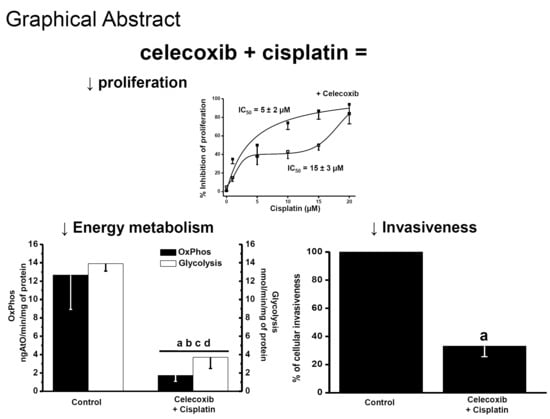
Non-Steroidal Anti-Inflammatory Drugs Increase Cisplatin, Paclitaxel, and Doxorubicin Efficacy against Human Cervix Cancer Cells
Abstract
1. Introduction
2. Results
2.1. Effect of NSAIDs and Canonical Chemotherapy Drugs on HeLa Cell Proliferation
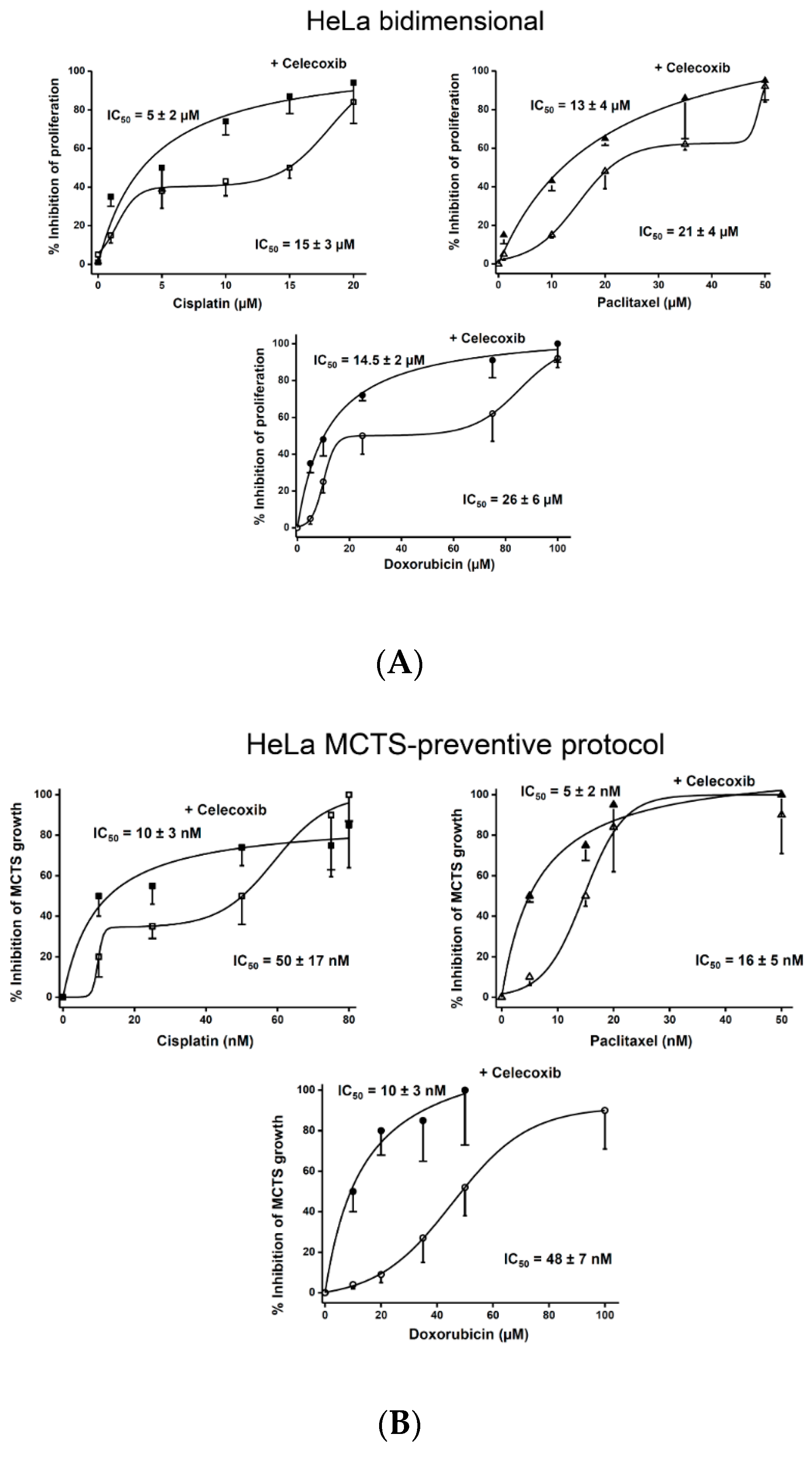
2.2. Synergism of NSAIDs or CasII-Gly with Chemotherapy Anticancer Drugs
2.3. Effect of NSAIDs on the Proliferation IC50 Values of Canonical Chemotherapy Drugs
2.4. Effect of Drug Combination on Growth of Non-Cancer Cells in Bidimensional Culture
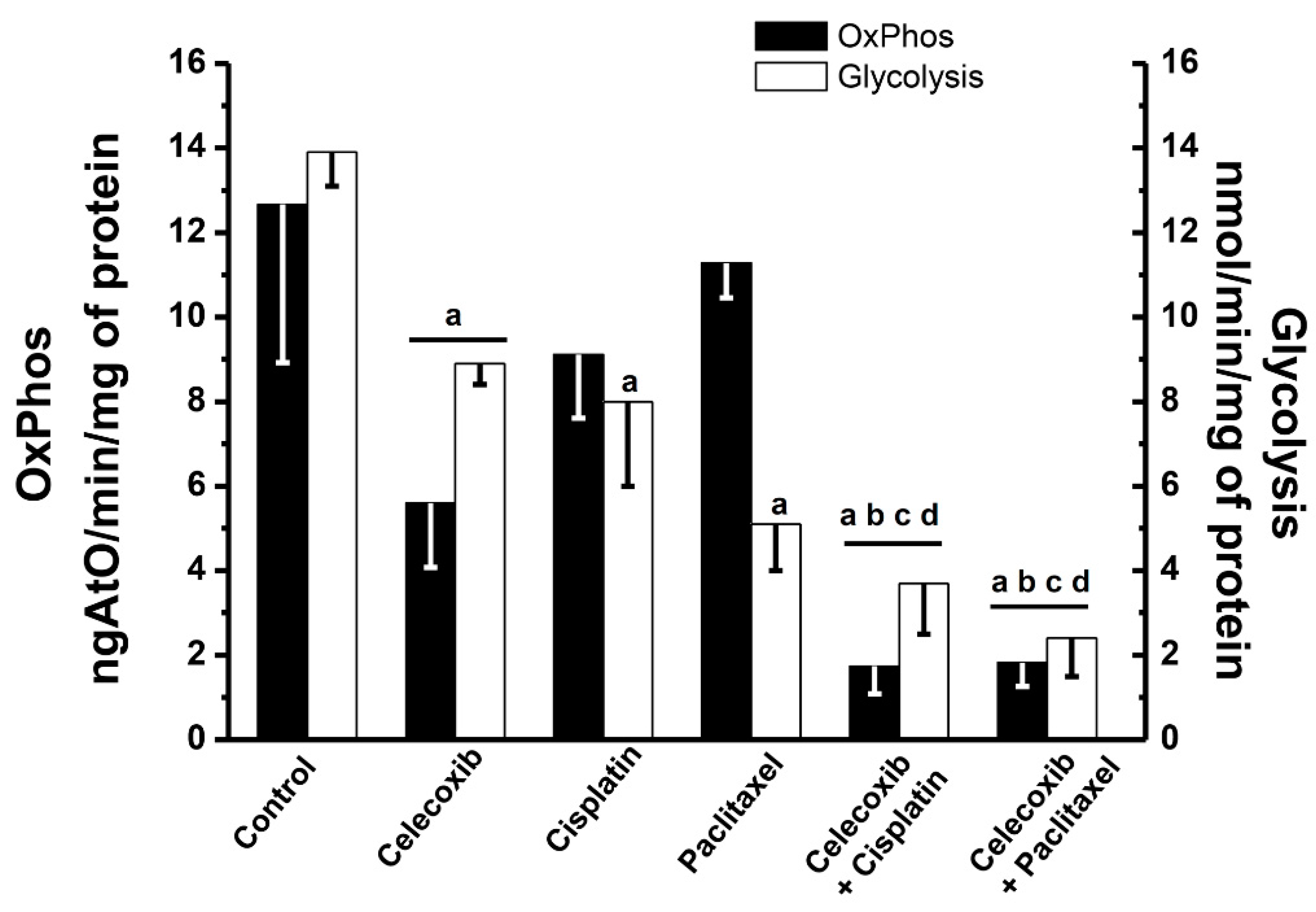
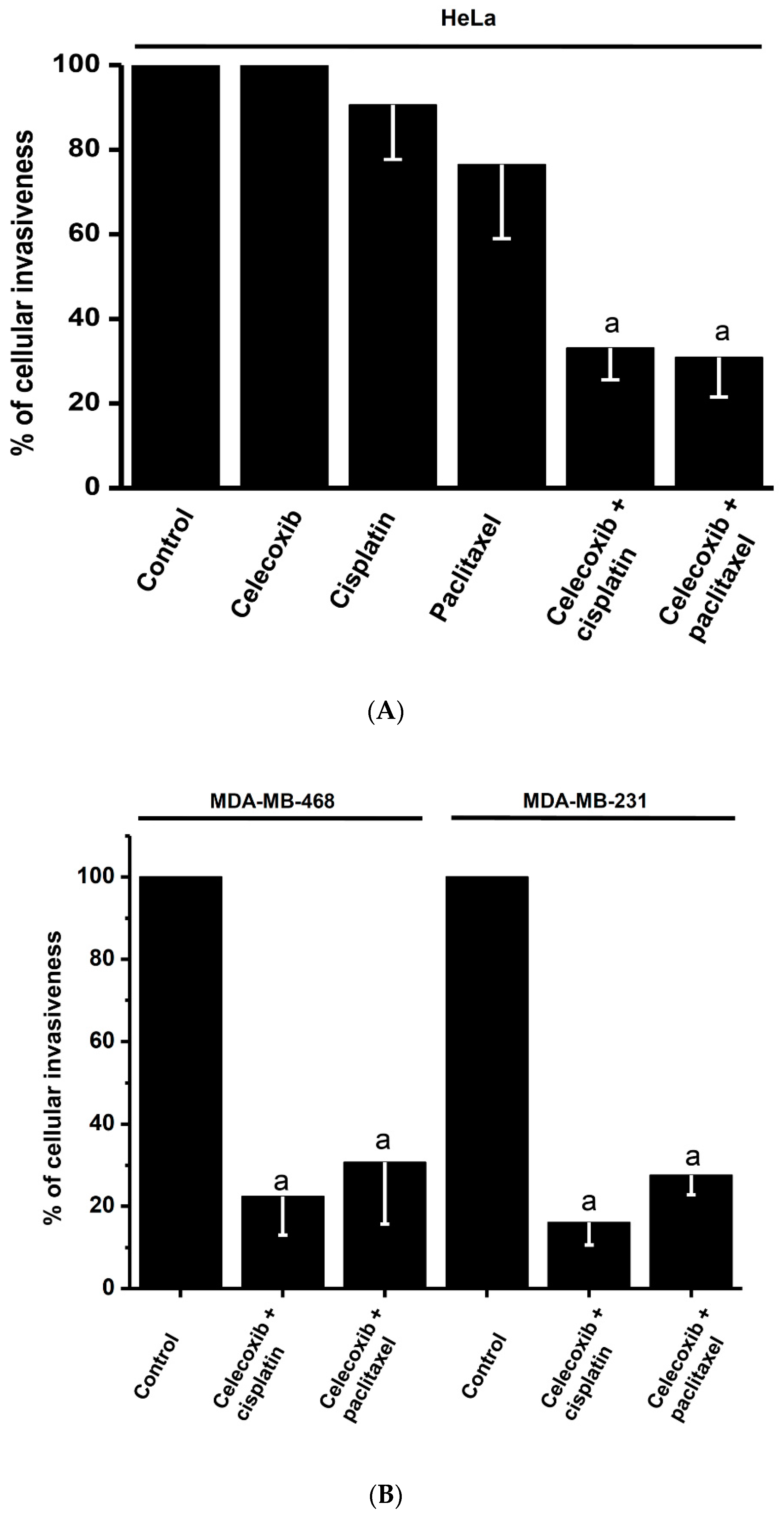
2.5. Effects of Synergistic Drug Combinations on Mitochondrial Function and Invasiveness in Bidimensional HeLa Cells
2.6. Effects of Synergistic Drug Combinations on Cancer Growth, Mitochondrial Function, and Invasiveness in Bidimensional and Tridimensional SiHa and U373 Cells
3. Discussion
3.1. NSAIDs as an Alternative to Decrease Proliferation of Cervical Cancer Cells
3.2. Supra-Additive Effects of NSAIDs and Paclitaxel or Cisplatin Combinations on Cancer Cell Growth, Energy Metabolism, and Invasiveness
3.3. Infra-Additive Effect of NSAIDs and Carboplatin or Cyclophosphamide on Cancer Cell Growth
4. Materials and Methods
4.1. Drugs
4.2. Cancer Cell Lines
4.3. Determination of Drug IC50 Values in Bidimensional Cultures
4.4. Multi-Cellular Tumor Spheroid (MCTS) Cultures
4.5. Determination of Drug IC50 Values in Tridimensional Spheroid Cultures
4.6. Analysis of Drug Toxicity by Assessing Bliss-Type Additivism, Resistance Index Ratio (RI), and Combination Index (CI) Value
4.7. Therapeutic Index Ratio (TI Ratio)
4.8. Determination of Glycolytic and OxPhos Fluxes in Bidimensional Cancer and Non-Cancer Cells Exposed to Drug Combinations
4.9. Cell Invasiveness Assays
4.10. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ambili, R. Toxicities of anticancer drugs and its management. Int. J. Basic Clin. Pharmacol. 2012, 1, 2–12. [Google Scholar] [CrossRef]
- Cadron, I.; Van Gorp, T.; Amant, F.; Leunen, K.; Neven, P.; Vergote, I. Chemotherapy for recurrent cervical cancer. Gynecol. Oncol. 2007, 107, S113–S118. [Google Scholar] [CrossRef]
- Pectasides, D.; Mylonakis, N.; Farmakis, D. Irinotecan and gemcitabine in patients with advanced non-small cell lung cancer, previously treated with cisplatin-based chemotherapy. A phase II study. Anticancer Res. 2003, 23, 4205–4211. [Google Scholar]
- Pectasides, D.; Pectasides, M.; Farmakis, D.; Nikolaou, M.; Koumpou, M.; Kostopoulou, V.; Mylonakis, N. Testicular function in patients with testicular cancer treated with bleomycin-etoposide-carboplatin (BEC(90)) combination chemotherapy. Eur. Urol. 2004, 45, 187–193. [Google Scholar] [CrossRef]
- Guardiola, E.; Pivot, X.; Tchicknavorian, X.; Magne, N.; Otto, J.; Thyss, A.; Schneider, M. Combination of cisplatin-doxorubicin-cyclophosphamide in adenocarcinoma of unknown primary site: A phase II trial. Am. J. Clin. Oncol. 2001, 24, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Loehrer, P.J., Sr.; Einhorn, L.H.; Elson, P.J.; Crawford, E.D.; Kuebler, P.; Tannock, I.; Raghavan, D.; Stuart-Harris, R.; Sarosdy, M.F.; Lowe, B.A.; et al. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: A cooperative group study. J. Clin. Oncol. 1992, 10, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.S.; Patel, C.J. A standard database for drug repositioning. Sci. Data 2017, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Novac, N. Challenges and opportunities of drug repositioning. Trends Pharmacol. Sci. 2013, 34, 267–272. [Google Scholar] [CrossRef]
- McCormack, P.L. Celecoxib: A review of its use for symptomatic relief in the treatment of osteoarthritis, rheumatoid arthritis and ankylosing spondylitis. Drugs 2011, 71, 2457–2489. [Google Scholar] [CrossRef]
- Bensen, W.G.; Fiechtner, J.J.; McMillen, J.I.; Zhao, W.W.; Yu, S.S.; Woods, E.M.; Hubbard, R.C.; Isakson, P.C.; Verburg, K.M.; Geis, G.S. Treatment of osteoarthritis with celecoxib, a cyclooxygenase-2 inhibitor: A randomized controlled trial. Mayo Clin. Proc. 1999, 74, 1095–1105. [Google Scholar] [CrossRef]
- Lev-Ari, S.; Strier, L.; Kazanov, D.; Madar-Shapiro, L.; Dvory-Sobol, H.; Pinchuk, I.; Marian, B.; Lichtenberg, D.; Arber, N. Celecoxib and curcumin synergistically inhibit the growth of colorectal cancer cells. Clin. Cancer Res. 2005, 11, 6738–6744. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Velázquez, S.C.; Robledo-Cadena, D.X.; Hernández-Reséndiz, I.; Gallardo-Pérez, J.C.; Moreno-Sánchez, R.; Rodríguez-Enríquez, S. Energy metabolism drugs block triple negative breast metastatic cancer cell phenotype. Mol. Pharm. 2018, 15, 2151–2164. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, R.; Rodríguez-Enríquez, S.; Pacheco-Velázquez, S.C.; Bortnik, V.; Moreno-Sánchez, R.; Ralph, S. Celecoxib inhibits mitochondrial O2 consumption, promoting ROS dependent death of murine and human metastatic cancer cells via the apoptotic signalling pathway. Biochem. Pharmacol. 2018, 154, 318–334. [Google Scholar] [CrossRef] [PubMed]
- Tsujii, M.; Kawano, S.; DuBois, R.N. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc. Natl. Acad. Sci. USA 1997, 94, 3336–3340. [Google Scholar] [CrossRef]
- Ralph, S.J.; Pritchard, R.; Rodríguez-Enríquez, S.; Moreno-Sánchez, R.; Ralph, R.K. Hitting the bull’s-eye in metastatic cancers-NSAIDs elevate ROS in mitochondria, inducing malignant cell death. Pharmaceuticals 2015, 8, 62–106. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Qin, Y.; Chen, X.; Li, D.; Wang, Q.; Zheng, H.; Gu, L.; Deng, C.; Xue, Y.; Zhu, D.; et al. Combination of celecoxib and PD184161 exerts synergistic inhibitory effects on gallbladder cancer cell proliferation. Oncol. Lett. 2017, 13, 3850–3858. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gowda, R.; Sharma, A.; Robertson, G.P. Synergistic inhibitory effects of Celecoxib and Plumbagin on melanoma tumor growth. Cancer Lett. 2017, 385, 243–250. [Google Scholar] [CrossRef]
- Liu, B.; Yan, S.; Qu, L.; Zhu, J. Celecoxib enhances anticancer effect of cisplatin and induces anoikis in osteosarcoma via PI3K/Akt pathway. Cancer Cell Int. 2017, 17, 1. [Google Scholar] [CrossRef]
- Boasberg, P.D.; Redfern, C.H.; Daniels, G.A.; Bodkin, D.; Garrett, C.R.; Ricart, A.D. Pilot study of PD-0325901 in previously treated patients with advanced melanoma, breast cancer, and colon cancer. Cancer Chemother. Pharmacol. 2011, 68, 547–552. [Google Scholar] [CrossRef]
- Haura, E.B.; Ricart, A.D.; Larson, T.G.; Stella, P.J.; Bazhenova, L.; Miller, V.A.; Cohen, R.B.; Eisenberg, P.D.; Selaru, P.; Wilner, K.D.; et al. A phase II study of PD-0325901, an oral MEK inhibitor, in previously treated patients with advanced non-small cell lung cancer. Clin. Cancer Res. 2010, 16, 2450–2457. [Google Scholar] [CrossRef]
- Arúajo, A.M.; Mendez, J.C.; Coelho, A.L.; Sousa, B.; Barata, F.; Figueiredo, A.; Amaro, T.; Azevedo, I.; Soares, M. Phase II study of celecoxib with cisplatin plus etoposide in extensive-stage small cell lung cancer. Cancer Investig. 2009, 27, 391–396. [Google Scholar] [CrossRef] [PubMed]
- El-Rayes, B.F.; Zalupski, M.M.; Shields, A.F.; Ferris, A.M.; Vaishampayan, U.; Heilbrun, L.K.; Venkatramannamoorthy, R.; Adsay, V.; Philip, P.A. A phase II study of celecoxib, gemcitabine, and cisplatin in advanced pancreatic cancer. Investig. New Drugs 2005, 23, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, T. Clinical application of drug delivery systems in cancer chemotherapy: Review of the efficacy and side effects of approved drugs. Biol. Pharm. Bull. 2013, 36, 715–718. [Google Scholar] [CrossRef]
- Backhus, L.M.; Petasis, N.A.; Uddin, J.; Schönthal, A.H.; Bart, R.D.; Lin, Y.; Starnes, V.A.; Bremner, R.M. Dimethyl celecoxib as a novel non-cyclooxygenase 2 therapy in the treatment of non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2005, 130, 1406–1412. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cianchi, F.; Cortesini, C.; Magnelli, L.; Fanti, E.; Papucci, L.; Schiavone, N.; Messerini, L.; Vanacci, A.; Capaccioli, S.; Perna, F.; et al. Inhibition of 5-lipoxygenase by MK886 augments the antitumor activity of celecoxib in human colon cancer cells. Mol. Cancer Ther. 2006, 5, 2716–2726. [Google Scholar] [CrossRef]
- Dai, Z.J.; Ma, X.B.; Kang, H.F.; Gao, J.; Min, W.L.; Guan, H.T.; Yan, D.; Wang-Feng, L.; Xi-Jing, W. Antitumor activity of the selective cyclooxygenase-2 inhibitor, celecoxib, on breast cancer in Vitro and in Vivo. Cancer Cell. Int. 2012, 12, 53. [Google Scholar] [CrossRef]
- Kardosh, A.; Soriano, N.; Liu, Y.T.; Uddin, J.; Petasis, N.A.; Hofman, F.M.; Chen, T.C.; Schönthal, A.H. Multitarget inhibition of drug-resistant multiple myeloma cell lines by dimethyl-celecoxib (DMC), a non-COX-2 inhibitory analog of celecoxib. Blood 2005, 106, 4330–4338. [Google Scholar] [CrossRef]
- Kardosh, A.; Wang, W.; Uddin, J.; Petasis, N.A.; Hofman, F.M.; Chen, T.C.; Schönthal, A.H. Dimethyl-celecoxib (DMC), a derivative of celecoxib that lacks cyclooxygenase-2-inhibitory function, potently mimics the anti-tumor effects of celecoxib on Burkitt’s lymphoma in vitro and in vivo. Cancer Biol. Ther. 2005, 4, 571–582. [Google Scholar] [CrossRef]
- Zhang, B.; Yan, Y.; Li, Y.; Zhang, D.; Zeng, J.; Wang, L.; Wang, M.; Lin, N. Dimethyl celecoxib sensitizes gastric cancer cells to ABT-737 via AIF nuclear translocation. J. Cell Mol. Med. 2016, 20, 2148–2159. [Google Scholar] [CrossRef]
- Schönthal, A.H. Antitumor properties of dimethyl-celecoxib, a derivative of celecoxib that does not inhibit cyclooxygenase-2: Implications for glioma therapy. Neurosurg. Focus 2006, 20, E2. [Google Scholar] [CrossRef]
- Gaffney, D.K.; Winter, K.; Dicker, A.P.; Miller, B.; Eifel, P.J.; Ryu, J.; Avizonis, V.; Fromm, M.; Small, W.; Greven, K. Efficacy and patterns of failure for locally advanced cancer of the cervix treated with celebrex (celecoxib) and chemoradiotherapy in RTOG 0128. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Carver, K.; Ming, X.; Juliano, R.L. Multicellular tumor spheroids as a model for assessing delivery of oligonucleotides in three dimensions. Mol. Ther. Nucleic Acids. 2014, 3, e153. [Google Scholar] [CrossRef] [PubMed]
- Katt, M.E.; Placone, A.L.; Wong, A.D.; Xu, Z.S.; Searson, P.C. In vitro tumor models: Advantages, disadvantages, variables, and selecting the right platform. Front. Bioeng. Biotechnol. 2016, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Hirschhaeuser, F.; Menne, H.; Dittfeld, C.; West, J.; Mueller-Klieser, W.; Kunz-Schughart, L.A. Multicellular tumor spheroids: An underestimated tool is catching up again. J. Biotechnol. 2010, 148, 3–15. [Google Scholar] [CrossRef]
- Senavirathna, L.K.; Fernando, R.; Maples, D.; Zheng, Y.; Polf, J.C.; Ranjan, A. Tumor Spheroids as an in vitro model for determining the therapeutic response to proton beam radiotherapy and thermally sensitive nanocarriers. Theranostics 2013, 3, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.W.; Lin, S.C.; Chen, K.F.; Lai, Y.Y.; Tsai, S.J. Induction of pyruvate dehydrogenase kinase-3 by hypoxia-inducible factor-1 promotes metabolic switch and drug resistance. J. Biol. Chem. 2008, 283, 28106–28114. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Gong, F.; Chen, Y.; Jiang, Y.; Liu, J.; Yu, M.; Zhang, S.; Wang, M.; Xiao, G.; Liao, H. Autophagy promotes paclitaxel resistance of cervical cancer cells: Involvement of Warburg effect activated hypoxia-induced factor 1-α-mediated signaling. Cell Death Dis. 2014, 5, e1367. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Zhou, Q.; Chai, W. Suppression of STN1 enhances the cytotoxicity of chemotherapeutic agents in cancer cells by elevating DNA damage. Oncol. Lett. 2016, 12, 800–808. [Google Scholar] [CrossRef]
- Pereira, P.; Berisha, N.; Bhupathiraju, N.; Fernandes, R.; Tomé, J.; Drain, C.M. Cancer cell spheroids are a better screen for the photodynamic efficiency of glycosylated photosensitizers. PLoS ONE 2017, 12, e0177737. [Google Scholar] [CrossRef]
- Lee, J.M.; Park, D.Y.; Yang, L.; Kim, E.J.; Ahrberg, C.D.; Lee, K.B.; Chung, B.G. Generation of uniform-sized multicellular tumor spheroids using hydrogel microwells for advanced drug screening. Sci. Rep. 2018, 8, 17145. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Esquivel, L.; Marín-Hernández, A.; Pavón, N.; Carvajal, K.; Moreno-Sánchez, R. Cardiotoxicity of copper-based antineoplastic drugs casiopeinas is related to inhibition of energy metabolism. Toxicol. Appl. Pharmacol. 2006, 212, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Marín-Hernández, A.; Gracia-Mora, I.; Ruiz-Ramírez, L.; Moreno-Sánchez, R. Toxic effects of copper-based antineoplastic drugs (Casiopeinas) on mitochondrial functions. Biochem. Pharmacol. 2003, 65, 1979–1989. [Google Scholar] [CrossRef]
- Hernández-Reséndiz, I.; Román-Rosales, A.; García-Villa, E.; López-Macay, A.; Pineda, E.; Saavedra, E.; Gallardo-Pérez, J.C.; Alvarez-Ríos, E.; Gariglio, P.; Moreno-Sánchez, R.; et al. Dual regulation of energy metabolism by p53 in human cervix and breast cancer cells. Biochim. Biophys. Acta 2015, 1853, 3266–3278. [Google Scholar] [CrossRef]
- Rodríguez-Enríquez, S.; Marín-Hernández, A.; Gallardo-Pérez, J.C.; Carreño-Fuentes, L.; Moreno-Sánchez, R. Targeting of cancer energy metabolism. Mol. Nutr. Food Res. 2009, 53, 29–48. [Google Scholar] [CrossRef]
- Sobolewski, C.; Rhim, J.; Legrand, N.; Muller, F.; Cerella, C.; Mack, F.; Chateauvieux, S.; Kim, J.G.; Yoon, A.Y.; Kim, K.W.; et al. 2,5-Dimethyl-celecoxib inhibits cell cycle progression and induces apoptosis in human leukemia cells. J. Pharmacol. Exp. Ther. 2015, 355, 308–328. [Google Scholar] [CrossRef]
- Mittler, F.; Obeïd, P.; Rulina, A.V.; Haguet, V.; Gidrol, X.; Balakirev, M.Y. High-content monitoring of drug effects in a 3D spheroid model. Front. Oncol. 2017, 7, 293. [Google Scholar] [CrossRef]
- Borisy, A.A.; Elliott, P.J.; Hurst, N.W.; Lee, M.S.; Lehar, J.; Price, E.R.; Serbedzija, G.; Zimmermann, G.R.; Foley, G.R.; Stockwell, B.R.; et al. Systematic discovery of multicomponent therapeutics. Proc. Natl. Acad. Sci. USA 2003, 100, 7977–7982. [Google Scholar] [CrossRef]
- Deepa, P.R.; Vandhana, S.; Jayanthi, U.; Krishnakumar, S. Therapeutic and toxicologic evaluation of anti-lipogenic agents in cancer cells compared with non-neoplastic cells. Basic Clin. Pharmacol. Toxicol. 2012, 110, 494–503. [Google Scholar] [CrossRef]
- Bai, H.; Chen, H.; Ren, C. Suppression of growth of Hela, EJ, SK-OV-3 and MDA-MB-231 cells by recombinant human NK4. Chin. J. Cancer Res. 2009, 21, 28–31. [Google Scholar] [CrossRef]
- LeBleu, V.S.; O’Connell, J.T.; Gonzalez Herrera, K.N.; Wikman, H.; Pantel, K.; Haigis, M.C.; de Carvalho, F.M.; Damascena, A.; Domingos Chinen, L.T.; Rocha, R.M.; et al. PGC-1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat. Cell Biol. 2014, 16, 992–1003. [Google Scholar] [CrossRef]
- Xia, C.; Chen, R.; Chen, J.; Qi, Q.; Pan, Y.; Du, L.; Xiao, G.; Jiang, S. Combining metformin and nelfinavir exhibits synergistic effects against the growth of human cervical cancer cells and xenograft in nude mice. Sci. Rep. 2017, 7, 43373. [Google Scholar] [CrossRef] [PubMed]
- Koraneekit, A.; Limpaiboon, T.; Sangka, A.; Boonsiri, P.; Daduang, S.; Daduang, J. Synergistic effects of cisplatin-caffeic acid induces apoptosis in human cervical cancer cells via the mitochondrial pathways. Oncol. Lett. 2018, 15, 7397–7402. [Google Scholar] [CrossRef]
- Jiang, B.; Sun, R.; Fang, S.; Qin, C.; Pan, X.; Peng, L.; Li, Y.; Li, G. Lnc-CC3 increases metastasis in cervical cancer by increasing Slug expression. Oncotarget 2016, 7, 41650–41661. [Google Scholar] [CrossRef]
- Advani, R.H.; Hong, F.; Horning, S.J.; Kahl, B.S.; Manola, J.; Swinnen, L.J.; Habermann, T.M.; Ganjoo, K. Cardiac toxicity associated with bevacizumab (Avastin) in combination with CHOP chemotherapy for peripheral T cell lymphoma in ECOG 2404 trial. Leuk. Lymphoma 2012, 53, 718–720. [Google Scholar] [CrossRef][Green Version]
- Finkel, K.W.; Foringer, J.R. Renal disease in patients with cancer. Nat. Clin. Pract. Nephrol. 2007, 3, 669–678. [Google Scholar] [CrossRef]
- Fridrik, M.A.; Jaeger, U.; Petzer, A.; Willenbacher, W.; Keil, F.; Lang, A.; Andel, J.; Burgstaller, S.; Krieguer, O.; Oberaigner, O.; et al. Cardiotoxicity with rituximab, cyclophosphamide, non-pegylated liposomal doxorubicin, vincristine and prednisolone compared to rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone in frontline treatment of patients with diffuse large B-cell lymphoma: A randomised phase-III study from the Austrian Cancer Drug Therapy Working Group [Ar be its geme in schaft Medikamentöse Tumor therapie AGMT](NHL-14). Eur. J. Cancer 2016, 58, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Boccia, R.V.; Cosgriff, T.M.; Headley, D.L.; Badarinath, S.; Dakhil, S.R. A phase II trial of FOLFOX6 and cetuximab in the first-line treatment of patients with metastatic colorectal cancer. Clin. Colorectal Cancer 2010, 9, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Spigel, D.R.; Greco, F.A.; Thompson, D.S.; Webb, C.; Rubinsak, J.; Inhorn, R.C.; Reeves, J., Jr.; Vazquez, E.R.; Lane, C.M.; Burris, H.A., 3rd; et al. Phase II study of cetuximab, docetaxel, and gemcitabine in patients with previously untreated advanced non-small-cell lung cancer. Clin. Lung Cancer 2010, 11, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Bafaloukos, D.; Linardou, H.; Aravantinos, G.; Papadimitriou, C.; Bamias, A.; Fountzilas, G.; Kalofonos, H.P.; Kosmidis, P.; Timotheadou, E.; Makatsoris, T.; et al. A randomized phase II study of carboplatin plus pegylated liposomal doxorubicin versus carboplatin plus paclitaxel in platinum sensitive ovarian cancer patients: A hellenic cooperative oncology group study. BMC Med. 2010, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Kunitoh, H.; Tamura, T.; Shibata, T.; Nakagawa, K.; Takeda, K.; Nishiwaki, Y.; Osaki, Y.; Noda, K.; Yokoyama, A.; Saijo, N. JCOG Lung Cancer Study Group, Tokyo, Japan. A phase-II trial of dose-dense chemotherapy in patients with disseminated thymoma: Report of a Japan Clinical Oncology Group trial (JCOG 9605). Br. J. Cancer 2009, 101, 1549–1554. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, A.J.; Reni, M.; Foppoli, M.; Martelli, M.; Pangalis, G.A.; Frezzato, M.; Cabras, M.G.; Fabbri, A.; Corazzelli, G.; Ilariucci, F.; et al. International Extranodal Lymphoma Study Group (IELSG). High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma: A randomised phase 2 trial. Lancet 2009, 374, 1512–1520. [Google Scholar] [CrossRef]
- Stinchcombe, T.E.; Bradford, D.S.; Hensing, T.A.; LaRocca, R.V.; Saleh, M.; Evans, T.; Bakri, K.; Socinski, M.A. A multicenter phase II trial of carboplatin and cetuximab for treatment of advanced nonsmall cell lung cancer. Cancer Investig. 2010, 28, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Gayvert, K.M.; Madhukar, N.S.; Elemento, O. A Data-driven approach to predicting successes and failures of clinical trials. Cell. Chem. Biol. 2016, 23, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Shimamura, T.; Hyodo, I.; Koizumi, W.; Doi, T.; Narahara, H.; Komatsu, Y.; Kato, T.; Saitoh, S.; Akiya, T.; et al. Phase I/II study of docetaxel and S-1 in patients with advanced gastric cancer. Br. J. Cancer 2006, 94, 1803–1808. [Google Scholar] [CrossRef]
- Goldberg, M.A.; Antin, J.H.; Guinan, E.C.; Rappeport, J.M. Cyclophosphamide cardiotoxicity: An analysis of dosing as a risk factor. Blood 1986, 68, 1114–1148. [Google Scholar] [CrossRef]
- Ichikawa, Y.; Ghanefar, M.; Bayeva, M.; Wu, R.; Khechaduri, A.; Naga, S.V.; Mutharasan, R.K.; Naik, T.J.; Ardehali, H. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J. Clin. Investig. 2014, 124, 617–630. [Google Scholar] [CrossRef]
- Mohebali, D.; Matos, J.; Chang, J.D. Gemcitabine induced cardiomyopathy: A case of multiple hit cardiotoxicity. ESC Heart Fail. 2017, 4, 71–74. [Google Scholar] [CrossRef]
- Shek, T.W.; Luk, I.S.; Ma, L.; Cheung, K.L. Paclitaxel-induced cardiotoxicity. An ultrastructural study. Arch. Pathol. Lab. Med. 1996, 120, 89–91. [Google Scholar]
- Gunter, B.R.; Butler, K.A.; Wallace, R.L.; Smith, S.M.; Harirforoosh, S. Non-steroidal anti-inflammatory drug-induced cardiovascular adverse events: A meta-analysis. J. Clin. Pharm. Ther. 2017, 42, 27–38. [Google Scholar] [CrossRef]
- Chuang, H.C.; Kardosh, A.; Gaffney, K.J.; Petasis, N.A.; Schönthal, A.H. COX-2 inhibition is neither necessary nor sufficient for celecoxib to suppress tumor cell proliferation and focus formation in vitro. Mol. Cancer 2008, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Wartenberg, M.; Fischer, K.; Hescheler, J.; Sauer, H. Redox regulation of P-glycoprotein-mediated multidrug resistance in multicellular prostate tumor spheroids. Int. J. Cancer 2000, 85, 267–274. [Google Scholar] [CrossRef]
- Baek, N.; Seo, O.W.; Kim, M.; Hulme, J.; An, S.S. Monitoring the effects of doxorubicin on 3D-spheroid tumor cells in real-time. OncoTargets Ther. 2016, 9, 7207–7218. [Google Scholar] [CrossRef] [PubMed]
- Gierse, J.K.; Koboldt, C.M.; Walker, M.C.; Seibert, K.; Isakson, P.C. Kinetic basis for selective inhibition of cyclo-oxygenase. Biochem. J. 1999, 339, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Enríquez, S.; Gallardo-Pérez, J.C.; Avilés-Salas, A.; Marín-Hernández, A.; Carreño-Fuentes, L.; Maldonado-Lagunas, V.; Moreno-Sánchez, R. Energy metabolism transition in multi-cellular human tumor spheroids. J. Cell Physiol. 2008, 216, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Zu, X.L.; Guppy, M. Cancer metabolism: Facts, fantasy, and fiction. Biochem. Biophys. Res. Commun. 2004, 313, 459–465. [Google Scholar] [CrossRef]
- Moreno-Sánchez, R.; Saavedra, E.; Rodríguez-Enríquez, S.; Gallardo-Pérez, J.C.; Quezada, H.; Westerhoff, H.V. Metabolic control analysis indicates a change of strategy in the treatment of cancer. Mitochondrion 2010, 10, 626–639. [Google Scholar] [CrossRef] [PubMed]
- Tallarida, R.J. Drug synergism: Its detection and applications. J. Pharmacol. Exp. Ther. 2001, 298, 865–872. [Google Scholar]
- Foucquier, J.; Guedj, M. Analysis of drug combinations: Current methodological landscape. Pharmacol. Res. Perspect. 2015, 3, e00149. [Google Scholar] [CrossRef]
- Lehár, J.; Krueger, A.S.; Avery, W.; Heilbut, A.M.; Johansen, L.M.; Price, E.R.; Rickles, R.J.; Short, G.F., 3rd; Staunton, J.E.; Jin, X.; et al. Synergistic drug combinations tend to improve therapeutically relevant selectivity. Nat. Biotechnol. 2009, 27, 659–666. [Google Scholar] [CrossRef]
- Pikman, Y.; Alexe, G.; Roti, G.; Conway, A.S.; Furman, A.; Lee, E.S.; Place, A.E.; Kim, S.; Saran, C.; Modiste, R.; et al. Synergistic drug combinations with a CDK4/6 inhibitor in t-cell acute lymphoblastic leukemia. Clin. Cancer Res. 2017, 23, 1012–1024. [Google Scholar] [CrossRef]
- Davila-Manzanilla, S.G.; Figueroa-de-Paz, Y.; Mejia, C.; Ruiz-Azuara, L. Synergistic effects between a copper-based metal Casiopeína III-ia and cisplatin. Eur. J. Med. Chem. 2017, 129, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, S.H.; Song, Y.C.; Song, Y.S. Celecoxib potentiates the anticancer effect of cisplatin on vulvar cancer, cells independently of cyclooxygenase. Ann. N. Y. Acad. Sci. 2009, 1171, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Kuhar, M.; Imran, S.; Singh, N. Celecoxib enhances the chemotherapeutic response of cisplatin and TNF-α in SiHa cells through reactive oxygen species-mediated mitochondrial pathway. Int. J. Biomed. Sci. 2007, 3, 176–184. [Google Scholar]
- Han, Y.; Chen, P.; Zhang, Y.; Lu, W.; Ding, W.; Luo, Y.; Wen, S.; Xu, R.; Liu, P.; Huang, P. Synergy between auranofin and celecoxib against colon cancer in vitro and in vivo trough a novel redox mediated mechanism. Cancers 2019, 11, 931. [Google Scholar] [CrossRef]
- Mutter, R.; Lu, B.; Carbone, D.P.; Csiki, I.; Moretti, L.; Johnson, D.H.; Morrow, J.D.; Sandler, A.B.; Shyr, Y.; Ye, F.; et al. A phase II study of celecoxib in combination with paclitaxel, carboplatin, and radiotherapy for patients with inoperable stage IIIA/B non-small cell lung cancer. Clin. Cancer Res. 2009, 15, 2158–2165. [Google Scholar] [CrossRef]
- Cervello, M.; Bachvarov, D.; Lampiasi, N.; Cusimano, A.; Azzolina, A.; McCubrey, J.A.; Montalto, G. Novel combination of sorafenib and celecoxib provides synergistic anti-proliferative and pro-apoptotic effects in human liver cancer cells. PLoS ONE 2013, 8, e65569. [Google Scholar] [CrossRef] [PubMed]
- Bassiouny, A.R.; Zaky, A.; Neenaa, H.M. Synergistic effect of celecoxib on 5-fluorouracil-induced apoptosis in hepatocellular carcinoma patients. Ann. Hepatol. 2010, 9, 410–418. [Google Scholar] [CrossRef]
- Moreno-Sánchez, R.; Hernández-Esquivel, L.; Rivero-Segura, N.A.; Marín-Hernández, A.; Neuzil, J.; Ralph, S.J.; Rodríguez-Enríquez, S. Reactive oxygen species are generated by the respiratory complex II--evidence for lack of contribution of the reverse electron flow in complex I. FEBS J. 2013, 280, 927–938. [Google Scholar] [CrossRef]
- Rodríguez-Enríquez, S.; Hernández-Esquivel, L.; Marín-Hernández, A.; Dong, L.F.; Akporiaye, E.T.; Neuzil, J.; Ralph, S.J.; Moreno-Sánchez, R. Molecular mechanism for the selective impairment of cancer mitochondrial function by a mitochondrially targeted vitamin E analogue. Biochim. Biophys. Acta 2012, 1817, 1597–1607. [Google Scholar] [CrossRef]
- Chiang, S.L.; Velmurugan, B.K.; Chung, C.M.; Lin, S.H.; Wang, Z.H.; Hua, C.H.; Tsai, M.H.; Kuo, T.M.; Yeh, K.T.; Chang, P.Y.; et al. Preventive effect of celecoxib use against cancer progression and occurrence of oral squamous cell carcinoma. Sci. Rep. 2017, 7, 6235. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, Y.; Zhou, Z.; Huang, M.; Deng, W.; Wang, Y.; Zhou, X.; Chen, L.; Li, Y.; Zeng, T.; et al. Celecoxib inhibits the epithelial-to-mesenchymal transition in bladder cancer via the miRNA-145/TGFBR2/Smad3 axis. Int. J. Mol. Med. 2019, 44, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Tang, X.H.; Xie, Y.K. Paclitaxel combined with harmine inhibits the migration and invasion of gastric cancer cells through downregulation of cyclooxygenase-2 expression. Oncol. Lett. 2015, 10, 1649–1654. [Google Scholar] [CrossRef]
- André, N.; Rome, A.; Coze, C.; Padovani, L.; Pasquier, E.; Camoin, L.; Gentet, J.C. Metronomic etoposide/cyclophosphamide/celecoxib regimen given to children and adolescents with refractory cancer: A preliminary monocentric study. Clin. Ther. 2008, 30, 1336–1340. [Google Scholar] [CrossRef]
- Dragovich, T.; Burris, H., 3rd; Loehrer, P.; Von Hoff, D.D.; Chow, S.; Stratton, S.; Green, S.; Obregon, Y.; Alvarez, I.; Gordon, M. Gemcitabine plus celecoxib in patients with advanced or metastatic pancreatic adenocarcinoma: Results of a phase II trial. Am. J. Clin. Oncol. 2008, 31, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Berenbaum, M.C. Criteria for analyzing interactions between biologically active agents. Adv. Cancer Res. 1981, 35, 269–335. [Google Scholar] [CrossRef]
- Goldoni, M.; Johansson, C. A mathematical approach to study combined effects of toxicants in vitro: Evaluation of the Bliss independence criterion and the Loewe additivity model. Toxicol. In Vitro 2007, 21, 759–769. [Google Scholar] [CrossRef]
- Longley, D.B.; Allen, W.L.; McDermott, U.; Wilson, T.R.; Latif, T.; Boyer, J.; Lynch, M.; Johnston, P.G. The roles of thymidylate synthase and p53 in regulating Fas-mediated apoptosis in response to antimetabolites. Clin. Cancer Res. 2004, 10, 3562–3571. [Google Scholar] [CrossRef][Green Version]
- Marín-Hernández, A.; Rodríguez-Enríquez, S.; Vital-González, P.A.; Flores-Rodríguez, F.L.; Macías-Silva, M.; Sosa-Garrocho, M.; Moreno-Sánchez, R. Determining and understanding the control of glycolysis in fast-growth tumor cells. Flux control by an over-expressed but strongly product-inhibited hexokinase. FEBS J. 2006, 273, 1975–1988. [Google Scholar] [CrossRef]
- Bergmeyer, H.U. Methods of Enzymatic Analysis; Verlag Chemie: Weinheim, Germany, 1983; p. 565. [Google Scholar] [CrossRef]
- Rodríguez-Enríquez, S.; Torres-Márquez, M.E.; Moreno-Sánchez, R. Substrate oxidation and ATP supply in AS-30D hepatoma cells. Arch. Biochem. Biophys. 2000, 375, 21–30. [Google Scholar] [CrossRef]
- Nakashima, R.A.; Paggi, M.G.; Pedersen, P.L. Contributions of glycolysis and oxidative phosphorylation to adenosine 5’-triphosphate production in AS-30D hepatoma cells. Cancer Res. 1984, 44, 5702–5706. [Google Scholar] [PubMed]
- Gallardo-Pérez, J.C.; Adán-Ladrón de Guevara, A.; Marín-Hernández, A.; Moreno-Sánchez, R.; Rodríguez-Enríquez, S. HPI/AMF inhibition halts the development of the aggressive phenotype of breast cancer stem cells. Biochim. Biophys. Acta Mol. Cell. Res. 2017, 1864, 1679–1690. [Google Scholar] [CrossRef] [PubMed]
- Klockars, A.J.; Hancock, G.R. Scheffé’s More Powerful Protected Post Hoc Procedure. J. Educ. Behav. Stat. 2000, 25, 13–19. [Google Scholar] [CrossRef]
- Krzywinski, M.; Altman, N. Points of significance: Analysis of variance and blocking. Nat. Methods 2014, 11, 699–700. [Google Scholar] [CrossRef]
- Henney, J.E. From the Food and Drug Administration. JAMA 2000, 283, 1131. [Google Scholar] [CrossRef]


| Bidimensional Cell Cultures | Tridimensional HeLa MCTS | |||||
|---|---|---|---|---|---|---|
| Drugs | Cancer | Non-cancer | Preventive (nM) | Curative (μM) | ||
| HeLa | 3T3 | MCF-10A | HFF1 | |||
| NSAIDs | ||||||
| Celecoxib | 55 ± 9 a,b,c | 119 ± 7 | >100 | 96 ± 13 | 1 ± 0.3 | 7.5 ± 2 |
| Dimethyl Celecoxib (DMC) | 48 ± 2 a,b | 69 ± 8.5 | >100 | 44 ± 11 | 10 ± 2 | 32 ± 10 |
| Non-canonical Drug | ||||||
| CasII-gly | 1.5 ± 0.9 a,b | 9 ± 2 | 17 ± 3 | N.D. | 30 ± 7.5 | 106 ± 2 |
| Canonical Anti-cancer Drugs | ||||||
| Cisplatin | 15 ± 3 a,b,c | 36 ± 3 | 82 ± 4 | 59 ± 26 | 50 ± 17 | 270 ± 20 |
| Paclitaxel | 21 ± 4 a,b | 80 ± 12 | 100 ± 18 | 68 ± 30 | 16 ± 5 | 26.5 ± 0.1 |
| Doxorubicin | 26 ± 6 b,c | 51 ± 18 | 82 ± 25 | 65 ± 19 | 48 ± 7 | 86 ± 5 |
| Gemcitabine | 30 ± 5 a,b | >2 mM | >1 mM | N.D. | 65 ± 17 | 128 ± 20 |
| Cyclophosphamide | 16 ± 3 a,b | >1 mM | 102 ± 35 | N.D. | 136 ± 87 | 315 ± 30 |
| Carboplatin | 1 ± 0.4 a mM | >5 mM | >1 mM | N.D. | 165 ± 28 | 287 ± 43 |
| Drug 1. | Assayed Doses (µM) | Drug 2 | Assayed Doses (µM) | C Values (BTA %) (Range) | Experimental Values (%) (Range) | Synergism (%) (Range) |
|---|---|---|---|---|---|---|
| Celecoxib | 5–10 | Cisplatin | 2–5 | 15.5 ± 4.5 (10–19) | 81 ± 14 (65–91) | 66 ± 10 (55–74] |
| 5–10 | Paclitaxel | 11–15 | 15 ± 3 (11–17) | 84 ± 5 (81–90) | 69 ± 8 (64–79] | |
| 5–10 | Doxorubicin | 10–20 | 14 ± 3 (11–17) | 71 ± 11 (58–79.5) | 57 ± 13 (42–68) | |
| 5–10 | Gemcitabine | 14–17 | 9 ± 3 (5–11) | 55.5 ± 6 (48–59) | 47 ± 8 (37–54) | |
| 5–10 | Cyclophosphamide | 10–20 | 9 ± 4 (4–13) | −(69 ± 28) (−(44–99)) | −(78 ± 24) (−(57–103)) | |
| 5–10 | Carboplatin | 5–15 | 7 ± 3 (4–10) | −(20 ± 8) (−(13.5–29)) | −(27 ± 11) (−(18–39)) | |
| DMC | 10–15 | Cisplatin | 2–5 | 15.5 ± 4.5 (10–19) | 69 ± 4 (65–74) | 54 ± 5 (48–58) |
| 20–25 | Paclitaxel | 20–21 | 15 ± 3 (11–17) | 52.5 ± 3 (49–54) | 38 ± 0.8 (37–38) | |
| 20–25 | Doxorubicin | 10–20 | 14 ± 3 (11–16) | 52 ± 7 (45.5–60) | 38 ± 10 (30–48) | |
| 10–25 | Gemcitabine | 10–17 | 16 ± 15 (5–34) | 49 ± 0.6 [48–50) | 33 ± 16 (15–45) | |
| 10–25 | Cyclophosphamide | 10–20 | 9 ± 4 (4–12) | −(17 ± 7) (−(10–25)) | −(26 ± 10) (−(20–37)) | |
| 10–25 | Carboplatin | 5–15 | 7 ± 3 (4–10) | −(37 ± 5.5) (−(33–43)) | −(44 ± 3.5) (−(40–47)) | |
| CasII-gly | 0.5–1 | Cisplatin | 5–10 | 15.5 ± 4.5 (10–19) | 54.5 ± 3 (51.5–56.5) | 39 ± 7 (32–46) |
| 0.5–1 | Paclitaxel | 10–20 | 15 ± 3 (11–17) | 56.5 ± 4 (53–61.5) | 42 ± 8 (36–50) | |
| 0.5–1 | Doxorubicin | 10–20 | 14 ± 3 (11–16) | 69 ± 6 (63–75) | 55 ± 8 (47.5–64) | |
| 0.3–1 | Gemcitabine | 5–10 | 16 ± 15 (5–34) | 40 ± 10 (33–51.5) | 24 ± 7 (18–31) | |
| 0.3–1 | Cyclophosphamide | 25–35 | 9 ± 4 (4–12) | 58 ± 10 (48–67) | 49 ± 10 (38–55) | |
| 0.3–1 | Carboplatin | 150 | 7 ± 3 (4–10) | 58 ± 6 (51–64) | 51 ± 9 (41–59) |
| HeLa Bidimensional Cultures | 3T3 Bidimensional Cultures | ||||
|---|---|---|---|---|---|
| Drug 1 | Assayed Doses (µM) | Drug 2 | Assayed Doses (µM) | RI Value (Range) | RI Value (Range) |
| Celecoxib | 5–7 | Cisplatin | 1–5 | 13 ± 11 (4.4–28) | 1.4 ± 0.7 (0.5–2.1) |
| 5–7 | Paclitaxel | 11–15 | 4 ± 2 (3.6–6) | 0.8 ± 0.2 (0.6–0.9) | |
| 5–10 | Doxorubicin | 15–20 | 7 ± 6 (3.7–16.5) | 1.3 ± 0.4 (0.9–1.7) | |
| DMC | 10–15 | Cisplatin | 4–5 | 6 ± 4 (3.1–12.6) | 1.2 ± 0.4 (0.7–1.4) |
| 20–25 | Paclitaxel | 20–21 | 7 ± 3.5 (4–11) | 0.5 ± 0.2 (0.3–0.6) | |
| 25 | Doxorubicin | 17 | 3 ± 0.4 (2.9–3.5) | 0.4 ± 0.1 (0.3–0.5) | |
| CasII-gly | 0.5–1 | Cisplatin | 5–10 | 18 ± 23 (5–61) | 0.4 ± 0.1 (0.3–0.5) |
| 0.5–1 | Paclitaxel | 10–20 | 10 ± 11 (3.2–27) | 0.5 ± 0.3 (0.3–0.8) | |
| 0.5–1 | Doxorubicin | 13 | 5 ± 4 (6.6–11) | 0.5 ± 0.1 (0.3–0.65) | |
| MCTS Preventive Protocol | |||||
| Drug 1 | Assayed Doses (nM) | Drug 2 | Assayed Doses (nM) | RI Value (Range) | |
| Celecoxib | 0.4–0.7 | Cisplatin | 10–30 | 3 ± 1 (2.2–5.5) | |
| 0.1–0.9 | Paclitaxel | 10–13 | 3 ± 0.8 (2.3–4.7) | ||
| 0.1–0.5 | Doxorubicin | 40 | 4.5 ± 2 (2.9–7.3) | ||
| DMC | 1–7 | Cisplatin | 30 | 6 ± 2 (3.6–8.3) | |
| 5 | Paclitaxel | 10–13 | 6 ± 3 (3.1–8.7) | ||
| 1 | Doxorubicin | 30–40 | 3 ± 0.4 (2.3–3) | ||
| CasII–gly | 10–15 | Cisplatin | 30 | 4 ± 1 (2–5) | |
| 2–3 | Paclitaxel | 13 | 4 ± 3 (2–8) | ||
| 20–25 | Doxorubicin | 25 | 4 ± 2 (2–7) | ||
| MCTS Curative Protocol | |||||
| Drug | Assayed Doses (µM) | Chemotherapy drugs | assayed doses (µM) | RI Value (Range) | |
| Celecoxib | 2–5 | Cisplatin | 3–5 | 4 ± 2 (2–7.4) | |
| 2 | Paclitaxel | 15–20 | 7 ± 5 (2–15) | ||
| 4 | Doxorubicin | 30–50 | 5 ± 3 (2–8) | ||
| DMC | 10–25 | Cisplatin | 2–5 | 4 ± 2 (2–6.9) | |
| 20 | Paclitaxel | 20–25 | 4 ± 2 (4–6) | ||
| 35 | Doxorubicin | 50 | 3 ± 1 (1.8–4) | ||
| CasII-gly | 11–12 | Cisplatin | 30 | 6 ± 4 (3–11) | |
| 20 | Paclitaxel | 15 | 1 ± 0.1 (0.9–1.3) | ||
| 30 | Doxorubicin | 10 | 3 ± 0.8 (2–4) | ||
| Preventive Protocol | ||||||
|---|---|---|---|---|---|---|
| Drug 1 | Assayed Doses (nM) | Drug 2 | Assayed Doses (nM) | C Values (BTA %) (Range) | Experimental Values (%) (Range) | Synergism (%) (Range) |
| Celecoxib | 0.4–0.7 | Cisplatin | 10–43 | 30.5 ± 4 (26–33) | 93 ± 3 (91–95.5) | 62 ± 6 (59–69) |
| 0.1–1 | Paclitaxel | 10–13 | 9 ± 1 (8–10) | 83 ± 0.7 (82.5–84) | 74.5 ± 1 (74–76) | |
| 0.1–0.5 | Doxorubicin | 20–40 | 31 ± 3 (27.5–33) | 83 ± 5 (77–86.5) | 52 ± 8 (43–58) | |
| 0.1–1 | Gemcitabine | 30–50 | 34 ± 6 (27–40) | −(23 ± 7) (−(16.5–30)) | −(58 ± 12) (−(44–66)) | |
| 0.1–1 | Cyclophosphamide | 10–100 | 20 ± 21 (4–44) | −(37 ± 25.5) (−(11–62)) | −(57 ± 8) (−(51–66)) | |
| 0.1–1 | Carboplatin | 100 | 19 ± 3 (17–22.5) | 33 ± 3 (29–34.5) | 14 ± 2.5 (12–16.5) | |
| DMC | 1–7 | Cisplatin | 10–43 | 33 ± 5 (29–38) | 81 ± 3 (79–85) | 48 ± 8 (41–56) |
| 5–6 | Paclitaxel | 10–13 | 13 ± 7 (5–17.5) | 31 ± 5 (26.5–36) | 18 ± 5 (12–22) | |
| 1–3 | Doxorubicin | 25–40 | 19 ± 10 (11–30.5) | 62.5 ± 6 (59–69) | 43 ± 5 (39–48) | |
| 1–10 | Gemcitabine | 30–50 | 33 ± 2 (31–34) | 58 ± 5 (53–63) | 25 ± 5 (20–28) | |
| 1–10 | Cyclophosphamide | 10–100 | 35 ± 5 (31–41) | 20 ± 6 (−(13–24) | −(15 ± 7) (−(7.5–20)) | |
| 1–10 | Carboplatin | 10–100 | 40.5 ± 2 (38–42) | 19 ± 0.3 (−(18–19)) | −(21 ± 1.5) (−(20–23)) | |
| CasII-gly | 10–17 | Cisplatin | 15–30 | 14 ± 4 (11–18.5) | 95 ± 0.5 (94–96) | 81 ± 4.5 (76–85) |
| 14–25 | Paclitaxel | 10–13 | 25 ± 2 (24–28) | 67 ± 5 (63–72) | 41.5 ± 2 (39–44) | |
| 10–25 | Doxorubicin | 20–40 | 31 ± 9 (22–39) | 62 ± 9 (52–69) | 31 ± 2 (30–34) | |
| 10–25 | Gemcitabine | 10–40 | 25 ± 0.6 (25–26) | 54 ± 4 (51–59) | 29 ± 4 (26–33) | |
| 5–25 | Cyclophosphamide | 28–110 | 25 ± 2 (23–28) | 10 ± 5 (5–13.5) | −(15 ± 3.5) (−(11.5–18.5)) | |
| 1–30 | Carboplatin | 50–90 | 33 ± 4 (28.5–37) | 68 ± 7.5 (62–76) | 35 ± 5 (29–39) | |
| Curative Protocol | ||||||
| Drug 1 | Assayed Doses (µM) | Drug 2 | Assayed Doses (µM) | C Value (BTA%) (Range) | Experimental Values (%) (Range) | Synergism (%) (Range) |
| Celecoxib | 2–5 | Cisplatin | 1–5 | 15.5 ± 4.5 (10–19) | 83 ± 12 (68.5–91) | 67 ± 8 (58–74) |
| 2–6 | Paclitaxel | 10–25 | 23 ± 4 (20–28) | 40 ± 8 (32–46.5) | 17 ± 7 (10–23) | |
| 2–4 | Doxorubicin | 30–50 | 23 ± 4 (19–27) | 59 ± 3 (56–63) | 37 ± 5 (32–41) | |
| 1–5 | Gemcitabine | 20–50 | 29 ± 4 (24–32) | 24 ± 7 (16.5–31) | −(4 ± 3) (−(1–8)) | |
| 4–7 | Cyclophosphamide | 75–115 | 27 ± 1.5 (26–29) | 23 ± 3.5 (19–25) | −(4 ± 2) (−(2–7)) | |
| 1–7 | Carboplatin | 60–190 | 45 ± 5 (40–48) | 25 ± 13 (16–40) | −(20 ± 11) (−(7–29)) | |
| DMC | 10–25 | Cisplatin | 1–5 | 24 ± 4 (22–28) | 71 ± 5 (66–75) | 47 ± 4 (44–51) |
| 20–30 | Paclitaxel | 10–25 | 33 ± 5 (29–38.5) | 52.5 ± 3 (49–54) | 20 ± 8 (10–25) | |
| 31–35 | Doxorubicin | 30–50 | 29 ± 9.5 (19–38) | 52 ± 7 (45.5–59) | 23 ± 16 (7–40) | |
| 31–35 | Gemcitabine | 20–50 | 30 ± 2 (28–32) | 20 ± 5) (16–25) | −(10 ± 3) (−(7–13)) | |
| 29–30 | Cyclophosphamide | 75–115 | 39 ± 6 (32–43) | 14.5 ± 5) (8–18) | −(24 ± 10) (−(14.5–35)) | |
| 22–25 | Carboplatin | 60–190 | 24.5 ± 4 (20–27) | 36 ± 0.9 (35–36.5) | 11 ± 3 (9–14) | |
| CasII-gly | 10–100 | Cisplatin | 15–30 | 23 ± 3 (20–25) | 97 ± 0.5 (97–97.5) | 74 ± 2 (72–76.5) |
| 20–60 | Paclitaxel | 10–25 | 15 ± 3 (11–17) | 64 ± 5 (61–69) | 50 ± 8 (44–58) | |
| 20–80 | Doxorubicin | 10 | 15 ± 4 (10–18) | 46 ± 2 (44.5–48) | 31 ± 6 (28–38) | |
| 50–90 | Gemcitabine | 20–40 | 24 ± 16 (5–34) | 65 ± 6 (58–70) | 40.5 ± 11 (32–53) | |
| 50–120 | Cyclophosphamide | 10–80 | 28 ± 2 (26–30) | 19 ± 3 (17–23) | −(9 ± 5) (−(3–13)) | |
| 20–120 | Carboplatin | 50–75 | 29 ± 2 (28–31) | 58 ± 6 (51–64) | 29 ± 6 (23.5–36) | |
| Chemotherapy Drug | HeLa | 3T3 | HFF1 |
|---|---|---|---|
| + Celecoxib (5–10 µM) | |||
| Cisplatin | 5 ± 2 a | 36 ± 4 | 75 ± 12 |
| Paclitaxel | 13 ± 4 | 52 ± 10 | 73 ± 18 |
| Doxorubicin | 14.5 ± 2 a | 54 ± 3 | 71 ± 7.3 |
| + DMC (15–25 µM) | |||
| Cisplatin | 5 ± 1 a | 28.5 ± 7 | 74.5 ± 9.5 |
| Paclitaxel | 13 ± 4 | 58.5 ± 17 | 66.5 ± 8.5 |
| Doxorubicin | 11 ± 4 a | 41 ± 5 | 62.5 ± 1.5 |
| + Cas-IIgly (0.5–1 µM) | |||
| Cisplatin | 9 ± 3 | 7.5 ± 6 | N.D |
| Paclitaxel | 17 ± 5 | 50 ± 20 | N.D |
| Doxorubicin | 12 ± 4 a | 38 ± 3 | N.D |
| Preventive Protocol | ||||
| Canonical Drug | IC50 (nM) | + Celecoxib (0.4–1 nM) | + DMC (1–10 nM) | + CasII-Gly (11–30 nM) |
| Cisplatin | 50 ± 17 | 10 ± 3 a | 24 ± 6 b | 8 ± 3 a |
| Paclitaxel | 16 ± 5 | 5 ± 2 b | 7.2 ± 3 b | 4.5 ± 1.5 a |
| Doxorubicin | 48 ± 7 | 10 ± 3 a | 31.2 ± 9 | 16.5 ± 4 b |
| Curative Protocol | ||||
| Canonical Drug | IC50 (µM) | + Celecoxib (2–6 µM) | + DMC (10–35 µM) | + CasII-gly (11–30 µM) |
| Cisplatin | 270 ± 20 | 10.5 ± 0.5 a | 148.5 ± 30 b | 11.3 ± 4 a |
| Paclitaxel | 26.5 ± 0.1 | 15 ± 3 b | 7.1 ± 2 a | 10 ± 2 b |
| Doxorubicin | 86 ± 5 | 10 ± 2 a | 77.4 ± 20 | 30 ± 9 a |
| NSAIDs | NSAIDs Concentrations (µM) | Chemotherapy Drugs | TI Ratio | |
|---|---|---|---|---|
| 3T3/HeLa | HFF1/HeLa | |||
| Celecoxib | 5 | Cisplatin | 36/5 = 7.2 | 75/5 = 15 |
| 5 | Paclitaxel | 52/13 = 4.0 | 73/13 = 5.6 | |
| 10 | Doxorubicin | 54/14.5 = 3.7 | 71/14.5 = 4.9 | |
| DMC | 15 | Cisplatin | 28.5/5 = 5.7 | 74.5/5 = 14.9 |
| 20 | Paclitaxel | 58.5/13 = 4.5 | 66.5/13 = 5.1 | |
| 25 | Doxorubicin | 41/11 = 3.6 | 62.5/11 = 5.7 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robledo-Cadena, D.X.; Gallardo-Pérez, J.C.; Dávila-Borja, V.; Pacheco-Velázquez, S.C.; Belmont-Díaz, J.A.; Ralph, S.J.; Blanco-Carpintero, B.A.; Moreno-Sánchez, R.; Rodríguez-Enríquez, S. Non-Steroidal Anti-Inflammatory Drugs Increase Cisplatin, Paclitaxel, and Doxorubicin Efficacy against Human Cervix Cancer Cells. Pharmaceuticals 2020, 13, 463. https://doi.org/10.3390/ph13120463
Robledo-Cadena DX, Gallardo-Pérez JC, Dávila-Borja V, Pacheco-Velázquez SC, Belmont-Díaz JA, Ralph SJ, Blanco-Carpintero BA, Moreno-Sánchez R, Rodríguez-Enríquez S. Non-Steroidal Anti-Inflammatory Drugs Increase Cisplatin, Paclitaxel, and Doxorubicin Efficacy against Human Cervix Cancer Cells. Pharmaceuticals. 2020; 13(12):463. https://doi.org/10.3390/ph13120463
Chicago/Turabian StyleRobledo-Cadena, Diana Xochiquetzal, Juan Carlos Gallardo-Pérez, Víctor Dávila-Borja, Silvia Cecilia Pacheco-Velázquez, Javier Alejandro Belmont-Díaz, Stephen John Ralph, Betsy Alejandra Blanco-Carpintero, Rafael Moreno-Sánchez, and Sara Rodríguez-Enríquez. 2020. "Non-Steroidal Anti-Inflammatory Drugs Increase Cisplatin, Paclitaxel, and Doxorubicin Efficacy against Human Cervix Cancer Cells" Pharmaceuticals 13, no. 12: 463. https://doi.org/10.3390/ph13120463
APA StyleRobledo-Cadena, D. X., Gallardo-Pérez, J. C., Dávila-Borja, V., Pacheco-Velázquez, S. C., Belmont-Díaz, J. A., Ralph, S. J., Blanco-Carpintero, B. A., Moreno-Sánchez, R., & Rodríguez-Enríquez, S. (2020). Non-Steroidal Anti-Inflammatory Drugs Increase Cisplatin, Paclitaxel, and Doxorubicin Efficacy against Human Cervix Cancer Cells. Pharmaceuticals, 13(12), 463. https://doi.org/10.3390/ph13120463