Paradoxical Anticonvulsant Effect of Cefepime in the Pentylenetetrazole Model of Seizures in Rats
Abstract
1. Introduction
2. Results
2.1. Absence of a Direct Proconvulsant Effect of CFP
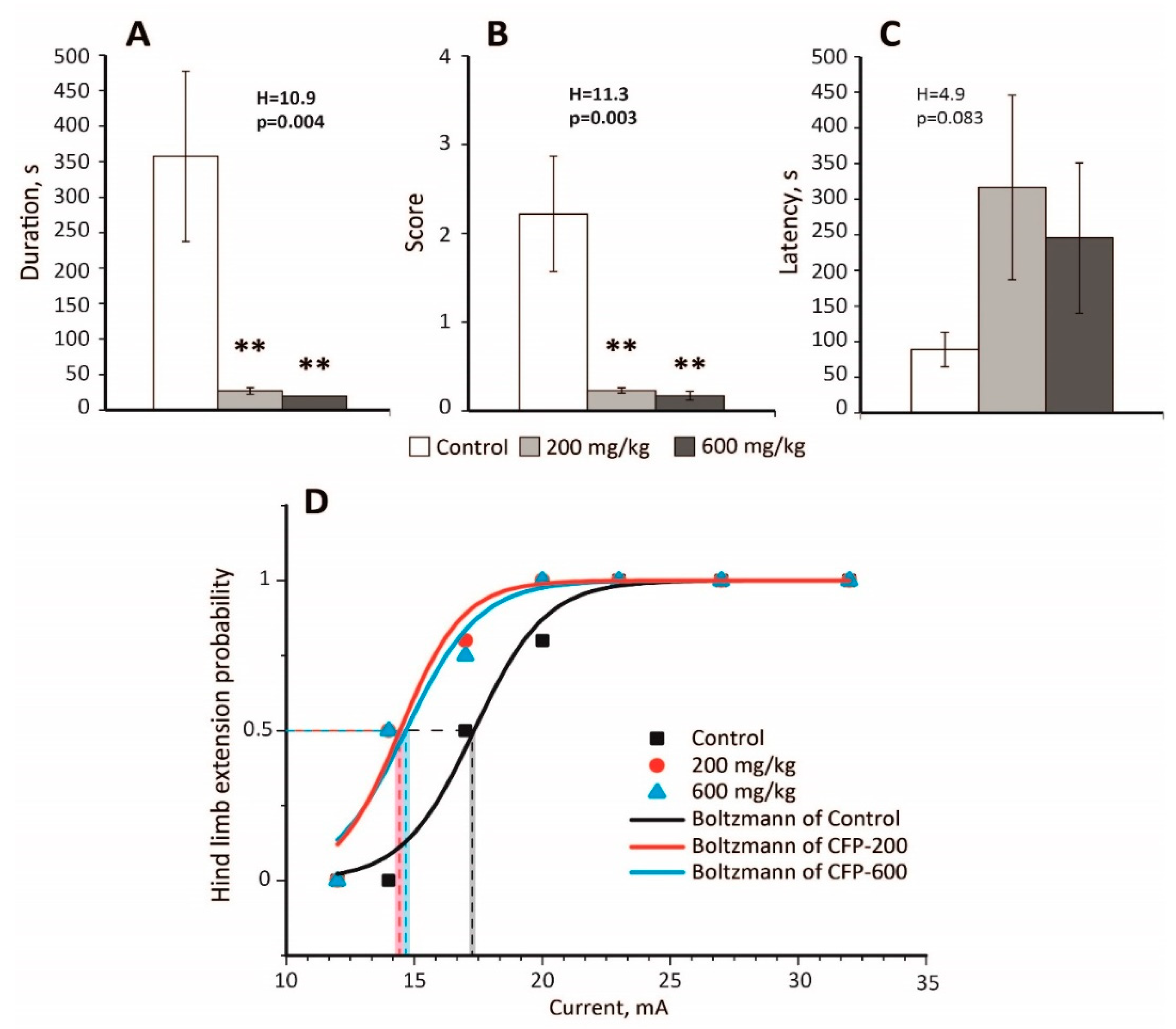
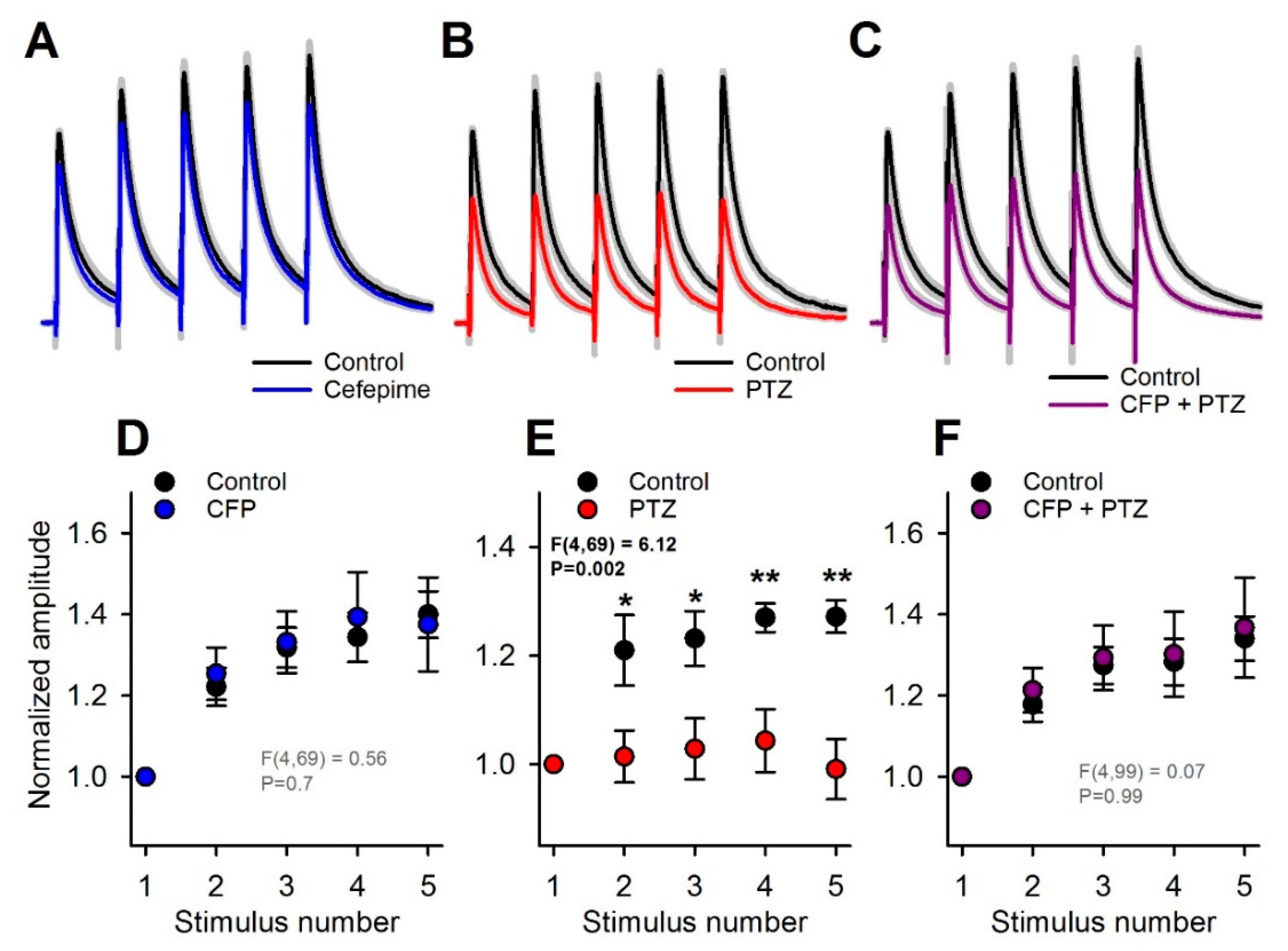
2.2. An Anticonvulsant Effect of CTP on PTZ-Induced Convulsions
2.3. Decreased Seizure Threshold in the MEST Test Following CFP Administration
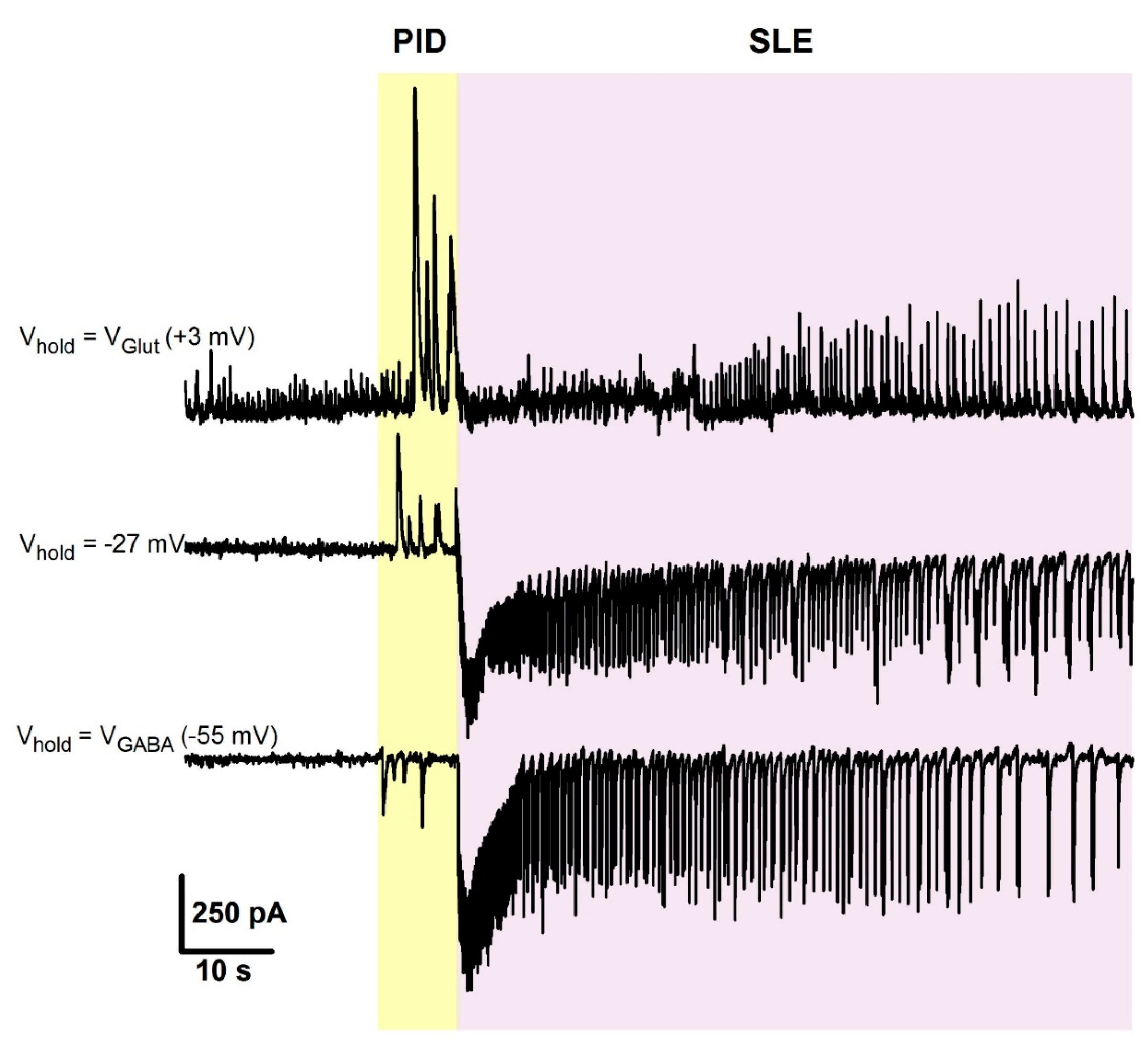
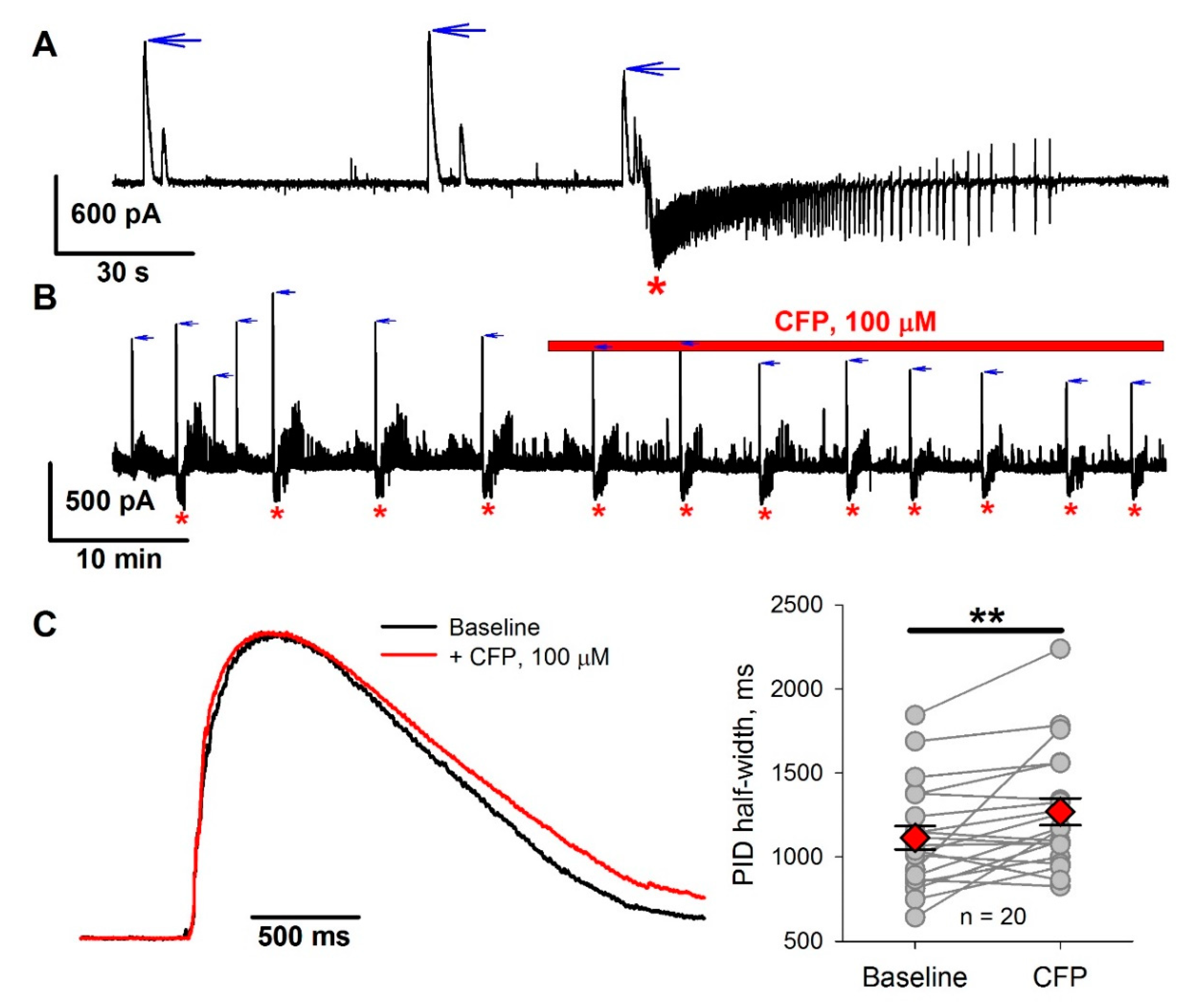
2.4. The Effect of CFP in the 4-Aminopyridine Model of Epileptiform Activity
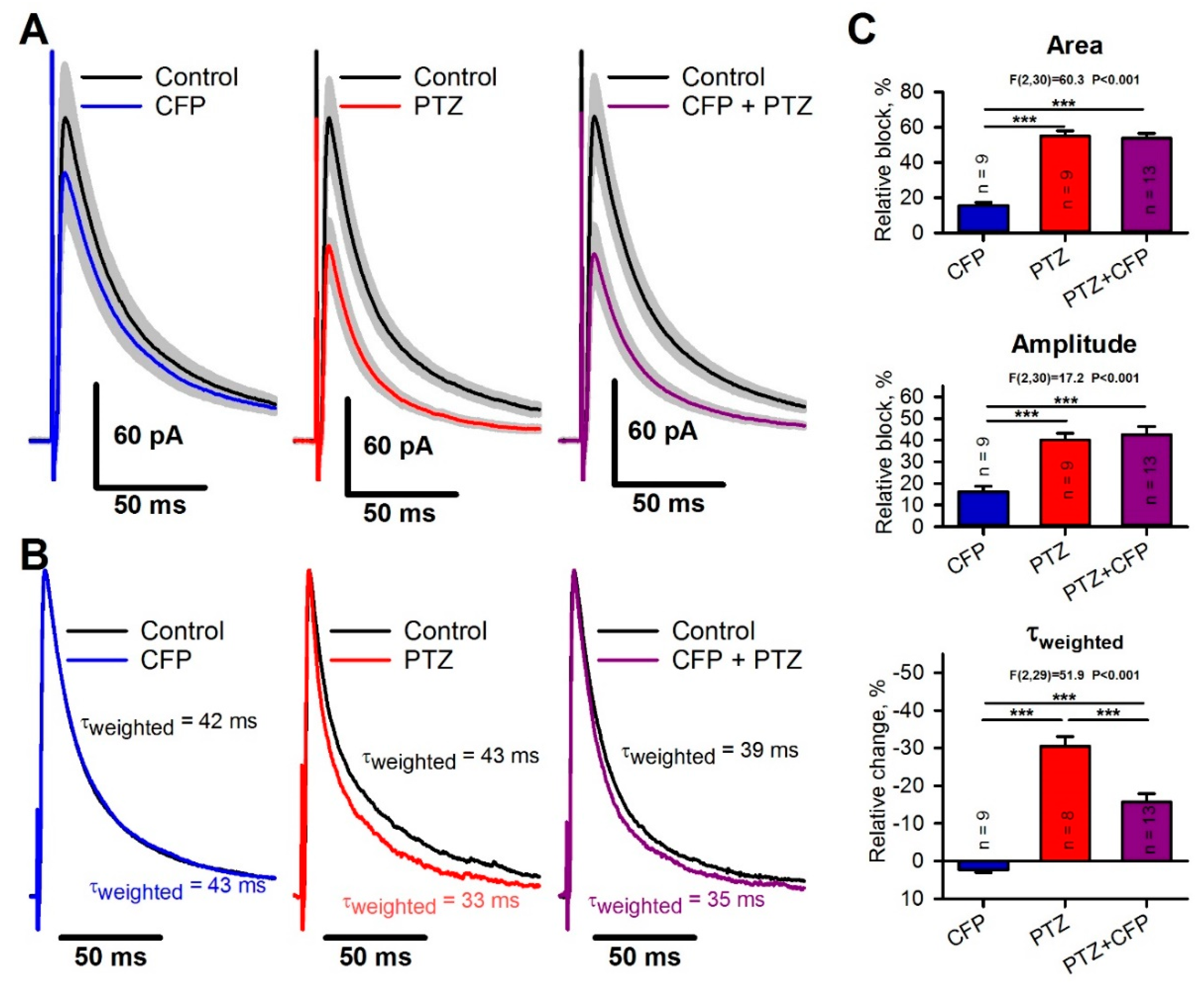
2.5. Occlusion of the Effects of PTZ and CFP on Inhibitory Synaptic Transmission
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. CFP Administration
4.3. Maximal Electroshock Threshold (MEST) Test
4.4. PTZ Test
4.5. Brain Slice Preparation
4.6. Whole-Cell Patch-Clamp Recordings
4.7. 4-Aminopyridine In Vitro Model of Epileptiform Activity
4.8. Statistics
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CFP | Cefepime |
| GABA | γ-Aminobutyric Acid |
| IPSC | Inhibitory Postsynaptic Current |
| MEST | Maximal Electroshock Threshold |
| PTZ | Pentylenetetrazole |
| PID | Preictal Discharge |
| SLE | Seizure-Like Event |
Appendix A
| mA | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | Probability |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | |||||||||||||
| 12 | - | - | - | - | 0% | ||||||||
| 14 | - | 0 | 0 | - | 0% | ||||||||
| 17 | - | x | x | 0 | 50% | ||||||||
| 20 | 0 | x | * | * | x | 80% | |||||||
| 23 | x | x | * | * | * | * | 100% | ||||||
| 27 | x | * | * | * | * | * | * | 100% | |||||
| 32 | * | * | * | * | * | * | * | 100% | |||||
| CFP-200 | |||||||||||||
| 12 | - | 0 | - | 0% | |||||||||
| 14 | - | x | 0 | x | 50% | ||||||||
| 17 | 0 | x | * | x | * | 80% | |||||||
| 20 | x | x | * | * | * | * | 100% | ||||||
| 23 | x | * | * | * | * | * | * | 100% | |||||
| 27 | x | * | * | * | * | * | * | 100% | |||||
| 32 | * | * | * | * | * | * | * | 100% | |||||
| CFP-600 | |||||||||||||
| 12 | - | - | - | 0 | 0% | ||||||||
| 14 | - | 0 | 0 | x | 25% | ||||||||
| 17 | 0 | x | x | * | 75% | ||||||||
| 20 | x | x | * | * | * | 100% | |||||||
| 23 | x | * | * | * | * | * | 100% | ||||||
| 27 | x | * | * | * | * | * | * | 100% | |||||
| 32 | x | * | * | * | * | * | * | * | 100% | ||||
References
- Johnson, H.C.; Walker, A.E. Intraventricular penicillin: A note of warning. J. Am. Med Assoc. 1945, 127, 217–219. [Google Scholar] [CrossRef]
- Walker, A.E.; Johnson, H.C.; Funderburk, W.H. Convulsive factor in commercial penicillin. Arch. Surg. 1945, 50, 69–73. [Google Scholar] [CrossRef]
- Alpay, H.; Altun, O.; Biyikli, N.K. Cefepime-induced non-convulsive status epilepticus in a peritoneal dialysis patient. Pediatr. Nephrol. 2004, 19, 445–447. [Google Scholar] [CrossRef] [PubMed]
- Arkaravichien, W.; Tamungklang, J.; Arkaravichien, T. Cefazolin induced seizures in hemodialysis patients. J. Med. Assoc. Thai 2006, 89, 1981–1983. [Google Scholar]
- Fernandez-Torre, J.L.; Martinez-Martinez, M.; Gonzalez-Rato, J.; Maestro, I.; Alonso, I.; Rodrigo, E.; Horcajada, J.P. Cephalosporin-induced nonconvulsive status epilepticus: Clinical and electroencephalographic features. Epilepsia 2005, 46, 1550–1552. [Google Scholar] [CrossRef]
- Lam, S.; Gomolin, I.H. Cefepime neurotoxicity: Case report, pharmacokinetic considerations, and literature review. Pharmacotherapy 2006, 26, 1169–1174. [Google Scholar] [CrossRef]
- Maganti, R.; Jolin, D.; Rishi, D.; Biswas, A. Nonconvulsive status epilepticus due to cefepime in a patient with normal renal function. Epilepsy Behav. 2006, 8, 312–314. [Google Scholar] [CrossRef]
- Ohtaki, K.; Matsubara, K.; Fujimaru, S.; Shimizu, K.; Awaya, T.; Suno, M.; Chiba, K.; Hayase, N.; Shiono, H. Cefoselis, a beta-lactam antibiotic, easily penetrates the blood-brain barrier and causes seizure independently by glutamate release. J. Neural Transm. 2004, 111, 1523–1535. [Google Scholar] [CrossRef]
- Sonck, J.; Laureys, G.; Verbeelen, D. The neurotoxicity and safety of treatment with cefepime in patients with renal failure. Nephrol. Dial. Transplant. 2008, 23, 966–970. [Google Scholar] [CrossRef]
- Yost, R.L.; Lee, J.D.; O‘Leary, J.P. Convulsions associated with sodium cefazolin: A case report. Am. Surg. 1977, 43, 417–420. [Google Scholar]
- De Sarro, A.; Zappala, M.; Chimirri, A.; Grasso, S.; De Sarro, G.B. Quinolones potentiate cefazolin-induced seizures in DBA/2 mice. Antimicrob. Agents Chemother. 1993, 37, 1497–1503. [Google Scholar] [CrossRef] [PubMed]
- Kamei, C.; Sunami, A.; Tasaka, K. Effects of certain antiepileptics on cephaloridine- and cefazolin-induced seizures in rats. Arch. Int. Pharmacodyn. Ther. 1983, 264, 220–231. [Google Scholar] [PubMed]
- Tamaddonfard, E.; Hamzeh Gooshchi, N.; Seiednejad-Yamchi, S. Central effect of crocin on penicillin-induced epileptiform activity in rats. Pharmacol. Rep. 2012, 64, 94–101. [Google Scholar] [CrossRef]
- Yamazaki, S.; Mochizuki, Y.; Terai, T.; Sugimoto, M.; Uchida, I.; Matsuoka, N.; Mutoh, S. Intracerebroventricular injection of the antibiotic cefoselis produces convulsion in mice via inhibition of GABA receptors. Pharmacol. Biochem. Behav. 2002, 74, 53–59. [Google Scholar] [CrossRef]
- Akula, K.K.; Dhir, A.; Kulkarni, S.K. Pro-convulsant effect of cefazolin sodium against pentylenetetrazol- or picrotoxin-induced convulsions in mice. Indian J. Exp. Biol. 2007, 45, 720–725. [Google Scholar] [PubMed]
- De Sarro, A.; Cecchetti, V.; Fravolini, V.; Naccari, F.; Tabarrini, O.; De Sarro, G. Effects of novel 6-desfluoroquinolones and classic quinolones on pentylenetetrazole-induced seizures in mice. Antimicrob. Agents Chemother. 1999, 43, 1729–1736. [Google Scholar] [CrossRef]
- Tanaka, A.; Takechi, K.; Watanabe, S.; Tanaka, M.; Suemaru, K.; Araki, H. Convulsive liability of cefepime and meropenem in normal and corneal kindled mice. Antimicrob. Agents Chemother. 2014, 58, 4380–4383. [Google Scholar] [CrossRef]
- Sugimoto, M.; Fukami, S.; Kayakiri, H.; Yamazaki, S.; Matsuoka, N.; Uchida, I.; Mashimo, T. The beta-lactam antibiotics, penicillin-G and cefoselis have different mechanisms and sites of action at GABA(A) receptors. Br. J. Pharmacol. 2002, 135, 427–432. [Google Scholar] [CrossRef]
- Antoniadis, A.; Muller, W.E.; Wollert, U. Inhibition of GABA and benzodiazepine receptor binding by penicillins. Neurosci. Lett. 1980, 18, 309–312. [Google Scholar] [CrossRef]
- Rossokhin, A.V.; Sharonova, I.N.; Bukanova, J.V.; Kolbaev, S.N.; Skrebitsky, V.G. Block of GABAA receptor ion channel by penicillin: Electrophysiological and modeling insights toward the mechanism. Mol. Cell. Neurosci. 2014, 63, 72–82. [Google Scholar] [CrossRef]
- Fujimoto, M.; Munakata, M.; Akaike, N. Dual mechanisms of GABAA response inhibition by beta-lactam antibiotics in the pyramidal neurones of the rat cerebral cortex. Br. J. Pharmacol. 1995, 116, 3014–3020. [Google Scholar] [CrossRef] [PubMed]
- Amakhin, D.V.; Soboleva, E.B.; Zaitsev, A.V. Cephalosporin antibiotics are weak blockers of GABAa receptor-mediated synaptic transmission in rat brain slices. Biochem. Biophys. Res. Commun. 2018, 499, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Chatellier, D.; Jourdain, M.; Mangalaboyi, J.; Ader, F.; Chopin, C.; Derambure, P.; Fourrier, F. Cefepime-induced neurotoxicity: An underestimated complication of antibiotherapy in patients with acute renal failure. Intensive Care Med. 2002, 28, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Chow, K.M.; Szeto, C.C.; Hui, A.C.; Wong, T.Y.; Li, P.K. Retrospective review of neurotoxicity induced by cefepime and ceftazidime. Pharmacotherapy 2003, 23, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Meillier, A.; Rahimian, D. Cefepime-induced encephalopathy with normal renal function. Oxf. Med. Case Rep. 2016, 2016, 118–120. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Kim, J.E.; Paek, Y.M.; Hong, K.S.; Cho, Y.J.; Cho, J.Y.; Park, H.K.; Koo, H.K.; Song, P. Cefepime-Induced Non-Convulsive Status Epilepticus (NCSE). J. Epilepsy Res. 2013, 3, 39–41. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Choi, J.Y.; Bae, E.K. Cefepime-induced Aphasic Status Epilepticus Mimicking Acute Stroke. J. Epilepsy Res. 2014, 4, 85–87. [Google Scholar] [CrossRef]
- Appa, A.A.; Jain, R.; Rakita, R.M.; Hakimian, S.; Pottinger, P.S. Characterizing Cefepime Neurotoxicity: A Systematic Review. In Open Forum Infect Diseases; Oxford University Press: London, UK, 2017; Volume 4. [Google Scholar] [CrossRef]
- Payne, L.E.; Gagnon, D.J.; Riker, R.R.; Seder, D.B.; Glisic, E.K.; Morris, J.G.; Fraser, G.L. Cefepime-induced neurotoxicity: A systematic review. Crit. Care 2017, 21, 276. [Google Scholar] [CrossRef]
- Yahav, D.; Paul, M.; Fraser, A.; Sarid, N.; Leibovici, L. Efficacy and safety of cefepime: A systematic review and meta-analysis. Lancet Infect. Dis. 2007, 7, 338–348. [Google Scholar] [CrossRef]
- Grill, M.F.; Maganti, R. Cephalosporin-induced neurotoxicity: Clinical manifestations, potential pathogenic mechanisms, and the role of electroencephalographic monitoring. Ann. Pharmacother. 2008, 42, 1843–1850. [Google Scholar] [CrossRef]
- Sutter, R.; Ruegg, S.; Tschudin-Sutter, S. Seizures as adverse events of antibiotic drugs: A systematic review. Neurology 2015, 85, 1332–1341. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Takechi, K.; Watanabe, S.; Tanaka, M.; Suemaru, K.; Araki, H. Comparison of the prevalence of convulsions associated with the use of cefepime and meropenem. Int. J. Clin. Pharm. 2013, 35, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Cock, H.R. Drug-induced status epilepticus. Epilepsy Behav. 2015, 49, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.; Uchida, I.; Mashimo, T.; Yamazaki, S.; Hatano, K.; Ikeda, F.; Mochizuki, Y.; Terai, T.; Matsuoka, N. Evidence for the involvement of GABA(A) receptor blockade in convulsions induced by cephalosporins. Neuropharmacology 2003, 45, 304–314. [Google Scholar] [CrossRef]
- Durand-Maugard, C.; Lemaire-Hurtel, A.S.; Gras-Champel, V.; Hary, L.; Maizel, J.; Prud’homme-Bernardy, A.; Andrejak, C.; Andrejak, M. Blood and CSF monitoring of cefepime-induced neurotoxicity: Nine case reports. J. Antimicrob. Chemother. 2012, 67, 1297–1299. [Google Scholar] [CrossRef] [PubMed]
- Amakhin, D.V.; Ergina, J.L.; Chizhov, A.V.; Zaitsev, A.V. Synaptic Conductances during Interictal Discharges in Pyramidal Neurons of Rat Entorhinal Cortex. Front. Cell. Neurosci. 2016, 10, 233. [Google Scholar] [CrossRef] [PubMed]
- Chizhov, A.V.; Amakhin, D.V.; Zaitsev, A.V. Mathematical model of Na-K-Cl homeostasis in ictal and interictal discharges. PLoS ONE 2019, 14, e0213904. [Google Scholar] [CrossRef]
- Chizhov, A.V.; Zefirov, A.V.; Amakhin, D.V.; Smirnova, E.Y.; Zaitsev, A.V. Minimal model of interictal and ictal discharges “Epileptor-2”. PLoS Comput. Biol. 2018, 14, e1006186. [Google Scholar] [CrossRef]
- Chizhov, A.V.; Amakhin, D.V.; Zaitsev, A.V. Computational model of interictal discharges triggered by interneurons. PLoS ONE 2017, 12, e0185752. [Google Scholar] [CrossRef]
- Jirsa, V.K.; Stacey, W.C.; Quilichini, P.P.; Ivanov, A.I.; Bernard, C. On the nature of seizure dynamics. Brain 2014, 137, 2210–2230. [Google Scholar] [CrossRef]
- Avoli, M.; D‘Antuono, M.; Louvel, J.; Kohling, R.; Biagini, G.; Pumain, R.; D‘Arcangelo, G.; Tancredi, V. Network and pharmacological mechanisms leading to epileptiform synchronization in the limbic system in vitro. Prog. Neurobiol. 2002, 68, 167–207. [Google Scholar] [CrossRef]
- Pernia-Andrade, A.J.; Goswami, S.P.; Stickler, Y.; Frobe, U.; Schlogl, A.; Jonas, P. A deconvolution-based method with high sensitivity and temporal resolution for detection of spontaneous synaptic currents in vitro and in vivo. Biophys. J. 2012, 103, 1429–1439. [Google Scholar] [CrossRef] [PubMed]
- Suemaru, K.; Yoshikawa, M.; Aso, H.; Watanabe, M. 5-Fluorouracil exacerbates cefepime-induced convulsions in pentylenetetrazol-kindled mice. Epilepsy Res. 2019, 157, 106195. [Google Scholar] [CrossRef] [PubMed]
- Shimada, J.; Hori, S.; Kanemitsu, K.; Shoji, Y.; Nakashio, S.; Yanagawa, A. A comparative study on the convulsant activity of carbapenems and beta-lactams. Drugs Exp. Clin. Res. 1992, 18, 377–381. [Google Scholar]
- Huang, R.Q.; Bell-Horner, C.L.; Dibas, M.I.; Covey, D.F.; Drewe, J.A.; Dillon, G.H. Pentylenetetrazole-induced inhibition of recombinant gamma-aminobutyric acid type A (GABA(A)) receptors: Mechanism and site of action. J. Pharmacol. Exp. Ther. 2001, 298, 986–995. [Google Scholar]
- Ramanjaneyulu, R.; Ticku, M.K. Interactions of pentamethylenetetrazole and tetrazole analogues with the picrotoxinin site of the benzodiazepine-GABA receptor-ionophore complex. Eur. J. Pharmacol. 1984, 98, 337–345. [Google Scholar] [CrossRef]
- Uyanikgil, Y.; Ozkeskek, K.; Cavusoglu, T.; Solmaz, V.; Tumer, M.K.; Erbas, O. Positive effects of ceftriaxone on pentylenetetrazol-induced convulsion model in rats. Int. J. Neurosci. 2016, 126, 70–75. [Google Scholar] [CrossRef]
- Jin, C.; Jung, I.; Ku, H.J.; Yook, J.; Kim, D.H.; Kim, M.; Cho, J.H.; Oh, C.H. Low convulsive activity of a new carbapenem antibiotic, DK-35C, as compared with existing congeners. Toxicology 1999, 138, 59–67. [Google Scholar] [CrossRef]
- Williams, P.D.; Bennett, D.B.; Comereski, C.R. Animal model for evaluating the convulsive liability of beta-lactam antibiotics. Antimicrob. Agents Chemother. 1988, 32, 758–760. [Google Scholar] [CrossRef]
- Rothstein, J.D.; Patel, S.; Regan, M.R.; Haenggeli, C.; Huang, Y.H.; Bergles, D.E.; Jin, L.; Dykes Hoberg, M.; Vidensky, S.; Chung, D.S.; et al. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature 2005, 433, 73–77. [Google Scholar] [CrossRef]
- Poulain, P.; Margineanu, D.G. Levetiracetam opposes the action of GABAA antagonists in hypothalamic neurones. Neuropharmacology 2002, 42, 346–352. [Google Scholar] [CrossRef]
- Burman, R.J.; Selfe, J.S.; Lee, J.H.; van den Berg, M.; Calin, A.; Codadu, N.K.; Wright, R.; Newey, S.E.; Parrish, R.R.; Katz, A.A.; et al. Excitatory GABAergic signalling is associated with benzodiazepine resistance in status epilepticus. Brain 2019, 142, 3482–3501. [Google Scholar] [CrossRef] [PubMed]
- Puskarjov, M.; Seja, P.; Heron, S.E.; Williams, T.C.; Ahmad, F.; Iona, X.; Oliver, K.L.; Grinton, B.E.; Vutskits, L.; Scheffer, I.E.; et al. A variant of KCC2 from patients with febrile seizures impairs neuronal Cl- extrusion and dendritic spine formation. EMBO Rep. 2014, 15, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Buchin, A.; Chizhov, A.; Huberfeld, G.; Miles, R.; Gutkin, B.S. Reduced Efficacy of the KCC2 Cotransporter Promotes Epileptic Oscillations in a Subiculum Network Model. J. Neurosci. 2016, 36, 11619–11633. [Google Scholar] [CrossRef] [PubMed]
- De Curtis, M.; Avoli, M. GABAergic networks jump-start focal seizures. Epilepsia 2016, 57, 679–687. [Google Scholar] [CrossRef] [PubMed]
- De Curtis, M.; Librizzi, L.; Uva, L.; Gnatkovsky, V. GABAA receptor-mediated networks during focal seizure onset and progression in vitro. Neurobiol. Dis. 2019, 125, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Librizzi, L.; Losi, G.; Marcon, I.; Sessolo, M.; Scalmani, P.; Carmignoto, G.; de Curtis, M. Interneuronal Network Activity at the Onset of Seizure-Like Events in Entorhinal Cortex Slices. J. Neurosci. 2017, 37, 10398–10407. [Google Scholar] [CrossRef]
- Miri, M.L.; Vinck, M.; Pant, R.; Cardin, J.A. Altered hippocampal interneuron activity precedes ictal onset. Elife 2018, 7. [Google Scholar] [CrossRef]
- Racusen, L.C.; McCrindle, B.W.; Christenson, M.; Fivush, B.; Fisher, R.S. Cyclosporine lowers seizure threshold in an experimental model of electroshock-induced seizures in Munich-Wistar rats. Life Sci. 1990, 46, 1021–1026. [Google Scholar] [CrossRef]
- Zaitsev, A.V.; Malkin, S.L.; Postnikova, T.Y.; Smolensky, I.V.; Zubareva, O.E.; Romanova, I.V.; Zakharova, M.V.; Karyakin, V.B.; Zavyalov, V. Ceftriaxone Treatment Affects EAAT2 Expression and Glutamatergic Neurotransmission and Exerts a Weak Anticonvulsant Effect in Young Rats. Int. J. Mol. Sci. 2019, 20, 5852. [Google Scholar] [CrossRef]
- Postnikova, T.Y.; Amakhin, D.V.; Trofimova, A.M.; Smolensky, I.V.; Zaitsev, A.V. Changes in Functional Properties of Rat Hippocampal Neurons Following Pentylenetetrazole-induced Status Epilepticus. Neuroscience 2019, 399, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Barbhaiya, R.H.; Forgue, S.T.; Gleason, C.R.; Knupp, C.A.; Pittman, K.A.; Weidler, D.J.; Movahhed, H.; Tenney, J.; Martin, R.R. Pharmacokinetics of cefepime after single and multiple intravenous administrations in healthy subjects. Antimicrob. Agents Chemother. 1992, 36, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Racine, R.J. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr. Clin. Neurophysiol. 1972, 32, 281–294. [Google Scholar] [CrossRef]
- Luttjohann, A.; Fabene, P.F.; van Luijtelaar, G. A revised Racine‘s scale for PTZ-induced seizures in rats. Physiol. Behav. 2009, 98, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Zaitsev, A.V.; Kim, K.K.; Vasilev, D.S.; Lukomskaya, N.Y.; Lavrentyeva, V.V.; Tumanova, N.L.; Zhuravin, I.A.; Magazanik, L.G. N-methyl-D-aspartate receptor channel blockers prevent pentylenetetrazole-induced convulsions and morphological changes in rat brain neurons. J. Neurosci. Res. 2015, 93, 454–465. [Google Scholar] [CrossRef]
- Vasilev, D.S.; Tumanova, N.L.; Kim, K.K.; Lavrentyeva, V.V.; Lukomskaya, N.Y.; Zhuravin, I.A.; Magazanik, L.G.; Zaitsev, A.V. Transient Morphological Alterations in the Hippocampus After Pentylenetetrazole-Induced Seizures in Rats. Neurochem. Res. 2018. [Google Scholar] [CrossRef]
- Perreault, P.; Avoli, M. Effects of low concentrations of 4-aminopyridine on CA1 pyramidal cells of the hippocampus. J. Neurophysiol. 1989, 61, 953–970. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amakhin, D.V.; Smolensky, I.V.; Soboleva, E.B.; Zaitsev, A.V. Paradoxical Anticonvulsant Effect of Cefepime in the Pentylenetetrazole Model of Seizures in Rats. Pharmaceuticals 2020, 13, 80. https://doi.org/10.3390/ph13050080
Amakhin DV, Smolensky IV, Soboleva EB, Zaitsev AV. Paradoxical Anticonvulsant Effect of Cefepime in the Pentylenetetrazole Model of Seizures in Rats. Pharmaceuticals. 2020; 13(5):80. https://doi.org/10.3390/ph13050080
Chicago/Turabian StyleAmakhin, Dmitry V., Ilya V. Smolensky, Elena B. Soboleva, and Aleksey V. Zaitsev. 2020. "Paradoxical Anticonvulsant Effect of Cefepime in the Pentylenetetrazole Model of Seizures in Rats" Pharmaceuticals 13, no. 5: 80. https://doi.org/10.3390/ph13050080
APA StyleAmakhin, D. V., Smolensky, I. V., Soboleva, E. B., & Zaitsev, A. V. (2020). Paradoxical Anticonvulsant Effect of Cefepime in the Pentylenetetrazole Model of Seizures in Rats. Pharmaceuticals, 13(5), 80. https://doi.org/10.3390/ph13050080





