Sustainability of Biosimilars in Europe: A Delphi Panel Consensus with Systematic Literature Review
Abstract
1. Introduction
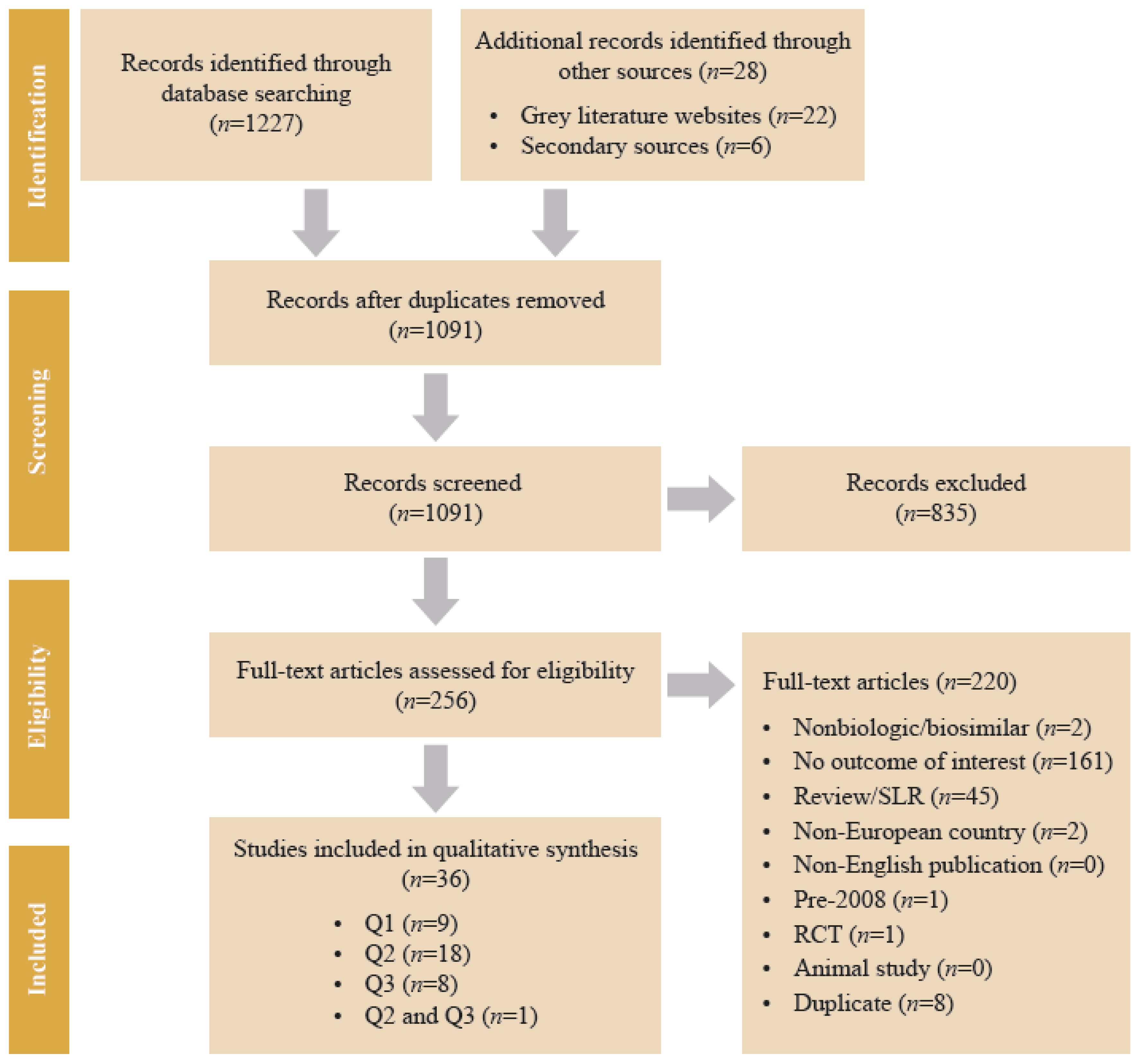
2. Methods
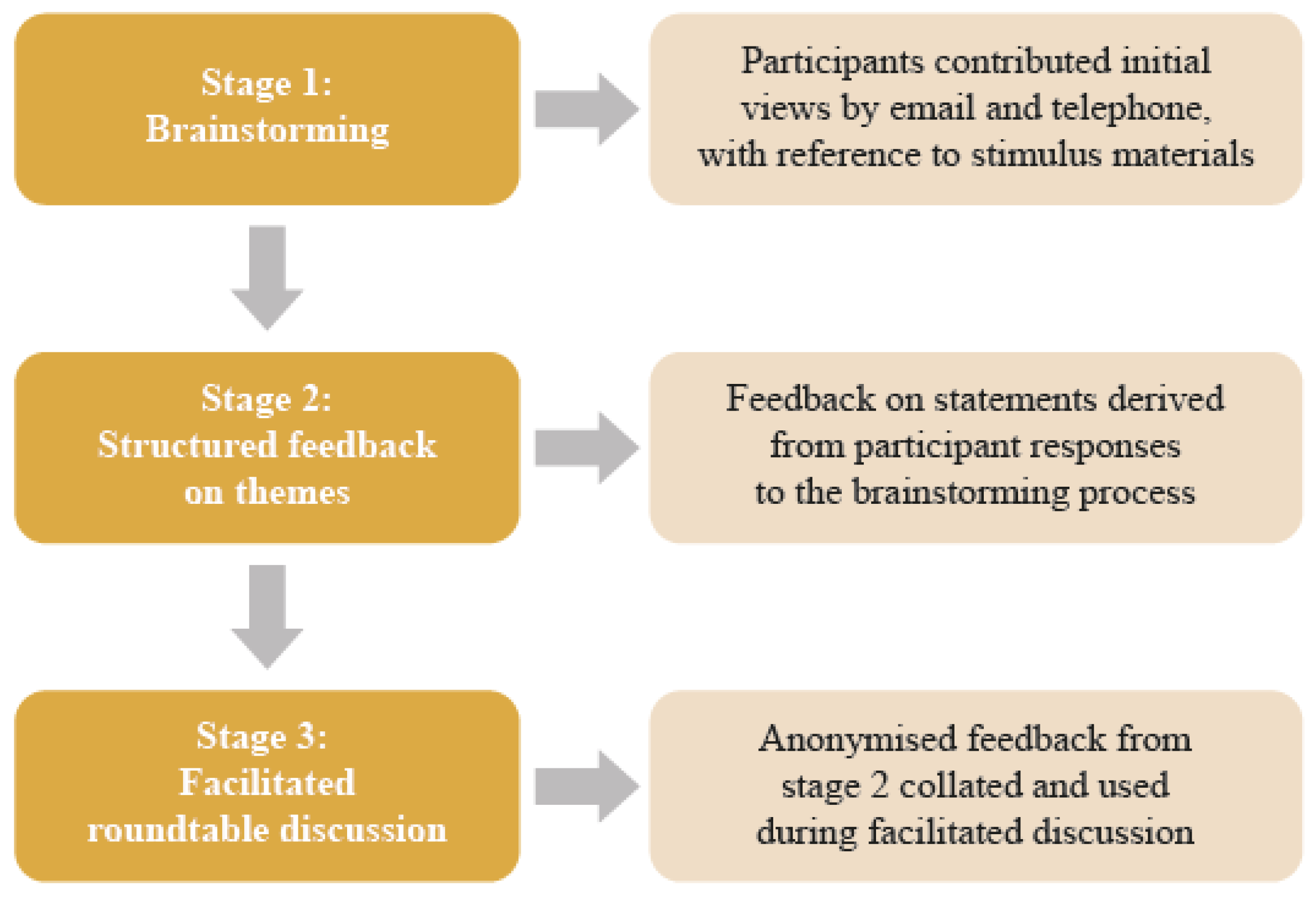
2.1. Design
2.2. Procedure
3. Results
3.1. Delphi Panel Consensus
3.1.1. A Multistakeholder Definition of Biosimilar Market Sustainability
- A sustainable biosimilar market means that…“All stakeholders, including patients, benefit from appropriate and reliable access to biological therapies. Competition leads to a long-term predictable price level, without compromising quality, while delivering savings that may be reinvested.”
3.1.2. Components of a Sustainable Biosimilar Market
- Deliver tangible and transparent benefits to the health care system, while
- Addressing the needs of all stakeholders
3.1.3. Drivers and Risks of a Sustainable Biosimilar Market (Competition and Incentives)
- Competition is a more effective mechanism to achieve a long-term predictable price level, compared to regulation
- There needs to be incentives for industry investment in future biosimilars
- Government and pricing bodies need to drive incentives
- Procurement processes should avoid monopolies and minimize patient discomfort and disruption to the health care system
- The principles for procurement should be defined by all stakeholders.
3.1.4. Drivers and Risks of a Sustainable Biosimilar Market (Procurement Processes)
3.2. Key Findings from the SLR
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| |
| |
| |
| |
| |
| |
Appendix B
Appendix C
| Topic | Source | References |
|---|---|---|
| Savings (implications for health system sustainability) | Systematic literature review/targeted additional search | Vulto A, et al. (2019) [18] |
| Sustainable competition | Systematic literature review | Mestre-Ferrandiz J, et al. (2016) [16] Dave CV, et al. (2017) [25] Dave CV, et al. (2018) [26] |
| Access and pricing | Targeted additional search | Moorkens E, et al. (2017) [17] Kawalec P, et al. (2017) [27] |
| Procurement/purchasing | Review of tender documents (2018) | Vulto A, et al. (2019) [18] |
| Patient safety/use | Targeted additional search | Tabernero J, et al. (2016) [4] EULAR PARE (2018) [3] |
Appendix D
| Presence of multiple suppliers on an ongoing basis—although there is no “correct” number of suppliers |
|
| Competition that is effective in reducing prices for biologics/biosimilars to a sustainable level |
|
| Shared decision making with payer, pharmacist, physician, and patients around biosimilar use |
|
| Reliable supply of biosimilars that meet appropriate standards for quality |
|
| Stability in procurement structure and approach |
|
| Avoidance of price erosion that leads to market exit and the emergence of monopolies or the consolidation of suppliers |
|
References
- Market Data Forecast. Biosimilars Market. Available online: https://www.marketdataforecast.com/market-reports/biosimilars-market (accessed on 13 May 2020).
- Technavio. Global Biosimilars Market 2018–2022. Available online: https://www.technavio.com/report/global-biosimilars-market-analysis-share-2018?tnplus (accessed on 13 May 2020).
- EULAR (European League Against Rheumatism) Standing Committee of People with Arthritis/Rheumatism in Europe (PARE). Biosimilars—Position Paper. Updating Position Statement from the European League against Rheumatism (EULAR) Standing Committee of People with Arthritis/Rheumatism in Europe (PARE). Available online: https://www.eular.org/myUploadData/files/biosimilars_paper_updated_2018_09_14_dw.pdf (accessed on 13 May 2020).
- Tabernero, J.; Vyas, M.; Giuliani, R.; Arnold, D.; Cardoso, F.; Casali, P.G.; Cervantes, A.; Eggermont, A.M.; Eniu, A.; Jassem, J.; et al. Biosimilars: A position paper of the European Society for Medical Oncology, with particular reference to oncology prescribers. ESMO Open 2016, 1, e000142. [Google Scholar] [CrossRef] [PubMed]
- EMA (European Medicines Agency). European Commission. Biosimilars in the EU. Information Guide for Healthcare Professionals. Available online: https://www.ema.europa.eu/en/documents/leaflet/biosimilars-eu-information-guide-healthcare-professionals_en.pdf (accessed on 13 May 2020).
- EMA (European Medicines Agency). Guidelines on Similar Biological Medicinal Products. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-similar-biological-medicinal-products-rev1_en.pdf (accessed on 13 May 2020).
- US FDA (Food and Drug Administration). Biosimilar and Interchangeable Products. Available online: https://www.fda.gov/drugs/biosimilars/biosimilar-and-interchangeable-products#generic (accessed on 13 May 2020).
- IQVIA Institute. The Impact of Biosimilar Competition in Europe. Available online: https://ec.europa.eu/docsroom/documents/38461 (accessed on 13 May 2020).
- Dutta, B.; Huys, I.; Vulto, A.G.; Simoens, S. Identifying key benefits in European off-patent biologics and biosimilar markets: It is not only about price! BioDrugs 2020, 34, 159–170. [Google Scholar] [CrossRef] [PubMed]
- IQVIA Institute. The Global Use of Medicine in 2019 and Outlook to 2023. Forecasts and Areas to Watch. Institute Report. Available online: https://www.iqvia.com/insights/the-iqvia-institute/reports/the-global-use-of-medicine-in-2019-and-outlook-to-2023 (accessed on 13 May 2020).
- IQVIA Institute. Advancing Biosimilar Sustainability in Europe. A Multi-Stakeholder Assessment. Institute Report. Available online: https://www.iqvia.com/insights/the-iqvia-institute/reports/advancing-biosimilar-sustainability-in-europe (accessed on 13 May 2020).
- Deloitte Development LLC. Winning with Biosimilars: Opportunities in Global Markets. Available online: https://www2.deloitte.com/content/dam/Deloitte/us/Documents/life-sciences-health-care/us-lshc-biosimilars-whitepaper-final.pdf (accessed on 13 May 2020).
- Danese, S.; Fiorino, G.; Raine, T.; Ferrante, M.; Kemp, K.; Kierkus, J.; Lakatos, P.L.; Mantzaris, G.; Van Der Woude, J.; Panes, J.; et al. ECCO position statement on the use of biosimilars for inflammatory bowel disease-an update. J. Crohns Colitis 2017, 11, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Digestive Cancers Europe. Position Paper on the Use of Biosimilar Medicines in Colorectal Cancer. Available online: https://digestivecancers.eu/Documents/Uploaded/468-Document-Positionpaperbiosimilarsfinal.pdf (accessed on 13 May 2020).
- EAHP (European Association of Hospital Pharmacists). EAHP Position Paper on Biosimilar Medicines. Available online: https://www.eahp.eu/content/position-paper-biosimilar-medicines-0 (accessed on 13 May 2020).
- Mestre-Ferrandiz, J.; Towse, A.; Berdud, M. Biosimilars: How can payers get long-term savings? Pharmacoeconomics 2016, 34, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Moorkens, E.; Vulto, A.G.; Huys, I.; Dylst, P.; Godman, B.; Keuerleber, S.; Claus, B.; Dimitrova, M.; Petrova, G.; Sović-Brkičić, L.; et al. Policies for biosimilar uptake in Europe: An overview. PLoS ONE 2017, 12, e0190147. [Google Scholar] [CrossRef] [PubMed]
- Vulto, A.; Cheesman, S.; Gonzalez-McQuire, S.; Lebioda, A.; Bech, A.; Hippenmeyer, J.; Lapham, K. Sustainable biosimilar procurement in Europe: A review of current policies and their potential impact. Value Health 2019, 22, S427. [Google Scholar] [CrossRef]
- Simon Kucher & Partners. Payers’ Price & Market Access Policies Supporting a Sustainable Biosimilar Medicines Market. Final Report. Available online: https://www.medicinesforeurope.com/wp-content/uploads/2016/09/Simon-Kucher-2016-Policy-requirements-for-a-sustainable-biosimilar-market-FINAL-report_for-publication2.pdf (accessed on 13 May 2020).
- Pugatch Consilium. Towards a Sustainable European Market for Off-Patent Biologics. Available online: https://www.pugatch-consilium.com/?p=2760 (accessed on 13 May 2020).
- Eubank, B.H.; Mohtadi, N.G.; Lafave, M.R.; Wiley, J.P.; Bois, A.J.; Boorman, R.S.; Sheps, D.M. Using the modified Delphi method to establish clinical consensus for the diagnosis and treatment of patients with rotator cuff pathology. BMC Med. Res. Methodol. 2016, 16, 56. [Google Scholar] [CrossRef] [PubMed]
- Hirschhorn, F. Reflections on the application of the Delphi method: Lessons from a case in public transport research. Int. J. Soc. Res. Methodol. 2019, 22, 309–322. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. (Eds.) The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). Available online: https://handbook-5-1.cochrane.org/front_page.htm (accessed on 13 May 2020).
- Centre for Reviews and Dissemination. Systematic Reviews. CRD’s Guidance for Undertaking Reviews in Health Care. Available online: https://www.york.ac.uk/media/crd/Systematic_Reviews.pdf (accessed on 13 May 2020).
- Dave, C.V.; Kesselheim, A.S.; Fox, E.R.; Qiu, P.; Hartzema, A. High generic drug prices and market competition: A retrospective cohort study. Ann. Intern. Med. 2017, 167, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Dave, C.V.; Pawar, A.; Fox, E.R.; Brill, G.; Kesselheim, A.S. Predictors of drug shortages and association with generic drug prices: A retrospective cohort study. Value Health 2018, 21, 1286–1290. [Google Scholar] [CrossRef] [PubMed]
- Kawalec, P.; Stawowczyk, E.; Tesar, T.; Skoupa, J.; Turcu-Stiolica, A.; Dimitrova, M.; Petrova, G.I.; Rugaja, Z.; Männik, A.; Harsanyi, A.; et al. Pricing and reimbursement of biosimilars in central and eastern European countries. Front. Pharmacol. 2017, 8, 288. [Google Scholar] [CrossRef] [PubMed]
- NHS (National Health Service). NHS Cuts Medicines Costs by Three Quarters of a Billion Pounds. Available online: https://www.england.nhs.uk/2019/08/nhs-cuts-medicines-costs-by-three-quarters-of-a-billion-pounds/ (accessed on 13 May 2020).
- Zorginstituut Nederland. GIPdatabank.nl. Available online: https://www.gipdatabank.nl/databank (accessed on 13 May 2020).
- Amgros. Price Negotiations and Effective Competition. Available online: https://amgros.dk/en/pharmaceuticals/price-negotiations-and-effective-competition/ (accessed on 13 May 2020).
- Strober, B.; Ryan, C.; van de Kerkhof, P.; Van Der Walt, J.; Kimball, A.B.; Barker, J.; Blauvelt, A.; Bourcier, M.; Carvalho, A.; Cohen, A.; et al. Recategorization of psoriasis severity: Delphi consensus from the International Psoriasis Council. J. Am. Acad. Dermatol. 2020, 82, 117–122. [Google Scholar] [CrossRef] [PubMed]
| |
| Biosimilars have the potential to reduce the cost of treatment; this, in turn, strengthens the sustainability of health care expenditure |  |
| Biosimilar-related savings must be tangible and transparent and should be reinvested efficiently; this may include addressing deficits, and funding innovative therapies, health care or other public services. Biosimilars have the potential to expand access |  |
| Providers (physicians and pharmacists) incur real costs when transitioning to a new biosimilar; transition should only occur if savings substantially exceed these transition costs and a portion of the savings are used to meet these costs |  |
| |
| Transitioning between biosimilars causes disruption to patient care and health care services. Unnecessary disruptions (i.e., frequent transitions and/or transitions that do not deliver tangible savings) should be minimized |  |
| Disruption caused by biosimilar transition may be unavoidable in some therapeutic areas (e.g., acute vs. chronic conditions); however, switch is not advisable if treatment duration is short |  |
| Disruption and transition costs occur in both hospital and out-of-hospital (including retail and home care) settings; these differences may need to be considered |  |
| |
| Policies and practices must encourage trust in biosimilar use among patients through effective communication between stakeholders |  |
| Language and messaging should be consistent among stakeholders and coordinated nationally |  |
| Clear guidance from regulators and clinical organisations at European and national levels is required to motivate multiple switches (i.e., following the initial transition from original biological to biosimilar) |  |
|  |
|  |
| |
| Increased competition leads to more rapid price reduction and, if procurement policies contribute to business continuity, a sustained lower price level |  |
| There is a need to develop better prospective indicators to warn about potential risk of de facto monopoly |  |
|  |
| New entrants may bring minor improvements (e.g., administration devices), although competition has been primarily price-focused and has led to a reduction in “value-add” (e.g., patient support programs) |  |
| Price-setting regulation, if needed to prevent predatory behaviour, should not aim primarily at the lowest possible prices but at long-term viability of a vibrant and competitive marketplace |  |
| |
| Continued investment in biosimilar development and market entry is important to generate competition for biological therapies for which no biosimilar is currently available and, to a lesser extent, therapies with biosimilars already available |  |
| Price expectations of policy and budget holders must reflect market opportunity, e.g., biosimilars of orphan therapies may require lower price discount levels |  |
| A stable, predictable price level enables manufacturers to make the long-term decisions that are required to invest in biosimilar development |  |
| |
| These bodies need to supply incentives that enable enough suppliers to survive free market onslaught; this may assure the continuity of long-term competition and sustainable discounts from originator biological therapy price levels |  |
|  |
| |
| The emergence of monopolies may lead to higher price levels and/or enhanced supply risks |  |
|  |
| Procurement design should aim to: | |
|  |
|  |
| |
| There should be a multistakeholder group that sets principles for policy and practice around biosimilar procurement |  |
| Patients and physicians should have an opportunity for their views to be represented (e.g., in a national forum) and patients should be informed of the rationale behind procurement decisions that impact on their care |  |
| There can be no one-size-fits-all approach to procurement, as the structure and characteristics of health care systems vary; however, there should be a consistent approach and a common set of guiding principles |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vulto, A.G.; Vanderpuye-Orgle, J.; van der Graaff, M.; Simoens, S.R.A.; Dagna, L.; Macaulay, R.; Majeed, B.; Lemay, J.; Hippenmeyer, J.; Gonzalez-McQuire, S. Sustainability of Biosimilars in Europe: A Delphi Panel Consensus with Systematic Literature Review. Pharmaceuticals 2020, 13, 400. https://doi.org/10.3390/ph13110400
Vulto AG, Vanderpuye-Orgle J, van der Graaff M, Simoens SRA, Dagna L, Macaulay R, Majeed B, Lemay J, Hippenmeyer J, Gonzalez-McQuire S. Sustainability of Biosimilars in Europe: A Delphi Panel Consensus with Systematic Literature Review. Pharmaceuticals. 2020; 13(11):400. https://doi.org/10.3390/ph13110400
Chicago/Turabian StyleVulto, Arnold G., Jackie Vanderpuye-Orgle, Martin van der Graaff, Steven R. A. Simoens, Lorenzo Dagna, Richard Macaulay, Beenish Majeed, Jeffrey Lemay, Jane Hippenmeyer, and Sebastian Gonzalez-McQuire. 2020. "Sustainability of Biosimilars in Europe: A Delphi Panel Consensus with Systematic Literature Review" Pharmaceuticals 13, no. 11: 400. https://doi.org/10.3390/ph13110400
APA StyleVulto, A. G., Vanderpuye-Orgle, J., van der Graaff, M., Simoens, S. R. A., Dagna, L., Macaulay, R., Majeed, B., Lemay, J., Hippenmeyer, J., & Gonzalez-McQuire, S. (2020). Sustainability of Biosimilars in Europe: A Delphi Panel Consensus with Systematic Literature Review. Pharmaceuticals, 13(11), 400. https://doi.org/10.3390/ph13110400





