Later-Line Treatment with Lorlatinib in ALK- and ROS1-Rearrangement-Positive NSCLC: A Retrospective, Multicenter Analysis
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Efficacy
2.2.1. ALK-Positive Patients
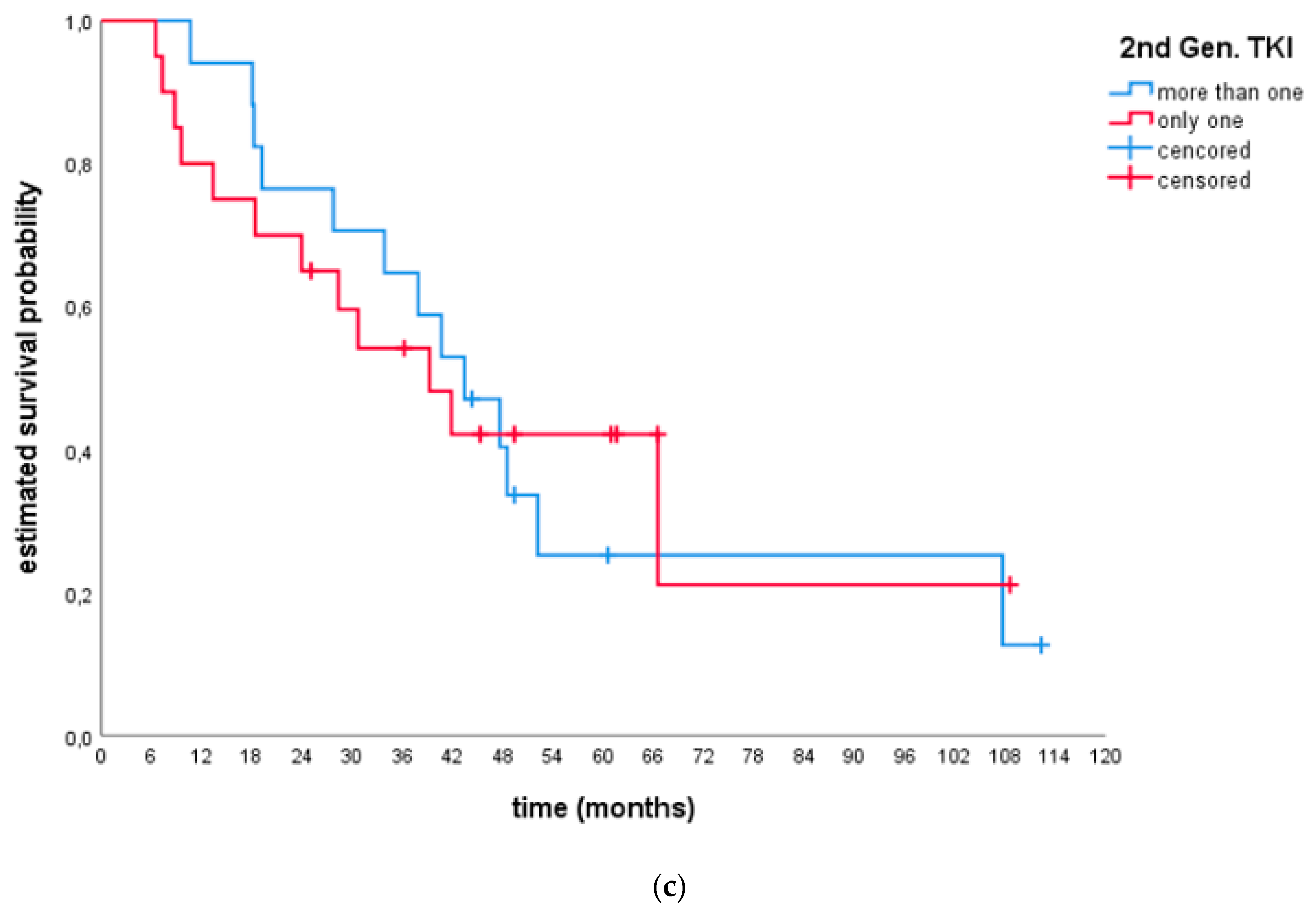
- Efficacy after only 1 s generation TKI and ≥2 s generation TKIs:
- Efficacy after alectinib and brigatinib:
2.2.2. ROS1-Positive Patients
2.3. Tolerability
3. Discussion
4. Materials and Methods
Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Soda, M.; Choi, Y.L.; Enomoto, M.; Takada, S.; Yamashita, Y.; Ishikawa, S.; Fujiwara, S.; Watanabe, H.; Kurashina, K.; Hatanaka, H.; et al. Identification of the transforming EML4-ALK fusion gene in non–small-cell lung cancer. Nature 2007, 448, 561–566. [Google Scholar] [CrossRef]
- Takeuchi, K.; Choi, Y.L.; Soda, M.; Inamura, K.; Togashi, Y.; Hatano, S.; Enomoto, M.; Takada, S.; Yamashita, Y.; Satoh, Y.; et al. Multiplex reverse transcription-PCR screening for EML4-ALK fusion transcripts. Clin. Cancer Res. 2008, 14, 6618–6624. [Google Scholar] [CrossRef]
- Koivunen, J.P.; Mermel, C.; Zejnullahu, K.; Murphy, C.; Lifshits, E.; Holmes, A.J.; Choi, H.G.; Kim, J.; Chiang, D.; Thomas, R.; et al. EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin. Cancer Res. 2008, 14, 4275–4283. [Google Scholar] [CrossRef]
- Mano, H. Non-solid oncogenes in solid tumors: EML4-ALK fusion genes in lung cancer. Cancer Sci. 2008, 99, 2349–2355. [Google Scholar] [CrossRef] [PubMed]
- Perner, S.; Wagner, P.L.; Demichelis, F.; Mehra, R.; Lafargue, C.J.; Moss, B.J.; Arbogast, S.; Soltermann, A.; Weder, W.; Giordano, T.J.; et al. EML4-ALK fusion lung cancer: A rare acquired event. Neoplasia 2008, 10, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.W.; Leung, E.L.; So, K.K.; Tam, I.Y.; Sihoe, A.D.; Cheng, L.C.; Ho, K.K.; Au, J.S.; Chung, L.P.; Pik Wong, M.; et al. The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer 2009, 115, 1723–1733. [Google Scholar] [CrossRef]
- Chong, C.R.; Bahcall, M.; Capelletti, M.; Kosaka, T.; Ercan, D.; Sim, T.; Sholl, L.M.; Nishino, M.; Johnson, B.E.; Gray, N.S.; et al. Identification of existing drugs that effectively target NTRK1 and ROS1 rearrangements in lung cancer. Clin. Cancer Res. 2017, 23, 204–213. [Google Scholar] [CrossRef]
- Katayama, R.; Lovely, C.M.; Shaw, A.T. Therapeutic targeting of anaplastic lymphoma kinase in lung cancer: A paradigm for precision cancer medicine. Clin. Cancer Res. 2015, 21, 2227–2235. [Google Scholar] [CrossRef]
- Zou, H.Y.; Li, Q.; Engstrom, L.D.; West, M.; Appleman, V.; Wong, K.A.; McTigue, M.; Deng, Y.L.; Liu, W.; Brooun, A.; et al. PF-06463922 is a potent and selective next-generation ROS1/ALK inhibitor capable of blocking crizotinib-resistant ROS1 mutations. Proc. Natl. Acad. Sci. USA 2015, 112, 3493–3498. [Google Scholar] [CrossRef]
- Song, Z.; Wang, M.; Zhang, A. Alectinib: A novel second generation anaplastic lymphoma kinase (ALK) inhibitor for overcoming clinically-acquired resistance. Acta Pharm. Sin. 2015, 5, 34–37. [Google Scholar] [CrossRef]
- Kodama, T.; Tsukaguchi, T.; Satoh, Y.; Yoshida, M.; Watanabe, Y.; Kondoh, O.; Sakamoto, H. Alectinib shows potent antitumor activity against RET-rearranged non–small-cell lung cancer. Mol. Cancer Ther. 2014, 13, 2910–2918. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Anjum, R.; Squillace, R.; Nadworny, S.; Zhou, T.; Keats, J.; Ning, Y.; Wardwell, S.D.; Miller, D.; Song, Y.; et al. The potent ALK inhibitor brigatinib (AP26113) overcomes mechanisms of resistance to first- and second-generation ALK inhibitors in preclinical models. Clin. Cancer Res. 2016, 22, 5527–5538. [Google Scholar] [CrossRef] [PubMed]
- Gainor, J.F.; Dardaei, L.; Yoda, S.; Friboulet, L.; Leshchiner, I.; Katayama, R.; Dagogo-Jack, I.; Gadgeel, S.; Schultz, K.; Singh, M.; et al. Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov. 2016, 6, 1118–1133. [Google Scholar] [CrossRef]
- Iwama, E.; Okamoto, I.; Harada, T.; Takayama, K.; Nakanishi, Y. Development of anaplastic lymphoma kinase (ALK) inhibitors and molecular diagnosis in ALK-rearrangement-positive lung cancer. OncoTargets Ther. 2014, 7, 375–385. [Google Scholar]
- Basit, S.; Ashraf, Z.; Lee, K.; Latif, M. First macrocyclic 3rd-generation ALK inhibitor for treatment of ALK/ROS1 cancer: Clinical and designing strategy update of lorlatinib. Eur. J. Med. Chem. 2017, 134, 348–356. [Google Scholar] [CrossRef]
- Zou, H.Y.; Friboulet, L.; Kodack, D.P.; Engstrom, L.D.; Li, Q.; West, M.; Tang, R.W.; Wang, H.; Tsaparikos, K.; Wang, J.; et al. PF-06463922, an ALK/ROS1 inhibitor, overcomes resistance to first and second generation ALK inhibitors in preclinical models. Cancer Cell 2015, 28, 70–81. [Google Scholar] [CrossRef]
- Cummings, M.D.; Sekharan, S. Structure-Based Macrocycle Design in Small-Molecule Drug Discovery and Simple Metrics to Identify Opportunities for Macrocyclization of Small-Molecule Ligands. J. Med. Chem. 2019, 62, 6843–6853. [Google Scholar] [CrossRef]
- Engelhardt, H.; Böse, D.; Petronczki, M.; Scharn, D.; Bader, G.; Baum, A.; Bergner, A.; Chong, E.; Döbel, S.; Egger, G.; et al. Start Selective and Rigidify: The Discovery Path toward a Next Generation of EGFR tyrosine kinase inhibitors. J. Med. Chem. 2019, 62, 10272–10293. [Google Scholar] [CrossRef]
- Shaw, A.T.; Kim, D.W.; Mehra, R.; Tan, D.S.; Felip, E.; Chow, L.Q.; Camidge, D.R.; Vansteenkiste, J.; Sharma, S.; De Pas, T.; et al. Ceritinib in ALK-rearranged non–small-cell lung cancer. N. Engl. J. Med. 2014, 370, 1189–1197. [Google Scholar] [CrossRef]
- Gainor, J.F.; Tan, D.S.; De Pas, T.; Solomon, B.J.; Ahmad, A.; Lazzari, C.; de Marinis, F.; Spitaleri, G.; Schultz, K.; Friboulet, L.; et al. Progression-free and overall survival in ALK-positive NSCLC patients treated with sequential crizotinib and ceritinib. Clin. Cancer Res. 2015, 21, 2745–2752. [Google Scholar] [CrossRef]
- Besse, B.; Solomon, B.J.; Felip, E.; Bauer, T.M.; Ou, S.-H.I.; Soo, R.A.; Camidge, D.R.; Chiari, R.; Gadgeel, S.M.; Riely, G.J.; et al. Lorlatinib in patients with previously treated ALK+ advanced non–small-cell lung cancer (NSCLC): Updated efficacy and safety. J. Clin. Oncol. 2018, 36, 9032. [Google Scholar] [CrossRef]
- Canale, M.; Pasini, L.; Bronte, G.; Delmonte, A.; Cravero, P.; Crinò, L.; Ulivi, P. Role of liquid biopsy in oncogene-addicted non–small-cell lung cancer. Transl. Lung Cancer Res. 2019, 8 (Suppl. 3), S265–S279. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network (NCCN) Guidelines on Non–Small-Cell Lung Cancer; Version 1; 2020; Available online: https://www.nccn.org/professionals/physician_gls/default.aspx (accessed on 6 November 2020).
- Lindeman, N.I.; Cagle, P.T.; Aisner, D.L.; Arcila, M.E.; Beasley, M.B.; Bernicker, E.H.; Colasacco, C.; Dacic, S.; Hirsch, F.R.; Kerr, K.; et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: Guidelines from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. J. Mol. Diagn. 2018, 20, 129–159. [Google Scholar]
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; Van Schil, P.E.; Hellmann, M.D.; et al. Metastatic non–small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Summary of Product Characteristics Lorviqua®. Available online: https://www.ema.europa.eu/en/documents/product-information/lorviqua-epar-product-information_en.pdf (accessed on 6 November 2020).
- Summary of Product Characteristics Lorbrena®. Available online: http://labeling.pfizer.com/ShowLabeling.aspx?id=11140 (accessed on 6 November 2020).
- Solomon, B.J.; Besse, B.; Bauer, T.M.; Felip, E.; Soo, R.A.; Camidge, D.R.; Chiari, R.; Bearz, A.; Lin, C.C.; Gadgeel, S.M.; et al. Lorlatinib in patients with ALK-positive non–small-cell lung cancer: Results from a global phase 2 study. Lancet Oncol. 2018, 19, 1654–1667. [Google Scholar] [CrossRef]
- Gobbini, E.; Chiari, R.; Pizzutillo, P.; Bordi, P.; Ghilardi, L.; Pilotto, S.; Osman, G.; Cappuzzo, F.; Cecere, F.; Riccardi, F.; et al. Real-world outcomes according to treatment strategies in ALK-rearranged non–small-cell lung cancer (NSCLC) patients: An Italian retrospective study. Clin. Transl. Oncol. 2020, 33, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; Shaw, A.T.; Besse, B. Impact of lorlatinib on patient-reported outcomes in patients with advanced ALK-positive or ROS1-positive non-small cell lung cancer. Lung Cancer 2020, 144, 10–19. [Google Scholar] [CrossRef]
- Zhu, V.W.; Lin, Y.-T.L.; Kim, D.-W.; Loong, H.H.; Nagasaka, M.; To, H.; Ang, Y.L.; Ock, C.Y.; Tchekmedyian, N.; Ou, S.I.; et al. An international Real-world Analysis of the Efficacy and safety of lorlatinib through early or expanded access preograms in patients with tyrosine kinase inhibitor-refractory ALK-positive or ROS1-positive NSCLC. JTO 2020, 15, 1484–1496. [Google Scholar] [PubMed]


| Patient Characteristics | ALK(+) | ROS(+) |
|---|---|---|
| N (%) | 37 (72.5%) | 14 (27.5%) |
| Age at metastatic diagnosis | ||
| Mean age (SD) at met. diagnosis | 53.0 (13.4) | 55.4 (16.4) |
| Range | 29–77 | 26–82 |
| Sex | ||
| Female | 24 (64.9%) | 7 (50.0%) |
| Male | 13 (35.1%) | 7 (50.0%) |
| Smoking history | ||
| Current | 3 (8.1%) | 1 (7.1%) |
| Former | 11 (29.7%) | 5 (35.7%) |
| Never-Smoker | 23 (62.2%) | 8 (57.1%) |
| Stage at initial diagnosis | ||
| Stage I | 1 (2.7%) | 1 (7.1%) |
| Stage II | 0 (0%) | 0 (0%) |
| Stage IIIa | 3 (8.1%) | 0 (0%) |
| Stage IIIb | 3 (8.1%) | 0 (0%) |
| Stage IV | 30 (81.1%) | 13 (92.9%) |
| Brain metastasis at diagnosis | ||
| Yes | 19 (51.4%) | 9 (64.3%) |
| No | 15 (40.5%) | 4 (28.6%) |
| Unknown | 3 (8.1%) | 1 (7.1%) |
| Method of detection | ||
| IHC | 13 (35.1%) | 5 (35.7%) |
| FISH | 17 (45.9%) | 7 (50.0%) |
| NGS | 1 (2.7%) | 0 (0%) |
| More than 1 | 6 (16.2%) | 2 (14.3%) |
| Histology | ||
| Adenocarcinoma | 35 (94.6%) | 13 (92.8) |
| Adeno-squamous | 1 (2.7%) | 1 (7.1%) |
| Squamous-cell carcinoma | 1 (2.7%) | 0 (0%) |
| Prior lines of therapy | ||
| 2 lines | 3 (8.1%) | 6 (42.9%) |
| 3 lines | 11 (29.7%) | 2 (14.3%) |
| 4 lines | 14 (37.8%) | 1 (7.1%) |
| 5 lines | 8 (21.6%) | 4 (28.6%) |
| 6 lines | 0 (0%) | 1 (7.1%) |
| 9 lines | 1 (2.7%) | 0 (0%) |
| Prior lines of TKI | ||
| 1 lines | 10 (27.0%) | 11 (78.6%) |
| 2 lines | 13 (35.1%) | 3 (21.4%) |
| 3 lines | 13 (35.1%) | 0 (0%) |
| 4 lines | 1 (2.7%) | 0 (0%) |
| Prior lines in detail | ||
| Alectinib | 14 (37.8%) | 0 (0%) |
| Brigatinib | 27 (73.0%) | 0 (0%) |
| Ceritinib | 21 (56.8%) | 3 (21.4%) |
| Crizotinib | 25 (67.6%) | 14 (100%) |
| At least one Chemotherapy | 10 (27.0%) | 8 (57.1%) |
| Efficacy Results of ALK Positive Patients | ||||||
| Results | Overall | 1 Prior TKI | 2 Prior TKI | ≥3 Prior TKI | Only One 2nd Gen TKI | ≥Two 2nd Gen TKI |
| N (%) | 37 (100%) | 10 (27.0%) | 13 (35.1%) | 14 (37.8%) | 20 (54.1%) | 17 (45.9%) |
| ORR1 | 43.2% (27.1; 60.5) | 40.0% (12.2; 73.8) | 53.8% (25.1; 80.8) | 35.7% (12.8; 64.9) | 50.0% (27.2; 72.8) | 35.3% (14.2; 61.7) |
| DCR1 | 56.8% (39.5; 72.9) | 60.0% (26.2; 87.8) | 69.2% (38.6; 90.9) | 42.9% (17.7; 71.1) | 60.0% (36.1; 80.9) | 52.9% (27.8; 77.0) |
| CR | 1 (2.7%) | 0 (0%) | 0 (0%) | 1 (7.1%) | 0 (0.0%) | 1 (5.9%) |
| PR | 15(40.5%) | 4 (40.0%) | 7 (53.8%) | 4 (28.6%) | 10 (50.0%) | 5 (29.4%) |
| SD | 5 (13.5%) | 2 (20.0%) | 2 (15.4%) | 1 (7.1%) | 3 (10.0%) | 3 (17.6%) |
| PD | 16 (43.2) | 4 (40.0%) | 4 (30.8%) | 8 (57.1%) | 8 (40.0%) | 8 (47.1%) |
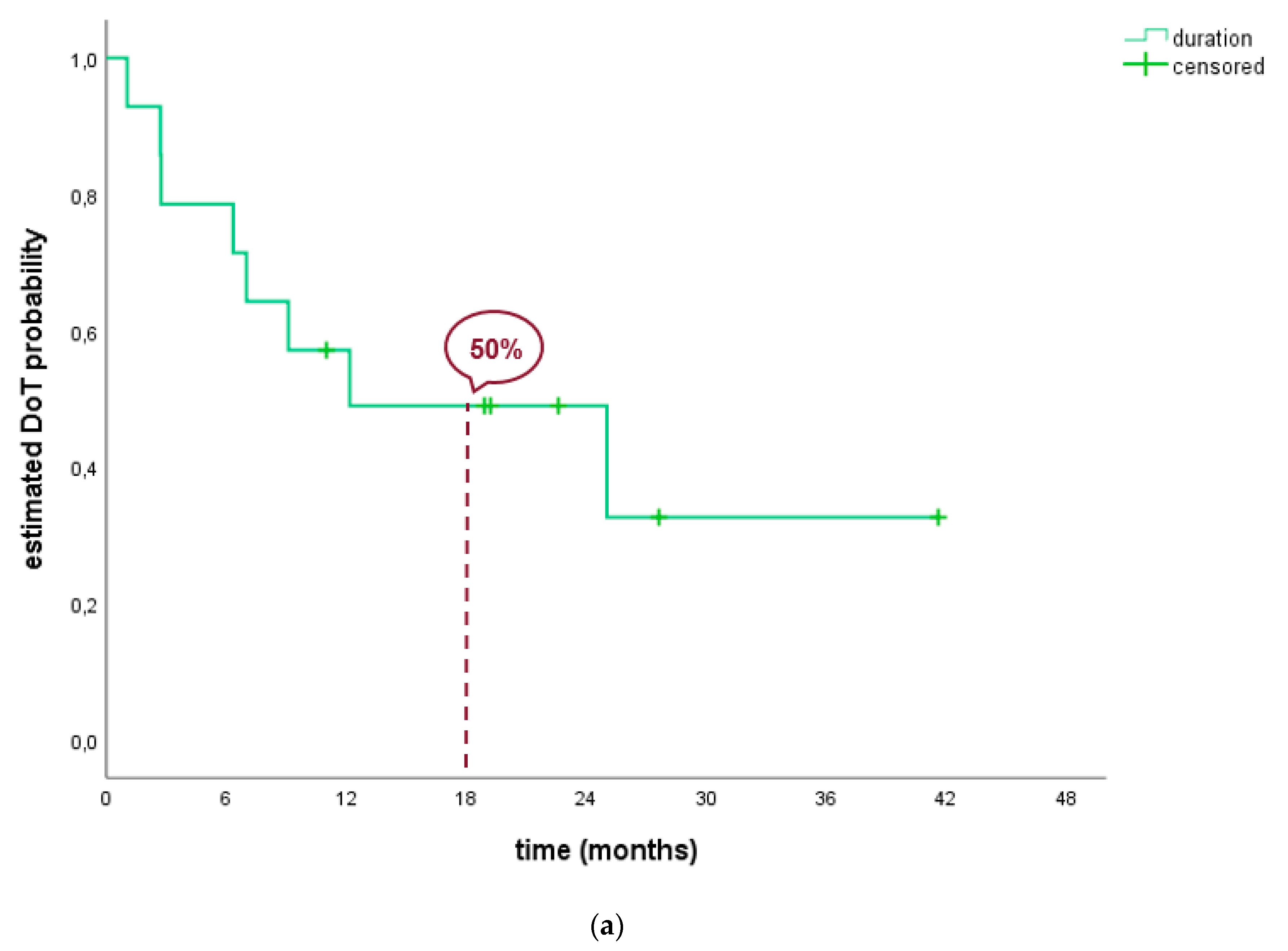
| Median DoT2 | 4.4 (1.3; 7.6) | 4.4 (0.5; 8.2) | NR | 3.0 (1.8; 4.2) | 4.4 (1.1; 7.7) | 4.4 (0.2; 8.6) |
| Median OS2 (since lorla start) | 10.2 (3.6; 16.8) | 6.4 (3.9; 9.0) | 31.2 (NR) | 7.1 (0.0; 20.2) | 7.9 (0.0; 29.7) | 10.2 (4.1; 16.2) |
| Median OS2 (since advanced diagnosis) | 41.8 (34.1; 49.5) | 28.3 (11.9; 44.8) | 66.5 (NR) | 40.6 (30.5–50.8) | 39.2 (21.7; 56.7) | 43.4 (30.9; 55.9) |
| Risk analysis of the efficacy data of ALK positive patients | ||||||
| Risk Analysis3 | Maturity | 3-Months | 6-Months | 12-Months | 2-Years | 5-Years |
| DoT rate | 70.3% | 62.2% | 45.9% | 31.7% | NR | NR |
| OS rate (since lorla start) | 67.6% | 73.0% | 62.2% | 45.4% | 35.1% | NR |
| OS rate (since advanced diagnosis) | 67.6% | 100% | 100% | 86.5% | 70.3% | 33.3% |
| Efficacy results of ROS1 positive patients | ||||||
| Results | Overall | 1 Prior TKI | 2 Prior TKI | |||
| N (%) | 14 (100%) | 11 (78.6%) | 3 (21.4%) | |||
| ORR 4 | 85.7% (57.2; 98.2) | 90.9% (58.7; 99.8) | 66.7% (0.9; 99.2) | |||
| DCR 1 | 92.9% (66.1; 99.8) | 100% (71.5; 100) | 69.2% (0.9; 99.2) | |||
| CR | 2 | 2 | 0 | |||
| PR | 10 | 8 | 2 | |||
| SD | 1 | 1 | 0 | |||
| PD | 1 | 0 | 1 | |||
| Median DoT 5 | 12.2 (0.0; 29.5) | 25.0 (8.0; 42.1) | 7.0 (0.0; 16.6) | |||
| Median OS 5 (since lorla start) | 20.0 (NR) | 17.6 (NR) | 24.4 (3.1; 45.6) | |||
| Median OS 5 (since advanced diagnosis) | 40.1 (NR) | 39.2 (NR) | 46.3 (38.7; 105.9) | |||
| Risk analysis of the efficacy data of ROS1 positive patients | ||||||
| Risk Analysis 6 | Maturity | 3-Months | 6-Months | 12-Months | 2-Years | 5-Years |
| DoT rate (SD) | 57.1% | 78.6% | 78.6% | 50.0% | 49.0% | NR |
| OS rate (SD) (since lorla start) | 42.9% | 92.9% | 92.9% | 61.4% | 50.8% | NR |
| OS rate (SD) (since advanced diagnosis) | 42.9% | 100% | 100% | 100% | 92.9% | 58.8% |
| Adverse Events | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| N = 51 | N (%) | N (%) | N (%) | N (%) |
| Hyperlipidemia | 8 (16%) | 8 (16%) | 4 (8%) | 5 (10%) |
| Hypercholesterolemia | 6 (12%) | 4 (8%) | 2 (4%) | 2 (4%) |
| Hypertriglyceridemia | 2 (4%) | 4 (8%) | 2 (4%) | 3 (6%) |
| Peripheral Edema | 5 (10%) | 1 (2%) | 1 (2%) | |
| Cognitive Effects | 1 (2%) | 2 (4%) | ||
| Diarrhea | 1 (2%) | 2 (4%) | ||
| Arthralgia | 1 (2%) | |||
| Fatigue | 1 (2%) | |||
| Peripheral Neuropathy | 1 (2%) | |||
| Other | 3 (6%) | 2 (4%) | ||
| Bloating | 1 (2%) | |||
| Muscle Weakness | 1 (2%) | |||
| Myalgia | 1 (2%) | |||
| Pneumonitis | 1 (2%) | |||
| Thrombosis | 1 (2%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hochmair, M.J.; Fabikan, H.; Illini, O.; Weinlinger, C.; Setinek, U.; Krenbek, D.; Prosch, H.; Rauter, M.; Schumacher, M.; Wöll, E.; et al. Later-Line Treatment with Lorlatinib in ALK- and ROS1-Rearrangement-Positive NSCLC: A Retrospective, Multicenter Analysis. Pharmaceuticals 2020, 13, 371. https://doi.org/10.3390/ph13110371
Hochmair MJ, Fabikan H, Illini O, Weinlinger C, Setinek U, Krenbek D, Prosch H, Rauter M, Schumacher M, Wöll E, et al. Later-Line Treatment with Lorlatinib in ALK- and ROS1-Rearrangement-Positive NSCLC: A Retrospective, Multicenter Analysis. Pharmaceuticals. 2020; 13(11):371. https://doi.org/10.3390/ph13110371
Chicago/Turabian StyleHochmair, Maximilian J., Hannah Fabikan, Oliver Illini, Christoph Weinlinger, Ulrike Setinek, Dagmar Krenbek, Helmut Prosch, Markus Rauter, Michael Schumacher, Ewald Wöll, and et al. 2020. "Later-Line Treatment with Lorlatinib in ALK- and ROS1-Rearrangement-Positive NSCLC: A Retrospective, Multicenter Analysis" Pharmaceuticals 13, no. 11: 371. https://doi.org/10.3390/ph13110371
APA StyleHochmair, M. J., Fabikan, H., Illini, O., Weinlinger, C., Setinek, U., Krenbek, D., Prosch, H., Rauter, M., Schumacher, M., Wöll, E., Wass, R., Brehm, E., Absenger, G., Bundalo, T., Errhalt, P., Urban, M., & Valipour, A. (2020). Later-Line Treatment with Lorlatinib in ALK- and ROS1-Rearrangement-Positive NSCLC: A Retrospective, Multicenter Analysis. Pharmaceuticals, 13(11), 371. https://doi.org/10.3390/ph13110371





