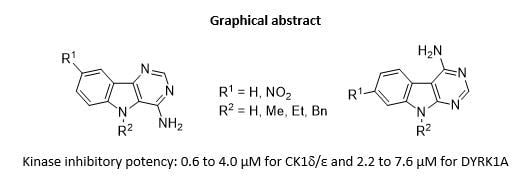
Exploring Kinase Inhibition Properties of 9H-pyrimido[5,4-b]- and [4,5-b]indol-4-amine Derivatives
Abstract
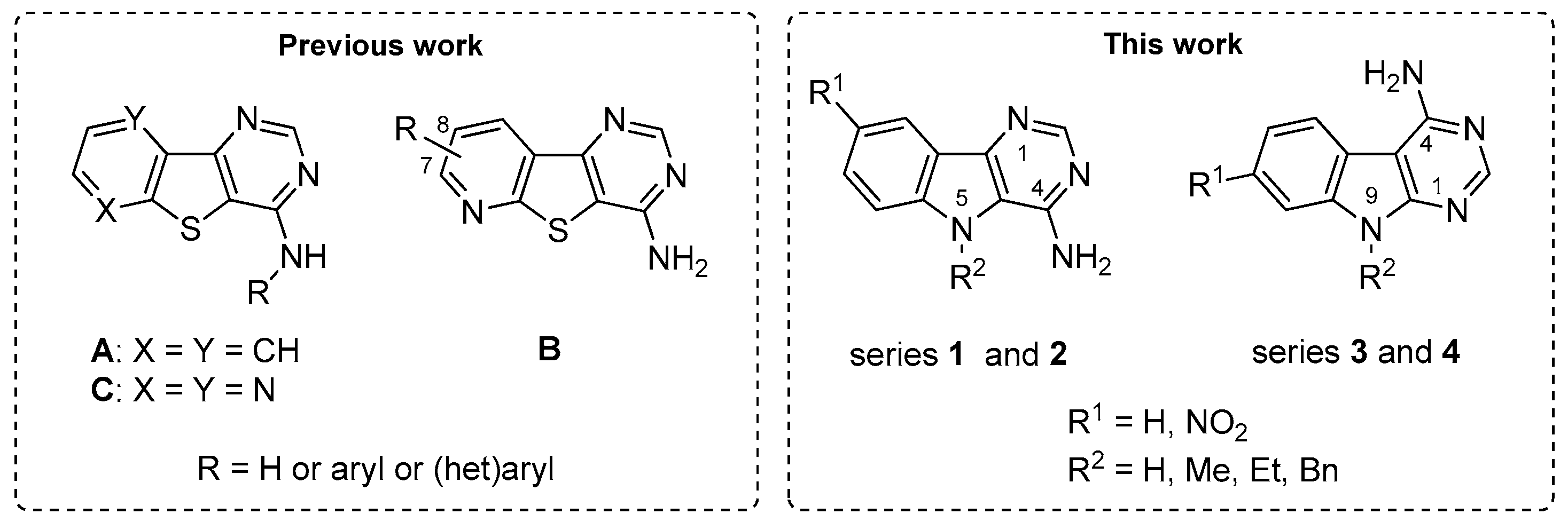
1. Introduction
2. Results
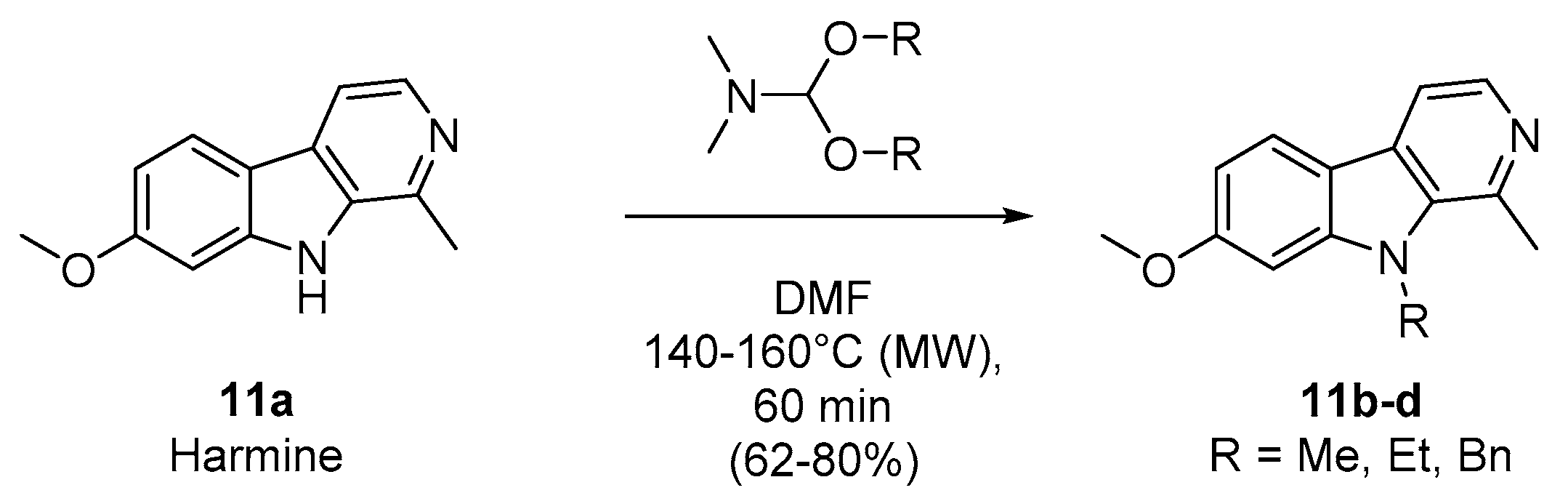
2.1. Chemistry
2.2. Biological Evaluation
3. Discussion
4. Materials and Methods
4.1. General Information
4.2. Chemistry
4.2.1. General Procedure for the Synthesis of 5H-Pyrimido[5,4-b]indol-4-amines (Series 1 and 2) and 5H-Pyrimido[4,5-b]indol-4-amines (Series 3 and 4).
4.2.2. General Procedure for the Synthesis of N-alkylated Harmine Derivatives (Compounds 11b–d).
4.3. In Vitro Kinase Preparation and Assays
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Manning, G.; Whyte, D.B.; Martinez, R.; Hunter, T.; Sudarsanam, S. The Protein Kinase Complement of the Human Genome. Science 2002, 298, 1912–1934. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, A.; Dufrasne, F.; Gelbcke, M.; Jabin, I.; Kiss, R.; Lamoral-Theys, D. DYRK1A kinase inhibitors with emphasis on cancer. Mini Rev. Med. Chem. 2012, 12, 1315–1329. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Martínez, P.; Zahonero, C.; Sánchez-Gómez, P. DYRK1A: The double-edged kinase as a protagonist in cell growth and tumorigenesis. Mol. Cell. Oncol. 2015, 2, 970048. [Google Scholar] [CrossRef] [PubMed]
- Duchon, A.; Herault, Y. DYRK1A, a Dosage-Sensitive Gene Involved in Neurodevelopmental Disorders, Is a Target for Drug Development in Down Syndrome. Front. Behav. Neurosci. 2016, 10, 87. [Google Scholar] [CrossRef] [PubMed]
- Branca, C.; Shaw, D.M.; Belfiore, R.; Gokhale, V.; Shaw, A.Y.; Foley, C.; Smith, B.; Hulme, C.; Dunckley, T.; Meechoovet, B.; et al. Dyrk1 inhibition improves Alzheimer’s disease-like pathology. Aging Cell 2017, 16, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Stotani, S.; Giordanetto, F.; Medda, F. DYRK1A inhibition as potential treatment for Alzheimer’s disease. Future Med. Chem. 2016, 8, 681–696. [Google Scholar] [CrossRef]
- Roskoski, R. Properties of FDA-approved small molecule protein kinase inhibitors: A 2020 update. Pharmacol. Res. 2020, 152, 104609. [Google Scholar] [CrossRef]
- Alexandre, F.-R.; Berecibar, A.; Wrigglesworth, R.; Besson, T. Efficient synthesis of thiazoloquinazolinone derivatives. Tetrahedron Lett. 2003, 44, 4455–4458. [Google Scholar] [CrossRef]
- Loidreau, Y.; Besson, T. Microwave-assisted thermal decomposition of formamide: A tool for coupling a pyrimidine ring with an aromatic partner. Tetrahedron 2011, 67, 4852–4857. [Google Scholar] [CrossRef]
- Foucourt, A.; Dubouilh-Benard, C.; Chosson, E.; Corbiere, C.; Buquet, C.; Iannelli, M.; Leblond, B.; Marsais, F.; Besson, T. Microwave-accelerated Dimroth rearrangement for the synthesis of 4-anilino-6-nitroquinazolines. Application to an efficient synthesis of a microtubule destabilizing agent. Tetrahedron 2010, 66, 4495–4502. [Google Scholar] [CrossRef]
- Loidreau, Y.; Dubouilh-Benard, C.; Marchand, P.; Nourrisson, M.-R.; Duflos, M.; Buquet, C.; Corbière, C.; Besson, T. Efficient New Synthesis ofN-Arylbenzo [b] furo [3,2-d] pyrimidin-4-amines and Their Benzo [b] thieno [3,2-d] pyrimidin-4-amine Analogues via a Microwave-Assisted Dimroth Rearrangement. J. Heterocycl. Chem. 2013, 50, 1187–1197. [Google Scholar] [CrossRef]
- Loidreau, Y.; Marchand, P.; Dubouilh-Benard, C.; Nourrisson, M.-R.; Duflos, M.; Loaëc, N.; Meijer, L.; Besson, T. Synthesis and biological evaluation of N-aryl-7-methoxybenzo [b] furo [3,2-d] pyrimidin-4-amines and their N-arylbenzo [b] thieno [3,2-d] pyrimidin-4-amine analogues as dual inhibitors of CLK1 and DYRK1A kinases. Eur. J. Med. Chem. 2013, 59, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Loidreau, Y.; Marchand, P.; Dubouilh-Benard, C.; Nourrisson, M.-R.; Duflos, M.; Lozach, O.; Loaëc, N.; Meijer, L.; Besson, T. Synthesis and biological evaluation of N-arylbenzo[b]thieno[3,2-d]pyrimidin-4-amines and their pyrido and pyrazino analogues as Ser/Thr kinase inhibitors. Eur. J. Med. Chem. 2012, 58, 171–183. [Google Scholar] [CrossRef]
- Loidreau, Y.; Deau, E.; Marchand, P.; Nourrisson, M.-R.; Logé, C.; Coadou, G.; Loaëc, N.; Meijer, L.; Besson, T. Synthesis and molecular modelling studies of 8-arylpyrido[3′,2′:4,5]thieno[3,2-d]pyrimidin-4-amines as multitarget Ser/Thr kinases inhibitors. Eur. J. Med. Chem. 2015, 92, 124–134. [Google Scholar] [CrossRef]
- Jarhad, D.B.; Mashelkar, K.K.; Kim, H.-R.; Noh, M.; Jeong, L.S. Dual-Specificity Tyrosine Phosphorylation-Regulated Kinase 1A (DYRK1A) Inhibitors as Potential Therapeutics. J. Med. Chem. 2018, 61, 9791–9810. [Google Scholar] [CrossRef]
- Czarna, A.; Wang, J.; Zelencova, D.; Liu, Y.; Deng, X.; Choi, H.G.; Zhang, T.; Zhou, W.; Chang, J.W.; Kildalsen, H.; et al. Novel Scaffolds for Dual Specificity Tyrosine-Phosphorylation-Regulated Kinase (DYRK1A) Inhibitors. J. Med. Chem. 2018, 61, 7560–7572. [Google Scholar] [CrossRef] [PubMed]
- Meijer, L.; Flajolet, M.; Greengard, P. Pharmacological inhibitors of glycogen synthase kinase 3. Trends Pharmacol. Sci. 2004, 25, 471–480. [Google Scholar] [CrossRef]
- Ryoo, S.-R.; Jeong, H.K.; Radnaabazar, C.; Yoo, J.-J.; Cho, H.-J.; Lee, H.-W.; Kim, I.-S.; Cheon, Y.-H.; Ahn, Y.S.; Chung, S.-H.; et al. DYRK1A-mediated Hyperphosphorylation of Tau. J. Boil. Chem. 2007, 282, 34850–34857. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, A.A.; Aulchenko, Y.S.; Isaacs, A.; Van Oosterhout, A.; Sleegers, K.; Hofman, A.; Van Broeckhoven, C.; Oostra, B.A.; Breteler, M.; Van Duijn, C.M. Cyclin-dependent kinase 5 is associated with risk for Alzheimer’s disease in a Dutch population-based study. J. Neurol. 2008, 255, 655–662. [Google Scholar] [CrossRef]
- Cozza, G.; Pinna, L.A. Casein kinases as potential therapeutic targets. Expert Opin. Ther. Targets 2015, 20, 319–340. [Google Scholar] [CrossRef]
- Li, G.; Yin, H.; Kuret, J. Casein Kinase 1 Delta Phosphorylates Tau and Disrupts Its Binding to Microtubules. J. Boil. Chem. 2004, 279, 15938–15945. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.; Medda, F.; Gokhale, V.; Dunckley, T.; Hulme, C. Recent Advances in the Design, Synthesis, and Biological Evaluation of Selective DYRK1A Inhibitors: A New Avenue for a Disease Modifying Treatment of Alzheimer’s? ACS Chem. Neurosci. 2012, 3, 857–872. [Google Scholar] [CrossRef] [PubMed]
- Becker, W.; Joost, H.-G. Structural and functional characteristics of Dyrk, a novel subfamily of protein kinases with dual specificity. Prog. Nucleic Acid Res. Mol. Biol. 1999, 62, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Aranda, S.; Laguna, A.; De La Luna, S. DYRK family of protein kinases: Evolutionary relationships, biochemical properties, and functional roles. FASEB J. 2010, 25, 449–462. [Google Scholar] [CrossRef]
- Tahtouh, T.; Elkins, J.M.; Filippakopoulos, P.; Soundararajan, M.; Burgy, G.; Durieu, E.; Cochet, C.; Schmid, R.; Lo, N.C.; Delhommel, F.; et al. Selectivity, Cocrystal Structures, and Neuroprotective Properties of Leucettines, a Family of Protein Kinase Inhibitors Derived from the Marine Sponge Alkaloid Leucettamine, B. J. Med. Chem. 2012, 55, 9312–9330. [Google Scholar] [CrossRef]
- Loidreau, Y.; Melissen, S.; Levacher, V.; Logé, C.; Graton, J.; Le Questel, J.-Y.; Besson, T. Study of N1-alkylation of indoles from the reaction of 2 (or 3) -aminoindole-3- (or 2) carbonitriles with DMF-dialkylacetals. Org. Biomol. Chem. 2012, 10, 4916. [Google Scholar] [CrossRef]
- Cao, R.; Fan, W.; Guo, L.; Ma, Q.; Zhang, G.; Li, J.; Chen, X.; Ren, Z.; Qiu, L. Synthesis and structure–activity relationships of harmine derivatives as potential antitumor agents. Eur. J. Med. Chem. 2013, 60, 135–143. [Google Scholar] [CrossRef]
- Cao, R.; Chen, Q.; Hou, X.; Chen, H.; Guan, H.; Ma, Y.; Peng, W.; Xu, A. Synthesis, acute toxicities, and antitumor effects of novel 9-substituted β-carboline derivatives. Bioorg. Med. Chem. 2004, 12, 4613–4623. [Google Scholar] [CrossRef]
- Balint, B.; Weber, C.; Cruzalegui, F.; Burbridge, M.; Kotschy, A. Structure-based design and synthesis of Harmine derivatives with different selectivity profiles in kinase vs monoamine oxidase inhibition. Chem. Med. Chem. 2017, 12, 932–939. [Google Scholar] [CrossRef]
- Du, H.; Tian, S.; Chen, J.; Gu, H.; Li, N.; Wang, J. Synthesis and biological evaluation of N9-substituted harmine derivatives as potential anticancer agents. Bioorg. Med. Chem. Lett. 2016, 26, 4015–4019. [Google Scholar] [CrossRef]
- Patel, K.; Gadewar, M.; Tripathi, R.; Prasad, S.; Patel, D.K. A review on medicinal importance, pharmacological activity and bioanalytical aspects of beta-carboline alkaloid “Harmine”. Asian Pac. J. Trop. Biomed. 2012, 2, 660–664. [Google Scholar] [CrossRef]
- Adayev, T.; Wegiel, J.; Hwang, Y.-W. Harmine is an ATP-competitive inhibitor for dual-specificity tyrosine phosphorylation-regulated kinase 1A (Dyrk1A). Arch. Biochem. Biophys. 2010, 507, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Baell, J.B.; Street, I.P.; Sleebs, B.E. Pyrido [3’,2’:4,5] Thieno [3,2-d] Pyrimidin-4-Ylamine Derivatives and Their Therapeutical Use. Patent WO 2012/131297A1, 4 October 2012. [Google Scholar]
- Primot, A.; Baratte, B.; Gompel, M.; Borgne, A.; Liabeuf, S.; Romette, J.-L.; Jho, E.-H.; Costantini, F.; Meijer, L. Purification of GSK-3 by Affinity Chromatography on Immobilized Axin. Protein Expr. Purif. 2000, 20, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Bach, S.; Knockaert, M.; Reinhardt, J.; Lozach, O.; Schmitt, S.; Baratte, B.; Koken, M.; Coburn, S.P.; Tang, L.; Jiang, T.; et al. Roscovitine Targets, Protein Kinases and Pyridoxal Kinase. J. Boil. Chem. 2005, 280, 31208–31219. [Google Scholar] [CrossRef]
- Reinhardt, J.; Ferandin, Y.; Meijer, L. Purification of CK1 by affinity chromatography on immobilised axin. Protein Expr. Purif. 2007, 54, 101–109. [Google Scholar] [CrossRef] [PubMed]
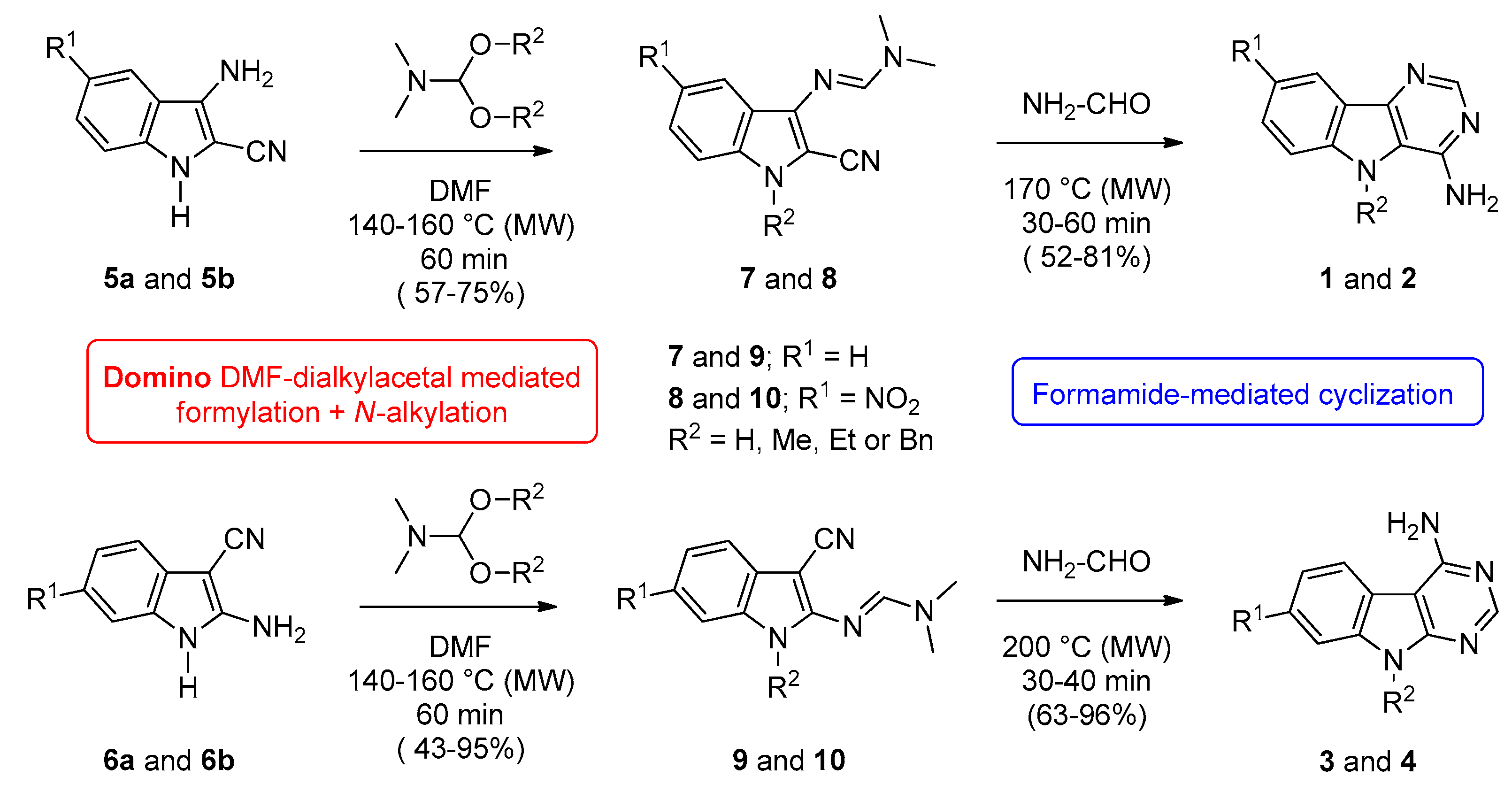
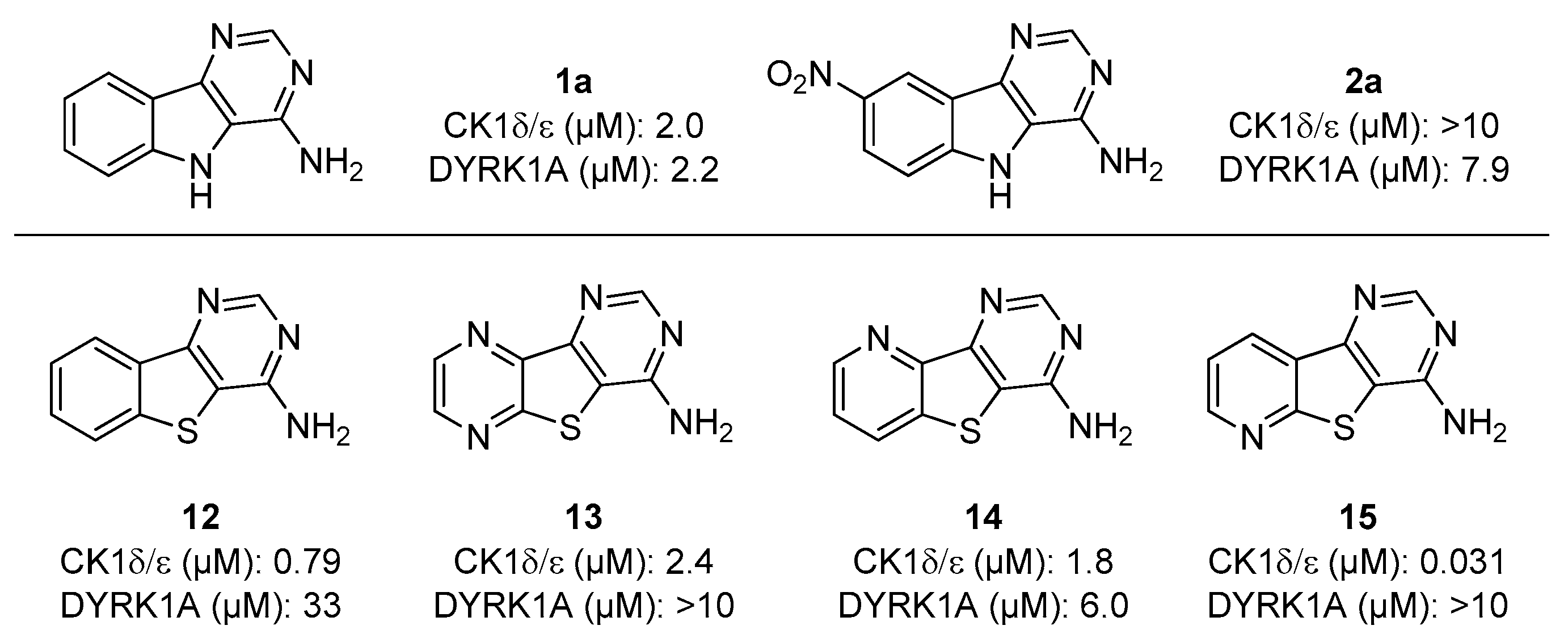
| Starting Indole | Temperature (°C) | Time (min) | Product | R1 | R2 | Yield (%) |
|---|---|---|---|---|---|---|
| 7a | 170 | 30 | 1a | H | H | 81 |
| 7b | 170 | 30 | 1b | H | Me | 58 |
| 7c | 170 | 30 | 1c | H | Et | 64 |
| 7d | 170 | 40 | 1d | H | Bn | 52 |
| 8a | 170 | 60 | 2a | NO2 | H | 65 |
| 8b | 170 | 40 | 2b | NO2 | Me | 75 |
| 8c | 170 | 30 | 2c | NO2 | Et | 72 |
| 8d | 170 | 30 | 2d | NO2 | Bn | 79 |
| 9a | 200 | 40 | 3a | H | H | 71 |
| 9b | 200 | 30 | 3b | H | Me | 85 |
| 9c | 200 | 30 | 3c | H | Et | 96 |
| 9d | 200 | 30 | 3d | H | Bn | 67 |
| 10a | 200 | 30 | 4a | NO2 | H | 67 |
| 10b | 200 | 30 | 4b | NO2 | Me | 80 |
| 10c | 200 | 30 | 4c | NO2 | Et | 63 |
| 10d | 200 | 30 | 4d | NO2 | Bn | 67 |
| N,N-dimethylformamide (DMF)-dialkylacetal (R) | Temperature (°C) | Product | Yield (%) |
|---|---|---|---|
| DMF-DMA (Me) | 140 | 11b | 79 |
| DMF-DEA (Et) | 160 | 11c | 80 |
| DMF-DBA (Bn) | 160 | 11d | 62 |
| Compound | R1 | R2 | CDK5/p25 | CK1δ/ε | DYRK1A | GSK-3α/β |
|---|---|---|---|---|---|---|
| 1a | H | H | >10 | 2.0 | 2.2 | >10 |
| 1b | H | Me | >10 | 4.0 | 5.8 | >10 |
| 1c | H | Et | >10 | 2.8 | 4.1 | >10 |
| 1d | H | Bn | >10 | 0.6 | >10 | >10 |
| 2a | NO2 | H | >10 | >10 | 7.6 | >10 |
| 2b | NO2 | Me | >10 | >10 | >10 | >10 |
| 2c | NO2 | Et | >10 | >10 | >10 | >10 |
| 2d | NO2 | Bn | >10 | >10 | >10 | >10 |
| 3a | H | H | 6 | 0.7 | 3.1 | >10 |
| 3b | H | Me | >10 | 2.5 | >10 | >10 |
| 3c | H | Et | >10 | 1.6 | 9.8 | >10 |
| 3d | H | Bn | >10 | 2.7 | >10 | >10 |
| 4a | NO2 | H | >10 | 3.5 | 7.6 | >10 |
| 4b | NO2 | Me | >10 | 2.8 | >10 | >10 |
| 4c | NO2 | Et | >10 | 1.6 | 5.9 | >10 |
| 4d | NO2 | Bn | >10 | >10 | >10 | >10 |
| 11a (Harmine) | R = H | >10 | 1.5 | 0.029 | >10 | |
| 11b | R = Me | >10 | >10 | 0.13 | >10 | |
| 11c | R = Et | >10 | >10 | 0.037 | >10 | |
| 11d | R = Bn | >10 | >10 | 0.059 | >10 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loidreau, Y.; Dubouilh-Benard, C.; Nourrisson, M.-R.; Loaëc, N.; Meijer, L.; Besson, T.; Marchand, P. Exploring Kinase Inhibition Properties of 9H-pyrimido[5,4-b]- and [4,5-b]indol-4-amine Derivatives. Pharmaceuticals 2020, 13, 89. https://doi.org/10.3390/ph13050089
Loidreau Y, Dubouilh-Benard C, Nourrisson M-R, Loaëc N, Meijer L, Besson T, Marchand P. Exploring Kinase Inhibition Properties of 9H-pyrimido[5,4-b]- and [4,5-b]indol-4-amine Derivatives. Pharmaceuticals. 2020; 13(5):89. https://doi.org/10.3390/ph13050089
Chicago/Turabian StyleLoidreau, Yvonnick, Carole Dubouilh-Benard, Marie-Renée Nourrisson, Nadège Loaëc, Laurent Meijer, Thierry Besson, and Pascal Marchand. 2020. "Exploring Kinase Inhibition Properties of 9H-pyrimido[5,4-b]- and [4,5-b]indol-4-amine Derivatives" Pharmaceuticals 13, no. 5: 89. https://doi.org/10.3390/ph13050089
APA StyleLoidreau, Y., Dubouilh-Benard, C., Nourrisson, M.-R., Loaëc, N., Meijer, L., Besson, T., & Marchand, P. (2020). Exploring Kinase Inhibition Properties of 9H-pyrimido[5,4-b]- and [4,5-b]indol-4-amine Derivatives. Pharmaceuticals, 13(5), 89. https://doi.org/10.3390/ph13050089