4.3.1. Spectroscopic Data
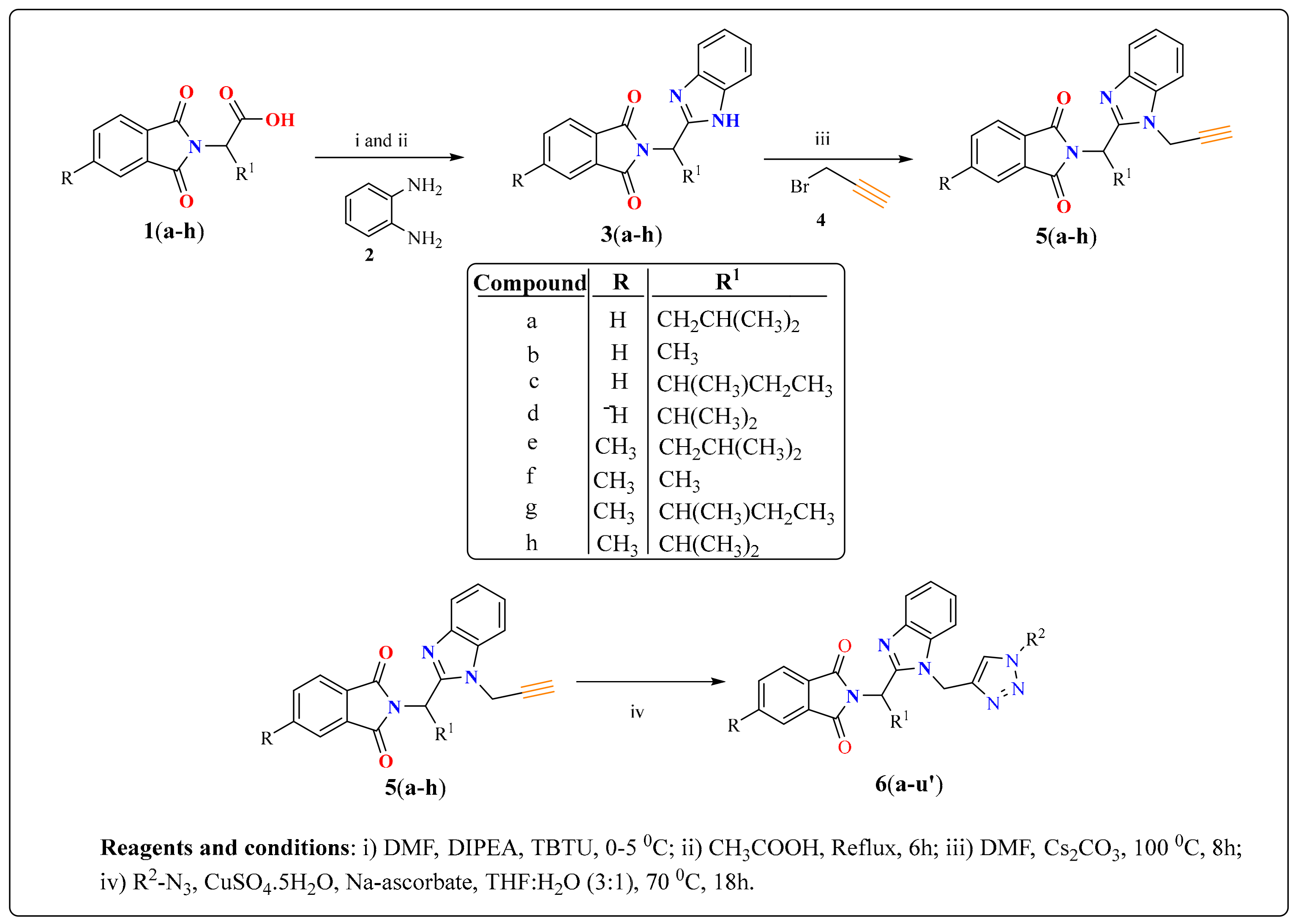
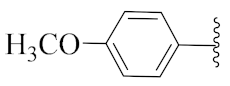
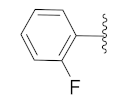
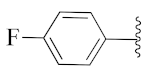
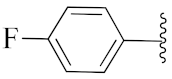
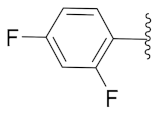
2-(1-(1-((1-(4-Fluorophenyl)-1H-1,2,3-triazol-4-yl)methyl)-1H-benzo[d]imidazol-2-yl)-3-methylbutyl)-5-methylisoindoline-1,3-dione (6a): White solid; m.p. 208–210 °C; Yield: 59%. HRMS m/z calcd. (M + H)+ 523.2180, found 523.2278. 1H NMR (400 MHz, CDCl3): δ 7.92 (d, J = 8.6 Hz, 1H), 7.48 (d, J = 7.6 Hz, 1H), 7.34–7.26 (m, 7H), 7.12–7.08 (m, 3H), 5.79 (dd, J = 10.9, 4.2 Hz, 1H), 5.51 (s, 2H), 2.87–2.80 (m, 1H), 2.39–2.32 (m, 1H), 2.29 (s, 3H), 1.60 (s, 1H), 1.05 (d, J = 6.4 Hz, 3H), 0.99 (d, J = 6.6 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 167.65, 167.54, 162.30 (d, J = 249.5 Hz), 151.25, 144.88 (d, J = 119.8 Hz), 142.28, 135.43, 134.64, 132.63, 131.25, 128.33, 123.67 (d, J = 6.4 Hz), 122.75, 121.82 (d, J = 8.4 Hz), 119.87 (d, J = 167.9 Hz), 116.53 (d, J = 23.3 Hz), 109.19, 45.19, 39.83, 38.80, 24.84, 23.44, 21.90, 21.72.
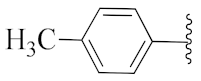
5-Methyl-2-(3-methyl-1-(1-((1-(p-tolyl)-1H-1,2,3-triazol-4-yl)methyl)-1H-benzo[d]imidazol-2-yl)butyl)isoindoline-1,3-dione (6b): White solid; m.p. 160–162 °C; Yield: 78% yield. HRMS m/z calcd. (M + H)+ 519.6089, found 519.2533. 1H NMR (400 MHz, CDCl3): δ 7.92 (d, J = 8.1 Hz, 1H), 7.47 (d, J = 7.5 Hz, 1H), 7.35–7.23 (m, 6H), 7.18 (s, 4H), 7.09 (s, 1H), 5.78 (dd, J = 10.8, 4.1 Hz, 1H), 5.49 (s, 2H), 2.86–2.79 (m, 1H), 2.39 (m, 1H), 2.36 (s, 3H), 2.24 (s, 3H), 1.59 (d, J = 6.1 Hz, 1H), 1.05 (d, J = 6.4 Hz, 3H), 0.98 (d, J = 6.6 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 167.56, 151.21, 145.33, 143.83, 142.21, 138.84, 135.52, 134.63, 133.95, 130.01, 128.18, 123.61, 123.19, 122.68, 120.59, 119.75, 118.75, 109.13, 45.05, 39.94, 38.80, 24.83, 23.47, 21.75, 21.12.
2-(1-(1-((1H-1,2,3-Triazol-4-yl)methyl)-1H-benzo[d]imidazol-2-yl)-3-methylbutyl)-5-methylisoindoline-1,3-dione (6c): Light yellow solid; m.p. 150–152 °C; Yield: 58%. HRMS m/z calcd. (M + H)+ 429.4864, found 429.2034. 1H NMR (400 MHz, CDCl3): δ 7.88 (dd, J = 6.3, 1.8 Hz, 1H), 7.57 (d, J = 7.6 Hz, 1H), 7.50 (s, 1H), 7.35 (d, J = 7.6 Hz, 1H), 7.36–7.24 (m, 5H), 5.79 (dd, J = 10.6, 4.7 Hz, 1H), 5.49 (s, 2H), 2.94–2.77 (m, 1H), 2.39 (s, 3H), 2.32–2.24 (m, 1H), 1.58 (s, 1H), 1.00 (d, J = 6.5 Hz, 3H), 0.93 (d, J = 6.7 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 167.85, 167.77, 151.23, 145.55, 141.87, 135.18, 134.77, 131.61, 128.66, 123.97, 123.58, 123.36, 122.77, 120.40, 109.69, 44.91, 39.40, 38.80, 24.87, 23.16, 21.89, 21.66.
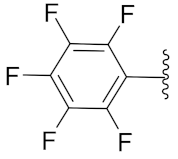
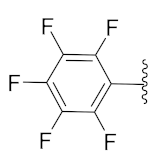
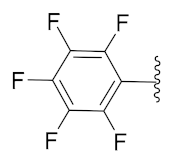
5-Methyl-2-(3-methyl-1-(1-((1-(perfluorophenyl)-1H-1,2,3-triazol-4-yl)methyl)-1H-benzo[d]imidazol-2-yl)butyl)isoindoline-1,3-dione (6d): Light brown solid; m.p. 128–130 °C; Yield: 69%. HRMS m/z calcd. (M + H)+ 595.5346, found 595.1882. 1H NMR (400 MHz, CDCl3): δ 7.86 (d, J = 7.2 Hz, 1H), 7.61 (d, J = 7.5 Hz, 1H), 7.53 (s, 1H), 7.44 (d, J = 7.4 Hz, 1H), 7.38 (s, 1H), 7.30–7.25 (m, 4H), 5.79 (dd, J = 10.3, 4.2 Hz, 1H), 5.56 (d, J = 6.3 Hz, 2H), 2.91–2.80 (m, 1H), 2.47 (d, J = 17.6 Hz, 3H), 2.34–2.24 (m, 1H), 1.58 (brs, 1H), 1.01 (d, J = 6.3 Hz, 3H), 0.96 (d, J = 6.1 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 167.90, 167.80, 151.16, 144.73 (d, J = 210.4 Hz), 142.31, 135.10, 134.85, 131.70, 128.72, 124.10 (d, J = 26.3 Hz), 123.47 (d, J = 20.0 Hz), 121.65 (d, J = 211.1 Hz), 109.41, 45.23, 39.19, 38.86, 24.94, 23.23, 21.97, 21.68.
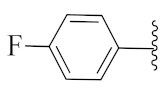
5-Methyl-2-(3-methyl-1-(1-((1-(4-(trifluoromethyl)phenyl)-1H-1,2,3-triazol-4-yl)methyl)-1H-benzo[d]imidazol-2-yl)butyl)isoindoline-1,3-dione (6e): White solid; m.p. 218–220 °C. Yield: 71%. HRMS m/z calcd. (M + H)+ 573.5803, found 573.2225. 1H NMR (400 MHz, CDCl3): δ 7.90 (d, J = 8.1 Hz, 1H), 7.66 (d, J = 8.4 Hz, 2H), 7.46 (dd, J = 17.9, 8.0 Hz, 3H), 7.27–7.21 (m, 7H), 5.77 (dd, J = 10.9, 4.1 Hz, 1H), 5.50 (s, 2H), 2.88–2.77 (m, 1H), 2.38–2.29 (m, 1H), 2.20 (s, 3H), 1.57 (brs, 1H), 1.03 (d, J = 6.6 Hz, 3H), 0.97 (d, J = 6.4 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 167.62, 167.48, 151.26, 145.50, 144.76, 142.35, 138.67, 135.43, 134.62, 131.23, 130.88, 130.55, 128.28, 126.91, 126.88, 124.83, 123.69, 123.65, 123.28, 122.80, 122.12, 120.80, 119.81, 118.82, 109.10, 45.21, 39.81, 38.81, 24.84, 23.44, 21.79, 21.71.
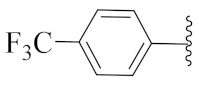
2-(1-(1-((1-Benzyl-1H-1,2,3-triazol-4-yl)methyl)-1H-benzo[d]imidazol-2-yl)-3-methylbutyl)-5-methylisoindoline-1,3-dione (6f): Yellow solid; m.p. 142–144 °C; Yield: 41%. HRMS m/z calcd. (M + H)+ 519.6089, found 519.2505. 1H NMR (400 MHz, CDCl3): δ 7.85 (d, J = 7.7 Hz, 1H), 7.57 (d, J = 7.6 Hz, 1H), 7.52 (s, 1H), 7.45 (d, J = 7.6 Hz, 1H), 7.30–7.28 (m, 4H), 7.27–7.21 (m, 4H), 7.09–7.06 (m, 1H), 7.00 (s, 2H), 5.76 (dd, J = 10.7, 4.5 Hz, 1H), 5.51 (d, J = 8.4 Hz, 1H), 5.44 (s, 1H), 5.28–5.09 (m, 2H), 2.87–2.80 (m, 1H), 2.47 (s, 3H), 2.26–2.21 (m, 1H), 1.82 (brs, 1H), 0.95 (dd, J = 16.5, 6.6 Hz, 6H). 13C NMR (100 MHz, CDCl3): δ 167.83, 167.73, 151.19, 145.51, 142.30, 135.25, 134.79, 134.18, 131.79, 129.13, 128.83, 127.95, 123.94, 123.40, 123.30, 122.53, 121.39, 120.57, 109.59, 54.04, 45.21, 39.61, 38.90, 24.93, 23.32, 22.15, 21.71.
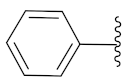
5-Methyl-2-(3-methyl-1-(1-((1-phenyl-1H-1,2,3-triazol-4-yl)methyl)-1H-benzo[d]imidazol-2-yl)butyl)isoindoline-1,3-dione (6g): Yellow solid; m.p. 67–69 °C; Yield: 57%. HRMS m/z calcd. (M + H)+ 505.5823, found 505.2346. 1H NMR (400 MHz, CDCl3): δ 7.93 (d, J = 7.6 Hz, 1H), 7.47 (d, J = 7.4 Hz, 1H), 7.43–7.37 (m, 3H), 7.35–7.25 (m, 8H), 7.12 (s, 1H), 5.79 (dd, J = 10.9, 4.2 Hz, 1H), 5.51 (s, 2H), 2.89–2.78 (m, 1H), 2.41–2.31 (m, 1H), 2.22 (s, 3H), 1.68 (brs, 1H), 1.05 (d, J = 6.4 Hz, 3H), 0.99 (d, J = 6.5 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 167.68, 167.56, 156.41, 151.27, 145.56, 144.03, 142.01, 136.25, 135.38, 134.68, 131.13, 129.63, 129.60, 128.83, 128.21, 123.81, 123.76, 123.35, 122.94, 120.61, 120.21, 119.84, 118.82, 115.60, 109.25, 45.13, 39.98, 38.78, 24.82, 23.39, 21.87, 21.73.
2-(1-(1-((1-(4-Methoxyphenyl)-1H-1,2,3-triazol-4-yl)methyl)-1H-benzo[d]imidazol-2-yl)-3-methylbutyl)-5-methylisoindoline-1,3-dione (6h): Yellow solid; m.p. 101–103 °C; Yield: 82%. HRMS m/z calcd. (M + H)+ 535.6083, found 535.2448. 1H NMR (400 MHz, CDCl3): δ 7.92 (d, J = 7.9 Hz, 1H), 7.48 (d, J = 7.5 Hz, 1H), 7.31–7.21 (m, 8H), 7.05 (s, 1H), 6.88 (d, J = 9.0 Hz, 1H), 5.79 (dd, J = 10.9, 4.1 Hz, 1H), 5.49 (s, 2H), 3.82 (s, 3H), 2.88–2.78 (m, 1H), 2.40–2.31 (m, 1H), 2.27 (s, 3H), 1.60 (brs, 1H), 1.05 (d, J = 6.4 Hz, 3H), 0.98 (d, J = 6.6 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 167.68, 167.57, 159.87, 151.30, 145.45, 143.91, 142.45, 135.49, 134.64, 131.22, 129.81, 128.31, 123.75, 123.63, 123.32, 122.72, 121.47, 120.72, 118.89, 114.52, 109.22, 55.71, 45.16, 39.98, 38.79, 24.83, 23.49, 21.95, 21.73.
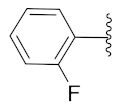
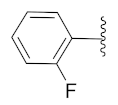
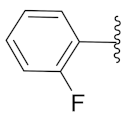
2-(1-(1-((1-(2-Fluorophenyl)-1H-1,2,3-triazol-4-yl)methyl)-1H-benzo[d]imidazol-2-yl)-3-methylbutyl)-5-methylisoindoline-1,3-dione (6i): Yellow solid; m.p. 138–140 °C; Yield: 67%. HRMS m/z calcd. (M + H)+ 523.5728, found 523.2246. 1H NMR (400 MHz, CDCl3): δ 7.88 (d, J = 8.3 Hz, 1H), 7.56–7.52 (m, 2H), 7.44–7.42 (m, 2H), 7.35–7.19 (m, 8H), 5.81 (dd, J = 10.8, 4.4 Hz, 1H), 5.52 (s, 2H), 2.91–2.78 (m, 1H), 2.33 (s, 3H), 1.64–1.53 (m, 1H), 1.23 (s, 1H), 1.03 (d, J = 6.4 Hz, 3H), 0.97 (d, J = 6.7 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 167.64 (d, J = 6.9 Hz), 151.67, 151.23, 145.43, 143.62, 142.32, 135.40, 134.64, 131.59, 130.30 (d, J = 7.7 Hz), 128.66, 125.04, 124.66, 124.40, 123.85, 123.47 (d, J = 17.8 Hz), 122.66, 122.37, 120.69, 117.04 (d, J = 20.1 Hz), 109.38, 45.17, 39.62, 38.81, 24.91, 23.39, 21.96, 21.74.
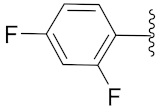
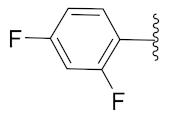
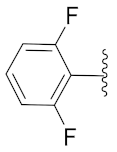
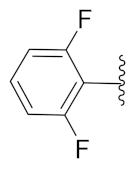
2-(1-(1-((1-(2,4-Difluorophenyl)-1H-1,2,3-triazol-4-yl)methyl)-1H-benzo[d]imidazol-2-yl)-3-methylbutyl)-5-methylisoindoline-1,3-dione (6j): Yellow solid; m.p. 72–74 °C; Yield: 50%. 1H NMR (400 MHz, CDCl3): δ 7.89 (s, 1H), 7.56–7.37 (m, 4H), 7.38 (d, J = 7.5 Hz, 2H), 7.26 (d, J = 11.9 Hz, 2H), 6.96 (d, J = 7.8 Hz, 2H), 5.84 (d, J = 4.8 Hz, 1H), 5.56 (s, 2H), 2.91 (brs, 1H), 2.37 (s, 3H), 1.85 (brs, 1H), 1.58 (s, 1H), 0.99 (dd, J = 22.8, 6.1 Hz, 6H). 13C NMR (100 MHz, CDCl3): δ 167.63, 162.44 (d, J = 264.6 Hz), 152.22, 151.22, 145.47, 143.01 (d, J = 142.5 Hz), 135.28, 134.68, 131.65, 128.72, 125.80 (d, J = 9.6 Hz), 123.91, 123.61, 123.41, 122.73, 122.39, 121.44, 120.74, 112.48 (d, J = 20.4 Hz), 109.40, 105.65, 105.40, 105.15, 45.17, 39.54, 38.85, 24.94, 23.37, 22.03, 21.74.
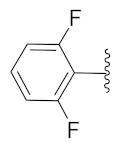
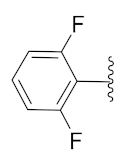
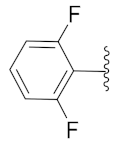
2-(1-(1-((1-(2,6-Difluorophenyl)-1H-1,2,3-triazol-4-yl)methyl)-1H-benzo[d]imidazol-2-yl)-3-methylbutyl)-5-methylisoindoline-1,3-dione (6k): White solid; m.p. 71–73 °C; Yield: 43%. HRMS m/z calcd. (M + H)+ 541.5632, found 541.2173. 1H NMR (400 MHz, CDCl3): δ 7.86 (d, J = 4.6 Hz, 1H), 7.60 (d, J= 7.4 Hz, 1H), 7.51 (s, 1H), 7.41 (d, J = 7.5 Hz, 2H), 7.37–7.31 (m, 2H), 7.25 (d, J = 3.9 Hz, 3H), 7.01 (t, J = 8.6 Hz, 2H), 5.82 (dd, J = 10.5, 4.6 Hz, 1H), 5.56 (s, 2H), 2.94–2.80 (m, 1H), 2.41 (s, 3H), 2.35–2.26 (m, 1H), 1.63–1.52 (m, 1H), 0.98 (dd, J = 22.6, 6.5 Hz, 6H). 13C NMR (100 MHz, CDCl3): δ 167.84, 156.51 (d, J = 257.0 Hz), 151.25, 145.67, 142.96, 142.20, 134.87, 131.59 (d, J = 14.6 Hz), 128.72, 124.21, 124.07, 123.69, 123.47, 122.79, 120.64, 112.49 (d, J = 19.5 Hz), 109.60, 45.12, 39.48, 38.91, 24.97, 23.27, 22.08, 21.74.
2-(3-Methyl-1-(1-((1-(perfluorophenyl)-1H-1,2,3-triazol-4-yl)methyl)-1H-benzo[d]imidazol-2-yl)butyl)isoindoline-1,3-dione (6l): Light brown solid; m.p. 100–102 °C; Yield: 65%. HRMS m/z calcd. (M + H)+ 581.5081, found 581.1750. 1H NMR (400 MHz, CDCl3): δ 7.92–7.85 (m, 1H), 7.78–7.74 (m, 2H), 7.70–7.76 (m, 2H), 7.43 (s, 1H), 7.35–7.31 (m, 1H), 7.25–7.29 (m, 2H), 5.84 (dd, J = 10.6, 4.7 Hz, 1H), 5.65–5.53 (m, 2H), 2.93–2.81 (m, 1H), 2.39–2.25 (m, 1H), 1.61 (s, 1H), 1.03 (d, J = 6.6 Hz, 3H), 0.98 (d, J = 6.5 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 167.76, 151.03, 143.68, 142.37, 135.10, 134.32, 131.39, 124.19, 123.66, 123.50, 122.79, 120.77, 109.34, 45.33, 39.22, 38.90, 24.96, 23.24, 21.71.
2-(3-Methyl-1-(1-((1-(4-(trifluoromethyl)phenyl)-1H-1,2,3-triazol-4-yl)methyl)-1H-benzo[d]imidazol-2-yl)butyl)isoindoline-1,3-dione (6m): White solid; m.p. 216–218 °C; Yield: 62%. HRMS m/z calcd. (M + H)+ 559.5537, found 559.2070. 1H NMR (400 MHz, CDCl3): δ 7.91 (d, J = 8.5 Hz, 1H), 7.66 (d, J = 8.4 Hz, 2H), 7.58–7.54 (m, J = 5.3, 2.9 Hz, 2H), 7.48 (d, J = 7.3 Hz, 3H), 7.32–7.22 (m, 5H), 5.80 (dd, J = 10.7, 4.4 Hz, 1H), 5.51 (s, 2H), 2.89–2.77 (m, 1H), 2.39–2.29 (m, 1H), 1.60 (s, 1H), 1.04 (d, J = 6.4 Hz, 3H), 0.98 (d, J = 6.5 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 167.52, 151.13, 144.66, 142.38, 138.68, 135.38, 134.10, 130.99, 130.66, 126.97, 126.93, 124.83, 123.71, 123.26, 122.82, 122.12, 120.82, 120.02, 119.05, 109.14, 45.37, 39.74, 38.88, 24.88, 23.42, 21.70.
2-(1-(1-((1-(2-Fluorophenyl)-1H-1,2,3-triazol-4-yl)methyl)-1H-benzo[d]imidazol-2-yl)-3-methylbutyl)isoindoline-1,3-dione (6n): Yellow solid; m.p. 107–109 °C; Yield: 78%. HRMS m/z calcd. (M + H)+ 509.5462, found 509.2106. 1H NMR (400 MHz, CDCl3): δ 7.85 (d, J = 8.0 Hz, 1H), 7.65 (dd, J = 5.1, 3.0 Hz, 2H), 7.60–7.46 (m, 4H), 7.38–7.10 (m, 7H), 5.84 (dd, J = 10.7, 4.4 Hz, 1H), 5.51 (s, 2H), 2.94–2.82 (m, 1H), 2.41–2.27 (m, 1H), 1.65–1.60 (brs, 1H), 1.03 (d, J = 6.5 Hz, 3H), 0.96 (d, J = 6.6 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 167.80, 152.94 (d, J = 251.5 Hz), 143.46, 142.29, 135.33, 134.12, 131.25, 130.40 (d, J = 7.7 Hz), 125.12 (d, J = 3.0 Hz), 124.67, 124.50, 123.55, 123.38, 122.65, 122.47 (d, J = 8.4 Hz), 120.64, 117.01 (d, J = 19.8 Hz), 109.43, 45.21, 39.50, 38.81, 24.90, 23.39, 21.73.
2-(1-(1-((1-(2,4-Difluorophenyl)-1H-1,2,3-triazol-4-yl)methyl)-1H-benzo[d]imidazol-2-yl)-3-methylbutyl)isoindoline-1,3-dione (6o): Yellow solid; m.p. 126–128 °C; Yield: 68%. HRMS m/z calcd. (M + H)+ 527.5367, found 527.2007. 1H NMR (400 MHz, CDCl3): δ 7.89 (d, J = 7.0 Hz, 1H), 7.71–7.67 (m, 2H), 7.65–7.58 (m, 2H), 7.55–7.44 (m, 2H), 7.34 (d, J = 7.1 Hz, 1H), 7.30–7.27 (m, 3H), 6.98–6.93 (m, 2H), 5.85 (dd, J = 10.4, 4.0 Hz, 1H), 5.55 (s, 2H), 2.95–2.83 (m, 1H), 2.40–2.27 (m, 1H), 1.69 (brs, 2H), 1.05 (d, J = 6.3 Hz, 3H), 0.99 (d, J = 6.5 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 167.59, 151.09, 142.96 (d, J = 132.2 Hz), 135.24, 134.13, 131.34, 125.90 (d, J = 9.9 Hz), 123.62, 123.43, 122.74, 122.44 (d, J = 7.4 Hz), 120.73, 112.53 (d, J = 19.2 Hz), 109.39, 105.65, 105.41, 105.39, 105.15, 45.25, 39.48, 38.86, 24.94, 23.35, 21.73.
2-(1-(1-((1-(2,6-Difluorophenyl)-1H-1,2,3-triazol-4-yl)methyl)-1H-benzo[d]imidazol-2-yl)-3-methylbutyl)isoindoline-1,3-dione (6p): Yellow solid; m.p. 198–200 °C; Yield: 65%. HRMS m/z calcd. (M + H)+ 527.5367, found 527.2014. 1H NMR (400 MHz, CDCl3): δ 7.83 (d, J = 4.4 Hz, 1H), 7.71 (s, 2H), 7.61 (s, 2H), 7.41–7.28 (m, 3H), 7.23 (d, J = 2.9 Hz, 2H), 6.98 (t, J = 8.2 Hz, 2H), 5.83 (dd, J = 9.8, 3.8 Hz, 1H), 5.54 (s, 2H), 2.93–2.77 (m, 1H), 2.34–2.23 (m, 1H), 1.58 (brs, 1H), 1.00 (d, J = 6.0 Hz, 3H), 0.95 (d, J = 6.2 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 167.79, 156.50 (d, J = 256.9 Hz), 151.13, 142.67 (d, J = 59.1 Hz), 135.24, 134.26, 131.65, 131.55, 131.45, 131.37, 124.24, 123.54, 123.48, 122.63, 120.66, 114.72, 112.51 (d, J = 22.8 Hz), 109.51, 45.30, 39.39, 38.89, 24.96, 23.30, 21.73.
2-(1-(1-((1-(2-Fluorophenyl)-1H-1,2,3-triazol-4-yl)methyl)-1H-benzo[d]imidazol-2-yl)-2-methylbutyl)-5-methylisoindoline-1,3-dione (6q): Light green solid; m.p. 114–116 °C; Yield: 60%. HRMS m/z calcd. (M + H)+ 523.5725, found 523.2258. 1H NMR (400 MHz, CDCl3): δ 7.86 (s, 1H), 7.66 (t, J = 29.0 Hz, 3H), 7.50 (s, 2H), 7.38 (s, 2H), 7.23 (d, J = 13.0 Hz, 4H), 5.76 (s, 1H), 5.57 (s, 1H), 5.44 (s, 1H), 3.57 (brs, 1H), 2.37 (s, 3H), 1.52 (brs, 1H), 1.11 (s, 1H), 0.94 (d, J = 22.6 Hz, 6H). 13C NMR (100 MHz, CDCl3): δ 167.78, 154.39, 145.70, 134.90, 131.66, 130.51, 128.67, 125.29, 124.57, 124.11, 123.86, 123.58, 122.91, 120.51, 117.20, 117.01, 27.01, 25.50, 22.08, 16.92, 15.54, 11.24, 10.58.
2-(1-(1-((1-(2,4-Difluorophenyl)-1H-1,2,3-triazol-4-yl)methyl)-1H-benzo[d]imidazol-2-yl)-2-methylbutyl)-5-methylisoindoline-1,3-dione (6r): Yellow solid; m.p. 89–91 °C; Yield: 50%. HRMS m/z calcd. (M + H)+ 541.5632, found 541.2164. 1H NMR (400 MHz, CDCl3): δ 7.84 (d, J = 4.8 Hz, 1H), 7.58–7.56 (m, 3H), 7.47 (s, 1H), 7.37 (d, J = 6.5 Hz, 2H), 7.25–7.18 (m, 2H), 6.93 (d, J = 7.6 Hz, 2H), 5.73–5.50 (m, 2H), 5.38 (d, J = 10.8 Hz, 1H), 3.41 (brs, 1H), 2.37 (s, 3H), 1.53 (d, J = 42.3 Hz, 1H), 1.15–1.10 (m, 1H), 1.00–0.85 (m, 6H). 13C NMR (100 MHz, CDCl3): δ 167.79, 150.43 (d, J = 16.4 Hz), 145.52, 143.83, 143.78, 142.53, 134.73, 130.18 (d, J = 297.2 Hz), 126.01, 125.91, 123.96, 123.43, 122.62, 120.68, 112.54 (d, J = 24.4 Hz), 109.77, 105.61, 105.36, 105.10, 51.81, 51.51, 39.19, 34.45, 33.94, 27.10, 25.45, 22.00, 17.02, 15.41, 11.13, 10.66.
5-Methyl-2-(2-methyl-1-(1-((1-(perfluorophenyl)-1H-1,2,3-triazol-4-yl)methyl)-1H-benzo[d]imidazol-2-yl)butyl)isoindoline-1,3-dione (6s): White solid; m.p. 196–198 °C; Yield: 64%. 1H NMR (400 MHz, CDCl3): δ 7.86–7.83 (m, 1H), 7.63 (d, J = 7.7 Hz, 1H), 7.55–7.52 (m, 2H), 7.45 (d, J = 7.6 Hz, 1H), 7.38 (dd, J = 6.1, 3.0 Hz, 1H), 7.24 (dd, J = 6.0, 3.2 Hz, 3H), 5.82–5.68 (m, 1H), 5.60 (dd, J = 16.8, 7.9 Hz, 1H), 5.35 (d, J = 11.0 Hz, 1H), 3.52–3.30 (m, 1H), 2.44 (s, 3H), 1.54–1.47 (m, 1H), 0.96–0.81 (m, 7H).13C NMR (100 MHz, CDCl3): δ 167.98, 167.90, 150.58, 150.38, 145.77, 143.99, 143.92, 142.57, 135.16, 134.87, 134.70, 134.57, 131.72, 128.73, 124.39, 124.09, 123.56, 123.49, 122.76, 120.78, 109.72, 77.48, 77.16, 76.84, 51.88, 51.66, 38.98, 34.42, 33.92, 27.02, 25.49, 22.03, 16.87, 15.45, 11.04, 10.59.
2-(2-Methyl-1-(1-((1-(perfluorophenyl)-1H-1,2,3-triazol-4-yl)methyl)-1H-benzo[d]imidazol-2-yl)butyl)isoindoline-1,3-dione (6t): Yellow solid; m.p. 86–88 °C; Yield: 77%. HRMS m/z calcd. (M + H)+ 581.5081, found 581.1716. 1H NMR (400 MHz, CDCl3): δ 7.82 (s, 2H), 7.74 (s, 2H), 7.65 (s, 2H), 7.56 (s, 1H), 7.37 (s, 1H), 7.23 (s, 2H), 5.71 (dd, J = 45.9, 26.5 Hz, 2H), 5.38 (s, 1H), 3.44 (s, 1H), 1.50 (s, 1H), 1.22 (s, 1H), 0.93 (d, J = 16.9 Hz, 6H). 13C NMR (100 MHz, CDCl3): δ 167.85, 150.52, 150.31, 143.89, 142.55, 141.05, 134.61, 134.32, 131.37, 124.48, 123.77, 123.56, 122.76, 120.77, 109.70, 51.94, 51.75, 38.94, 34.44, 33.92, 26.99, 25.50, 16.83, 15.48, 11.04, 10.57.
2-(2-Methyl-1-(1-((1-(pyridin-4-yl)-1H-1,2,3-triazol-4-yl)methyl)-1H-benzo[d]imidazol-2-yl)butyl)isoindoline-1,3-dione (6u): White solid; m.p. 101–103 °C; Yield: 42%. HRMS m/z calcd. (M + H)+ 492.5438, found 492.2151. 1H NMR (400 MHz, CDCl3): δ 8.63 (brs, 1H), 7.80 (s, 1H), 7.71 (d, J = 6.1 Hz, 1H), 7.60 (s, 2H), 7.49 (s, 3H), 7.32 (d, J = 5.9 Hz, 2H), 7.31 (s, 2H), 5.67–5.48 (m, 2H), 5.38 (dd, J = 10.1, 4.1 Hz, 1H), 3.36 (s, 1H), 1.57 (d, J = 80.9 Hz, 1H), 1.26–1.11 (m, 1H), 1.05 (d, J = 6.0 Hz, 1H), 0.94–0.87 (m, 6H). 13C NMR (100 MHz, CDCl3): δ 167.60, 167.55, 150.31, 150.17, 144.61, 142.48, 141.17, 134.93, 134.78, 134.22, 131.08, 127.54, 123.54, 123.41, 122.74, 120.73, 119.58, 109.61, 51.91, 51.47, 39.39, 34.51, 34.03, 27.16, 25.42, 17.17, 15.41, 11.19, 10.73.
2-(1-(1-((1-(2-Fluorophenyl)-1H-1,2,3-triazol-4-yl)methyl)-1H-benzo[d]imidazol-2-yl)-2-methylbutyl)isoindoline-1,3-dione (6v): Yellow solid; m.p. 85–87 °C; Yield: 79%. HRMS m/z calcd. (M + H)+ 509.5462, found 509.2094. 1H NMR (400 MHz, CDCl3): δ 7.88–7.86 (m, 1H), 7.73 (d, J = 3.1 Hz, 2H), 7.61 (s, 4H), 7.39–7.36 (m, 2H), 7.25–7.15 (m, 4H), 5.75–5.52 (m, 2H), 5.42 (d, J = 10.7 Hz, 1H), 3.45 (brs, 1H), 1.56 (d, J = 42.6 Hz, 1H), 1.17 (s, 1H), 1.02–0.91 (m, 6H). 13C NMR (100 MHz, CDCl3): δ 167.70, 153.08 (d, J = 253.3 Hz), 150.36 (d, J = 16.5 Hz), 143.63, 142.56, 134.88, 134.75, 134.63, 134.16, 131.33, 130.42 (d, J = 7.8 Hz), 125.20 (d, J = 3.4 Hz), 124.84, 124.67, 123.79, 123.51, 123.46, 122.84, 122.65, 120.73, 117.02 (d, J = 19.9 Hz), 109.80, 51.92, 51.63, 39.26, 34.45, 33.95, 27.11, 25.48, 17.03, 15.46, 11.14, 10.68.
2-(1-(1-((1-(2,4-Difluorophenyl)-1H-1,2,3-triazol-4-yl)methyl)-1H-benzo[d]imidazol-2-yl)-2-methylbutyl)isoindoline-1,3-dione (6w): Yellow solid; m.p. 86–88 °C; Yield: 75%. HRMS m/z calcd. (M + H)+ 527.5367, found 527.1997. 1H NMR (400 MHz, CDCl3): δ 7.88 (s, 1H), 7.74 (s, 2H), 7.64 (s, 4H), 7.41 (s, 1H), 7.25 (s, 2H), 7.02–6.91 (q, 2H), 5.82–5.51 (m, 2H), 5.44 (d, J = 10.4 Hz, 1H), 3.44 (brs, 1H), 1.55 (brs, 1H), 1.24–1.06 (m, 1H), 1.04–0.87 (m, 6H). 13C NMR (100 MHz, CDCl3): δ 167.72, 162.53 (d, J = 264.4 Hz), 153.60 (d, J = 266.1 Hz), 150.51, 142.41, 134.66, 134.26, 131.35, 126.08 (d, J = 9.4 Hz), 123.58, 122.88, 120.68, 112.64 (d, J = 22.6 Hz), 109.94, 105.66, 105.41, 105.16, 53.59, 51.80, 51.59, 39.25, 34.59, 34.02, 27.05, 25.51, 16.95, 15.51, 11.15, 10.58.
2-(1-(1-((1-(2,6-Difluorophenyl)-1H-1,2,3-triazol-4-yl)methyl)-1H-benzo[d]imidazol-2-yl)-2-methylbutyl)isoindoline-1,3-dione (6x): White solid; m.p. 59–61 °C; Yield: 43%. HRMS m/z calcd. (M + H)+ 527.5367, found 527.2021. 1H NMR (400 MHz, CDCl3): δ 7.87 (s, 1H), 7.78 (s, 2H), 7.66 (s, 2H), 7.44 (d, J = 19.2 Hz, 2H), 7.25 (d, J = 6.1 Hz, 3H), 7.04 (dd, J = 13.9, 7.5 Hz, 2H), 5.83–5.73 (m, 1H), 5.62–5.58 (m, 1H), 5.41 (dd, J = 10.2, 5.1 Hz, 1H), 3.50–3.41 (m, 1H), 2.15 (brs, 1H), 1.51 (s, 1H), 0.98–0.89 (m, 6H). 13C NMR (100 MHz, CDCl3): δ 167.84, 157.98, 155.44, 150.42, 143.31, 142.61, 134.19, 131.45, 123.58, 120.82, 120.74, 112.62, 112.42, 109.97, 109.66, 52.03, 51.84, 39.20, 34.46, 33.91, 16.79, 15.46, 11.01, 10.61.
2-(1-(1-((1-(4-Fluorophenyl)-1H-1,2,3-triazol-4-yl)methyl)-1H-benzo[d]imidazol-2-yl)ethyl)isoindoline-1,3-dione (6y): White solid; m.p. 241–243 °C; Yield: 74%. HRMS m/z calcd. (M + H)+ 467.4665, found 467.1627. 1H NMR (400 MHz, CDCl3): δ 7.91 (d, J = 8.0 Hz, 1H), 7.61–7.49 (m, 4H), 7.39–7.25 (m, 5H), 7.19 (s, 1H), 7.10 (t, J = 8.3 Hz, 2H), 5.88 (q, J = 6.8 Hz, 1H), 5.47 (s, 2H), 2.09 (d, J = 6.9 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 167.24, 162.39 (d, J = 249.5 Hz), 151.19, 144.16, 142.33, 135.59, 134.11, 132.63, 131.13, 123.70, 123.22, 122.78, 121.99 (d, J = 8.1 Hz), 120.77, 119.29 (d, J = 6.1 Hz), 116.60 (d, J = 22.8 Hz), 109.14, 42.80, 42.74, 39.87, 17.10.
2-(1-(1-((1-(p-Tolyl)-1H-1,2,3-triazol-4-yl)methyl)-1H-benzo[d]imidazol-2-yl)ethyl)isoindoline-1,3-dione (6z): Yellow solid; m.p. 118–120 °C; Yield: 65%. HRMS m/z calcd. (M + H)+ 463.5026, found 463.1884. 1H NMR (400 MHz, CDCl3): δ 7.92 (d, J = 8.4 Hz, 1H), 7.62–7.48 (m, 4H), 7.36–7.25 (m, 5H), 7.23–7.14 (m, 4H), 5.88 (q, 1H), 5.47 (s, 2H), 2.37 (s, 3H), 2.09 (d, J = 6.9 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 167.26, 151.24, 143.79, 142.30, 139.00, 135.63, 134.09, 131.08, 130.08, 123.67, 123.24, 122.75, 120.73, 119.95, 119.07, 109.19, 42.73, 39.98, 21.14, 17.10.
2-(1-(1-((1-Phenyl-1H-1,2,3-triazol-4-yl)methyl)-1H-benzo[d]imidazol-2-yl)ethyl)isoindoline-1,3-dione (6a’): Brown solid; m.p. 187–189 °C; Yield: 51%. HRMS m/z calcd. (M + H)+ 449.4760, found 449.1713. 1H NMR (400 MHz, CDCl3): δ 7.88 (dd, J = 6.0, 2.0 Hz, 1H), 7.61 (d, J = 5.2 Hz, 2H), 7.54 (d, J = 3.1 Hz, 2H), 7.47–7.31 (m, 8H), 5.91 (dd, J = 13.8, 6.8 Hz, 1H), 5.52 (d, J= 6.6 Hz, 2H), 2.13 (d, J = 6.9 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 167.23, 151.21, 143.84, 141.95, 136.26, 135.46, 134.12, 131.04, 129.61, 128.90, 123.80, 123.23, 122.91, 120.54, 120.00, 119.14, 109.28, 42.67, 39.92, 21.12, 17.03.
2-(1-(1-((1-(Perfluorophenyl)-1H-1,2,3-triazol-4-yl)methyl)-1H-benzo[d]imidazol-2-yl)ethyl)isoindoline-1,3-dione (6b’): White solid; m.p. 215–217 °C; Yield: 62%. HRMS m/z calcd. (M + H)+ 539.4283, found 539.1247. 1H NMR (400 MHz, CDCl3): δ 7.88 (dd, J = 6.0, 2.0 Hz, 1H), 7.76–7.73 (m, 2H), 7.69–7.67 (m, 2H), 7.42 (s, 1H), 7.29 (dd, J = 7.9, 3.1 Hz, 3H), 5.91 (q, 1H), 5.52 (d, J = 6.6 Hz, 2H), 2.10 (d, J = 6.9 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 167.45, 151.15, 143.54, 142.31, 140.87, 139.25, 136.70, 135.25, 134.32, 131.49, 124.28, 123.65, 123.41, 122.78, 120.70, 109.28, 42.82, 39.33, 17.11.
2-(1-(1-((1-(2-Fluorophenyl)-1H-1,2,3-triazol-4-yl)methyl)-1H-benzo[d]imidazol-2-yl)ethyl)isoindoline-1,3-dione (6c’): White solid; m.p. 186–188 °C; Yield: 64%. HRMS m/z calcd. (M + H)+ 467.4665, found 467.1624. 1H NMR (400 MHz, CDCl3): δ 7.82 (s, 1H), 7.62–7.56 (m, 3H), 7.55–7.47 (m, 3H), 7.29 (s, 1H), 7.23 (s, 2H), 7.15 (d, J = 6.3 Hz, 2H), 5.87 (d, J = 4.5 Hz, 1H), 5.44 (s, 2H), 2.06 (d, J = 4.5 Hz, 3H).13C NMR (100 MHz, CDCl3): δ 167.28, 150.98, 142.76 (d, J = 120.9 Hz), 135.47, 134.11, 131.37, 130.42 (d, J = 8.0 Hz), 125.12 (d, J = 3.4 Hz), 124.51, 123.63, 123.38, 123.33, 122.75, 122.51 (d, J = 8.3 Hz), 120.58, 117.04 (d, J = 19.8 Hz), 42.74, 39.65, 17.09.
2-(1-(1-((1-(2,4-Difluorophenyl)-1H-1,2,3-triazol-4-yl)methyl)-1H-benzo[d]imidazol-2-yl)ethyl)isoindoline-1,3-dione (6d’): White solid; m.p. 195–197 °C; Yield: 43%. HRMS m/z calcd. (M + H)+ 485.4569, found 485.1532. 1H NMR (400 MHz, CDCl3): δ 7.82 (d, J = 28.8 Hz, 2H), 7.66 (s, 2H), 7.58 (s, 2H), 7.45 (d, J = 19.8 Hz, 2H), 7.35–7.16 (m, 3H), 6.93 (s, 1H), 5.89 (s, 1H), 5.46 (s, 2H), 2.07 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 167.25, 151.18, 142.85 (d, J = 129.4 Hz), 135.41, 134.10, 131.41, 125.80, 123.59, 123.31), 122.60 (d, J = 23.1 Hz), 120.61, 112.49 (d, J = 18.8 Hz), 109.29, 105.40, 42.74, 39.55, 17.08.
2-(1-(1-((1-Benzyl-1H-1,2,3-triazol-4-yl)methyl)-1H-benzo[d]imidazol-2-yl)ethyl)isoindoline-1,3-dione (6e’): Yellow solid; m.p. 181–183 °C; Yield: 61%. HRMS m/z calcd. (M + H)+ 463.5026, found 463.1881. 1H NMR (400 MHz, CDCl3): δ 7.85 (d, J = 7.5 Hz, 1H), 7.65 (s, 4H), 7.39–7.20 (m, 7H), 7.07 (d, J = 4.3 Hz, 2H), 6.98 (s, 1H), 5.85 (q, 1H), 5.38 (s, 2H), 5.12 (q, J = 31.3, 14.9 Hz, 2H), 2.06 (d, J = 6.9 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 167.32, 151.25, 143.30, 142.25, 135.45, 134.19, 131.51, 129.11, 128.80, 127.97, 123.43, 123.27, 122.56, 121.45, 120.53, 109.48, 54.00, 42.78, 39.74, 17.17.
2-(1-(1-((1-(2,6-Difluorophenyl)-1H-1,2,3-triazol-4-yl)methyl)-1H-benzo[d]imidazol-2-yl)ethyl)isoindoline-1,3-dione (6f’): Green solid; m.p. 94–96 °C; Yield: 54%. HRMS m/z calcd. (M + H)+ 485.4569, found 485.1531. 1H NMR (400 MHz, CDCl3): δ 7.67 (d, J = 34.1 Hz, 1H), 7.39 (s, 3H), 7.24 (s, 2H), 7.00 (s, 3H), 5.92 (s, 1H), 5.52 (s, 2H), 2.11 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 167.49, 156.48 (d, J = 256.5 Hz), 134.35, 131.59 (d, J = 20.5 Hz), 128.86, 126.99, 123.81, 123.49, 120.58, 114.76, 112.57 (d, J = 19.0 Hz), 29.76, 22.77, 17.05, 14.24.
2-(1-(1-((1H-1,2,3-Triazol-4-yl)methyl)-1H-benzo[d]imidazol-2-yl)ethyl)isoindoline-1,3-dione (6g’): White solid; m.p. 97–99 °C; Yield: 57%. HRMS m/z calcd. (M + H)+ 373.3800, found 373.1433. 1H NMR (400 MHz, DMSO): δ 7.77–7.73 (m, 2H), 7.71–7.67 (m, 2H), 7.63 (d, J = 4.9 Hz, 1H), 7.48–7.29 (m, 2H), 7.20–7.12 (m, 2H), 5.90 (s, 1H), 5.37 (dd, J = 41.7, 16.5 Hz, 2H), 1.86 (d, J = 6.6 Hz, 3H). 13C NMR (100 MHz, DMSO): δ 167.66, 152.15, 142.16, 136.15, 134.90, 131.76, 123.44, 123.08, 122.30, 119.77, 110.91, 79.71, 42.85, 17.25.
2-(1-(1-((1-(4-Fluorophenyl)-1H-1,2,3-triazol-4-yl)methyl)-1H-benzo[d]imidazol-2-yl)ethyl)-5-methylisoindoline-1,3-dione (6h’): White solid; m.p. 222–224 °C; Yield: 64%. HRMS m/z calcd. (M + H)+ 481.4930, found 481.1785. 1H NMR (400 MHz, CDCl3): δ 7.88 (d, J = 7.6 Hz, 1H), 7.44 (d, J = 7.8 Hz, 1H), 7.35–7.21 (m, 8H), 7.08 (dd, J = 16.9, 8.7 Hz, 3H), 5.82 (q, J = 6.7 Hz, 1H), 5.43 (s, 2H), 2.25 (s, 3H), 2.04 (d, J = 6.9 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 167.21, 151.31, 144.82 (d, J = 125.8 Hz), 142.28, 135.60, 134.63, 131.99 (d, J = 124.4 Hz), 128.48, 123.64, 122.97 (d, J = 45.8 Hz), 121.83 (d, J = 8.4 Hz), 119.12, 116.54 (d, J = 23.2 Hz), 109.12, 42.62, 39.89, 31.01, 21.89, 17.05.
5-Methyl-2-(1-(1-((1-(p-tolyl)-1H-1,2,3-triazol-4-yl)methyl)-1H-benzo[d]imidazol-2-yl)ethyl)isoindoline-1,3-dione (6i’): White solid; m.p. 216–218 °C; Yield: 83%. HRMS m/z calcd. (M + H)+ 477.5292, found 477.2035. 1H NMR (400 MHz, CDCl3): δ 7.87 (d, J = 7.8 Hz, 1H), 7.42 (d, J = 8.1 Hz, 1H), 7.24 (m, 5H), 7.15 (d, J = 2.4 Hz, 4H), 7.10 (s, 1H), 5.80 (q, J = 6.9 Hz, 1H), 5.40 (d, J = 2.7 Hz 2H), 2.33 (s, 3H), 2.20 (s, 3H), 2.03 (d, J = 7.0 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 167.35, 167.16, 151.38, 145.38, 143.75, 142.21, 138.82, 135.64, 134.58, 134.03, 131.28, 129.99, 128.40, 123.57, 123.16, 122.63, 120.55, 119.68, 118.83, 109.23, 42.53, 39.91, 21.84, 21.09, 17.03.
5-Methyl-2-(1-(1-((1-(4-(trifluoromethyl)phenyl)-1H-1,2,3-triazol-4-yl)methyl)-1H-benzo[d]imidazol-2-yl)ethyl)isoindoline-1,3-dione (6j’): White solid; m.p. 265–267 °C; Yield: 74%. HRMS m/z calcd. (M + H)+ 477.5292, found 477.2035. 1H NMR (400 MHz, CDCl3): δ 7.91 (d, J = 7.6 Hz, 1H), 7.67 (d, J = 8.5 Hz, 2H), 7.50 (d, J = 8.5 Hz, 2H), 7.43 (d, J = 8.1 Hz, 1H), 7.34–7.22 (m, 6H), 5.83 (q, J = 6.9 Hz, 1H), 5.46 (s, 2H), 2.21 (s, 3H), 2.06 (d, J = 7.0 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 167.39, 167.18, 151.31, 145.50, 144.68, 142.35, 138.69, 135.60, 134.64, 131.35, 128.43, 126.94, 123.75, 123.60, 123.23, 122.84, 120.84, 119.85, 118.91, 109.05, 42.66, 39.89, 21.81, 17.07.
2-(1-(1-((1H-1,2,3-Triazol-4-yl)methyl)-1H-benzo[d]imidazol-2-yl)ethyl)-5-methylisoindoline-1,3-dione (6k’): Brown solid; m.p. 100–102 °C; Yield: 54%. HRMS m/z calcd. (M + H)+ 387. 4066, found 387.1605. 1H NMR (400 MHz, CDCl3): δ 7.86 (d, J = 7.1 Hz, 1H), 7.54 (d, J = 7.5 Hz, 1H), 7.47 (s, 1H), 7.32 (d, J = 7.5 Hz, 1H), 7.25 (t, J = 6.5 Hz, 4H), 5.84 (q, J = 13.4, 6.5 Hz 1H), 5.41 (s, 2H), 2.35 (s, 3H), 2.04 (d, J = 6.8 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 167.54, 151.35, 145.59, 141.88, 135.44, 134.80, 131.76, 128.81, 123.91, 123.63, 123.34, 122.80, 120.45, 109.55, 42.49, 39.43, 21.96, 17.08.
5-Methyl-2-(1-(1-((1-phenyl-1H-1,2,3-triazol-4-yl)methyl)-1H-benzo[d]imidazol-2-yl)ethyl)isoindoline-1,3-dione (6l’): White solid; m.p. 185–187 °C; Yield: 63%. HRMS m/z calcd. (M + H)+ 463.5026, found 463.1893. 1H NMR (400 MHz, CDCl3): δ 7.89 (d, J = 7.8 Hz, 1H), 7.43 (d, J = 8.1 Hz, 1H), 7.40–7.34 (m, 3H), 7.33–7.22 (m, 8H), 7.16 (s, 1H), 5.82 (q, J = 6.9 Hz, 1H), 5.48–5.37 (m, 2H), 2.20 (s, 3H), 2.05 (d, J = 6.9 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 167.32, 167.14, 151.31, 145.40, 143.92, 142.16, 136.23, 135.60, 134.57, 131.21, 129.53, 128.70, 128.34, 123.58, 123.15, 122.67, 120.58, 119.73, 118.82, 109.15, 42.50, 39.90, 21.79, 16.99.
2-(1-(1-((1-Benzyl-1H-1,2,3-triazol-4-yl)methyl)-1H-benzo[d]imidazol-2-yl)ethyl)-5-methylisoindoline-1,3-dione (6m’): Green solid; m.p. 142–144 °C; Yield: 74%. HRMS m/z calcd. (M+H)+ 477.5292, found 477.2048. 1H NMR (400 MHz, CDCl3): δ 7.76 (d, J = 7.3 Hz, 1H), 7.51–7.33 (m, 3H), 7.32–7.08 (m, 7H), 7.08–6.91 (m, 3H), 5.77 (d, J = 6.8 Hz, 1H), 5.29 (s, 2H), 5.07 (q, J = 15.0 Hz, 2H), 2.40 (s, 3H), 1.99 (d, J = 6.7 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 167.50, 167.40, 151.36, 145.50, 143.28, 142.16, 135.39, 134.79, 134.29, 131.89, 129.07, 128.94, 128.74, 127.90, 123.83, 123.39, 123.19, 122.52, 121.61, 120.41, 109.60, 53.95, 42.72, 39.68, 22.12, 17.15.
5-Methyl-2-(1-(1-((1-(pyridin-4-yl)-1H-1,2,3-triazol-4-yl)methyl)-1H-benzo[d]imidazol-2-yl)ethyl)isoindoline-1,3-dione (6n’): White solid; m.p. 236–238 °C; Yield: 75%. HRMS m/z calcd. (M + H)+ 463.4906, found 464.1820. 1H NMR (400 MHz, CDCl3): δ 8.63 (d, J = 5.2 Hz, 2H), 7.91 (d, J = 8.0 Hz, 1H), 7.72 (d, J = 8.1 Hz, 1H), 7.48 (d, J = 7.5 Hz, 1H), 7.41–7.36 (m, 1H), 7.34–7.27 (m, 6H), 5.86 (q, J = 6.9 Hz, 1H), 5.49 (s, 2H), 2.29 (s, 3H), 2.08 (d, J = 7.0 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 167.37, 167.20, 151.31, 149.95, 145.65, 144.64, 142.31, 141.25, 135.57, 134.70, 131.43, 128.49, 127.26, 124.01, 123.72, 123.67, 123.24, 122.82, 120.79, 119.08, 109.09, 42.65, 39.85, 21.91, 17.07.
2-(1-(1-((1-(2-Fluorophenyl)-1H-1,2,3-triazol-4-yl)methyl)-1H-benzo[d]imidazol-2-yl)ethyl)-5-methylisoindoline-1,3-dione (6o’): White solid; m.p. 185–187 °C; Yield: 64%. 1H NMR (400 MHz, CDCl3): δ 7.87 (d, J = 7.4 Hz, 1H), 7.51 (dd, J = 13.7, 7.5 Hz, 2H), 7.41 (d, J = 12.7 Hz, 2H), 7.36–7.23 (m, 5H), 7.17 (dd, J = 17.5, 9.2 Hz, 2H), 5.86 (q, J = 13.6, 6.7 Hz, 1H), 5.46 (s, 2H), 2.31 (s, 3H), 2.06 (d, J = 6.9 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 167.38, 167.27, 152.89 (d, J = 251.9 Hz), 151.32, 145.43, 143.50, 142.27, 135.59, 134.65, 131.66, 130.33 (d, J = 7.7 Hz), 128.76, 125.08, 125.05, 124.67 (d, J = 9.9 Hz), 124.35, 123.78, 123.58, 123.31, 122.67, 122.35 (d, J = 8.6 Hz), 120.65, 117.05 (d, J = 20.0 Hz), 109.32, 42.63, 39.71, 21.96, 17.07.
2-(1-(1-((1-(2,4-Difluorophenyl)-1H-1,2,3-triazol-4-yl)methyl)-1H-benzo[d]imidazol-2-yl)ethyl)-5-methylisoindoline-1,3-dione (6p’): White solid; m.p. 206–208 °C; Yield: 52%. HRMS m/z calcd. (M + H)+ 499.4835, found 499.1699. 1H NMR (400 MHz, CDCl3): δ 7.85 (d, J = 7.5 Hz, 1H), 7.53 (d, J = 7.5 Hz, 1H), 7.47 (d, J = 5.9 Hz, 1H), 7.40 (d, J = 5.8 Hz, 2H), 7.35 (d, J = 7.5 Hz, 1H), 7.31–7.21 (m, 3H), 6.93 (t, J = 8.1 Hz, 2H), 5.84 (q, 1H), 5.45 (s, 2H), 2.35 (s, 3H), 2.05 (d, J = 6.6 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 167.38, 167.27, 154.14, 151.64, 151.32, 144.54 (d, J = 183.5 Hz), 142.27, 135.59, 134.65, 131.66, 130.36, 130.29, 128.76, 125.77 (d, J = 9.8 Hz), 124.72, 124.62, 124.35, 123.78, 123.58, 123.31, 122.67, 122.36 (d, J = 7.5 Hz), 120.65, 117.15, 116.95, 112.46 (d, J = 19.5 Hz), 109.32, 42.63, 39.71, 21.96, 17.07.
2-(1-(1-((1-(2,6-Difluorophenyl)-1H-1,2,3-triazol-4-yl)methyl)-1H-benzo[d]imidazol-2-yl)ethyl)-5-methylisoindoline-1,3-dione (6q’): Green solid; m.p.166–168 °C; Yield: 52%. HRMS m/z calcd. (M + H)+ 499.4835, found 499.1698. 1H NMR (400 MHz, CDCl3): δ 7.87 (s, 1H), 7.60 (d, J = 7.5 Hz, 1H), 7.50 (s, 1H), 7.41 (d, J = 7.9 Hz, 2H), 7.35 (s, 2H), 7.29–7.24 (m, 2H), 7.02 (t, J = 8.4 Hz, 2H), 5.90 (d, J = 6.4 Hz, 1H), 5.52 (s, 2H), 2.41 (s, 3H), 2.10 (d, J = 6.3 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 167.62, 167.51, 156.45 (d, J = 257.1 Hz), 145.71, 134.90, 131.67 (d, J = 18.4 Hz), 128.83, 123.94 (d, J = 17.2 Hz), 123.43, 122.90, 120.52, 114.72, 112.52 (d, J = 20.4 Hz), 109.71, 42.58, 39.63, 29.77, 22.08, 17.14, 14.24.
2-(2-Methyl-1-(1-((1-(perfluorophenyl)-1H-1,2,3-triazol-4-yl)methyl)-1H-benzo[d]imidazol-2-yl)propyl)isoindoline-1,3-dione (6r’): Yellow solid; m.p. 170–172 °C; Yield: 59%. HRMS m/z calcd. (M + H)+ 567.4815, found 567.1564. 1H NMR (400 MHz, CDCl3): δ 7.73 (dd, J = 5.1, 3.1 Hz, 1H), 7.73–7.71 (m, 2H), 7.66–7.61 (m, 2H), 7.60 (s, 1H), 7.38–7.31 (m, 1H), 7.20–7.18 (m, 2H), 5.65 (dd, J = 53.0, 16.7 Hz, 2H), 5.29 (d, J = 10.8 Hz, 1H), 3.64–3.55 (m, 1H), 0.99 (dd, J = 6.4, 4.1 Hz, 6H). 13C NMR (100 MHz, CDCl3): δ 167.80, 150.27, 143.80, 142.54, 134.63, 134.33, 131.34, 124.52, 123.52, 122.70, 120.70, 109.67, 53.23, 38.93, 28.45, 20.87, 19.47.
2-(1-(1-((1-(2-Fluorophenyl)-1H-1,2,3-triazol-4-yl)methyl)-1H-benzo[d]imidazol-2-yl)-2-methylpropyl)isoindoline-1,3-dione (6s’): Yellow solid; m.p. 115–117 °C; Yield: 62%. HRMS m/z calcd. (M + H)+ 495.1937, found 495.5196. 1H NMR (400 MHz, CDCl3): δ 7.92–7.86 (m, 1H), 7.73 (dd, J = 5.3, 3.0 Hz, 2H), 7.64–7.57 (m, 4H), 7.44–7.32 (m, 2H), 7.29–7.14 (m, 4H), 5.63 (dd, J = 55.0, 16.8 Hz, 2H), 5.35 (d, J = 10.8 Hz, 1H), 3.69–3.59 (m, 1H), 1.10 (d, J = 6.6 Hz, 3H), 1.02 (d, J = 6.6 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 167.66, 153.05 (d, J = 251.2 Hz), 150.19, 143.06 (d, J = 115.3 Hz), 134.77, 134.18, 131.27, 130.42 (d, J = 7.8 Hz), 125.20, 125.17, 124.65, 123.52, 122.70, 120.74, 117.02 (d, J = 20.0 Hz), 109.74, 53.09, 39.31, 28.48, 21.08, 19.48.
2-(1-(1-((1-(2,4-Difluorophenyl)-1H-1,2,3-triazol-4-yl)methyl)-1H-benzo[d]imidazol-2-yl)-2-methylpropyl)isoindoline-1,3-dione (6t’): Yellow solid; m.p. 100–102 °C; Yield: 59%. HRMS m/z calcd. (M + H)+ 513.5101, found 513.1841. 1H NMR (400 MHz, CDCl3): δ 7.84 (d, J = 8.0 Hz, 1H), 7.69 (dd, J = 4.0, 3.0 Hz, 2H), 7.62–7.47 (m, 4H), 7.36 (d, J = 4.7 Hz, 1H), 7.30–7.15 (m, 2H), 6.92 (d, J = 6.0 Hz, 2H), 5.59 (dd, J = 51.8, 16.8 Hz, 2H), 5.32 (d, J = 10.8 Hz, 1H), 3.70–3.49 (m, 1H), 1.06 (d, J = 6.3 Hz, 3H), 0.98 (d, J = 6.3 Hz, 3H). 13C NMR (100 MHz, CDCl3): δ 177.92, 167.65, 162.50 (d, J = 253.1 Hz), 153.49 (d, J = 241.9 Hz), 150.15, 143.70, 142.45, 134.76, 134.63, 134.21, 131.25, 126.00 (d, J = 9.9 Hz), 123.47, 122.74, 122.66, 121.43, 120.64, 112.56 (d, J = 19.4 Hz), 109.73, 105.61, 105.37, 105.11, 68.62, 53.62, 53.07, 39.18, 28.43, 27.85, 22.22, 21.04, 19.45.
2-(1-(1-((1-(2,6-Difluorophenyl)-1H-1,2,3-triazol-4-yl)methyl)-1H-benzo[d]imidazol-2-yl)-2-methylpropyl)isoindoline-1,3-dione (6u’): Yellow solid; m.p. 91–93 °C; Yield: 50%. HRMS m/z calcd. (M + H)+ 513.5101, found 513.1866. 1H NMR (400 MHz, CDCl3): δ 7.84 (s, 1H), 7.75 (s, 2H), 7.64 (s, 2H), 7.42 (d, J = 20.8 Hz, 3H), 7.24 (s, 3H), 7.01 (t, J = 7.1 Hz, 2H), 5.66 (dd, J = 63.4, 15.7 Hz, 2H), 5.31 (d, J = 8.6 Hz, 1H), 3.63 (brs, 1H), 0.99 (d, J = 1.4 Hz, 6H). 13C NMR (100 MHz, CDCl3): δ 167.83, 156.60 (d, J = 255.5 Hz), 150.35, 142.78 (d, J = 71.6 Hz), 134.65, 134.33, 131.74, 131.65, 131.55, 131.31, 124.51, 123.59, 122.75, 120.66, 112.55 (d, J = 19.5 Hz), 109.86, 53.16, 39.15, 28.46, 20.85, 19.52.