From Ethnomedicine to Plant Biotechnology and Machine Learning: The Valorization of the Medicinal Plant Bryophyllum sp.
Abstract
1. Introduction
2. Bryophyllum sp. Secondary Metabolites as Antioxidants and Anticancer Agents
2.1. Phenolic Compounds
2.1.1. Phenolic Acids
2.1.2. Flavonoids
2.2. Bufadienolides
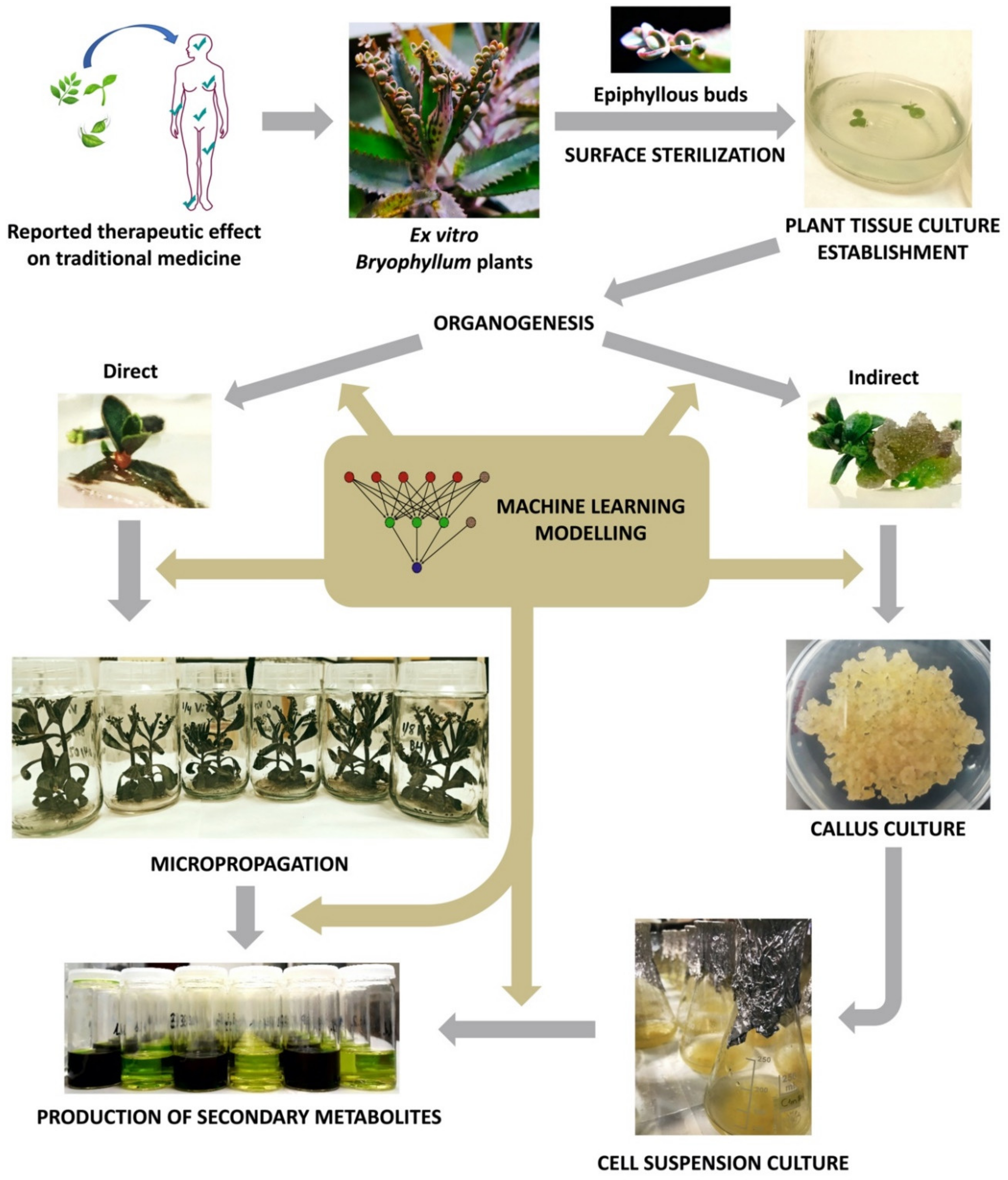
3. Plant Tissue Culture for Sustainable Valorization of Bioactive Compounds of Bryophyllum sp.
3.1. PTC Establishment
Plant Culture Media Composition
3.2. Organogenesis and Plant Regeneration
3.3. Micropropagation
3.4. Establishment of Plant Suspension-Cultured Cells (PSCCs)
3.5. Enhancement of Phenolic Compounds Production from Bryophyllum sp. via Elicitation
4. Machine Learning for Optimizing the Biotechnological Valorization of Bryophyllum sp.
5. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Adanson, M. Familles des Plantes par M. Adanson; chez Vincent: Paris, France, 1763. [Google Scholar]
- Akulova-Barlow, Z. Kalanchoe. Cactus Succul. J. 2009, 81, 268–276. [Google Scholar] [CrossRef]
- Smith, G.; Volmer, P.A. Kalanchoe species poisoning in pets. Vet. Med. 2004, 99, 933–936. [Google Scholar]
- Descoings, B. Le genre Kalanchoe structure et définition par Bernard Descoings. Le J. Bot. 2006, 33, 3–28. [Google Scholar]
- Salisbury, R.A. Crassulaceae Bryophyllum salisb. Parad. Londinensis 1805, 1, 3. [Google Scholar]
- Baker, J.G. Notes on a Collection of Flowering Plants made by L. Kitching, Esq., in Madagascar in 1879. Bot. J. Linn. Soc. 1881, 18, 264–281. [Google Scholar] [CrossRef]
- Chernetskyy, M.A. The role of morpho-anatomical traits of the leaves in the taxonomy of Kalanchoideae Berg. subfamily (Crassulaceae DC.). Mod. Phytomorphology 2012, 1, 15–18. [Google Scholar]
- Gehrig, H.H.; Rösicke, H.; Kluge, M. Detection of DNA polymorphisms in the genus Kalanchoe by RAPD-PCR fingerprint and its relationships to infrageneric taxonomic position and ecophysiological photosynthetic behaviour of the species. Plant Sci. 1997, 125, 41–51. [Google Scholar] [CrossRef]
- Hamburger, M.; Potterat, O.; Fürer, K.; Simões-Wüst, A.P.; Von Mandach, U. Bryophyllum pinnatum - Reverse engineering of an anthroposophic herbal medicine. Nat. Prod. Commun. 2017, 12, 1359–1364. [Google Scholar] [CrossRef]
- Cushman, J.C. Crassulacean acid metabolism: Recent advances and future opportunities. Funct. Plant Biol. 2005, 32, 375–380. [Google Scholar] [CrossRef]
- Garcês, H.M.P.; Koenig, D.; Townsley, B.T.; Kim, M.; Sinha, N.R. Truncation of LEAFY COTYLEDON1 protein is required for asexual reproduction in Kalanchoë daigremontiana. Plant Physiol. 2014, 165, 196–206. [Google Scholar] [CrossRef][Green Version]
- Garcês, H.; Sinha, N. The “Mother of Thousands” (Kalanchoë daigremontiana): A plant model for asexual reproduction and CAM studies. Cold Spring Harb. Protoc. 2009, 4, 1–9. [Google Scholar]
- García-Pérez, P.; Barreal, M.E.; Rojo-De Dios, L.; Cameselle-Teijeiro, J.F.; Gallego, P.P. Bioactive natural products from the genus Kalanchoe as cancer chemopreventive agents: A review. In Studies in Natural Products Chemistry; Rahman, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 61, pp. 49–84. ISBN 9780444641830. [Google Scholar]
- Boxall, S.F.; Kadu, N.; Dever, L.V.; Knerová, J.; Waller, J.L.; Gould, P.J.D.; Hartwell, J. Kalanchoë PPC1 is essential for crassulacean acid metabolism and the regulation of core circadian clock and guard cell signaling genes. Plant Cell 2020, 32, 1136–1160. [Google Scholar] [CrossRef] [PubMed]
- Gehrig, H.; Gaußmann, O.; Marx, H.; Schwarzott, D.; Kluge, M. Molecular phylogeny of the genus Kalanchoe (Crassulaceae) inferred from nucleotide sequences of the ITS-1 and ITS-2 regions. Plant Sci. 2001, 160, 827–835. [Google Scholar] [CrossRef]
- Kulka, R.G. Cytokinins inhibit epiphyllous plantlet development on leaves of Bryophyllum (Kalanchoë) marnierianum. J. Exp. Bot. 2006, 57, 4089–4098. [Google Scholar] [CrossRef]
- Garcês, H.M.P.; Champagne, C.E.M.; Townsley, B.T.; Park, S.; Malhó, R.; Pedroso, M.C.; Harada, J.J.; Sinha, N.R. Evolution of asexual reproduction in leaves of the genus Kalanchoë. Proc. Natl. Acad. Sci. USA 2007, 104, 15578–15583. [Google Scholar]
- Rodriguez, B.K. Daigremontiana as a Model Plant for the Study of Auxin Effects in Plant Morphology. J. Plant Biochem. Physiol. 2014, 02, 1–3. [Google Scholar] [CrossRef]
- Pasternak, T.; Dudits, D. Epigenetic clues to better understanding of the asexual embryogenesis In planta and In vitro. Front. Plant Sci. 2019, 10, 1–5. [Google Scholar] [CrossRef]
- Zhong, T.; Zhu, C.; Zeng, H.; Han, L. Analysis of gene expression in Kalanchoe daigremontiana leaves during plantlet formation under drought stress. Electron. J. Biotechnol. 2013, 16. [Google Scholar]
- Kulka, R.G. Hormonal control of root development on epiphyllous plantlets of Bryophyllum (Kalanchoë) marnierianum: Role of auxin and ethylene. J. Exp. Bot. 2008, 59, 2361–2370. [Google Scholar] [CrossRef]
- Herrera, I.; Nassar, J.M. Reproductive and recruitment traits as indicators of the invasive potential of Kalanchoe daigremontiana (Crassulaceae) and Stapelia gigantea (Apocynaceae) in a Neotropical arid zone. J. Arid Environ. 2009, 73, 978–986. [Google Scholar] [CrossRef]
- Herrando-Moraira, S.; Vitales, D.; Nualart, N.; Gómez-Bellver, C.; Ibáñez, N.; Massó, S.; Cachón-Ferrero, P.; González-Gutiérrez, P.A.; Guillot, D.; Herrera, I.; et al. Global distribution patterns and niche modelling of the invasive Kalanchoe × houghtonii (Crassulaceae). Sci. Rep. 2020, 10, 3143. [Google Scholar] [CrossRef]
- Kolodziejczyk-Czepas, J.; Nowak, P.; Wachowicz, B.; Piechocka, J.; Głowacki, R.; Moniuszko-Szajwaj, B.; Stochmal, A. Antioxidant efficacy of Kalanchoe daigremontiana bufadienolide-rich fraction in blood plasma in vitro. Pharm. Biol. 2016, 54, 3182–3188. [Google Scholar] [CrossRef] [PubMed]
- Ojewole, J.A.O. Antinociceptive, anti-inflammatory and antidiabetic effects of Bryophyllum pinnatum (Crassulaceae) leaf aqueous extract. J. Ethnopharmacol. 2005, 99, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Kamboj, A.; Saluja, A.K. Bryophyllum pinnatum (Lam.) Kurz.: Phytochemical and pharmacological profile: A review. Pharmacogn. Rev. 2009, 3, 364–374. [Google Scholar]
- Akinpelu, D.A. Antimicrobial activity of Bryophyllum pinnatum leaves. Fitoterapia 2000, 71, 193–194. [Google Scholar] [CrossRef]
- Mawla, F.; Khatoon, S.; Rehana, F.; Jahan, S.; Moshiur, M.R.; Hossain, S.; Haq, W.M.; Rahman, S.; Debnath, K.; Rahmatullah, M. Ethnomedicinal plants of folk medicinal practitioners in four villages of natore and rajshahi districts, bangladesh. Am. J. Sustain. Agric. 2012, 6, 406–416. [Google Scholar]
- Hsieh, Y.J.; Yang, M.Y.; Leu, Y.L.; Chen, C.; Wan, C.F.; Chang, M.Y.; Chang, C.J. Kalanchoe tubiflora extract inhibits cell proliferation by affecting the mitotic apparatus. BMC Complement. Altern. Med. 2012, 12. [Google Scholar] [CrossRef]
- Abebe, W. An Overview of Ethiopian Traditional Medicinal Plants Used for Cancer Treatment. European J. Med. Plants 2016, 14, 1–16. [Google Scholar] [CrossRef]
- Supratman, U.; Fujita, T.; Akiyama, K.; Hayashi, H.; Murakami, A.; Sakai, H.; Koshimizu, K.; Ohigashi, H. Anti-tumor promoting activity of bufadienolides from Kalanchoe pinnata and K. daigremontiana × tubiflora. Biosci. Biotechnol. Biochem. 2001, 65, 947–949. [Google Scholar] [CrossRef]
- Nguelefack, T.B.; Nana, P.; Atsamo, A.D.; Dimo, T.; Watcho, P.; Dongmo, A.B.; Tapondjou, L.A.; Njamen, D.; Wansi, S.L.; Kamanyi, A. Analgesic and anticonvulsant effects of extracts from the leaves of Kalanchoe crenata (Andrews) Haworth (Crassulaceae). J. Ethnopharmacol. 2006, 106, 70–75. [Google Scholar] [CrossRef]
- Kamgang, R.; Youmbi Mboumi, R.; Foyet Fondjo, A.; Fokam Tagne, M.A.; Mengue N’dillé, G.P.R.; Ngogang Yonkeu, J. Antihyperglycaemic potential of the water-ethanol extract of Kalanchoe crenata (Crassulaceae). J. Nat. Med. 2008, 62, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Akinsulire, O.R.; Aibinu, I.E.; Adenipekun, T.; Adelowotan, T.; Odugbemi, T. In vitro antimicrobial activity of crude extracts from plants Bryophyllum pinnatum and Kalanchoe crenata. African J. Tradit. Complement. Altern. Med. 2007, 4, 338–344. [Google Scholar] [CrossRef]
- Malan, D.F.; Neuba, D.F.R. Traditional practices and medicinal plants use during pregnancy by Anyi-Ndenye women (Eastern Côte d’Ivoire). Afr. J. Reprod. Health 2011, 15, 85–93. [Google Scholar] [PubMed]
- Süsskind, M.; Thürmann, P.A.; Lüke, C.; Jeschke, E.; Tabali, M.; Matthes, H.; Ostermann, T. Adverse drug reactions in a complementary medicine hospital: A prospective, intensified surveillance study. Evidence-based Complement. Altern. Med. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Richwagen, N.; Lyles, J.T.; Dale, B.L.F.; Quave, C.L. Antibacterial activity of Kalanchoe mortagei and K. fedtschenkoi against ESKAPE pathogens. Front. Pharmacol. 2019, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cumberbatch, A. An Ethonobotanical Survey of Medicinal Plant Usage in Salvador de Bahia, Brazil. CGI Gr. 2011. Available online: https://radar.auctr.edu/islandora/object/sc.gstem%3A2011_cumberbatch_ashli (accessed on 21 October 2020).
- Costa, S.S.; Muzitano, M.F.; Camargo, L.M.M.; Coutinho, M.A.S. Therapeutic potential of Kalanchoe species: Flavonoids and other secondary metabolites. Nat. Prod. Commun. 2008, 3, 2151–2164. [Google Scholar] [CrossRef]
- Suárez, F.S.; Ramirez, A.M.; López-Marure, R.; Gutiérrez, R.M.P. In vitro cytotoxic potential and apoptotic activity of bufadienolide-rich fraction from leaves of Kalanchoe mortagei against human HeLa cancer cells. Int. J. Ayurvedic Med. 2018, 9, 25–33. [Google Scholar]
- Vera-Marin, B.; Sánchez-Sáen, M. Plantas medicinales y predictibilidad de uso en algunas veredas del corregimiento de San Cristóbal (Antioquia), Colombia. Actual. Biológicas 2016, 38, 167–180. [Google Scholar]
- Herawati, M.H.; Husin, N. Berbagai jenis tumbuhan yang berkhasiat sebagai obat kecacingan. Media Penelit. dan Pengemb. Kesehat. 2000, 10. Available online: isis://www.neliti.com/publications/158068/berbagai-jenis-tumbuhan-yang-berkhasiat-sebagai-obat-kecacingan (accessed on 21 October 2020).
- Rajsekhar, P.B.; Arvind Bharani, R.S.; Ramachandran, M.; Jini Angel, K.; Rajsekhar, S.P.V. The “wonder plant” Kalanchoe pinnata (linn.) pers.: A review. J. Appl. Pharm. Sci. 2016, 6, 151–158. [Google Scholar] [CrossRef]
- Rahmatullah, M.; Mollik, M.A.H.; Ali, M.; Abbas, M.F.B.; Jahan, R.; Khatun, A.; Seraj, S.; Ahsan, S. An ethnomedicinal survey of vitbilia village in sujanagar sub-district of pabna district, Bangladesh. Am. J. Sustain. Agric. 2010, 4, 302–308. [Google Scholar]
- Lans, C.A. Ethnomedicines used in Trinidad and Tobago for urinary problems and diabetes mellitus. J. Ethnobiol. Ethnomed. 2006, 2, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, R.; Saha, M.R.; Rahman, M.A.; Islam, M.A.U. Ethnomedicinal Survey of Plants in the Southern District Noakhali, Bangladesh. Bangladesh Pharm. J. 2015, 17, 205–214. [Google Scholar] [CrossRef]
- Fernandes, J.M.; Cunha, L.M.; Azevedo, E.P.; Lourenço, E.M.G.; Fernandes-Pedrosa, M.F.; Zucolotto, S.M. Kalanchoe laciniata and Bryophyllum pinnatum: An updated review about ethnopharmacology, phytochemistry, pharmacology and toxicology. Rev. Bras. Farmacogn. 2019, 29, 529–558. [Google Scholar] [CrossRef]
- Sen, P.; Dollo, M.; Choudhury, M.D.; Choudhury, D. Documentation of traditional herbal knowledge of Khamptis of Arunachal Pradesh. Indian J. Tradit. Knowl. 2008, 7, 438–442. [Google Scholar]
- Khan, M.A.; Islam, M.K.; Siraj, M.A.; Saha, S.; Barman, A.K.; Awang, K.; Rahman, M.M.; Shilpi, J.A.; Jahan, R.; Islam, E.; et al. Ethnomedicinal survey of various communities residing in Garo Hills of Durgapur, Bangladesh. J. Ethnobiol. Ethnomed. 2015, 11. [Google Scholar] [CrossRef]
- Okwu, D.E.; Nnamdi, F.U. Two novel flavonoids from Bryophyllum pinnatum and their antimicrobial activity. J. Chem. Pharm. Res. 2011, 3, 1–10. [Google Scholar]
- Budi, V.; Sihotang, L. Ethnomedicinal study of the Sundanese people at the Bodogol area, Gede Pangrango Mountain National Park, West Java. Gard. Bull. Singapore 2011, 63, 527–534. [Google Scholar]
- Namukobe, J.; Kasenene, J.M.; Kiremire, B.T.; Byamukama, R.; Kamatenesi-Mugisha, M.; Krief, S.; Dumontet, V.; Kabasa, J.D. Traditional plants used for medicinal purposes by local communities around the Northern sector of Kibale National Park, Uganda. J. Ethnopharmacol. 2011, 136, 236–245. [Google Scholar] [CrossRef]
- Lai, Z.R.; Peng, W.H.; Ho, Y.L.; Huang, S.C.; Huang, T.H.; Lai, S.C.; Ku, Y.R.; Tsai, J.C.; Wang, C.Y.; Chang, Y.S. Analgesic and anti-inflammatory activities of the methanol extract of Kalanchoe gracilis (L.) DC stem in mice. Am. J. Chin. Med. 2010, 38, 529–546. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Huang, S.C.; Zhang, Y.; Lai, Z.R.; Kung, S.H.; Chang, Y.S.; Lin, C.W. Antiviral ability of Kalanchoe gracilis leaf extract against Enterovirus 71 and coxsackievirus A16. Evidence-based Complement. Altern. Med. 2012, 2012, 503165. [Google Scholar]
- Milad, R. Genus Kalanchoe (Crassulaceae): A review of its ethnomedicinal, botanical, chemical and pharmacological properties. European J. Med. Plants 2014, 4, 86–104. [Google Scholar] [CrossRef]
- Al-Snafi, A. The chemical constituents and pharmacological effects of Bryophyllum calycinum. A review. Int. J. Pharma Sci. Res. 2013, 4, 171–176. [Google Scholar]
- De Araújo, E.R.D.; Félix-Silva, J.; Xavier-Santos, J.B.; Fernandes, J.M.; Guerra, G.C.B.; de Araújo, A.A.; Araújo, D.F.d.S.; de Santis Ferreira, L.; da Silva Júnior, A.A.; Fernandes-Pedrosa, M.d.F.; et al. Local anti-inflammatory activity: Topical formulation containing Kalanchoe brasiliensis and Kalanchoe pinnata leaf aqueous extract. Biomed. Pharmacother. 2019, 113, 108721. [Google Scholar]
- Huang, H.C.; Huang, G.J.; Liaw, C.C.; Yang, C.S.; Yang, C.P.; Kuo, C.L.; Tseng, Y.H.; Wang, S.Y.; Chang, W.T.; Kuo, Y.H. A new megastigmane from Kalanchoe tubiflora (Harvey) Hamet. Phytochem. Lett. 2013, 6, 379–382. [Google Scholar] [CrossRef]
- Anisimov, M.M.; Gerasimenko, N.I.; Chaikina, E.L.; Serebryakov, Y.M. Biological activity of metabolites of the herb Kalanchoe daigremontiana (Hamet de la Bathie) Jacobs et Perr. Biol. Bull. 2009, 36, 568–574. [Google Scholar] [CrossRef]
- Ürményi, F.G.G.; Saraiva, G.d.N.; Casanova, L.M.; Matos, A.d.S.; de Magalhães Camargo, L.M.; Romanos, M.T.V.; Costa, S.S. Anti-HSV-1 and HSV-2 Flavonoids and a New Kaempferol Triglycoside from the Medicinal Plant Kalanchoe daigremontiana. Chem. Biodivers. 2016, 13, 1707–1714. [Google Scholar] [CrossRef]
- Mahata, S.; Maru, S.; Shukla, S.; Pandey, A.; Mugesh, G.; Das, B.C.; Bharti, A.C. Anticancer property of Bryophyllum pinnata (Lam.) Oken. leaf on human cervical cancer cells. BMC Complement. Altern. Med. 2012, 12, 15. [Google Scholar] [CrossRef]
- García-Pérez, P.; Lozano-Milo, E.; Landín, M.; Gallego, P.P. Combining medicinal plant in vitro culture with machine learning technologies for maximizing the production of phenolic compounds. Antioxidants 2020, 9, 210. [Google Scholar] [CrossRef]
- Bogucka-Kocka, A.; Zidorn, C.; Kasprzycka, M.; Szymczak, G.; Szewczyk, K. Phenolic acid content, antioxidant and cytotoxic activities of four Kalanchoë species. Saudi J. Biol. Sci. 2016, 25, 622–630. [Google Scholar] [CrossRef] [PubMed]
- García-Pérez, P.; Losada-Barreiro, S.; Bravo-Díaz, C.; Gallego, P.P. Exploring the use of Bryophyllum as natural source of bioactive compounds with antioxidant activity to prevent lipid oxidation of fish oil-in-water emulsions. Plants 2020, 9, 1012. [Google Scholar] [CrossRef] [PubMed]
- Maharani, R.; Fajriah, S.; Hardiawan, R.; Supratman, U. Insecticidal bufadienolides from the leaves of Kalanchoe daigremontiana (Crassulaceae). Proceeding Int. Semin. Chem. 2008, 11, 236–239. [Google Scholar]
- Supratman, U.; Fujita, T.; Akiyama, K.; Hayash, H. New insecticidal bufadienolide, bryophyllin C, from Kalanchoe pinnata. Biosci. Biotechnol. Biochem. 2000, 64, 1310–1312. [Google Scholar] [CrossRef]
- Supratman, U.; Fujita, T.; Akiyama, K.; Hayashi, H. Insecticidal compounds from Kalanchoe daigremontiana x tubiflora. Phytochemistry 2001, 58, 311–314. [Google Scholar] [CrossRef]
- Kolodziejczyk-Czepas, J.; Stochmal, A. Bufadienolides of Kalanchoe species: An overview of chemical structure, biological activity and prospects for pharmacological use. Phytochem. Rev. 2017, 16, 1155–1171. [Google Scholar] [CrossRef]
- Bopda, O.S.M.; Longo, F.; Bella, T.N.; Edzah, P.M.O.; Taïwe, G.S.; Bilanda, D.C.; Tom, E.N.L.; Kamtchouing, P.; Dimo, T. Antihypertensive activities of the aqueous extract of Kalanchoe pinnata (Crassulaceae) in high salt-loaded rats. J. Ethnopharmacol. 2014, 153, 400–407. [Google Scholar] [CrossRef]
- Kolodziejczyk-Czepas, J.; Sieradzka, M.; Moniuszko-Szajwaj, B.; Pecio, Ł.; Ponczek, M.B.; Nowak, P.; Stochmal, A. Bufadienolides from Kalanchoe daigremontiana as thrombin inhibitors—In vitro and in silico study. Int. J. Biol. Macromol. 2017, 99, 141–150. [Google Scholar] [CrossRef]
- Kuo, P.C.; Kuo, T.H.; Su, C.R.; Liou, M.J.; Wu, T.S. Cytotoxic principles and α-pyrone ring-opening derivatives of bufadienolides from Kalanchoe hybrida. Tetrahedron 2008, 64, 3392–3396. [Google Scholar] [CrossRef]
- Yadav, N.P.; Dixit, V.K. Hepatoprotective activity of leaves of Kalanchoe pinnata Pers. J. Ethnopharmacol. 2003, 86, 197–202. [Google Scholar] [CrossRef]
- Menon, N.; Sparks, J.; Omoruyi, F. Hypoglycemic and hypocholesterolemic activities of the aqueous preparation of Kalanchoe pinnata leaves in streptozotocin-induced diabetic rats. Asian Pac. J. Trop. Biomed. 2015, 5, 3–9. [Google Scholar] [CrossRef]
- Bartwal, A.; Mall, R.; Lohani, P.; Guru, S.K.; Arora, S. Role of Secondary Metabolites and Brassinosteroids in Plant Defense Against Environmental Stresses. J. Plant Growth Regul. 2013, 32, 216–232. [Google Scholar] [CrossRef]
- Nile, S.H.; Park, S.W. Edible berries: Bioactive components and their effect on human health. Nutrition 2014, 30, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Fürer, K.; Simões-Wüst, A.P.; Von Mandach, U.; Hamburger, M.; Potterat, O. Bryophyllum pinnatum and related species used in anthroposophic medicine: Constituents, pharmacological activities, and clinical efficacy. Planta Med. 2016, 82, 930–941. [Google Scholar] [CrossRef]
- Stefanowicz-Hajduk, J.; Asztemborska, M.; Krauze-Baranowska, M.; Godlewska, S.; Gucwa, M.; Moniuszko-Szajwaj, B.; Stochmal, A.; Ochocka, J.R. Identification of flavonoids and bufadienolides and cytotoxic effects of Kalanchoe daigremontiana extracts on human cancer cell lines. Planta Med. 2020, 86, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Casanova, J.M.; dos Santos Nascimento, L.B.; Casanova, L.M.; Leal-Costa, M.V.; Costa, S.S.; Tavares, E.S. Differential distribution of flavonoids and phenolic acids in leaves of Kalanchoe delagoensis Ecklon & Zeyher (Crassulaceae). Microsc. Microanal. 2020, 1–8. [Google Scholar]
- Prasad, A.K.; Kumar, S.; Iyer, S.V.; Sudani, R.J.; Vaidya, S.K. Pharmacognostical, Phytochemical and Pharmacological Review on Bryophyllum pinnata. Int. J. Pharm. Biol. Arch. 2012, 3, 423–433. [Google Scholar]
- García-Pérez, P.; Losada-Barreiro, S.; Gallego, P.P.; Bravo-Díaz, C. Adsorption of gallic acid, propyl gallate and polyphenols from Bryophyllum extracts on activated carbon. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- García-Pérez, P.; Lozano-Milo, E.; Gallego, P.P.; Tojo, C.; Losada-Barreiro, S.; Bravo-Díaz, C. Plant antioxidants in food emulsions. In Some New Aspects of Colloidal Systems in Foods; Milani, J., Ed.; IntechOpen: Rijeka, Croatia, 2018; pp. 11–29. [Google Scholar]
- Chernetskyy, M.; Woźniak, A.; Skalska-Kamińska, A.; Żuraw, B.; Blicharska, E.; Rejdak, R.; Donica, H.; Weryszko-Chmielewska, E. Structure of leaves and phenolic acids in Kalanchoë daigremontiana Raym.-Hamet & H. Perrier. Acta Sci. Pol. Hortorum Cultus 2018, 17, 137–155. [Google Scholar]
- Bonache, M.A.; Moreno-Fernández, S.; Miguel, M.; Sabater-Muñoz, B.; González-Muñiz, R. Small Library of Triazolyl Polyphenols Correlating Antioxidant Activity and Stability with Number and Position of Hydroxyl Groups. ACS Comb. Sci. 2018, 20, 694–699. [Google Scholar] [CrossRef]
- Özçelik, B.; Kartal, M.; Orhan, I. Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharm. Biol. 2011, 49, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Cueva, C.; Moreno-Arribas, M.V.; Martín-Álvarez, P.J.; Bills, G.; Vicente, M.F.; Basilio, A.; Rivas, C.L.; Requena, T.; Rodríguez, J.M.; Bartolomé, B. Antimicrobial activity of phenolic acids against commensal, probiotic and pathogenic bacteria. Res. Microbiol. 2010, 161, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Mudnic, I.; Modun, D.; Rastija, V.; Vukovic, J.; Brizic, I.; Katalinic, V.; Kozina, B.; Medic-Saric, M.; Boban, M. Antioxidative and vasodilatory effects of phenolic acids in wine. Food Chem. 2010, 119, 1205–1210. [Google Scholar] [CrossRef]
- Gomes, C.A.; Girão Da Cruz, T.G.; Andrade, J.L.; Milhazes, N.; Borges, F.; Marques, M.P.M. Anticancer Activity of Phenolic Acids of Natural or Synthetic Origin: A Structure-Activity Study. J. Med. Chem. 2003, 46, 5395–5401. [Google Scholar] [CrossRef] [PubMed]
- El Abdellaoui, S.; Destandau, E.; Toribio, A.; Elfakir, C.; Lafosse, M.; Renimel, I.; André, P.; Cancellieri, P.; Landemarre, L. Bioactive molecules in Kalanchoe pinnata leaves: Extraction, purification, and identification. Anal. Bioanal. Chem. 2010, 398, 1329–1338. [Google Scholar] [CrossRef]
- Omojokun, O.S.; Oboh, G.; Ademiluyi, A.O.; Oladele, J.O.; Boligon, A.A. Impact of drying processes on Bryophyllum pinnatum phenolic constituents and its anti-inflammatory and antioxidative activities in human erythrocytes. J. Food Biochem. 2020, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fürer, K.; Raith, M.; Brenneisen, R.; Mennet, M.; Simões-Wüst, A.P.; Von Mandach, U.; Hamburger, M.; Potterat, O. Two new flavonol glycosides and a metabolite profile of Bryophyllum pinnatum, a phytotherapeutic used in obstetrics and gynaecology. Planta Med. 2013, 79, 1565–1571. [Google Scholar] [CrossRef]
- Bä, W.; Dettner, K.; Pfeifer, P. Intra- and Interspecific Allelochemical Effects in Three Kalanchoe-Species (Crassulaceae). Zeitschrift fur Naturforsch. Sect. C J. Biosci. 1997, 52, 441–449. [Google Scholar]
- Katrucha, E.M.; Lopes, J.; Paim, M.; dos Santos, J.C.; Siebert, D.A.; Micke, G.A.; Vitali, L.; Alberton, M.D.; Tenfen, A. Phenolic profile by HPLC-ESI-MS/MS and enzymatic inhibitory effect of Bryophyllum delagoense. Nat. Prod. Res. 2020, 0, 1–4. [Google Scholar] [CrossRef]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Ekiert, H.; Ali, H.M.; Elshikh, M.S.; Abdel-Salam, E.M.; El-Esawi, M.; El-Ansary, D.O. Bioactivities of Traditional Medicinal Plants in Alexandria. Evidence-Based Complement. Altern. Med. 2018, 2018. [Google Scholar] [CrossRef]
- Chibli, L.A.; Rodrigues, K.C.M.; Gasparetto, C.M.; Pinto, N.C.C.; Fabri, R.L.; Scio, E.; Alves, M.S.; Del-Vechio-Vieira, G.; Sousa, O.V. Anti-inflammatory effects of Bryophyllum pinnatum (Lam.) Oken ethanol extract in acute and chronic cutaneous inflammation. J. Ethnopharmacol. 2014, 154, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, L.B.d.S.; de Aguiar, P.F.; Leal-Costa, M.V.; Coutinho, M.A.S.; Borsodi, M.P.G.; Rossi-Bergmann, B.; Tavares, E.S.; Costa, S.S. Optimization of Aqueous Extraction from Kalanchoe pinnata Leaves to Obtain the Highest Content of an Anti-inflammatory Flavonoid using a Response Surface Model. Phytochem. Anal. 2018, 29, 308–315. [Google Scholar]
- Nielsen, A.H.; Olsen, C.E.; Møller, B.L. Flavonoids in flowers of 16 Kalanchoë blossfeldiana varieties. Phytochemistry 2005, 66, 2829–2835. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.Y.; Li, Q.; Bi, K.S. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Carotenoids and flavonoids contribute to nutritional protection against skin damage from sunlight. Mol. Biotechnol. 2007, 37, 26–30. [Google Scholar] [CrossRef]
- Mandić, L.; Sadžak, A.; Strasser, V.; Baranović, G.; Jurašin, D.D.; Sikirić, M.D.; Šegota, S. Enhanced protection of biological membranes during lipid peroxidation: Study of the interactions between flavonoid loaded mesoporous silica nanoparticles and model cell membranes. Int. J. Mol. Sci. 2019, 20, 2709. [Google Scholar] [CrossRef]
- Cherrak, S.A.; Mokhtari-Soulimane, N.; Berroukeche, F.; Bensenane, B.; Cherbonnel, A.; Merzouk, H.; Elhabiri, M. In vitro antioxidant versus metal ion chelating properties of flavonoids: A structure-activity investigation. PLoS ONE 2016, 11, 1–21. [Google Scholar] [CrossRef]
- García-Pérez, P.; Losada-Barreiro, S.; Gallego, P.P.; Bravo-Díaz, C. Cyclodextrin-elicited Bryophyllum suspension cultured cells: Enhancement of the production of bioactive compounds. Int. J. Mol. Sci. 2019, 20, 5180. [Google Scholar] [CrossRef]
- Tatsimo, S.J.N.; Tamokou, J.D.D.; Havyarimana, L.; Csupor, D.; Forgo, P.; Hohmann, J.; Kuiate, J.R.; Tane, P. Antimicrobial and antioxidant activity of kaempferol rhamnoside derivatives from Bryophyllum pinnatum. BMC Res. Notes 2012, 5, 1–6. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Orhan, D.D.; Özçelik, B.; Özgen, S.; Ergun, F. Antibacterial, antifungal, and antiviral activities of some flavonoids. Microbiol. Res. 2010, 165, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Plochmann, K.; Korte, G.; Koutsilieri, E.; Richling, E.; Riederer, P.; Rethwilm, A.; Schreier, P.; Scheller, C. Structure-activity relationships of flavonoid-induced cytotoxicity on human leukemia cells. Arch. Biochem. Biophys. 2007, 460, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Lai, C.S.; Ho, C.T. Anti-inflammatory activity of natural dietary flavonoids. Food Funct. 2010, 1, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Testai, L. Flavonoids and mitochondrial pharmacology: A new paradigm for cardioprotection. Life Sci. 2015, 135, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Babu, P.V.A.; Liu, D.; Gilbert, E.R. Recent advances in understanding the anti-diabetic actions of dietary flavonoids. J. Nutr. Biochem. 2013, 24, 1777–1789. [Google Scholar] [CrossRef]
- Muzitano, M.F.; Tinoco, L.W.; Guette, C.; Kaiser, C.R.; Rossi-Bergmann, B.; Costa, S.S. The antileishmanial activity assessment of unusual flavonoids from Kalanchoe pinnata. Phytochemistry 2006, 67, 2071–2077. [Google Scholar] [CrossRef]
- Ferreira, R.T.; Coutinho, M.A.S.; Malvar, D.D.C.; Costa, E.A.; Florentino, I.F.; Costa, S.S.; Vanderlinde, F.A. Mechanisms underlying the antinociceptive, antiedematogenic, and anti-inflammatory activity of the main flavonoid from Kalanchoe pinnata. Evidence-Based Complement. Altern. Med. 2014, 2014. [Google Scholar] [CrossRef]
- Ogungbamila, F.O.; Onawunmi, G.O.; Adeosun, O. A new acylated flavan-3-ol from Bryophyllum pinnatum. Nat. Prod. Lett. 1997, 10, 201–203. [Google Scholar] [CrossRef]
- Nascimento, L.B.D.S.; Leal-Costa, M.V.; Menezes, E.A.; Lopes, V.R.; Muzitano, M.F.; Costa, S.S.; Tavares, E.S. Ultraviolet-B radiation effects on phenolic profile and flavonoid content of Kalanchoe pinnata. J. Photochem. Photobiol. B Biol. 2015, 148, 73–81. [Google Scholar] [CrossRef]
- Henn, D.; Venter, A.; Botha, C. In vitro cytotoxicity induced by the bufadienolides 1α,2α-epoxyscillirosidine and lanceotoxin b on rat myocardial and mouse neuroblastoma cell lines. Toxins (Basel). 2019, 11, 14. [Google Scholar] [CrossRef]
- Oufir, M.; Seiler, C.; Gerodetti, M.; Gerber, J.; Fürer, K.; Mennet-von Eiff, M.; Elsas, S.M.; Brenneisen, R.; Von Mandach, U.; Hamburger, M.; et al. Quantification of Bufadienolides in Bryophyllum pinnatum Leaves and Manufactured Products by UHPLC-ESIMS/MS. Planta Med. 2015, 81, 1190–1197. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.L.; Hsu, Y.L.; Wu, T.S.; Bastow, K.F.; Lee, K.H. Kalanchosides A-C, new cytotoxic bufadienolides from the aerial parts of Kalanchoe gracilis. Org. Lett. 2006, 8, 5207–5210. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lin, X.; Yang, Z.; Zhang, W.; Ren, T.; Qu, F.; Wang, Y.; Zhang, N.; Tang, X. A bufadienolide-loaded submicron emulsion for oral administration: Stability, antitumor efficacy and toxicity. Int. J. Pharm. 2015, 479, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Bick, R.J.; Poindexter, B.J.; Sweney, R.R.; Dasgupta, A. Effects of Chan Su, a traditional Chinese medicine, on the calcium transients of isolated cardiomyocytes: Cardiotoxicity due to more than Na, K-ATPase blocking. Life Sci. 2002, 72, 699–709. [Google Scholar] [CrossRef]
- McKenzie, R.A.; Franke, F.P.; Dunster, P.J. The toxicity to cattle and bufadienolide content of six Bryophyllum species. Aust. Vet. J. 1987, 64, 298–301. [Google Scholar] [CrossRef]
- McKenzie, R.A.; Franke, F.P.; Dunster, P.J. The toxicity for cattle of bufadienolide cardiac glycosides from Bryophyllum tubiflorum flowers. Aust. Vet. J. 1989, 66, 374–376. [Google Scholar] [CrossRef]
- Gao, H.; Popescu, R.; Kopp, B.; Wang, Z. Bufadienolides and their antitumor activity. Nat. Prod. Rep. 2011, 28, 953–969. [Google Scholar] [CrossRef]
- Li, F.; Weng, Y.; Wang, L.; He, H.; Yang, J.; Tang, X. The efficacy and safety of bufadienolides-loaded nanostructured lipid carriers. Int. J. Pharm. 2010, 393, 204–212. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhao, C.; Wu, W.Y.; Fan, T.Y.; Li, N.G.; Chen, M.; Duan, J.A.; Shi, Z.H. Total synthesis, chemical modification and structure-activity relationship of bufadienolides. Eur. J. Med. Chem. 2020, 189, 112038. [Google Scholar] [CrossRef]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef]
- Moniuszko-Szajwaj, B.; Pecio, Ł.; Kowalczyk, M.; Stochmal, A. New bufadienolides isolated from the roots of Kalanchoe daigremontiana (Crassulaceae). Molecules 2016, 21, 243. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, T.; Haruna, M.; Yan, X.-Z.; Chang, J.-J.; Lee, K.-H. Antitumor agents, 110, Bryophyllin B, a novel potent cytotoxic bufadienolide from Bryophyllum pinnatum. J. Nat. Prod. 1989, 52, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Stefanowicz-Hajduk, J.; Hering, A.; Gucwa, M.; Hałasa, R.; Soluch, A.; Kowalczyk, M.; Stochmal, A.; Ochocka, R. Biological activities of leaf extracts from selected Kalanchoe species and their relationship with bufadienolides content. Pharm. Biol. 2020, 58, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Lin, M.K.; Yang, H.L.; Hseu, Y.C.; Liaw, C.C.; Tseng, Y.H.; Tsuzuki, M.; Kuo, Y.H. Cardenolides and bufadienolide glycosides from Kalanchoe tubiflora and evaluation of cytotoxicity. Planta Med. 2013, 79, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Marchev, A.S.; Yordanova, Z.P.; Georgiev, M.I. Green (cell) factories for advanced production of plant secondary metabolites. Crit. Rev. Biotechnol. 2020, 0, 1–16. [Google Scholar] [CrossRef]
- El Sheikha, A.F. Medicinal plants: Ethno-uses to biotechnology era. In Biotechnology and Production of Anti-Cancer Compounds; Malik, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–38. [Google Scholar]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 3670–3695. [Google Scholar] [CrossRef]
- Eibl, R.; Meier, P.; Stutz, I.; Schildberger, D.; Hühn, T.; Eibl, D. Plant cell culture technology in the cosmetics and food industries: Current state and future trends. Appl. Microbiol. Biotechnol. 2018, 102, 8661–8675. [Google Scholar] [CrossRef]
- Karuppusamy, S. A review on trends in production of secondary metabolites from higher plants by in vitro tissue, organ and cell cultures. J. Med. Plants Res. 2009, 3, 1222–1239. [Google Scholar]
- Thorpe, T.A. History of plant tissue culture. Mol. Biotechnol. 2007, 37, 169–180. [Google Scholar] [CrossRef]
- Su, Y.H.; Tang, L.P.; Zhao, X.Y.; Zhang, X.S. Plant cell totipotency: Insights into cellular reprogramming. J. Integr. Plant Biol. 2020, 00. [Google Scholar]
- George, E.F.; Hall, M.A.; De Klerk, G.-J. Plant tissue culture procedure-background. In Plant Propagation by Tissue Culture; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1–28. [Google Scholar]
- Da Silva, J.A.T.; Tanaka, M. Thin cell layers: The technique. In Plant Cell Culture: Essential Methods.; Davey, M.R., Anthony, P., Eds.; John Wiley & Sons: Chichester, UK, 2010; pp. 25–37. [Google Scholar]
- García-Pérez, P.; Lozano-Milo, E.; Landin, M.; Gallego, P.P. Machine Learning technology reveals the concealed interactions of phytohormones on medicinal plant in vitro organogenesis. Biomolecules 2020, 10, 746. [Google Scholar] [CrossRef] [PubMed]
- Kulus, D. Micropropagation of Kalanchoe tubiflora (Harvey) Hamet. Nauk. Przyr. Technol. 2015, 9. [Google Scholar] [CrossRef]
- Naz, S.; Javad, S.; Ilyas, S.; Ali, A. An efficient protocol for rapid multiplication of Bryophyllum pinnatum and Bryophyllum daigremontianum. Pakistan J. Bot. 2009, 41, 2347–2355. [Google Scholar]
- Frello, S.; Venerus, E.; Serek, M. Regeneration of various species of Crassulaceae, with special reference to Kalanchoë. J. Hortic. Sci. Biotechnol. 2002, 77, 204–208. [Google Scholar] [CrossRef]
- Mohammed, S.U.B.; Choi, K.-S.; Kim, T.-R.; In, J.-G.; Yang, D.-C. Plant regeneration from leaf explants of Kalanchoe daigremontiana Hamet & Perrier. Korean J. Med. Crop Sci. 2006, 14, 293–298. [Google Scholar]
- Kefu, Z.; Hai, F.; San, Z.; Jie, S. Study on the salt and drought tolerance of Suaeda salsa and Kalanchoe daigremontiana under iso-osmotic salt and water stress. Plant Sci. 2003, 165, 837–844. [Google Scholar] [CrossRef]
- George, E.F.; Hall, M.A.; De Klerk, G.-J. The components of plant tissue culture media I: Macro-and micro-nutrients. In Plant Propagation by Tissue Culture; Springer: Berlin/Heidelberg, 2008; pp. 65–113. [Google Scholar]
- Nezami-Alanagh, E.; Garoosi, G.A.; Haddad, R.; Maleki, S.; Landín, M.; Gallego, P.P. Design of tissue culture media for efficient Prunus rootstock micropropagation using artificial intelligence models. Plant Cell. Tissue Organ Cult. 2014, 117, 349–359. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Ikenganyia, E.E.; Anikwe, M.A.N.; Omeje, T.E.; Adinde, J.O. Plant tissue culture regeneration and aseptic techniques. Asian J. Biotechnol. Bioresour. Technol. 2017, 1–6. [Google Scholar] [CrossRef]
- Gamborg, O.L.; Murashige, T.; Thorpe, T.A.; Vasil, I.K. Plant tissue culture media. In Vitro 1976, 12, 473–478. [Google Scholar] [CrossRef]
- Nezami-Alanagh, E.; Garoosi, G.A.; Landín, M.; Gallego, P.P. Computer-based tools provide new insight into the key factors that cause physiological disorders of pistachio rootstocks cultured in vitro. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Phillips, G.C.; Garda, M. Plant tissue culture media and practices: An overview. Vitr. Cell. Dev. Biol. Plant 2019, 55, 242–257. [Google Scholar] [CrossRef]
- Pereira, P.N.; Cushman, J.C. Exploring the relationship between crassulacean acid metabolism (CAM) and mineral nutrition with a special focus on nitrogen. Int. J. Mol. Sci. 2019, 20, 4363. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.N.; Smith, J.A.C.; Mercier, H. Nitrate enhancement of CAM activity in two Kalanchoë species is associated with increased vacuolar proton transport capacity. Physiol. Plant. 2017, 160, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.R.A.; Ferreira, M.G.R.; Guimarães, M.C.M.; Lima, R.A.; Oliveira, C. Callogenesis in leaves of Kalanchoe pinnata Lam. by 2, 4-D and BA action. Rev. Bras. Plantas Med. 2014, 16, 760–764. [Google Scholar] [CrossRef][Green Version]
- García-Pérez, P.; Lozano-Milo, E.; Landin, M.; Gallego, P.P. Machine Learning unmasked nutritional imbalances on the medicinal plant Bryophyllum sp. cultured in vitro. Front. Plant Sci. 2020, 11, 576177. [Google Scholar] [CrossRef]
- Niedz, R.P.; Evens, T.J. A solution to the problem of ion. Nat. Methods 2006, 3, 34945. [Google Scholar] [CrossRef]
- Wilson, S.A.; Roberts, S.C. Recent advances towards development and commercialization of plant cell culture processes for the synthesis of biomolecules. Plant Biotechnol. J. 2012, 10, 249–268. [Google Scholar] [CrossRef]
- Namdeo, A.G. Plant cell elicitation for production of secondary metabolites: A review. Rev. Lit. Arts Am. 2007, 1, 69–79. [Google Scholar]
- Ramirez-Estrada, K.; Vidal-Limon, H.; Hidalgo, D.; Moyano, E.; Golenioswki, M.; Cusidó, R.M.; Palazon, J. Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules 2016, 21, 182. [Google Scholar] [CrossRef]
- Yue, W.; Ming, Q.L.; Lin, B.; Rahman, K.; Zheng, C.J.; Han, T.; Qin, L.P. Medicinal plant cell suspension cultures: Pharmaceutical applications and high-yielding strategies for the desired secondary metabolites. Crit. Rev. Biotechnol. 2016, 36, 215–232. [Google Scholar] [CrossRef] [PubMed]
- Giri, C.C.; Zaheer, M. Chemical elicitors versus secondary metabolite production in vitro using plant cell, tissue and organ cultures: Recent trends and a sky eye view appraisal. Plant Cell. Tissue Organ Cult. 2016, 126, 1–18. [Google Scholar] [CrossRef]
- Vasconsuelo, A.; Boland, R. Molecular aspects of the early stages of elicitation of secondary metabolites in plants. Plant Sci. 2007, 172, 861–875. [Google Scholar] [CrossRef]
- Narayani, M.; Srivastava, S. Elicitation: A stimulation of stress in in vitro plant cell/tissue cultures for enhancement of secondary metabolite production. Phytochem. Rev. 2017, 16, 1227–1252. [Google Scholar] [CrossRef]
- Ochoa-Villarreal, M.; Howat, S.; Hong, S.M.; Jang, M.O.; Jin, Y.W.; Lee, E.K.; Loake, G.J. Plant cell culture strategies for the production of natural products. BMB Rep. 2016, 49, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Gago, J.; Martínez-Núñez, L.; Landín, M.; Gallego, P.P. Artificial neural networks as an alternative to the traditional statistical methodology in plant research. J. Plant Physiol. 2010, 167, 23–27. [Google Scholar] [CrossRef]
- Gago, J.; Martínez-Núñez, L.; Landin, M.; Flexas, J.; Gallego, P.P. Modeling the effects of light and sucrose on in vitro propagated plants: A multiscale system analysis using artificial intelligence technology. PLoS ONE 2014, 9, e85989. [Google Scholar] [CrossRef]
- Olden, J.D.; Lawler, J.J.; Poff, N.L. Machine learning methods without tears: A primer for ecologists. Q. Rev. Biol. 2008, 83, 171–193. [Google Scholar] [CrossRef]
- Landin, M.; Rowe, R.C. Artificial neural networks technology to model, understand, and optimize drug formulations. In Formulation Tools for Pharmaceutical Development; Woodhead Publishing Limited: Cambridge, UK, 2013; pp. 7–37. ISBN 9781907568992. [Google Scholar]
- Gallego, P.P.; Gago, J.; Landín, M. Artificial Neural Networks Technology to Model and Predict Plant Biology Process. In Artificial Neural Networks; Suzuki, K., Ed.; IntechOpen: Rijeka, Croatia, 2011. [Google Scholar]
- Lu, Y.; Shao, D.; Shi, J.; Huang, Q.; Yang, H.; Jin, M. Strategies for enhancing resveratrol production and the expression of pathway enzymes. Appl. Microbiol. Biotechnol. 2016, 100, 7407–7421. [Google Scholar] [CrossRef]
- Sharma, A.; Verma, P.; Mathur, A.; Mathur, A. Genetic engineering approach using early Vinca alkaloid biosynthesis genes led to increased tryptamine and terpenoid indole alkaloids biosynthesis in differentiating cultures of Catharanthus roseus. Protoplasma 2018, 255, 425–435. [Google Scholar] [CrossRef]
- Farag, M.A.; Mekky, H.; El-Masry, S. Metabolomics driven analysis of Erythrina lysistemon cell suspension culture in response to methyl jasmonate elicitation Erythrina lysistemon cell culture metabolomics. J. Adv. Res. 2016, 7, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Cho, Y.U.; Kim, K.H.; Lee, D.Y. Distinctive metabolomic responses of Chlamydomonas reinhardtii to the chemical elicitation by methyl jasmonate and salicylic acid. Process Biochem. 2016, 51, 1147–1154. [Google Scholar] [CrossRef]
- Abenavoli, L.; Milanovic, M.; Procopio, A.C.; Spampinato, G.; Maruca, G.; Perrino, E.V.; Mannino, G.C.; Fagoonee, S.; Luzza, F.; Musarella, C.M. Ancient wheats: Beneficial effects on insulin resistance. Minerva Med. 2020. [Google Scholar] [CrossRef]
- Benmeziane-Derradji, F.; Derradji, E.-F.; Djermoune-Arkoub, L. Antioxidant activities and beneficial health effects of some dried fruits commonly consumed in Algeria: A review. Euro-Mediterranean J. Environ. Integr. 2019, 4, 28. [Google Scholar] [CrossRef]

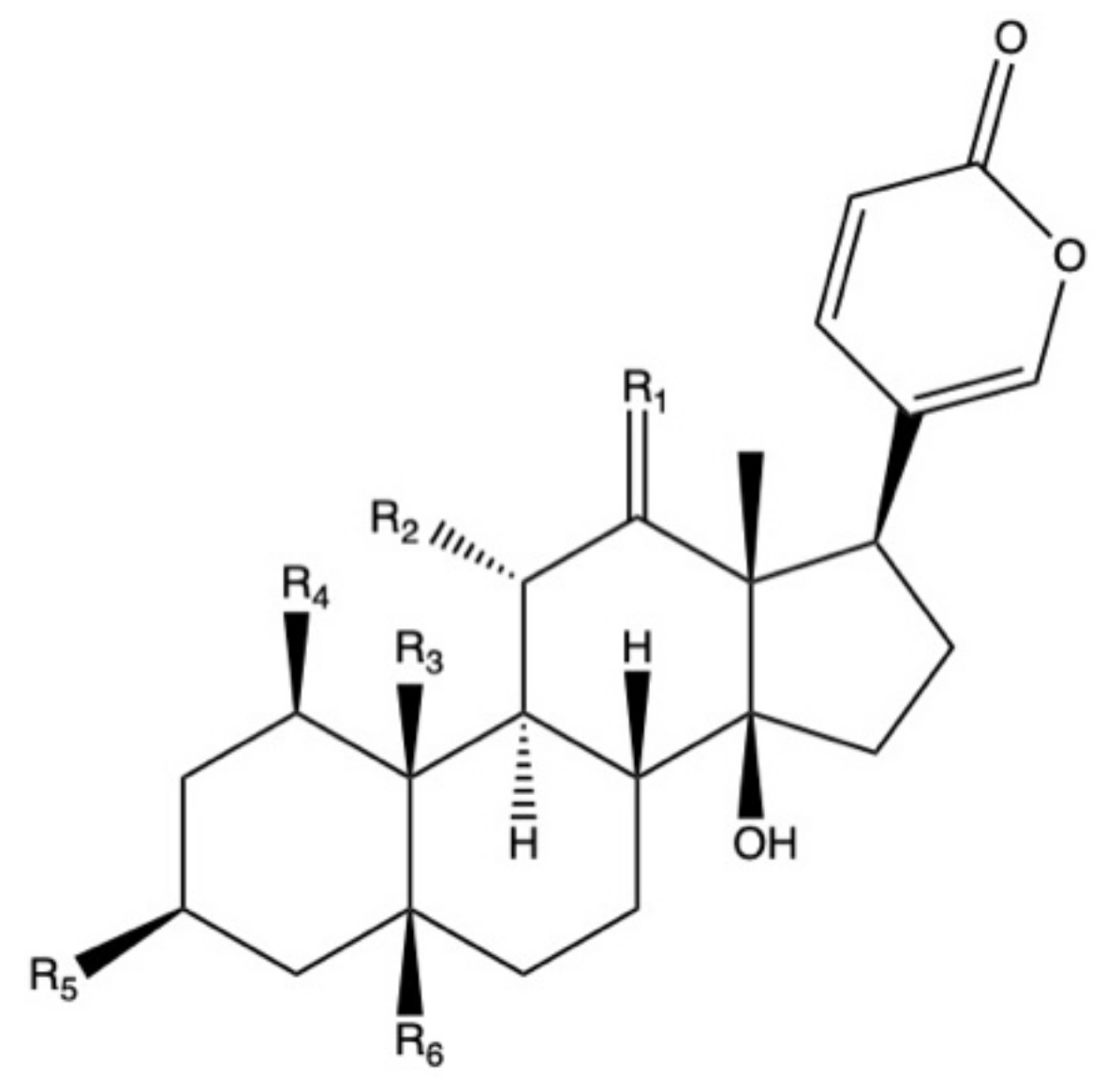
| Species | Ethnobotanical Uses | Plant Organ | Locations 1 | References |
|---|---|---|---|---|
| B. crenatum (Andr.) Baker | Wounds, smallpox, otitis, cough, asthma, palpitations, headache, abscesses, convulsions, general debility, diabetes, obstetrics and gynecology, vermifuge, abortion, antimicrobial treatment | Leaves Roots | Africa | [32,33,34,35] |
| B. daigremontianum Raym.-Hamet et Perr. | Leucorrhea, dysmenorrheal, carminative, psychic agitation, anxiety, restlessness | Leaves | Bangladesh | [28,36] |
| B. fedtschenkoi Raym.-Hamet et Perr. | Analgesic, cytotoxic, antimicrobial treatment | Leaves Aerial parts Woody stems | Brazil | [37,38,39] |
| B. mortagei (Raym.-Hamet et Perr.) G.E. Wickens | Digestive disorders, neoplastic diseases, vermifuge, antimicrobial treatment | Aerial parts Flowers Roots | Mexico, Colombia, Indonesia | [37,40,41,42] |
| B. pinnatum (Lam.) Oken | Wounds, burns, coughs, earache, headache, muscle pain, asthma, bronchitis, pneumonia, arthritis, rheumatism, ulcers, diabetes, urinary bladder stones, dysentery, diarrhea, vermifuge, antibacterial, insect bites, fevers, menstrual disorders, nausea, tumors, gynecology | Leaves Roots | Nigeria, Uganda, Madagascar, India, China, Vietnam, Bangladesh, Australia, Brazil, Peru, Trinidad and Tobago | [43,44,45,46,47,48,49,50,51,52] |
| B. serratum (Mann. and Boit.) Blanco | Pain, inflammation, fever, antiviral | Stems | Taiwan | [53,54] |
| B. tubiflorum Harv. | Wounds, epilepsy, vermifuge, neoplastic diseases | Leaves | Brazil, Ethiopia | [29,30] |
| Subfamily | Compound 1 | Species 2 | References |
|---|---|---|---|
| Cinnamic acids | p-Coumaric acid | BD, BP, BT | [63,82,88,89,90] |
| Caffeic acid | BD, BP, BT | [63,79,88,91] | |
| Chlorogenic acid | BD, BT | [63,92] | |
| Ferulic acid | BD, BP, BT | [26,63,82,92,93] | |
| Benzoic acids | p-Hydroxybenzoic acid | BD, BP, BT | [91] |
| Protocatechuic acid | BD, BP, BT | [26,63,82,91,93] | |
| Vanillic acid | BT | [58,78] | |
| Gallic acid | BD, BP, BT | [63,78,82,88,90,91,93] | |
| Syringic acid | BD, BP, BT | [63,78,90] |
| Subfamily | Compound 1 | Species 2 | References |
|---|---|---|---|
| Flavanones | Naringenin | BT | [92] |
| Flavones | Luteolin | BP | [89,94,111] |
| Apigenin | BP, BT | [50,78] | |
| 4’,5-dihydroxy-3’,8-dimethoxyflavone | BP | [109,112] | |
| Acacetin | BP | [90] | |
| Diosmetin | BP | [90] | |
| Afzelin | BP | [102] | |
| Galangustin | BT | [58] | |
| Hispidulin | BT | [92] | |
| Flavonols | Quercetin | BD, BP, BT | [58,77,78,88,89,92,94,95,109] |
| Kaempferol | BD, BP, BT | [77,78,88,89,90,92,102,109,112] | |
| Quercitrin | BP | [109,112] | |
| Myricetin | BD, BP, BT | [77,90,92] | |
| Rutin | BP | [89,94] | |
| Isorhamnetin | BD, BP | [77,88] | |
| Kaempferitrin | BP | [102] | |
| Herbacetin | BT | [58] | |
| Patuletin | BD | [77] | |
| Isoquercetin | BT | [92] | |
| Aromadendrin | BT | [92] | |
| Galangin | BT | [92] | |
| Flavanols | Catechin | BP | [89] |
| Epicatechin | BT | [92] | |
| Epigallocatechin | BP | [111] |
| Species 1 | Plant Organ | Bufadienolides | Bioactivities 2 | References |
|---|---|---|---|---|
| BD | Roots | 11α,19-dihydroksytelocinobufagin, bersaldegenin-1-acetate, bersaldegenin-1,3,5-orthoacetate, 19-(acetyloxy)-3β,5β,11α,14-tetrahydroxyl-12-oxo-bufa-20,22-dienolide and 19-(acetyloxy)-1b,3b,5b,14-tetrahydroxyl-bufa-20,22-dienolide | Moderate antioxidant activity using in vitro blood plasma model under peroxynitrite-induced oxidative stress. Effective for prevention of lipid hydroperoxides generation and thiobarbituric acid-reactive substances (TBARS) | [24] |
| BP | Leaves | Bryophyllin A and C | Insecticidal against silkworm larvae | [66] |
| BH | Leaves | Bryophyllin A and C, bersaldegenin-1-acetate, bersaldegenin-3-acetate, bersaldegenin-1,3,5-orthoacetate, daigremontianin, methyl daigremoniate | Insecticidal against silkworm larvae, except for bersaldegenin-1-acetate. Cytotoxic effect of bersaldegenin-1,3,5-orthoacetate and daigremontianin against induced Raji cell line (Burkitt’s lymphoma); inhibition of Epstein–Barr virus | [31,67] |
| BH | Whole plant | Kalanhybrins A, B and C, bersaldegenin-1-acetate, bersaldegenin-3-acetate | Cytotoxic activity of bersaldegenin derivatives against human breast MCF-7 cancer cell line, human lung carcinoma NCI-H460 and glioblastoma SF-268 cell line | [71] |
| BD | Roots | Kalandaigremosides A-H | nd | [124] |
| BP | Whole plant | Bryophyllin A and B, bersaldegenin-3-acetate | Cytotoxic effect against keratin-forming tumor KB cell line, adenocarcinomic human alveolar basal epithelial A-549 cell line and human ileocecal carcinoma HCT-8 cell line | [125] |
| BP, BD, BT | Leaves (BD, BP) and stems (BT) | BP, BT: bersaldegenin-1-acetate, bersaldegenin-3-acetate, bersaldegenin-1,3,5-orthoacetate, bryophyllin A. BD: Bersaldegenin-1,3,5-orthoacetate | nd | [114] |
| BD | Leaves | Bersaldegenin-1,3,5-orthoacetate, daigremontianin | Insecticidal against silkworm larvae | [65] |
| BP | Leaves | Bersaldegenin-1-acetate, bersaldegenin-3-acetate, bersaldegenin-1,3,5-orthoacetate, bryophyllin A | nd | [90] |
| BD, BP | Leaves | BD: Bersaldegenin-1-acetate, bersaldegenin-2-acetate, bersaldegenin-1,3,5-orthoacetate, bryophyllin A, daigremontianin. BP: Bersaldegenin-1-acetate, bersaldegenin-2-acetate, bersaldegenin-3-acetate, bersaldegenin-4-acetate, bersaldegenin-5-acetate, bersaldegenin-1,3,5-orthoacetate, bryophyllin A | Cytotoxic activity against human ovarian cancer SKOV-3 cell line, cervical adenocarcinoma HeLa S3 cell line and malignant melanoma A375 cell line. Antimicrobial activity against Corynebacterium diphtheriae, Staphylococcus aureus, Staphylococcus epidermidis, and Enterococcus hirae | [77,126] |
| BT | Whole plant | Kalantubosides A and B, bryophyllin A, bersaldegenin-1-acetate, bersaldegenin-1,3,5-orthoacetate | Cytotoxic effect against adenocarcinomic human alveolar basal epithelial A-549 cell line, promyelocytic leukemia HL-60 cell line, oral adenosquamous carcinoma Cal-27 cell line, and melanoma A2058 cell line | [127] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Pérez, P.; Lozano-Milo, E.; Landin, M.; Gallego, P.P. From Ethnomedicine to Plant Biotechnology and Machine Learning: The Valorization of the Medicinal Plant Bryophyllum sp. Pharmaceuticals 2020, 13, 444. https://doi.org/10.3390/ph13120444
García-Pérez P, Lozano-Milo E, Landin M, Gallego PP. From Ethnomedicine to Plant Biotechnology and Machine Learning: The Valorization of the Medicinal Plant Bryophyllum sp. Pharmaceuticals. 2020; 13(12):444. https://doi.org/10.3390/ph13120444
Chicago/Turabian StyleGarcía-Pérez, Pascual, Eva Lozano-Milo, Mariana Landin, and Pedro P. Gallego. 2020. "From Ethnomedicine to Plant Biotechnology and Machine Learning: The Valorization of the Medicinal Plant Bryophyllum sp." Pharmaceuticals 13, no. 12: 444. https://doi.org/10.3390/ph13120444
APA StyleGarcía-Pérez, P., Lozano-Milo, E., Landin, M., & Gallego, P. P. (2020). From Ethnomedicine to Plant Biotechnology and Machine Learning: The Valorization of the Medicinal Plant Bryophyllum sp. Pharmaceuticals, 13(12), 444. https://doi.org/10.3390/ph13120444






