Efficacy of Panax ginseng Meyer Herbal Preparation HRG80 in Preventing and Mitigating Stress-Induced Failure of Cognitive Functions in Healthy Subjects: A Pilot, Randomized, Double-Blind, Placebo-Controlled Crossover Trial
Abstract
1. Introduction
2. Results
2.1. Study Participants, Their Disposition, Baseline Variables, and Treatment Compliance
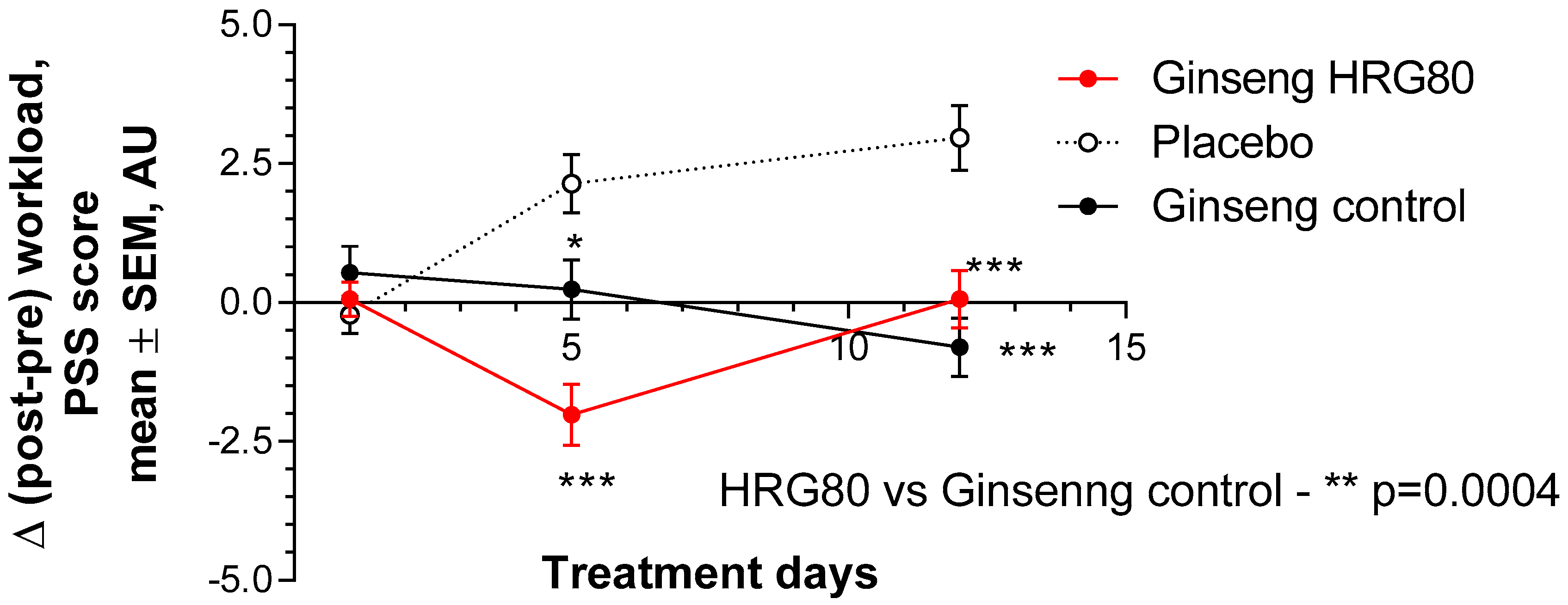
2.2. Treatment Efficacy and Safety
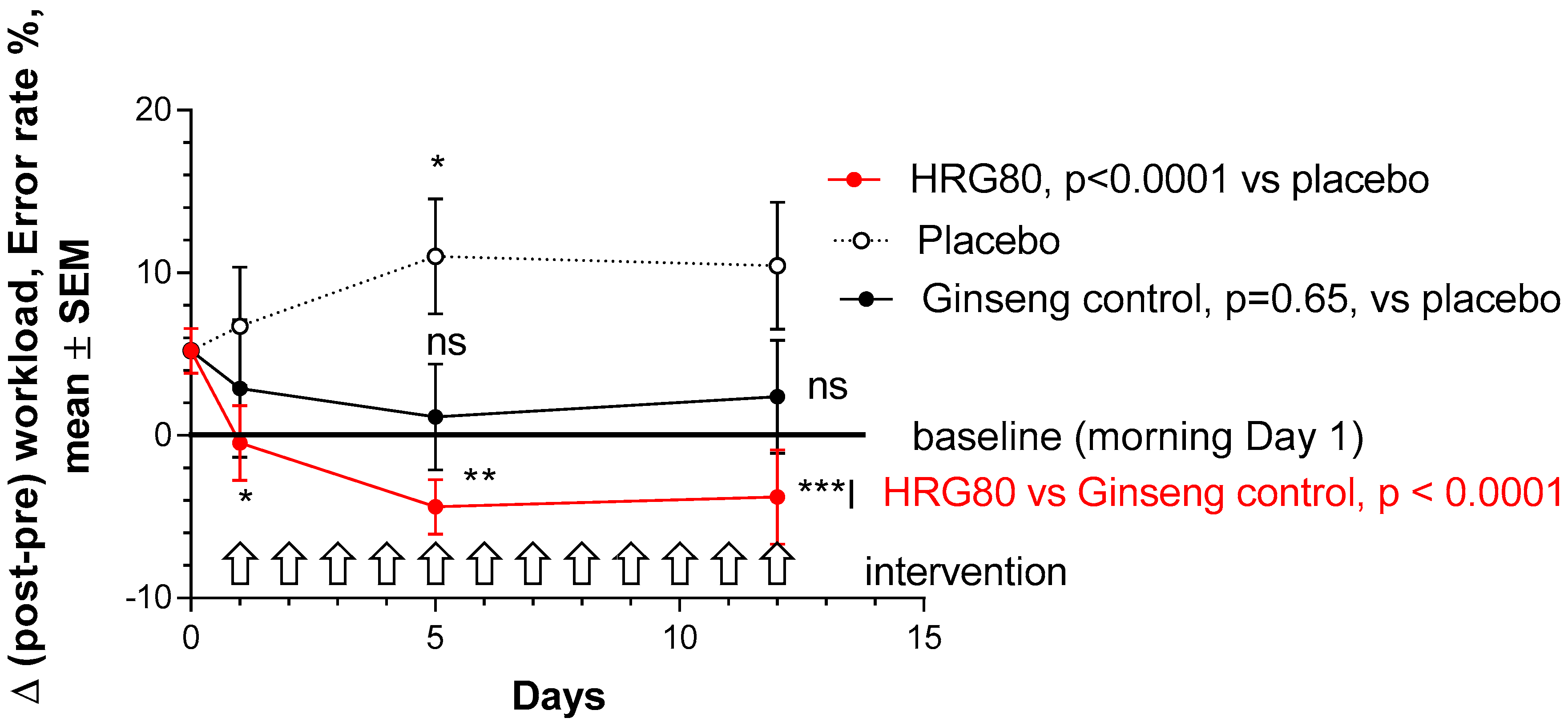
2.2.1. Primary Efficacy Endpoint
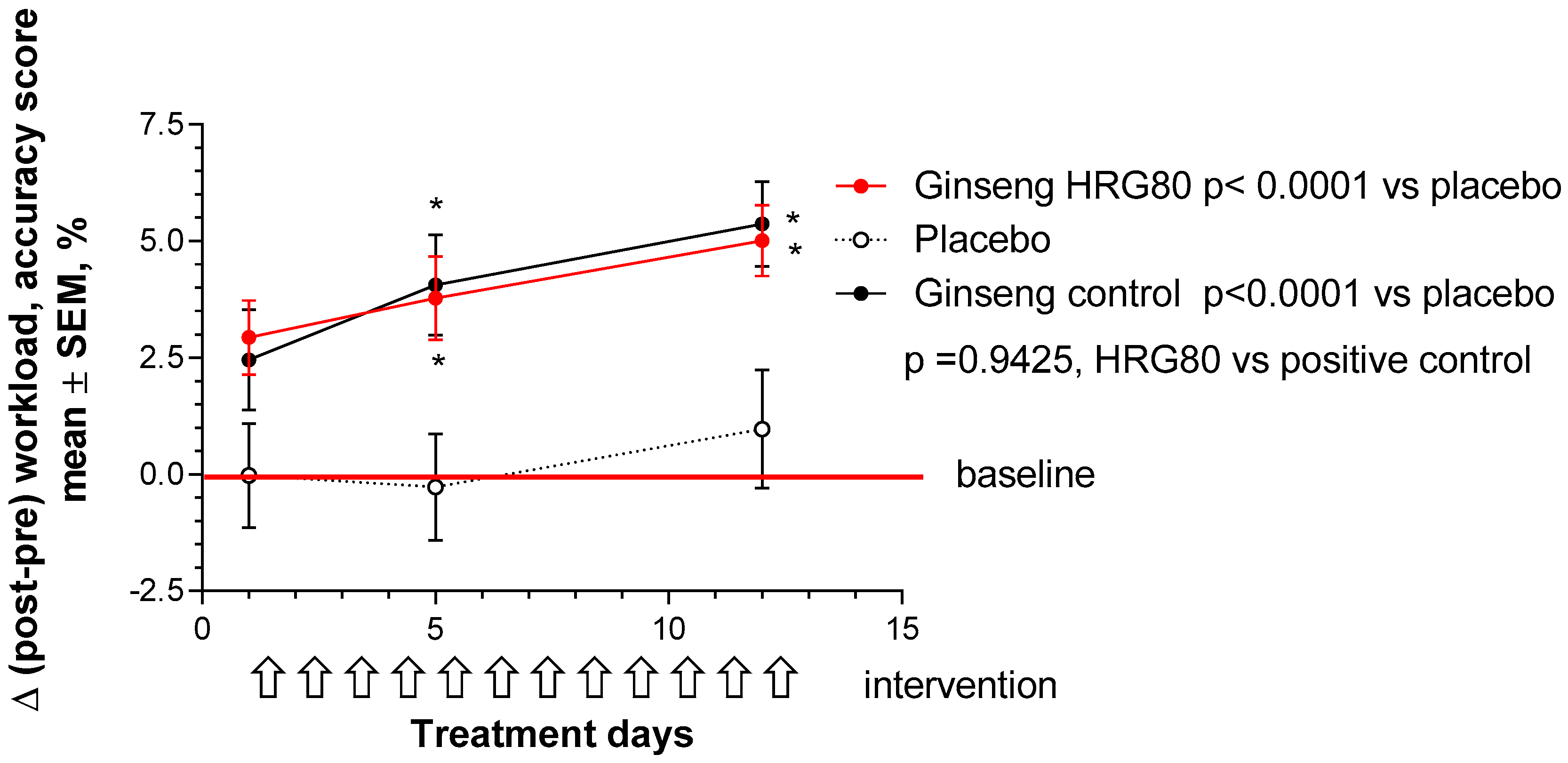
2.2.2. Secondary Efficacy Endpoints
3. Discussion
4. Materials and Methods
4.1. Participant Eligibility
4.2. Study-Population Selection
4.2.1. Recruitment and Screening
4.2.2. Inclusion Criteria
4.2.3. Exclusion Criteria
4.2.4. Participant Withdrawal
4.3. Study Design
4.4. Intervention and Comparator
4.4.1. Doses and Treatment Regimens
4.4.2. Randomization and Blinding
4.4.3. Allocation Concealment
4.4.4. Implementation and Blinding
4.4.5. Evaluation of Compliance
4.5. Study Procedures and Follow-Up
4.5.1. Phase 0, Screening, and Training Visits 1–5
4.5.2. Phase I, Treatment, and Assessment Visits 6–8
4.5.3. Phase II, Treatment, and Assessment Visits 9–11
4.5.4. Phase III, Treatment, and Assessment Visits 12–14
4.6. Efficacy and Safety Evaluation
4.6.1. Primary Efficacy Outcome
4.6.2. Primary Efficacy Endpoint
- change between baseline (morning, before treatment, and stressful workload) and final workload, as presented by the error-rate score, Δ (post–pre) workload;
- change between baseline and 1, 5, and 12 days of treatment, as presented by Δ error rate score; and
- differences between HRG80, positive control, and placebo at 1, 5, and 12 days of treatment in error-rate score change from baseline, Δ (post–pre) workload.
4.6.3. Secondary Efficacy Outcomes
- accuracy score obtained by a computerized memory test (MT) for assessment of cognitive functions (learning, memory, and attention), which is expressed as the number of correct responses during 2 min expressed in % (a scale from 0 to 100%); and
- perceived-stress (PS) score that is the only empirically established index of general stress appraisal.
4.6.4. Secondary Efficacy Endpoints
- change between baseline (morning, before treatment, and stressful workload) and final workload in PS and MT scores, Δ (post–pre) workload;
- change between baseline and 1, 5, and 12 days of treatment in Δ PS and MT scores; and
- differences between HRG80, positive control, and placebo at 1, 5, and 12 days of treatment in PS and MT scores changes from baseline.
4.6.5. Other Endpoints
- change between baseline (morning, before treatment, and stressful workload) and final workload in heart rate;
- change between baseline and 1, 5, and 12 days of treatment in heart rate; and
- differences between HRG80, positive control, and placebo at 1, 5, and 12 days of treatment in heart-rate changes from baseline.
4.6.6. Safety Outcomes
4.7. Statistical Analysis
Sample-Size Considerations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
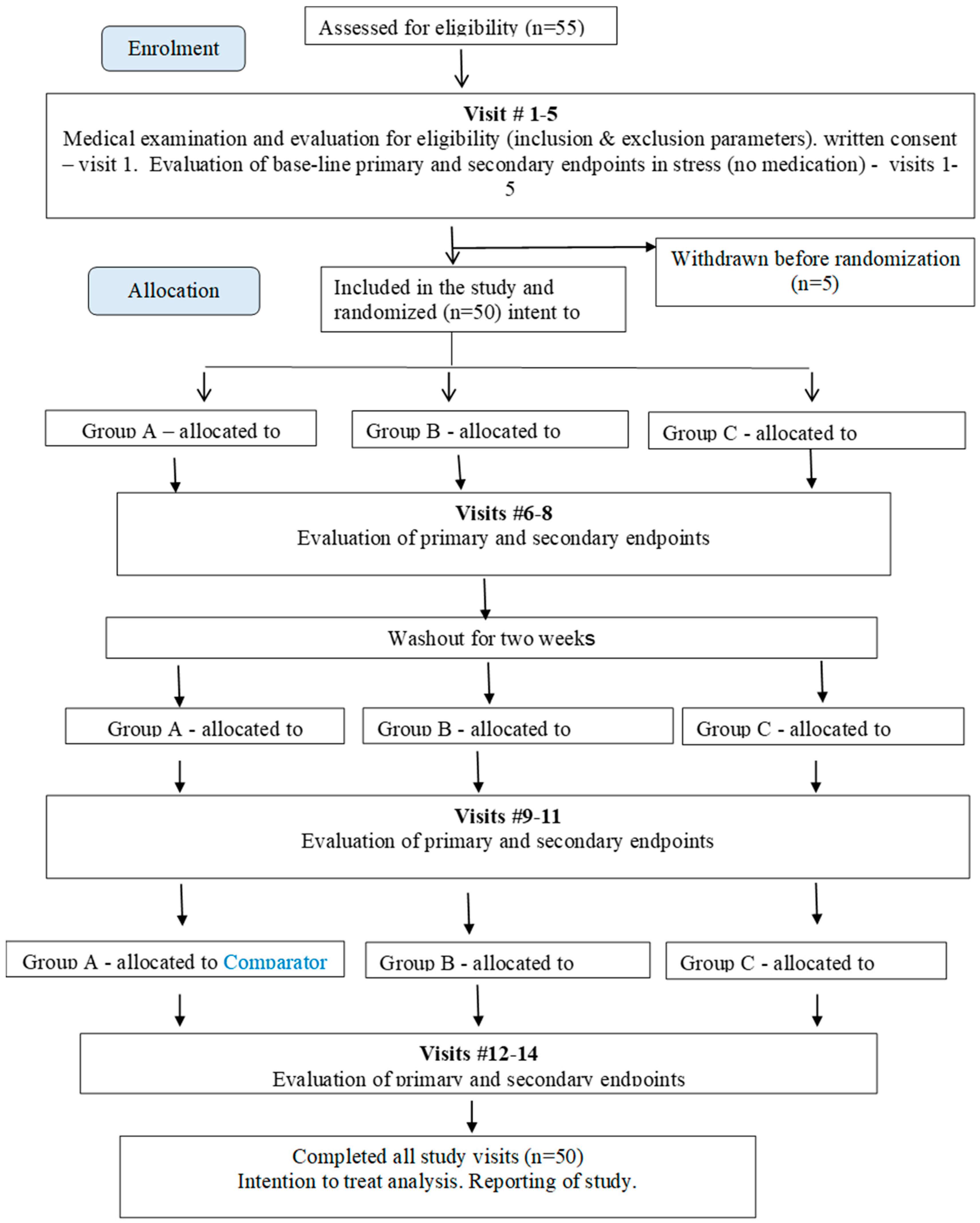
Appendix A. Study Design
Appendix B. Identity and Quantity Testing of Active Markers

- ginsenoside Rg1 (purity ≥98%, Extrasynthese, ref. 0101S), retention time 18.4 min;
- ginsenoside Re (purity ≥98%, Extrasynthese, ref. 0103S), retention time 19.5 min;
- ginsenoside Rf (purity ≥98%, Extrasynthese, ref. 0107S), retention time 46.6 min;
- ginsenoside Rh1 (purity ≥90%, Sigma-Aldrich, ref. 56805), retention time 51.3 min;
- ginsenoside Rg2 (purity ≥94%, Sigma-Aldrich, ref. 08171), retention time 52.1 min;
- ginsenoside Rb1 (purity ≥98%, Extrasynthese, ref. 0105S), retention time 55.5 min;
- ginsenoside Rc (purity ≥98%, Extrasynthese, ref. 0106S), retention time 58.0 min;
- ginsenoside Rb2 (purity ≥90%, Extrasynthese, ref. 0104S), retention time 60.5 min;
- ginsenoside Rd (purity ≥98%, Extrasynthese, ref. 0102S), retention time 65.5 min;
- ginsenoside Rg6 (purity ≥90%, Sigma-Aldrich, ref. 43019), retention time 71.1 min;
- ginsenoside Rh4 (purity ≥90%, Sigma-Aldrich), retention time 71.5 min;
- ginsenosides Rg3 (S/R) (purity ≥98%, Sigma-Aldrich, ref. SML0184), retention time 72.0–72.3 min;
- ginsenoside PPT (purity ≥98%, Extrasynthese, ref. 2307), retention time 72.7 min;
- ginsenoside Rk1 (purity ≥95%, Sigma-Aldrich, ref. 42754), retention time 74.7 min;
- ginsenoside CK (purity ≥95%, Sigma-Aldrich, ref. PHL80461), retention time 75.1 min;
- ginsenoside Rg5 (purity ≥90%, Sigma-Aldrich, ref. 43016), retention time 75.1 min;
- ginsenoside Rh2 (purity ≥97%, Sigma-Aldrich, ref. 73658), retention time 75.9 min;
- ginsenoside Rh3 (purity ≥90%, Sigma-Aldrich, ref. 43084), retention time 83.5 min; and
- ginsenoside PPD (purity ≥97%, Sigma-Aldrich, ref. P0031), retention time 87.9 min).
HRG80 Purity
| Parameters | Specification |
|---|---|
| Loss on drying (IR balance) | <10% |
| Granulometry | <300 µm |
| Mycotoxin (external lab) | 0 (aseptic culture) |
| Pesticide (external lab) | 0 (aseptic culture) |
| Ginsenoside content * | Typical value 12–15% |
| Rare-ginsenoside content ** | Typical value 10–12% |
| DNA identification test (PCR) | Panax ginseng |
| Heavy metal (external lab) | <10 ppm |
| Al (external lab) | <5 ppm |
| Pb (external lab) | <1 ppm |
| As (external lab) | <0.2 ppm |
| Cd (external lab) | <0.1 ppm |
| Hg (external lab) | <0.1 ppm |
Appendix C. Efficacy Outcome Measures
Appendix C.1. Primary Outcome Measure
- ○
- TN score: total number of items processed for 4 min 40 s;
- ○
- E1 score: omission errors—failure to respond to target;
- ○
- E2 score: commission errors—errors made when responses given to nontargets;
- ○
- E = E1 + E2: total errors;
- ○
- E%: error percentage—allows for the interpretation of performance accuracy and carefulness; and
- ○
- TP: total performance = TN − E—measures overall test performance.
Appendix C.2. Secondary Outcome Measures
| Age | N | Mean | S.D. |
| 18–29 | 645 | 14.2 | 6.2 |
| 30–44 | 750 | 13.0 | 6.2 |
| 45–54 | 285 | 12.6 | 6.1 |
| 55–64 | 282 | 11.9 | 6.9 |
| 65 and older | 296 | 12.0 | 6.3 |
Appendix C.3. Safety Measures
- 0 = mild (awareness of signs or symptoms, but easily tolerated),
- 0 = moderate (sufficient discomfort to interfere with normal activities), and
- 0 = severe (incapacitating, with inability to perform normal activities).
Appendix D. Supplementary Figures
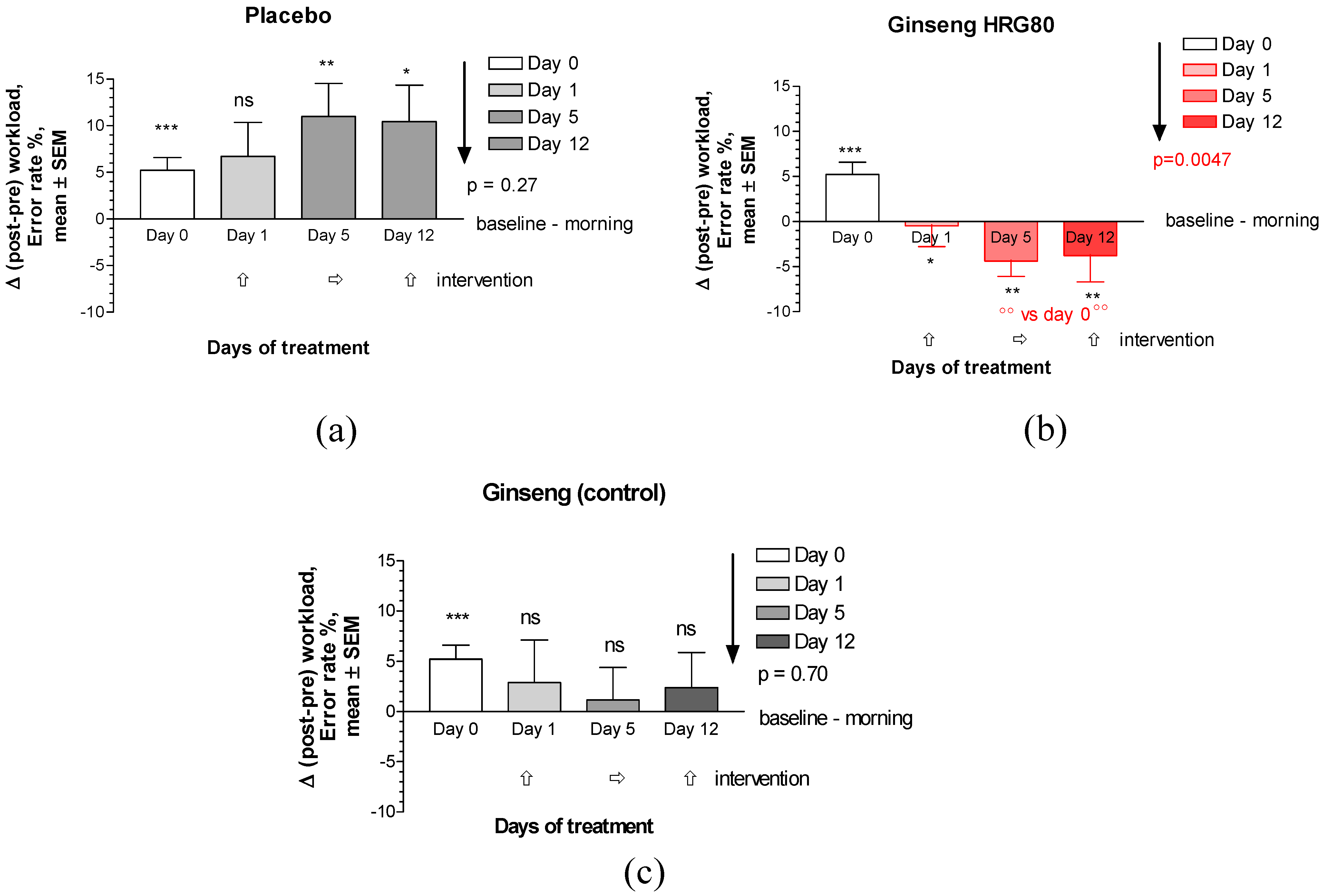
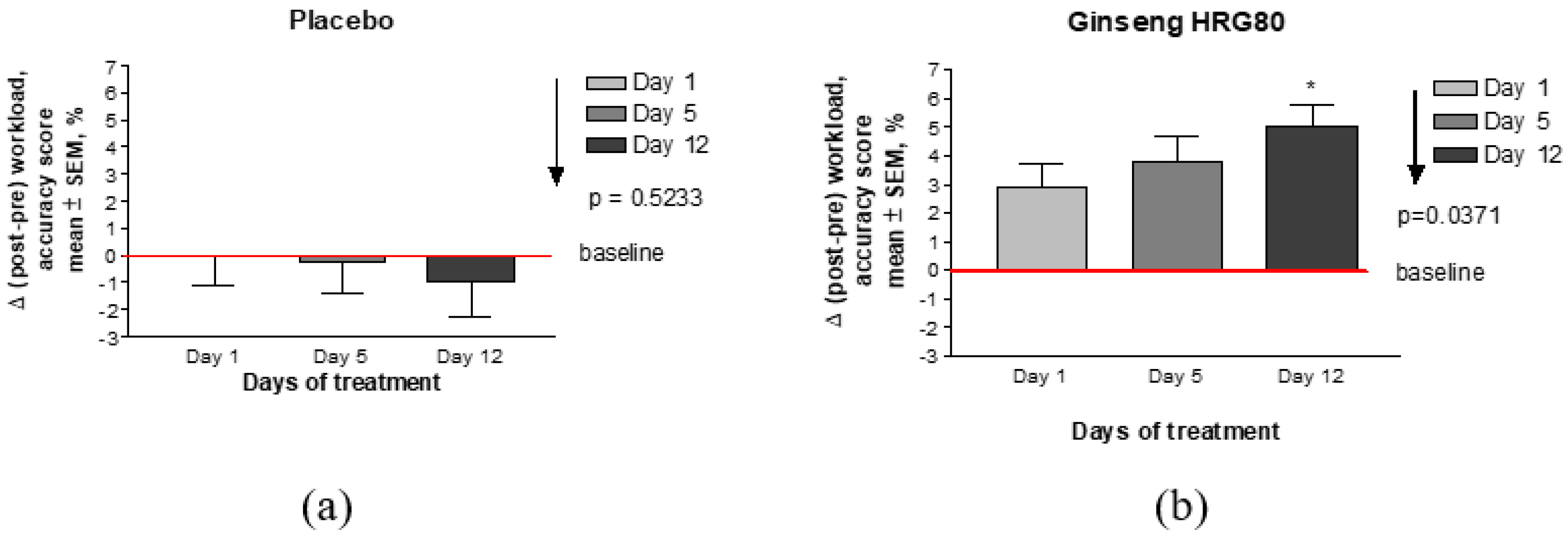
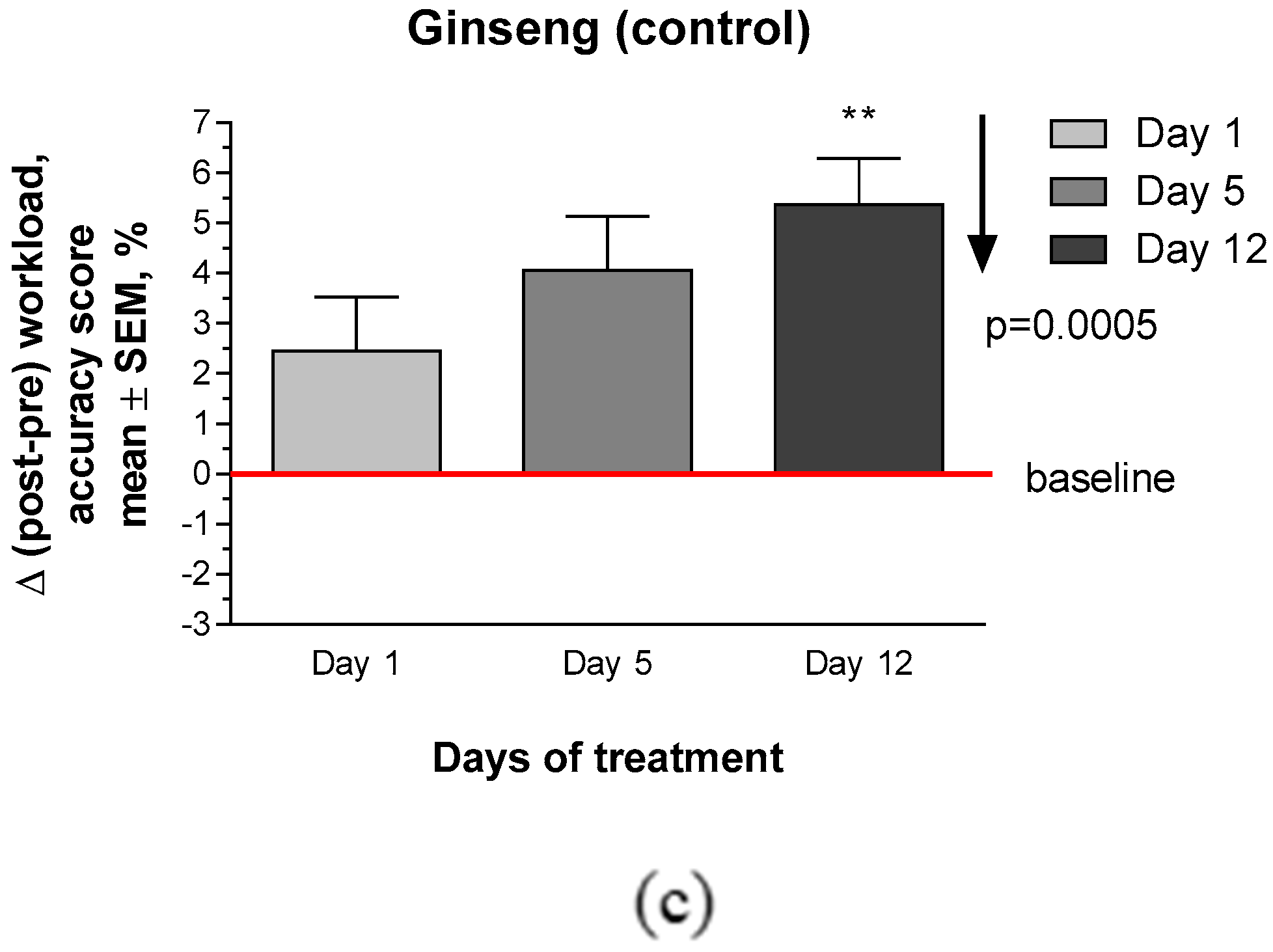
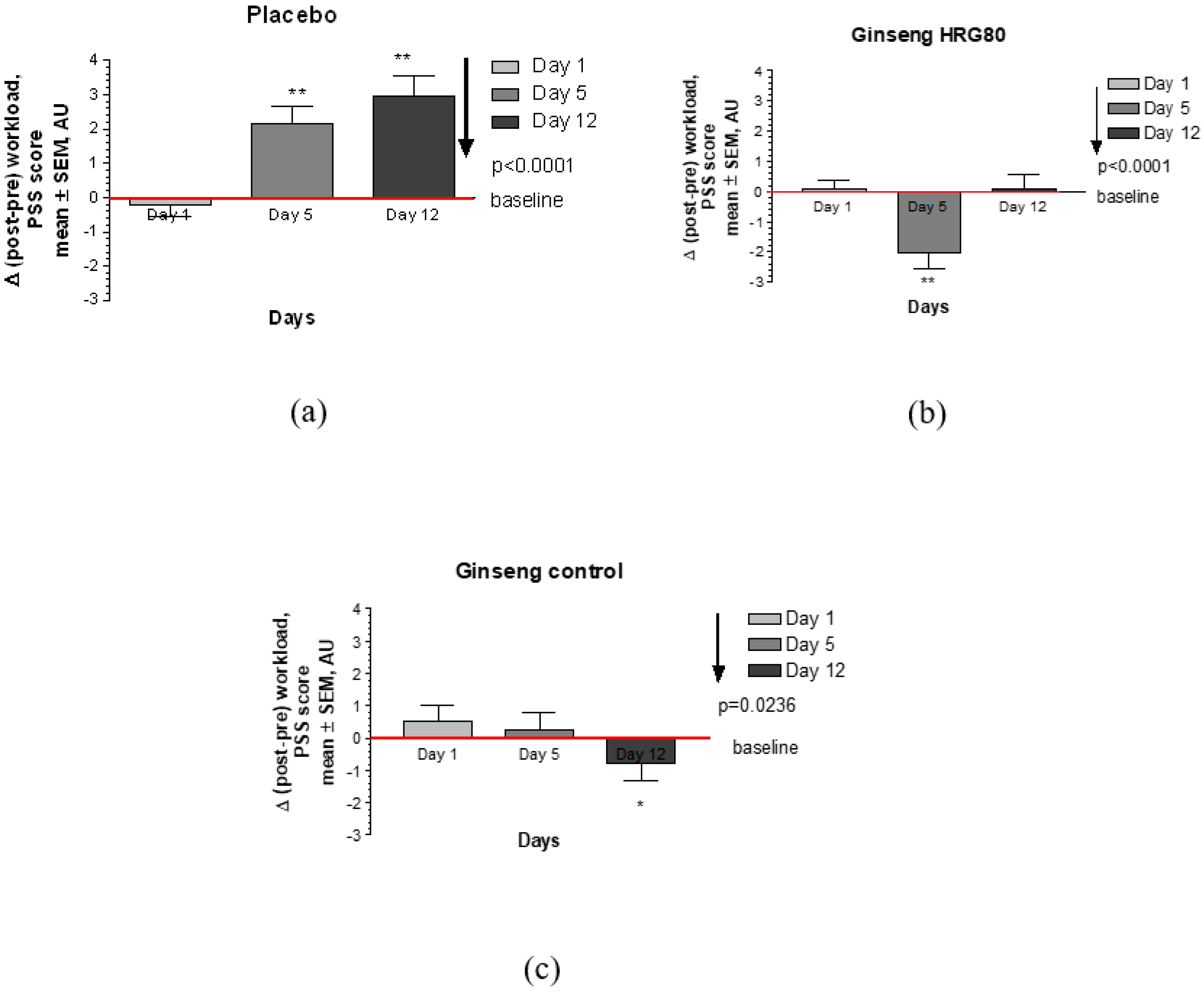
Appendix E. CONSORT Checklist
| Section/Topic | Item No. | Checklist Item | Reported on Page No. |
|---|---|---|---|
| Title and abstract | |||
| 1a | Identification as a randomized trial in the title. | 1 | |
| 1b | Structured summary of trial design, methods, results, and conclusions (for specific guidance, see CONSORT for abstracts). | 1 | |
| Introduction | |||
| Background and objectives. | 2a | Scientific background and explanation of rationale | 2 |
| 2b | Specific objectives or hypotheses. | 2,8 | |
| Methods | |||
| Trial design. | 3a | Description of trial design (such as parallel, factorial), including allocation ratio. | 9, Appendix A, Table 1 |
| 3b | Important changes to methods after trial commencement (such as eligibility criteria), with reasons. | n/a | |
| Participants. | 4a | Eligibility criteria for participants. | 9 |
| 4b | Settings and locations where the data were collected. | 8.9 | |
| Interventions. | 5 | Interventions for each group with sufficient details to allow replication, including how and when they were actually administered. | 9,10, Appendix B |
| Outcomes. | 6a | Completely defined prespecified primary and secondary outcome measures, including how and when they were assessed. | 12,13, Table 1, Appendix C |
| 6b | Any changes to trial outcomes after the trial commenced, with reasons. | N/a | |
| Sample size. | 7a | How sample size was determined. | 14 |
| 7b | When applicable, explanation of any interim analyses and stopping guidelines. | N/a | |
| Randomization: | |||
| Sequence generation. | 8a | Method used to generate random-allocation sequence. | 10 |
| 8b | Type of randomization, details of any restriction (such as blocking and block size). | 10 | |
| Allocation-concealment mechanism. | 9 | Mechanism used to implement random-allocation sequence (such as sequentially numbered containers), describing any steps taken to conceal the sequence until interventions were assigned. | 10 |
| Implementation. | 10 | Who generated the random-allocation sequence, who enrolled participants, and who assigned participants to interventions. | 10 |
| Blinding. | 11a | If done, who was blinded after assignment to interventions (for example, participants, care providers, those assessing outcomes) and how. | 10,11 |
| 11b | If relevant, description of intervention similarity. | 10 | |
| Statistical methods. | 12a | Statistical methods used to compare groups for primary and secondary outcomes. | 13 |
| 12b | Methods for additional analyses, such as subgroup and adjusted analyses. | Appendix D | |
| Results | |||
| Participant flow (diagram is strongly recommended). | 13a | For each group, numbers of participants who were randomly assigned, received intended treatment, and were analyzed for the primary outcome. | 3, Figure 1, Figure 2 and Figure 3, Table 1 |
| 13b | For each group, losses and exclusions after randomization, with reasons. | 3 | |
| Recruitment. | 14a | Dates defining periods of recruitment and follow-up. | N |
| 14b | Why the trial ended or was stopped. | /a | |
| Baseline data. | 15 | Table showing baseline demographic and clinical characteristics for each group. | Table 1 |
| Analyzed numbers. | 16 | For each group, number of participants (denominator) included in each analysis, and whether analysis was by originally assigned groups. | 3, Figure 1, Figure 2 and Figure 3, Table 1 |
| Outcomes and estimation. | 17a | For each primary and secondary outcome, results for each group, and the estimated effect size and its precision (such as 95% confidence interval) | Figure 1, Figure 2 and Figure 3, Appendix D |
| 17b | For binary outcomes, presentation of both absolute and relative effect sizes is recommended. | Figure 1, Figure 2 and Figure 3, Appendix D | |
| Ancillary analyses. | 18 | Results of any other performed analyses, including subgroup and adjusted analyses, distinguishing prespecified from exploratory. | N/a |
| Harm. | 19 | All-important harmful or unintended effects in each group. | 6 |
| Discussion | |||
| Limitations. | 20 | Trial limitations addressing sources of potential bias, imprecision, and, if relevant, multiplicity of analyses. | 8 |
| Generalizability. | 21 | Generalizability (external validity, applicability) of trial findings. | 8 |
| Interpretation. | 22 | Interpretation consistent with results, balancing benefits and harmful effects, and considering other relevant evidence. | 8 |
| Other information | |||
| Registration. | 23 | Registration number and name of trial registry. | 6 |
| Protocol. | 24 | Where full trial protocol can be accessed, if available. | 14 |
| Funding. | 25 | Sources of funding and other support (such as supply of drugs), role of funders. | 14 |
References
- National Pharmacopoeia Committee. Pharmacopoeia of the People’s Republic of China, English ed.; National Pharmacopoeia Committee: Beijing, China, 2010. [Google Scholar]
- World Health Organization. Radix ginseng. In WHO Monographs on Selected Medicinal Plants; World Health Organization: Geneva, Switzerland, 1999; Volume 1, pp. 168–182. [Google Scholar]
- EMA/HMPC/321232/2012. Assessment Report on Panax ginseng C.A. Meyer, Radix. Based on Article 16d (1), Article 16f and Article 16h of Directive 2001/83/EC as Amended (Traditional Use); European Medicines Agency: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Lee, S.M.; Bae, B.S.; Park, H.W.; Ahn, N.G.; Cho, B.G.; Cho, Y.L.; Kwak, Y.S. Characterization of Korean Red Ginseng (Panax ginseng Meyer): History, preparation method, and chemical composition. J. Ginseng Res. 2015, 39, 384–391. [Google Scholar] [CrossRef]
- Im, D.S.; Nah, S.Y. Yin and Yang of ginseng pharmacology: Ginsenosides vs. gintonin. Acta Pharmacol. Sin. 2013, 34, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Jung, S.W.; Lee, B.H.; Kim, H.J.; Hwang, S.H.; Kim, H.K.; Nah, S.Y. Ginseng pharmacology: A new paradigm based on gintonin-lysophosphatidic acid receptor interactions. Front. Pharmacol. 2015, 6, 245. [Google Scholar] [CrossRef] [PubMed]
- Yue, P.Y.; Mak, N.K.; Cheng, Y.K.; Leung, K.W.; Ng, T.B.; Fan, D.T.; Yeung, H.W.; Wong, R.N. Pharmacogenomics and the Yin/Yang actions of ginseng: Anti-tumor, angiomodulating and steroid-like activities of ginsenosides. Chin. Med. 2007, 2, 6. [Google Scholar] [CrossRef]
- Radad, K.; Gille, G.; Liu, L.; Rausch, W.D. Use of ginseng in medicine with emphasis on neurodegenerative disorders. J. Pharmacol. Sci. 2006, 100, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Brekhman, I.I.; Dardymov, I.V. New substances of plant origin which increase nonspecific resistance. Annu. Rev. Pharmacol. 1969, 9, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A. Understanding adaptogenic activity: Specificity of the pharmacological action of adaptogens and other phytochemicals. Ann. N. Y. Acad. Sci. 2017, 1401, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.; Hambardzumyan, M.; Hovhanissyan, A.; Wikman, G. The adaptogens rhodiola and schizandra modify the response to immobilization stress in rabbits by suppressing the increase of phosphorylated stress-activated protein kinase, nitric oxide and cortisol. Drug Target Insights 2007, 2, 39–54. [Google Scholar] [CrossRef]
- Panossian, A.; Seo, E.J.; Efferth, T. Novel molecular mechanisms for the adaptogenic effects of herbal extracts on isolated brain cells using systems biology. Phytomedicine 2018, 50, 257–284. [Google Scholar] [CrossRef]
- Liao, L.Y.; He, Y.F.; Li, L.; Meng, H.; Dong, Y.M.; Yi, F.; Xiao, P.G. A preliminary review of studies on adaptogens: Comparison of their bioactivity in TCM with that of ginseng-like herbs used worldwide. Chin. Med. 2018, 13, 57. [Google Scholar] [CrossRef]
- Leung, K.W.; Wong, A.S. Pharmacology of ginsenosides: A literature review. Chin. Med. 2010, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Rhee, D.K. Effects of ginseng on stress-related depression, anxiety, and the hypothalamic-pituitary-adrenal axis. J. Ginseng Res. 2017, 41, 589–594. [Google Scholar] [CrossRef]
- Geng, J.; Dong, J.; Ni, H.; Lee, M.S.; Wu, T.; Jiang, K.; Wang, G.; Zhou, A.L.; Malouf, R. Ginseng for cognition. Cochrane Database Syst. Rev. 2010, 12, CD007769. [Google Scholar] [CrossRef]
- Lee, M.S.; Yang, E.J.; Kim, J.I.; Ernst, E. Ginseng for cognitive function in Alzheimer’s disease: A systematic review. J. Alzheimers Dis. 2009, 18, 339–344. [Google Scholar] [CrossRef]
- Choi, J.; Kim, T.H.; Choi, T.Y.; Lee, M.S. Ginseng for health care: A systematic review of randomized controlled trials in Korean literature. PLoS ONE 2013, 8, e59978. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.Y.; Farooqui, T.; Koh, H.L.; Farooqui, A.A.; Ling, E.A. Protective effects of ginseng on neurological disorders. Front. Aging Neurosci. 2015, 7, 129. [Google Scholar] [CrossRef] [PubMed]
- Hallstrom, C.; Fulder, S.; Carruthers, M. Effect of ginseng on the performance of nurses on night duty. Comp. Med. East West 1982, 6, 277–282. [Google Scholar] [CrossRef]
- Kim, H.G.; Cho, J.H.; Yoo, S.R.; Lee, J.S.; Han, J.M.; Lee, N.H.; Ahn, Y.C.; Son, C.G. Antifatigue effects of Panax ginseng C.A. Meyer: A randomised, double-blind, placebo-controlled trial. PLoS ONE 2013, 8, e61271. [Google Scholar] [CrossRef] [PubMed]
- Arring, N.M.; Millstine, D.; Marks, L.A.; Nail, L.M. Ginseng as a treatment for fatigue: A systematic review. J. Altern. Complement. Med. 2018, 24, 624–633. [Google Scholar] [CrossRef]
- Reay, J.L.; Kennedy, D.O.; Scholey, A.B. Effects of Panax ginseng, consumed with and without glucose, on blood glucose levels and cognitive performance during sustained ‘mentally demanding’ tasks. J. Psychopharmacol. 2006, 20, 771–781. [Google Scholar] [CrossRef]
- Kennedy, D.O.; Scholey, A.B.; Wesnes, K.A. Dose dependent changes in cognitive performance and mood following acute administration of Ginseng to healthy young volunteers. Nutr. Neurosci. 2001, 4, 295–310. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O.; Reay, J.L.; Scholey, A.B. Effects of 8 weeks administration of Korean panax ginseng extract on the mood and cognitive performance of healthy individuals. J. Ginseng Res. 2007, 31, 34–43. [Google Scholar]
- Kennedy, D.O.; Scholey, A.B. Ginseng: Potential for the enhancement of cognitive performance and mood. Pharmacol. Biochem. Behav. 2003, 75, 687–700. [Google Scholar] [CrossRef]
- Yeo, H.B.; Yoon, H.K.; Lee, H.J.; Kang, S.G.; Jung, K.Y.; Kim, L. Effects of Korean Red Ginseng on cognitive and motor function: A Double-blind, Randomized, Placebo-controlled Trial. J. Ginseng Res. 2012, 36, 190–197. [Google Scholar] [CrossRef]
- Kaneko, H.; Nakanishi, K. Proof of the mysterious efficacy of ginseng: Basic and clinical trials: Clinical effects of medical ginseng, Korean red ginseng: Specifically, its anti-stress action for prevention of disease. J. Pharmacol. Sci. 2004, 95, 158–162. [Google Scholar] [CrossRef]
- Park, K.; Jin, H.; Rhee, H.Y.; Kim, S.; Lee, S.E.; Kim, Y.O.; Kim, G.S.; Kim, S.Y.; Yim, S.V.; Choi, Y.C. A randomized, double-blind, placebo-controlled clinical trial of Korean ginseng as a functional food in mild cognitive impairment. Alzheimers Dement. 2013, 9, P804. [Google Scholar] [CrossRef]
- Lee, S.T.; Chu, K.; Sim, J.Y.; Heo, J.H.; Kim, M. Panax ginseng enhances cognitive performance in Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2008, 22, 222–226. [Google Scholar] [CrossRef]
- Heo, J.H.; Lee, S.T.; Chu, K.; Oh, M.J.; Park, H.J.; Shim, J.Y.; Kim, M. An open-label trial of Korean red ginseng as an adjuvant treatment for cognitive impairment in patients with Alzheimer’s disease. Eur. J. Neurol. 2008, 15, 865–868. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.H.; Lee, S.T.; Oh, M.J.; Park, H.J.; Shim, J.Y.; Chu, K.; Kim, M. Improvement of cognitive deficit in Alzheimer’s disease patients by long term treatment with Korean red ginseng. J. Ginseng Res. 2011, 35, 457–461. [Google Scholar] [CrossRef]
- European Directorate for the Quality of Medicines & Health Care. Ginseng radix. In European Pharmacopoeia, Monograph 01/2008:1523; European Directorate for the Quality of Medicines & Health Care: Strasburg, France, 2008. [Google Scholar]
- Chen, X.J.; Zhang, X.J.; Shui, Y.M.; Wan, J.B.; Gao, J.L. Anticancer activities of protopanaxadiol-and protopanaxatriol-type ginsenosides and their metabolites. Evid. Based Complement. Alternat. Med. 2016, 2016, 5738694. [Google Scholar] [CrossRef]
- He, D.-T.; Wang, B.; Chen, L.M. Research progress on pharmacological effects of ginsenoside. J. Liaoning Univ. Tradit. Chin. Med. 2012, 14, 118–121. [Google Scholar]
- Ren, Y.; Wang, J.L.; Zhang, X.; Wang, H.; Ye, Y.; Song, L.; Wang, Y.J.; Tu, M.J.; Wang, W.W.; Yang, L.; et al. Antidepressant-like effects of ginsenoside Rg2 in a chronic mild stress model of depression. Brain Res. Bull. 2017, 134, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Raza, S.H.; Maryam, A.; Setzer, W.N.; Braidy, N.; Nabavi, S.F.; De Oliveira, M.R.; Nabavi, S.M. Ginsenoside Rb1 as a neuroprotective agent: A review. Brain Res. Bull. 2016, 125, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Chen, Y.; Shi, J.; Wang, R.; Yang, Y.; Yang, L.; Zhao, S.; Wang, Z. Biosynthesis of rare 20(R)-protopanaxadiol/protopanaxatriol type ginsenosides through Escherichia coli engineered with uridine diphosphate glycosyltransferase genes. J. Ginseng Res. 2019, 43, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Shin, B.K.; Kwon, S.W.; Park, J.H. Chemical diversity of ginseng saponins from Panax ginseng. J. Ginseng Res. 2015, 39, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Choi, B.R.; Kim, Y.C.; Choi, D.J.; Lee, Y.S.; Kim, G.S.; Baek, N.I.; Kim, S.Y.; Lee, D.Y. Comprehensive profiling and quantification of ginsenosides in the root, stem, leaf, and berry of Panax ginseng by UPLC-QTOF/MS. Molecules 2017, 22, 2147. [Google Scholar] [CrossRef]
- Jakaria, M.; Haque, M.E.; Kim, J.; Cho, D.Y.; Kim, I.S.; Choi, D.K. Active ginseng components in cognitive impairment: Therapeutic potential and prospects for delivery and clinical study. Oncotarget 2018, 9, 33601–33620. [Google Scholar] [CrossRef]
- Pan, W.; Xue, B.; Yang, C.; Miao, L.; Zhou, L.; Chen, Q.; Cai, Q.; Liu, Y.; Liu, D.; He, H.; et al. Biopharmaceutical characters and bioavailability improving strategies of ginsenosides. Fitoterapia 2018, 129, 272–282. [Google Scholar] [CrossRef]
- Akao, T.; Kida, H.; Kanaoka, M.; Hattori, M.; Kobashi, K. Intestinal bacterial hydrolysis is required for the appearance of compound K in rat plasma after oral administration of ginsenoside Rb1 from Panax ginseng. J. Pharm. Pharmacol. 1998, 50, 1155–1160. [Google Scholar] [CrossRef]
- Yu, S.; Zhou, X.; Li, F.; Xu, C.; Zheng, F.; Li, J.; Zhao, H.; Dai, Y.; Liu, S.; Feng, Y. Microbial transformation of ginsenoside Rb1, Re and Rg1 and its contribution to the improved anti-inflammatory activity of ginseng. Sci. Rep. 2017, 7, 138. [Google Scholar] [CrossRef]
- Zhou, S.S.; Xu, J.; Zhu, H.; Wu, J.; Xu, J.D.; Yan, R.; Li, X.Y.; Liu, H.H.; Duan, S.M.; Wang, Z.; et al. Gut microbiota-involved mechanisms in enhancing systemic exposure of ginsenosides by coexisting polysaccharides in ginseng decoction. Sci. Rep. 2016, 6, 22474. [Google Scholar] [CrossRef]
- Xue, P.; Yao, Y.; Yang, X.S.; Feng, J.; Ren, G.X. Improved antimicrobial effect of ginseng extract by heat transformation. J. Ginseng Res. 2017, 41, 180–187. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, L.; Liu, G.Y.; Liu, J.X.; Li, T. Pharmacokinetics and brain distribution of ginsenosides after administration of sailuotong. China J. Chin. Mater. Med. 2014, 39, 316–321. [Google Scholar]
- Zheng, M.M.; Xu, F.X.; Li, Y.J.; Xi, X.Z.; Cui, X.W.; Han, C.C.; Zhang, X.L. Study on transformation of ginsenosides in different methods. Biomed. Res. Int. 2017, 2017, 8601027. [Google Scholar] [CrossRef]
- Hasegawa, H.; Uchiyama, M. Antimetastatic efficacy of orally administered ginsenoside Rb1 in dependence on intestinal bacterial hydrolyzing potential and significance of treatment with an active bacterial metabolite. Planta Med. 1998, 64, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.; Kim, D.H.; Ji, G.E. Transformation of ginsenosides Rb2 and Rc from Panax ginseng by food microorganisms. Biol. Pharm. Bull. 2005, 28, 2102–2105. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.; Ji, G.E. Transformation of ginsenosides Rb1 and Re from Panax ginseng by food microorganisms. Biotechnol. Lett. 2005, 27, 765–771. [Google Scholar] [CrossRef]
- Quan, K.; Liu, Q.; Wan, J.Y.; Zhao, Y.J.; Guo, R.Z.; Alolga, R.N.; Li, P.; Qi, L.W. Rapid preparation of rare ginsenosides by acid transformation and their structure-activity relationships against cancer cells. Sci. Rep. 2015, 5, 8598. [Google Scholar] [CrossRef]
- Adil, M.; Jeong, B.R. In vitro cultivation of Panax ginseng C.A. Meyer. Ind. Crops Prod. 2018, 122, 239–251. [Google Scholar] [CrossRef]
- Davies, K.M.; Deroles, S.C. Prospects for the use of plant cell cultures in food biotechnology. Curr. Opin. Biotechnol. 2014, 26, 133–140. [Google Scholar] [CrossRef]
- Choi, S.Y.; Cho, C.W.; Lee, Y.; Kim, S.S.; Lee, S.H.; Kim, K.T. Comparison of ginsenoside and phenolic ingredient contents in hydroponically-cultivated ginseng leaves, fruits, and roots. J. Ginseng Res. 2012, 36, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Yang, D. Production of functional Korean ginseng by selenium supplement in hydroponic system. In Proceedings of the XXVI International Horticultural Congress: The Future for Medicinal and Aromatic Plants, Toronto, ON, Canada, 11–17 August 2002; Volume 629, pp. 307–311. [Google Scholar]
- Kim, G.S.; Lee, S.E.; Noh, H.J.; Kwon, H.; Lee, S.W.; Kim, S.Y.; Kim, Y.B. Effects of natural bioactive products on the growth and ginsenoside contents of Panax ginseng cultured in an aeroponic system. J. Ginseng Res. 2012, 36, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Lee, O.R.; Kim, K.T.; Yang, D.C. High frequency of plant regeneration through cyclic secondary somatic embryogenesis in Panax ginseng. J. Ginseng Res. 2012, 36, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.R.; Lee, S.H.; Jang, G.Y.; Hwang, I.G.; Kim, H.Y.; Woo, K.S.; Lee, J.; Jeong, H.S. Changes in ginsenoside compositions and antioxidant activities of hydroponic-cultured ginseng roots and leaves with heating temperature. J. Ginseng Res. 2014, 38, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, K.; Tanaka, M.; Yamaguti, K.; Kajimoto, O.; Kuratsune, H.; Watanabe, Y. Mental fatigue caused by prolonged cognitive load associated with sympathetic hyperactivity. Behav. Brain Funct. 2011, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Steinborn, M.B.; Langner, R.; Flehmig, H.C.; Huestegge, L. Methodology of performance scoring in the d2 sustained-attention test: Cumulative-reliability functions and practical guidelines. Psychol. Assess. 2018, 30, 339–357. [Google Scholar] [CrossRef]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A global measure of perceived stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef]
- Cohen, S.; Williamson, G. Perceived stress in a probability sample of the United States. In The Social Psychology of Health: Claremont Symposium on Applied Social Psychology; Spacapan, S., Oskamp, S., Eds.; Sage: Newbury Park, CA, USA, 1988; pp. 31–67. [Google Scholar]
- Dimpfel, W.; Schombert, L.; Panossian, A. Panax ginseng preparations enhance long term potentiation in rat hippocampal slices by glutamatergic NMDA and kainate receptor mediated transmission. Pharmaceuticals 2020. submitted for publication. [Google Scholar]
- Dimpfel, W. Study in Progress. Available online: https://clinicaltrials.gov/ct2/show/NCT04167449 (accessed on 27 March 2020).
- Panossian, A.; Wikman, G. Evidence-based efficacy of adaptogens in fatigue, and molecular mechanisms related to their stress-protective activity. Curr. Clin. Pharmacol. 2009, 4, 198–219. [Google Scholar] [CrossRef]
- Lieberman, H.R. The effects of ginseng, ephedrine, and caffeine on cognitive performance, mood and energy. Nutr. Rev. 2001, 59, 91–102. [Google Scholar] [CrossRef]
- Panossian, A.G. Adaptogens: Tonic herbs for fatigue and stress. Altern. Complement. Ther. 2003, 9, 327–332. [Google Scholar] [CrossRef]
- Panossian, A.; Wikman, G. Effects of adaptogens on the central nervous 760 system and the molecular mechanisms associated with their stress-protective activity. Pharmaceuticals 2010, 3, 188–224. [Google Scholar] [CrossRef] [PubMed]
| Age | Heart Rate | CP Score | MT Score | PS Score | |
|---|---|---|---|---|---|
| Number of subjects | 50 | 50 | 50 | 50 | 50 |
| Mean | 37.46 | 56 | 25.62 | 88.58 | 16.2 |
| Std. deviation | 8.016 | 64.50 | 17.34 | 6.419 | 2.241 |
| Std. error | 1.134 | 68.00 | 2.453 | 0.9078 | 0.3169 |
| Lower 95% CI | 35.18 | 66.95 | 20.69 | 86.76 | 15.56 |
| Upper 95% CI | 39.74 | 70.13 | 30.55 | 90.4 | 16.84 |
| Normality Test | |||||
| KS distance | 0.0873 | 0.08834 | 0.1734 | 0.1149 | 0.2156 |
| p-value | >0.10 | >0.10 | 0.0989 | >0.10 | 0.0192 |
| Passed normality test (* = 0.05)? | Yes | Yes | Yes | Yes | No |
| p-value summary | ns | ns | ns | ns | * |
| Coefficient of variation | 21.40% | 8.17% | 67.69% | 7.25% | 13.83% |
| Ginsenosides | HRG80 (mg/g *) | Arkopharma (mg/g) | Placebo | |
|---|---|---|---|---|
| Major ginsenosides | Rg1 | 1.26 | 2.97 | 0 |
| Re | 1.40 | 2.77 | 0 | |
| Rb1 | 2.73 | 3.62 | 0 | |
| Rc | 2.32 | 3.68 | 0 | |
| Rb2 | 1.56 | 3.26 | 0 | |
| Rd | 4.53 | 0.99 | 0 | |
| Rf | 0.00 | 0.55 | 0 | |
| Rare ginsenosides | Rg2 | 1.83 | 0.23 | 0 |
| Rg6 | 5.04 | 0.23 | 0 | |
| Rh4 | 9.60 | 1.97 | 0 | |
| Rg3 | 8.43 | 2.22 | 0 | |
| Rk1 | 9.97 | 0.04 | 0 | |
| C(k) | 17.59 | 0.23 | 0 | |
| Rh2 | 2.56 | 2.19 | 0 | |
| Rh3 | 6.04 | 0.39 | 0 | |
| PPD | 1.11 | 0.50 | 0 | |
| Total | 76.0 = 7.6% | 25.8 = 2.6% | 0 | |
| Top 7 | 13.8 | 17.8 | 0 | |
| Rare | 62.2 = 6.2% | 8.0 = 0.8% | 0 | |
| Proportion | Rare: Major | 82:18 | 31:69 | 0 |
| Visit 1 Phase 0 No Treatment | Visits 2–5 No Treatment | Visits 6–8 Phase I Treatment Days 1–14 | Washout Period 12 Days | Visits 9–11 Phase II Treatment Days 1–14 | Washout Period 12 Days | Visits 12–14 Phase III Treatment Days 1–14 | |
|---|---|---|---|---|---|---|---|
| Information | + | ||||||
| Informed Consent | + | ||||||
| Clinical Examination | + | ||||||
| Enrollment and Allocation to IP | + | + Visit 6 | |||||
| Test days | 1 | 2–5 | 8, 12, 21 | 24–37 | 38, 42, 49 | 52–65 | 66, 70, 77 |
| PSSAQ score | + * | + * | + * | + * | |||
| MAT Tests scores | + * | + * | + * | + * | + * | ||
| d2 test of attention | + * | + * | + * | + * | + * | ||
| Hearth rate | + * | + * | + * | ||||
| Treatment | + | + | + | ||||
| IP accountability | + | + | + | ||||
| Adverse Events | + | + | + |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariage, P.-A.; Hovhannisyan, A.; Panossian, A.G. Efficacy of Panax ginseng Meyer Herbal Preparation HRG80 in Preventing and Mitigating Stress-Induced Failure of Cognitive Functions in Healthy Subjects: A Pilot, Randomized, Double-Blind, Placebo-Controlled Crossover Trial. Pharmaceuticals 2020, 13, 57. https://doi.org/10.3390/ph13040057
Mariage P-A, Hovhannisyan A, Panossian AG. Efficacy of Panax ginseng Meyer Herbal Preparation HRG80 in Preventing and Mitigating Stress-Induced Failure of Cognitive Functions in Healthy Subjects: A Pilot, Randomized, Double-Blind, Placebo-Controlled Crossover Trial. Pharmaceuticals. 2020; 13(4):57. https://doi.org/10.3390/ph13040057
Chicago/Turabian StyleMariage, Pierre-Antoine, Areg Hovhannisyan, and Alexander G. Panossian. 2020. "Efficacy of Panax ginseng Meyer Herbal Preparation HRG80 in Preventing and Mitigating Stress-Induced Failure of Cognitive Functions in Healthy Subjects: A Pilot, Randomized, Double-Blind, Placebo-Controlled Crossover Trial" Pharmaceuticals 13, no. 4: 57. https://doi.org/10.3390/ph13040057
APA StyleMariage, P.-A., Hovhannisyan, A., & Panossian, A. G. (2020). Efficacy of Panax ginseng Meyer Herbal Preparation HRG80 in Preventing and Mitigating Stress-Induced Failure of Cognitive Functions in Healthy Subjects: A Pilot, Randomized, Double-Blind, Placebo-Controlled Crossover Trial. Pharmaceuticals, 13(4), 57. https://doi.org/10.3390/ph13040057






