | 0.009 ± 0.002 | - | - |
| 7 | Benzoic acid | 24.65 | 1253 * | - | 0.039 ± 0.003 | - |
| 8 | Menthol | 25.24 | 1264 * | 0.0035 ± 0.0004 | - | - |
| 9 | Octanoic acid | 25.52 | 1270 * | 0.013 ± 0.003 | 0.026 ± 0.002 | <0.001 |
| 10 | Carvacrol | 28.54 | 1340 * | 0.13 ± 0.04 | 0.1256 ± 0.00004 | 0.155 |
| 11 | Copaene | 30.49 | 1385 | 0.013 ± 0.001 | - | - |
| 12 | β-Caryophyllene | 32.39 | 1430 | 0.034 ± 0.002 | - | - |
| 13 | Decanoic acid | 33.91 | 1466 * | 0.198 ± 0.001 | 0.125 ± 0.002 | <0.001 |
| 14 | D-(-)-Citramalic acid | 35.04 | 1494 * | - | 0.077 ± 0.003 | - |
| 15 | Epicubebol | 35.47 | 1504 | 0.048 ± 0.003 | - | - |
| 16 | Malic acid | 35.79 | 1512 * | 0.033 ± 0.002 | 0.115 ± 0.002 | <0.001 |
| 17 | Dihydroactinidiolide | 36.83 | 1539 | 0.052 ± 0.002 | - | - |
| 18 | trans-Nerolidol | 38.09 | 1571 | 0.048 ± 0.001 | - | - |
| 19 | Spathulenol | 38.79 | 1589 | 0.22 ± 0.01 | - | - |
| 20 | Caryophyllene oxide | 39.03 | 1595 | 0.22 ± 0.01 | - | - |
| 21 | Dodecanoic acid | 41.55 | 1662 * | 0.15 ± 0.04 | 0.066 ± 0.002 | <0.001 |
| 22 | Oplopanone | 44.49 | 1755 | 0.507 ± 0.004 | - | - |
| 23 | Azelaic acid | 45.78 | 1803 * | - | 0.371 ± 0.003 | - |
| 24 | Neophytadiene | 46.35 | 1845 | 0.907 ± 0.001 | 2.71 ± 0.1 | <0.001 |
| 25 | Myristic acid | 46.53 | 1858 * | 1.4 ± 0.1 | 1.14 ± 0.01 | 0.092 |
| 26 | Hexadecanoic acid, methyl ester | 47.51 | 1936 | 0.351 ± 0.004 | - | - |
| 27 | Pentadecanoic acid | 47.78 | 1960 * | 0.44 ± 0.02 | 0.27 ± 0.01 | <0.001 |
| 28 | Gallic acid | 48.09 | 1988 * | - | 0.12 ± 0.01 | - |
| 29 | Undecanedioic acid | 48.29 | 2006 * | - | 0.154 ± 0.003 | - |
| 30 | Palmitelaidic acid | 48.58 | 2039 * | 0.16 ± 0.01 | - | - |
| 31 | Palmitic Acid | 48.79 | 2061 * | 4.6 ± 0.1 | 5.7 ± 0.2 | 0.015 |
| 32 | Linoleic acid, methyl ester | 49.21 | 2109 | 0.644 ± 0.001 | - | - |
| 33 | Heptadecanoic acid | 49.59 | 2157 * | 0.8 ± 0.1 | - | - |
| 34 | Caffeic acid | 49.62 | 2161 * | - | 0.822 ± 0.003 | - |
| 35 | Phytol | 49.85 | 2190 * | 1.1 ± 0.1 | 0.55 ± 0.01 | <0.001 |
| 36 | Docosane | 49.99 | 2210 | - | 0.42 ± 0.02 | - |
| 37 | Linoleic acid | 50.12 | 2229 * | 5.0 ± 0.1 | 4.3 ± 0.1 | 0.016 |
| 38 | α-Linolenic acid | 50.17 | 2236 * | 2.2 ± 0.2 | 2.4 ± 0.1 | 0.121 |
| 39 | Stearic acid | 50.31 | 2256 * | 2.30 ± 0.04 | 1.5 ± 0.1 | <0.001 |
| 40 | Tricosane | 50.68 | 2311 | - | 1.0 ± 0.1 | - |
| 41 | Tetracosane | 51.30 | 2411 | 1.6 ± 0.1 | 1.9 ± 0.1 | 0.025 |
| 42 | Arachidic acid | 51.55 | 2454 * | 3.3 ± 0.2 | 1.664 ± 0.005 | <0.001 |
| 43 | Pentacosane | 51.89 | 2511 | 1.6 ± 0.02 | 3.20 ± 0.04 | <0.001 |
| 44 | Heneicosanoic acid | 52.13 | 2552 * | 0.37 ± 0.02 | 0.56 ± 0.04 | <0.001 |
| 45 | Hexacosane | 52.48 | 2612 | 1.58 ± 0.01 | 4.36 ± 0.02 | <0.001 |
| 46 | Behenic acid | 52.73 | 2651 * | 1.21 ± 0.05 | 1.4 ± 0.1 | 0.084 |
| 47 | 3-Methylhexacosane | 52.95 | 2686 | - | 0.47 ± 0.02 | - |
| 48 | Heptacosane | 53.12 | 2712 | 2.6 ± 0.1 | 5.0 ± 0.3 | <0.001 |
| 49 | Tricosanoic acid | 53.39 | 2750 * | 0.82 ± 0.03 | 0.84 ± 0.02 | 0.373 |
| 50 | Octacosane | 53.83 | 2812 | 1.6 ± 0.01 | 4.4 ± 0.1 | <0.001 |
| 51 | Lignoceric acid | 54.12 | 2849 * | 1.3 ± 0.1 | 1.04 ± 0.01 | 0.023 |
| 52 | Squalene | 54.18 | 2856 | 1.3 ± 0.1 | 0.434 ± 0.02 | <0.001 |
| 53 | 2-Methyl-octacosane | 54.31 | 2873 | - | 0.592 ± 0.01 | - |
| 54 | 3-Methyloctacosane | 54.41 | 2886 | - | 0.52 ± 0.01 | - |
| 55 | Nonacosane | 54.63 | 2912 | 3.1 ± 0.1 | 5.14 ± 0.03 | <0.001 |
| 56 | 1-Hexacosanol | 55.01 | 2953 * | 1.6 ± 0.1 | 1.3 ± 0.1 | 0.091 |
| 57 | Hexacosanoic acid | 55.95 | 3048 * | - | 1.4 ± 0.1 | - |
| 58 | Nonacosanal | 56.07 | 3059 * | 0.75 ± 0.04 | - | - |
| 59 | Untriacontane | 56.67 | 3112 | 2.4 ± 0.2 | - | - |
| 60 | α-Tocopherol | 57.56 | 3182 | - | 1.09 ± 0.04 | - |
| 61 | Cholesterol | 58.11 | 3221 * | 0.39 ± 0.01 | - | - |
| 62 | Campesterol | 59.78 | 3325 * | 2.00 ± 0.01 | 0.41 ± 0.02 | <0.001 |
| 63 | Stigmasterol | 60.31 | 3353 * | 3.9 ± 0.2 | - | - |
| 64 | β-Sitosterol | 61.40 | 3416 * | 7.5 ± 0.5 | 4.8 ± 0.3 | <0.001 |
| Total identified compounds | 61.0 ± 0.5 | 63 ± 1 | 0.482 | |||
| Monoterpene hydrocarbons | 0.06 ± 0.002 | - | - | |||
| Oxygen-containing monoterpenes | 0.021 ± 0.002 | - | - | |||
| Sesquiterpene hydrocarbons | 0.38 ± 0.03 | 0.1256 ± 0.00004 | <0.001 | |||
| Oxygen-containing sesquiterpenes | 0.824 ± 0.002 | - | - | |||
| Diterpene hydrocarbons | 3.4 ± 0.2 | 3.7 ± 0.1 | 0.151 | |||
| Sterol | 13.9 ± 0.3 | 5.2 ± 0.3 | <0.001 | |||
| Fatty acids | 24.8 ± 0.5 | 23 ± 1 | 0.362 | |||
| Long-chain hydrocarbons | 14.57 ± 0.03 | 27.1 ± 0.3 | <0.001 | |||
| Long-chain alcohols | 1.6 ± 0.1 | 1.3 ± 0.1 | 0.018 | |||
| Others | 1.4 ± 0.04 | 2.61 ± 0.04 | <0.001 | |||
| Organic Extracts | Aqueous Extracts | Controls | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n-Hexane | DCM | EtOAc | Acetone | MeOH | Infusion | Decoction | Ampicilin | Imipenem | Vancomycin | Fluconazol | ||||||||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| Gram-negative bacteria | ||||||||||||||||||||||
| Escherichia coli | 20 | >20 | 10 | >20 | 5 | >20 | 10 | >20 | >20 | >20 | >20 | >20 | >20 | >20 | <0.15 | <0.15 | <0.0078 | <0.0078 | nt | nt | nt | nt |
| Klebsiella pneumoniae | 20 | >20 | 20 | >20 | 10 | >20 | 10 | >20 | >20 | >20 | >20 | >20 | 20 | >20 | 10 | 20 | <0.0078 | <0.0078 | nt | nt | nt | nt |
| Morganella morganii | 20 | >20 | 5 | >20 | 5 | >20 | 10 | >20 | >20 | >20 | >20 | >20 | >20 | >20 | 20 | >20 | <0.0078 | <0.0078 | nt | nt | nt | nt |
| Proteus mirabilis | 20 | >20 | 20 | >20 | 10 | >20 | 10 | >20 | >20 | >20 | >20 | >20 | >20 | >20 | <0.15 | <0.15 | <0.0078 | <0.0078 | nt | nt | nt | nt |
| Pseudomonas aeruginosa | 20 | >20 | 10 | >20 | 10 | >20 | 10 | >20 | >20 | >20 | >20 | >20 | >20 | >20 | >20 | >20 | 0.5 | 1 | nt | nt | nt | nt |
| Gram-positive bacteria | ||||||||||||||||||||||
| Enterococcus faecalis | 10 | >20 | 2.5 | >20 | 10 | >20 | 5 | >20 | >20 | >20 | >20 | >20 | >20 | >20 | <0.15 | <0.15 | nt | nt | <0.0078 | <0.0078 | nt | nt |
| Listeria monocytogenes | >20 | >20 | 2.5 | >20 | 20 | >20 | 20 | >20 | >20 | >20 | >20 | >20 | >20 | >20 | <0.15 | <0.15 | <0.0078 | <0.0078 | nt | nt | nt | nt |
| MRSA | 10 | >20 | 5 | >20 | 5 | >20 | 5 | >20 | 20 | >20 | >20 | >20 | >20 | >20 | <0.15 | <0.15 | nt | nt | <0.0078 | <0.0078 | nt | nt |
| MSSA | 10 | >20 | 5 | >20 | 5 | >20 | 2.5 | >20 | 20 | >20 | >20 | >20 | >20 | >20 | <0.15 | <0.15 | nt | nt | 0.25 | 0.5 | nt | nt |
| Yeasts | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC |
| Candida albicans | 10 | >20 | 5 | >20 | 10 | >20 | 5 | >20 | >20 | >20 | >20 | >20 | >20 | >20 | nt | nt | nt | nt | nt | nt | 0.06 | 0.06 |
| NCI H460 | HepG2 | HeLa | MCF-7 | PLP2 | ||
|---|---|---|---|---|---|---|
| Organic | n-Hexane | 70 ± 2 c | 47 ± 3 c | 58 ± 5 e | 29 ± 2 d | 104 ± 7 c |
| DCM | 48 ± 4 d | 36.7 ± 0.6 c | 51 ± 4 e | 21 ± 2 e | 100 ± 8 c | |
| EtOAc | 192 ± 4 a | 226 ± 15 a | 85 ± 6 d | 51 ± 5 c | 166 ± 9 b | |
| Acetone | 142 ± 10 b | 82 ± 4 b | 98 ± 5 cd | 50 ± 4 c | 195 ± 15 a | |
| MeOH | > 400 | > 400 | 111 ± 9 c | 76 ± 5 b | >400 | |
| Aqueous | Infusion | >400 | >400 | 305 ± 23 b | 247 ± 5 a | >400 |
| Decoction | >400 | >400 | 319 ± 12 a | >400 | >400 | |
| Fraction of n-hexane | FH1 | 105 ± 7 h | 206 ± 12 e | 150 ± 5 d | 110 ± 3 fg | 326 ± 17 b |
| FH2 | 142 ± 5 g | 344 ± 10 ab | 180 ± 7 c | 140 ± 7 e | >400 | |
| FH3 | 184 ± 15 ef | 308 ± 19 c | 212 ± 6 b | 182 ± 9 c | >400 | |
| FH4 | 243 ± 16 a | 233 ± 3 d | 240 ± 3 a | 222 ± 5 b | >400 | |
| FH5 | 238 ± 4 ab | 359 ± 7 a | 232 ± 5 a | 239 ± 4 a | >400 | |
| FH6 | 250 ± 1 a | 327 ± 16 bc | 235 ± 5 a | 237 ± 8 a | >400 | |
| FH7 | 197 ± 12 de | 159 ± 6 gh | 213 ± 8 b | 179 ± 9 c | 343 ± 22 ab | |
| FH8 | 222 ± 5 bc | 168 ± 5 fg | 241 ± 2 a | 121 ± 10 f | 356 ± 11 a | |
| FH9 | 206 ± 11 cd | 159 ± 3 gh | 181 ± 8 c | 171 ± 4 cd | 342 ± 10 ab | |
| FH10 | 211 ± 5 cd | 141 ± 4 hi | 170 ± 9 c | 181 ± 5 c | 331 ± 6 ab | |
| FH11 | 100 ± 7 h | 93 ± 4 j | 105 ± 9 f | 106 ± 10 g | 269 ± 6 c | |
| FH12 | 128 ± 5 g | 131 ± 6 i | 127 ± 9 e | 111 ± 3 fg | >400 | |
| FH13 | 168 ± 12 f | 187 ± 12 ef | 176 ± 12 c | 160 ± 7 d | >400 | |
| FH14 | >400 | 338 ± 13 b | >400 | >400 | >400 | |
| Fraction of DCM | FD1 | >400 | >400 | >400 | >400 | >400 |
| FD2 | 236 ± 11 c | 278 ± 9 ab | 178 ± 12 c | 146 ± 11 c | >400 | |
| FD3 | >400 | >400 | >400 | >400 | >400 | |
| FD4 | >400 | >400 | >400 | >400 | >400 | |
| FD5 | 128 ± 4 e | 146 ± 4 f | 116 ± 4 e | 89 ± 8 d | 267 ± 13 * | |
| FD6 | 135 ± 4 e | 141 ± 3 f | 113 ± 4 e | 81 ± 3 d | >400 | |
| FD7 | 170 ± 2 d | 187 ± 16 e | 150 ± 3 d | 139 ± 5 c | 302 ± 18 * | |
| FD8 | 225 ± 9 c | 212 ± 3 d | 174 ± 9 c | 151 ± 4 bc | >400 | |
| FD9 | 238 ± 4 c | 205 ± 7 de | 178 ± 9 c | 144 ± 9 c | >400 | |
| FD10 | 341 ± 9 a | 298 ± 19 a | 241 ± 5 a | 181 ± 9 a | >400 | |
| FD11 | 291 ± 9 b | 253 ± 8 c | 220 ± 5 b | 149 ± 3 bc | >400 | |
| FD12 | 292 ± 17 b | 256 ± 11 c | 223 ± 14 b | 144 ± 3 c | >400 | |
| FD13 | 336 ± 10 a | 271 ± 4 bc | 254 ± 6 a | 161 ± 4 b | >400 | |
| FD14 | >400 | >400 | >400 | >400 | >400 | |
| FD15 | >400 | >400 | >400 | >400 | >400 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho, J.; Barros, L.; Dias, M.I.; Finimundy, T.C.; Amaral, J.S.; Alves, M.J.; Calhelha, R.C.; Santos, P.F.; Ferreira, I.C.F.R. Echinacea purpurea (L.) Moench: Chemical Characterization and Bioactivity of Its Extracts and Fractions. Pharmaceuticals 2020, 13, 125. https://doi.org/10.3390/ph13060125
Coelho J, Barros L, Dias MI, Finimundy TC, Amaral JS, Alves MJ, Calhelha RC, Santos PF, Ferreira ICFR. Echinacea purpurea (L.) Moench: Chemical Characterization and Bioactivity of Its Extracts and Fractions. Pharmaceuticals. 2020; 13(6):125. https://doi.org/10.3390/ph13060125
Chicago/Turabian StyleCoelho, Joana, Lillian Barros, Maria Inês Dias, Tiane C. Finimundy, Joana S. Amaral, Maria José Alves, Ricardo C. Calhelha, P. F. Santos, and Isabel C.F.R. Ferreira. 2020. "Echinacea purpurea (L.) Moench: Chemical Characterization and Bioactivity of Its Extracts and Fractions" Pharmaceuticals 13, no. 6: 125. https://doi.org/10.3390/ph13060125
APA StyleCoelho, J., Barros, L., Dias, M. I., Finimundy, T. C., Amaral, J. S., Alves, M. J., Calhelha, R. C., Santos, P. F., & Ferreira, I. C. F. R. (2020). Echinacea purpurea (L.) Moench: Chemical Characterization and Bioactivity of Its Extracts and Fractions. Pharmaceuticals, 13(6), 125. https://doi.org/10.3390/ph13060125











