The Effects of Maternal Metformin Treatment on Late Prenatal and Early Postnatal Development of the Offspring Are Modulated by Sex
Abstract
1. Introduction
2. Results
2.1. Effects of Maternal Metformin Treatment on Late Prenatal Development and Neonatal Features
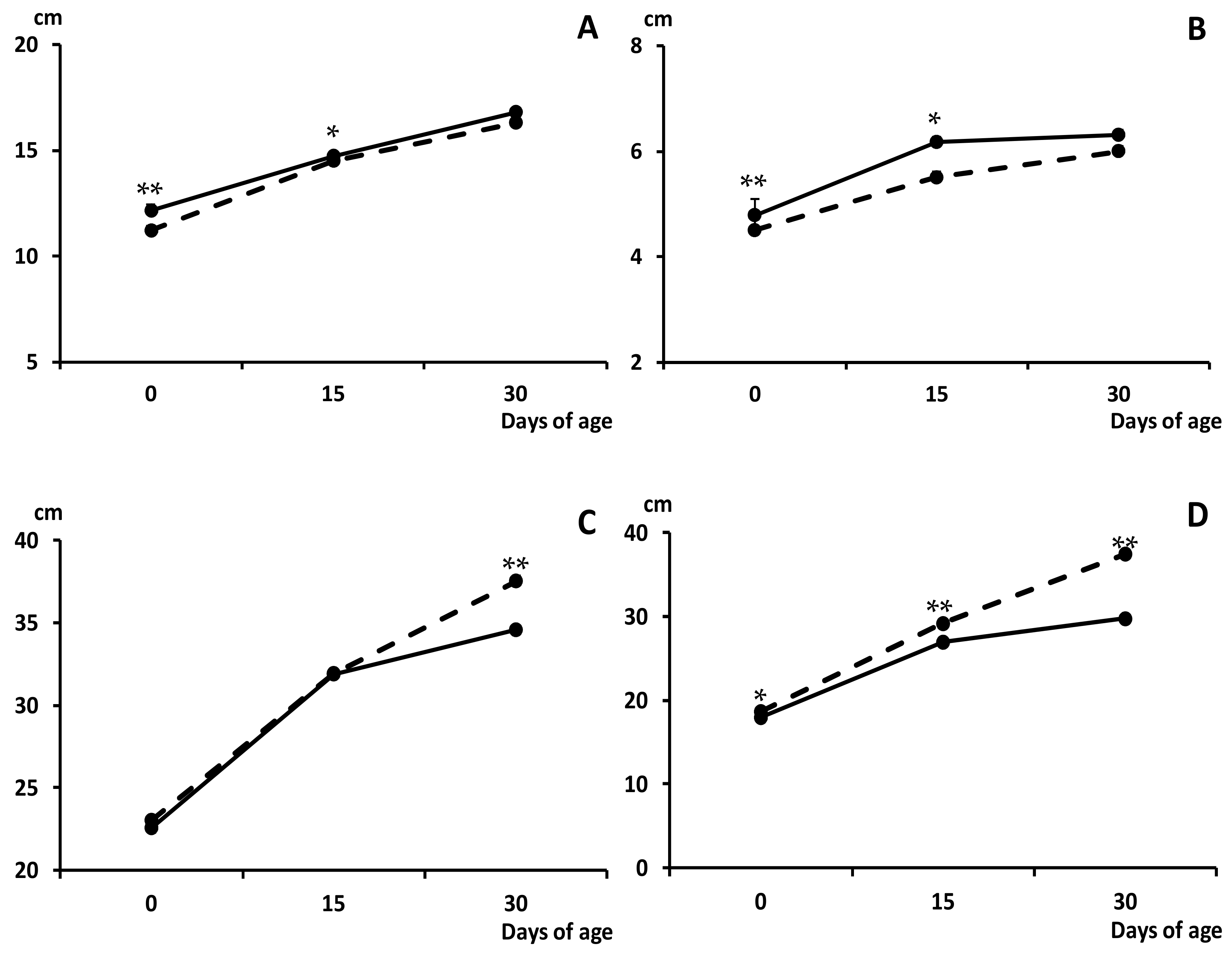
2.2. Effects of Maternal Metformin Treatment on Early Postnatal Development and Body Composition
3. Discussion
4. Material and Methods
4.1. Ethics Statement
4.2. Animal Handling and Experimental Procedure
4.3. Assessment of Neonatal Features and Early Postnatal Development of Piglets
4.4. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bhutta, Z.A.; Das, J.K.; Rizvi, A.; Gaffey, M.F.; Walker, N.; Horton, S.; Webb, P.; Lartey, A. E Black, REvidence-based interventions for improvement of maternal and child nutrition: What can be done and at what cost? Lancet 2013, 382, 452–477. [Google Scholar] [CrossRef]
- Aski, S.K.; Akbari, R.; Hantoushzadeh, S.; Ghotbizadeh, F. A bibliometric analysis of Intrauterine Growth Restriction research. Placenta 2020, 95, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, S.; Kyle, P.M. Aetiology and pathogenesis of IUGR. Best Pract. Res. Clin. Obstet. Gynaecol. 2009, 23, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Ghidini, A. Idiopathic fetal growth restriction: A pathophysiologic approach. Obstet. Gynecol. Surv. 1996, 51, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Bulnes, A.; Astiz, S.; Parraguez, V.H.; Garcia-Contreras, C.; Vazquez-Gomez, M. Empowering translational research in Fetal Growth Restriction: Sheep and swine animal models. Curr. Pharm. Biotechnol. 2016, 17, 848–855. [Google Scholar] [CrossRef]
- Zeitlin, J.; Ancel, P.Y.; Saurel-Cubizolles, M.J.; Papiernik, E. The relationship between intrauterine growth restriction and preterm delivery: An empirical approach using data from a European case-control study. BJOG Int. J. Obstet. Gynaecol. 2000, 107, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Bulnes, A.; Parraguez, V.H.; Berlinguer, F.; Barbero, A.; Garcia-Contreras, C.; Lopez-Tello, J.; Pesantez-Pacheco, J.L.; Martinez-Ros, P. The impact of prenatal environment on postnatal life and performance: Future perspectives for prevention and treatment. Theriogenology 2020, 150, 15–19. [Google Scholar] [CrossRef]
- Gonzalez-Bulnes, A.; Ovilo, C.; Lopez-Bote, C.J.; Astiz, S.; Ayuso, M.; Perez-Solana, M.L.; Sanchez-Sanchez, R.; Torres-Rovira, L. Gender-specific early postnatal catch-up growth after intrauterine growth retardation by food restriction in swine with obesity/leptin resistance. Reproduction 2012, 144, 269–278. [Google Scholar] [CrossRef]
- Gonzalez-Bulnes, A.; Astiz, S.; Ovilo, C.; Lopez-Bote, C.J.; Torres-Rovira, L.; Barbero, A.; Ayuso, M.; Garcia-Contreras, C.; Vazquez-Gomez, M. Developmental Origins of Health and Disease in swine: Implications for animal production and biomedical research. Theriogenology 2016, 86, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.J. Metformin: Historical overview. Diabetologia 2017, 60, 1566–1576. [Google Scholar] [CrossRef]
- Lindsay, R.S.; Loeken, M.R. Metformin use in pregnancy: Promises and uncertainties. Diabetologia 2017, 60, 1612–1619. [Google Scholar] [CrossRef]
- Hyer, S.; Balani, J.; Shehata, H. Metformin in pregnancy: Mechanisms and clinical applications. Int. J. Mol. Sci. 2018, 19, 1954. [Google Scholar] [CrossRef] [PubMed]
- Stumvoll, M.; Nurjhan, N.; Perriello, G.; Dailey, G.; Gerich, J.E. Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. N. Engl. J. Med. 1995, 333, 550–554. [Google Scholar] [CrossRef]
- Simmons, D. Safety considerations with pharmacological treatment of gestational diabetes mellitus. Drug Saf. 2015, 38, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Contreras, C.; Vazquez-Gomez, M.; Pesantez-Pacheco, J.L.; Torres-Rovira, L.; Heras-Molina, A.; Encinas, T.; Astiz, S.; Gonzalez-Bulnes, A. Maternal metformin treatment improves developmental and metabolic traits of IUGR fetuses. Biomolecules 2019, 9, 166. [Google Scholar] [CrossRef]
- American Diabetes Association. Management of diabetes in pregnancy: Standards of medical care in diabetes 2019. Diabetes Care 2019, 42, S165–S172. [Google Scholar] [CrossRef]
- Douglas, S.L.; Edwards, S.A.; Kyriazakis, I. Are all piglets born lightweight alike? Morphological measurements as predictors of postnatal performance. J. Anim. Sci. 2016, 94, 3510–3518. [Google Scholar] [CrossRef]
- Hales, J.; Moustsen, V.A.; Nielsen, M.B.F.; Hansen, C.F. Individual physical characteristics of neonatal piglets affect preweaning survival of piglets born in a noncrated system. J. Anim. Sci. 2013, 91, 4991–5003. [Google Scholar] [CrossRef]
- Foxcroft, G.R.; Dixon, W.T.; Novak, S.; Putman, C.T.; Town, S.C.; Vinsky, M.D. The biological basis for prenatal programming of postnatal performance in pigs. J. Anim. Sci. 2006, 84, E105–E112. [Google Scholar] [CrossRef]
- Huting, A.M.S.; Sakkas, P.; Wellock, I.; Almond, K.; Kyriazakis, I. Once small always small? To what extent morphometric characteristics and post-weaning starter regime affect pig lifetime growth performance. Porcine Health Manag. 2018, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Vanky, E.; Stridsklev, S.; Heimstad, R.; Romundstad, P.; Skogøy, K.; Kleggetveit, O.; Hjelle, S.; von Brandis, P.; Eikeland, T.; Flo, K.; et al. Metformin versus placebo from first trimester to delivery in polycystic ovary syndrome: A randomized, controlled multicenter study. J. Clin. Endocrinol. Metab. 2010, 95, E448–E455. [Google Scholar] [CrossRef]
- Hanem, L.G.E.; Stridsklev, S.; Júlíusson, P.B.; Salvesen, Ø.; Roelants, M.; Carlsen, S.M.; Ødegård, R.; Vanky, E. Metformin use in PCOS pregnancies increases the risk of offspring overweight at 4 years of age: Follow-up of two RCTs. J. Clin. Endocrinol. Metab. 2018, 103, 1612–1621. [Google Scholar] [CrossRef]
- Bartholomeusz, H.H.; Courchesne, E.; Karns, C.M. Relationship between head circumference and brain volume in healthy normal toddlers, children, and adults. Neuropediatrics 2002, 33, 239–241. [Google Scholar] [CrossRef]
- Charles, B.; Norris, R.; Xiao, X.; Hague, W. Population pharmacokinetics of metformin in late pregnancy. Ther. Drug Monit. 2006, 28, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Vanky, E.; Zahlsen, K.; Spigset, O.; Carlsen, S.M. Placental passage of metformin in women with polycystic ovary syndrome. Fertil. Steril. 2005, 83, 1575–1578. [Google Scholar] [CrossRef]
- Łabuzek, K.; Suchy, D.; Gabryel, B.; Bielecka, A.; Liber, S.; Okopień, B. Quantification of metformin by the HPLC method in brain regions, cerebrospinal fluid and plasma of rats treated with lipopolysaccharide. Pharmacol. Rep. 2010, 62, 956–965. [Google Scholar] [CrossRef]
- Rehfeldt, C.; Lefaucheur, L.; Block, J.; Stabenow, B.; Pfuhl, R.; Otten, W.; Metges, C.C.; Kalbe, C. Limited and excess protein intake of pregnant gilts differently affects body composition and cellularity of skeletal muscle and subcutaneous adipose tissue of newborn and weanling piglets. Eur. J. Nutr. 2012, 51, 151–165. [Google Scholar] [CrossRef]
- Rehfeldt, C.; Stabenow, B.; Pfuhl, R.; Block, J.; Nurnberg, G.; Otten, W.; Metges, C.C.; Kalbe, C. Effects of limited and excess protein intakes of pregnant gilts on carcass quality and cellular properties of skeletal muscle and subcutaneous adipose tissue in fattening pigs. J. Anim. Sci. 2012, 90, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Patience, J.F.; Rossoni-Serão, M.C.; Gutiérrez, N.A. A review of feed efficiency in swine: Biology and application. J. Anim. Sci. Biotechnol. 2015, 6, 33. [Google Scholar] [CrossRef]
- Witczak, C.A.; Mokelke, E.A.; Boullion, R.; Wenzel, J.; Keisler, D.H.; Sturek, M. Noninvasive measures of body fat percentage in male Yucatan swine. Comp. Med. 2005, 55, 445–451. [Google Scholar]
- Dyson, M.C.; Alloosh, M.; Vuchetich, J.P.; Mokelke, E.A.; Sturek, M. Components of metabolic syndrome and coronary artery disease in female Ossabaw swine fed excess atherogenic diet. Comp. Med. 2006, 56, 35–45. [Google Scholar]
- Christoffersen, B.O.; Grand, N.; Golozoubova, V.; Svendsen, O.; Raun, K. Gender-associated differences in metabolic syndrome-related parameters in Gottingen Minipigs. Comp. Med. 2007, 57, 493–504. [Google Scholar]
- Breier, B.H.; Vickers, M.H.; Ikenasio, B.A.; Chan, K.Y.; Wong, W.P.S. Fetal programming of appetite and obesity. Mol. Cell. Endocrinol. 2001, 185, 73–79. [Google Scholar] [CrossRef]
- Hales, C.N.; Ozanne, S.E. The dangerous road of catch-up growth. J. Physiol. 2003, 547, 5–10. [Google Scholar] [CrossRef]
- Ross, M.G.; Desai, M. Gestational programming: Population survival effects of drought and famine during pregnancy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R25–R33. [Google Scholar] [CrossRef]
- Ibañez, L.; Ong, K.; Dunger, D.B.; de Zegher, F. Early development of adiposity and insulin resistance after catch-up weight gain in small-forgestational-age children. J. Clin. Endocrinol. Metabol. 2006, 91, 2153–2158. [Google Scholar] [CrossRef] [PubMed]
- Barbero, A.; Astiz, S.; Lopez-Bote, C.J.; Perez-Solana, M.L.; Ayuso, M.; Garcia-Real, I.; Gonzalez-Bulnes, A. Maternal malnutrition and offspring sex determine juvenile obesity and metabolic disorders in a swine model of leptin resistance. PLoS ONE 2013, 8, e78424. [Google Scholar] [CrossRef]
- Óvilo, C.; Gonzalez-Bulnes, A.; Benítez, R.; Ayuso, M.; Barbero, A.; Perez-Solana, M.L.; Barragán, C.; Astiz, S.; Fernández, A.; López-Bote, C. Prenatal programming in an obese swine model: Sex-related effects of maternal energy restriction on morphology, metabolism and hypothalamic gene expression. Br. J. Nutr. 2014, 111, 735–746. [Google Scholar] [CrossRef]
- Astiz, S.; Gonzalez-Bulnes, A.; Astiz, I.; Barbero, A.; Perez-Solana, M.L.; Garcia-Real, I. Advanced onset of puberty after metformin therapy in swine with thrifty genotype. Exp. Physiol. 2014, 99, 1241–1252. [Google Scholar] [CrossRef]
- Glueck, C.J.; Goldenberg, N.; Pranikoff, J.; Loftspring, M.; Sieve, L.; Wang, P. Height, weight, and motor-social development during the first 18 months of life in 126 infants born to 109 mothers with polycystic ovary syndrome who conceived on and continued metformin through pregnancy. Hum. Reprod. 2004, 19, 1323–1330. [Google Scholar] [CrossRef]
- Ijäs, H.; Vääräsmäki, M.; Saarela, T.; Keravuo, R.; Raudaskoski, T. A follow-up of a randomised study of metformin and insulin in gestational diabetes mellitus: Growth and development of the children at the age of 18 months. BJOG Int. J. Obstet. Gynaecol. 2015, 122, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Rowan, J.A.; Rush, E.C.; Plank, L.D.; Lu, J.; Obolonkin, V.; Coat, S.; Hague, W.M. Metformin in gestational diabetes: The offspring follow-up (MiG TOFU): Body composition and metabolic outcomes at 7–9 years of age. BMJ Open Diabetes Res. Care 2018, 6, e000456. [Google Scholar] [CrossRef]
- Rowan, J.A.; Rush, E.C.; Obolonkin, V.; Battin, M.; Wouldes, T.; Hague, W.M. Metformin in gestational diabetes: The offspring follow-up (MiG TOFU): Body composition at 2 years of age. Diabetes Care 2011, 34, 2279–2284. [Google Scholar] [CrossRef]
- Gonzalez-Bulnes, A.; Torres-Rovira, L.; Astiz, S.; Ovilo, C.; Sanchez-Sanchez, R.; Gomez-Fidalgo, E.; Perez-Solana, M.; Martin-Lluch, M.; Garcia-Contreras, C.; Vazquez-Gomez, M. Fetal Sex Modulates Developmental Response to Maternal Malnutrition. PLoS ONE 2015, 10, e0142158. [Google Scholar] [CrossRef]
- Cogollos, L.; Garcia-Contreras, C.; Vazquez-Gomez, M.; Astiz, S.; Sanchez-Sanchez, R.; Gomez-Fidalgo, E.; Ovilo, C.; Isabel, B.; Gonzalez-Bulnes, A. Effects of fetal genotype and sex on developmental response to maternal malnutrition. Reprod. Fertil. Dev. 2017, 29, 1155–1168. [Google Scholar] [CrossRef]
- Aiken, C.E.; Ozanne, S.E. Sex differences in developmental programming models. Reproduction 2013, 145, R1–R13. [Google Scholar] [CrossRef]
- Garcia-Contreras, C.; Vazquez-Gomez, M.; Barbero, A.; Pesantez, J.L.; Zinellu, A.; Berlinguer, F.; Gonzalez-Añover, P.; Gonzalez, J.; Encinas, T.; Torres-Rovira, L.; et al. Polyphenols and IUGR pregnancies: Effects of maternal hydroxytyrosol supplementation on placental gene expression and fetal antioxidant status, DNA-methylation and phenotype. Int. J. Mol. Sci. 2019, 20, 1187. [Google Scholar] [CrossRef]
- Vazquez-Gomez, M.; Garcia-Contreras, C.; Torres-Rovira, L.; Pesantez, J.L.; Gonzalez-Añover, P.; Gómez-Fidalgo, E.; Sánchez-Sánchez, R.; Ovilo, C.; Isabel, B.; Astiz, S.; et al. Polyphenols and IUGR pregnancies: Maternal hydroxytyrosol supplementation improves prenatal and early-postnatal growth and metabolism of the offspring. PLoS ONE 2017, 12, e0177593. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Contreras, C.; Vázquez-Gómez, M.; Pardo, Z.; Heras-Molina, A.; Encinas, T.; Torres-Rovira, L.; Astiz, S.; Nieto, R.; Óvilo, C.; Gonzalez-Bulnes, A.; et al. Polyphenols and IUGR pregnancies: Effects of maternal hydroxytyrosol supplementation on hepatic fat accretion and energy and fatty acids profile of fetal tissues. Nutrients 2019, 11, 1534. [Google Scholar] [CrossRef]

| Parameter | Group C | Group METF | ||
|---|---|---|---|---|
| Females | Males | Females | Males | |
| Body weight (g) | 1.13 ± 0.03 | 1.15 ± 0.03 | 1.11 ± 0.03 | 1.16 ± 0.03 |
| Body length (cm) | 23.27 ± 0.34 a | 23.03 ± 0.28 e | 23.72 ± 0.30 b | 23.92 ± 0.29 f |
| Occipito-nasal length (cm) | 11.14 ± 0.22 e | 11.31 ± 0.19 e | 11.93 ± 0.13 f,1 | 12.27 ± 0.16 f,2 |
| Biparietal diameter (cm) | 4.57 ± 0.08 a | 4.58 ± 0.07 a | 4.77 ± 0.05 b | 4.77 ± 0.07 b |
| Thoracic circumference (cm) | 23.25 ± 0.29 c | 22.48 ± 0.29 | 22.20 ± 0.19 d,1 | 22.64 ± 0.27 2 |
| Abdominal circumference (cm) | 18.76 ± 0.31 a | 18.63 ± 0.30 | 17.83 ± 0.23 b | 18.28 ± 0.30 |
| Parameter (g) | Group C | Group METF | ||
|---|---|---|---|---|
| Females | Males | Females | Males | |
| Body | 4986.76 ± 112.65 | 4895.59 ± 137.63 | 4728.75 ± 74.11 | 4777.13 ± 88.72 |
| Head | 689.67 ± 9.23 | 666.71 ± 12.68 | 653.42 ± 10.45 | 645.22 ± 6.99 |
| Carcass | 3053.83 ± 51.04 | 3219.43 ± 98.94 | 3048.53 ± 110.45 | 2955.56 ± 104.3 |
| Total viscerae | 835.00 ± 17.76 | 755.29 ± 24.49 | 895.0 ± 16.88 | 849.59 ± 16.64 |
| Heart | 32.17 ± 0.50 | 30.57 ± 0.62 | 35.38 ± 0.77 | 35.09 ± 1.08 |
| Lungs | 71.67 ± 1.65 | 81.14 ± 3.21 | 79.66 ± 1.47 | 77.44 ± 1.36 |
| Liver | 131.00 ± 1.63 | 131.86 ± 3.11 | 132.10 ± 2.62 | 131.56 ± 2.52 |
| Intestine | 429.17 ± 10.80 | 352.86 ± 17.31 | 439.72 ± 9.70 | 411.48 ± 7.88 |
| Kidneys | 28.33 ± 0.38 | 29.71 ± 0.60 a | 28.36 ± 0.47 | 27.00 ± 0.43 b |
| Spleen | 11.42 ± 0.18 | 16.39 ± 0.67 | 15.45 ± 0.50 | 13.95 ± 0.52 |
| Pancreas | 4.99 ± 0.28 | 6.11 ± 0.13 | 5.57 ± 0.20 | 5.32 ± 0.18 |
| Adrenal glands | 0.71 ± 0.02 | 0.95 ± 0.02 | 0.85 ± 0.02 | 0.88 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Contreras, C.; Vazquez-Gomez, M.; Pesantez-Pacheco, J.L.; Heras-Molina, A.; Encinas, T.; Astiz, S.; Gonzalez-Bulnes, A. The Effects of Maternal Metformin Treatment on Late Prenatal and Early Postnatal Development of the Offspring Are Modulated by Sex. Pharmaceuticals 2020, 13, 363. https://doi.org/10.3390/ph13110363
Garcia-Contreras C, Vazquez-Gomez M, Pesantez-Pacheco JL, Heras-Molina A, Encinas T, Astiz S, Gonzalez-Bulnes A. The Effects of Maternal Metformin Treatment on Late Prenatal and Early Postnatal Development of the Offspring Are Modulated by Sex. Pharmaceuticals. 2020; 13(11):363. https://doi.org/10.3390/ph13110363
Chicago/Turabian StyleGarcia-Contreras, Consolacion, Marta Vazquez-Gomez, José Luis Pesantez-Pacheco, Ana Heras-Molina, Teresa Encinas, Susana Astiz, and Antonio Gonzalez-Bulnes. 2020. "The Effects of Maternal Metformin Treatment on Late Prenatal and Early Postnatal Development of the Offspring Are Modulated by Sex" Pharmaceuticals 13, no. 11: 363. https://doi.org/10.3390/ph13110363
APA StyleGarcia-Contreras, C., Vazquez-Gomez, M., Pesantez-Pacheco, J. L., Heras-Molina, A., Encinas, T., Astiz, S., & Gonzalez-Bulnes, A. (2020). The Effects of Maternal Metformin Treatment on Late Prenatal and Early Postnatal Development of the Offspring Are Modulated by Sex. Pharmaceuticals, 13(11), 363. https://doi.org/10.3390/ph13110363





