Attenuation of Spatial Memory in 5xFAD Mice by Halting Cholinesterases, Oxidative Stress and Neuroinflammation Using a Cyclopentanone Derivative
Abstract
1. Introduction
2. Results
2.1. Behavioral Studies
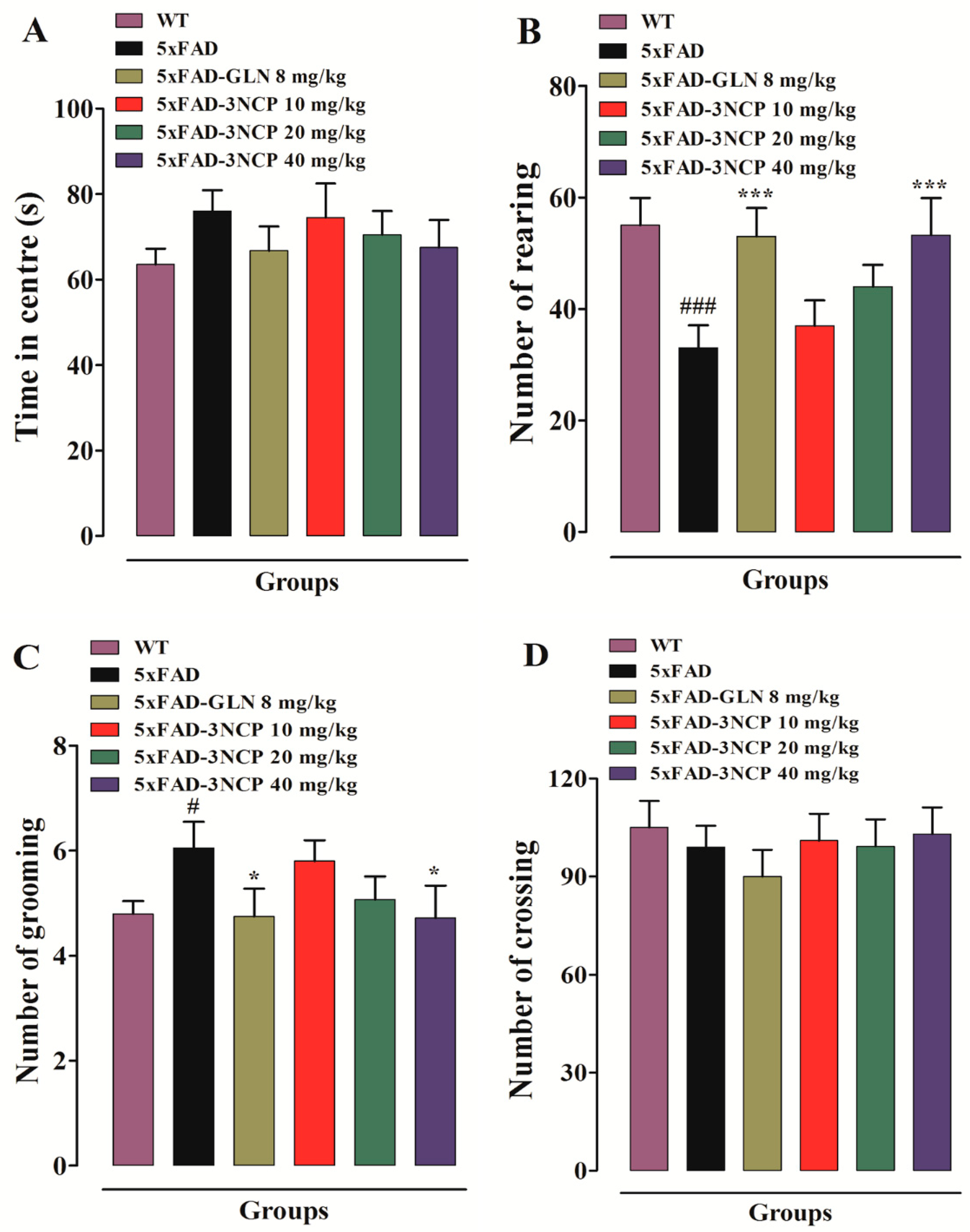
2.1.1. Open Field Test
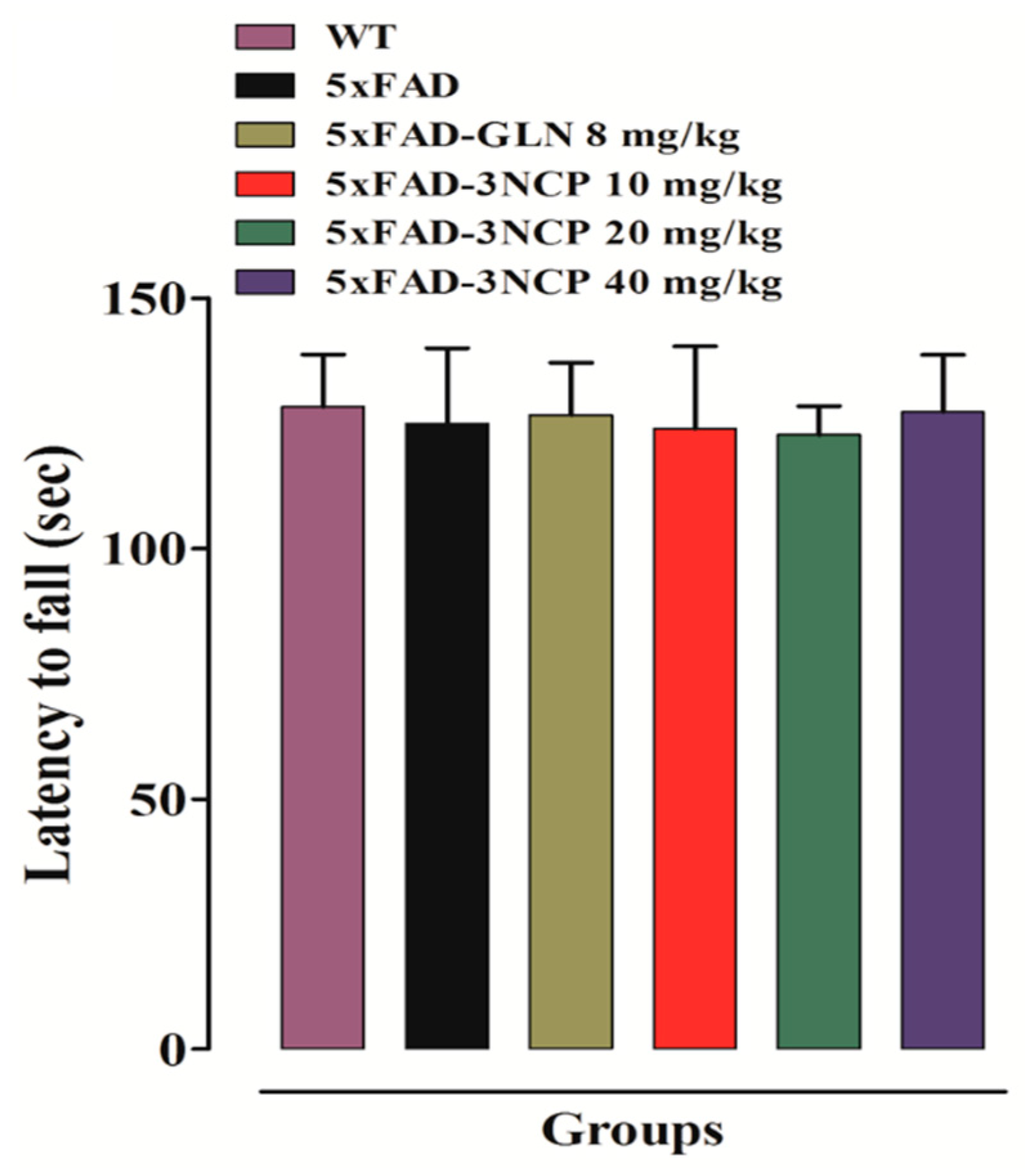
2.1.2. Effect of Cyclopentanone Derivative “3NCP” in the Rotarod Test
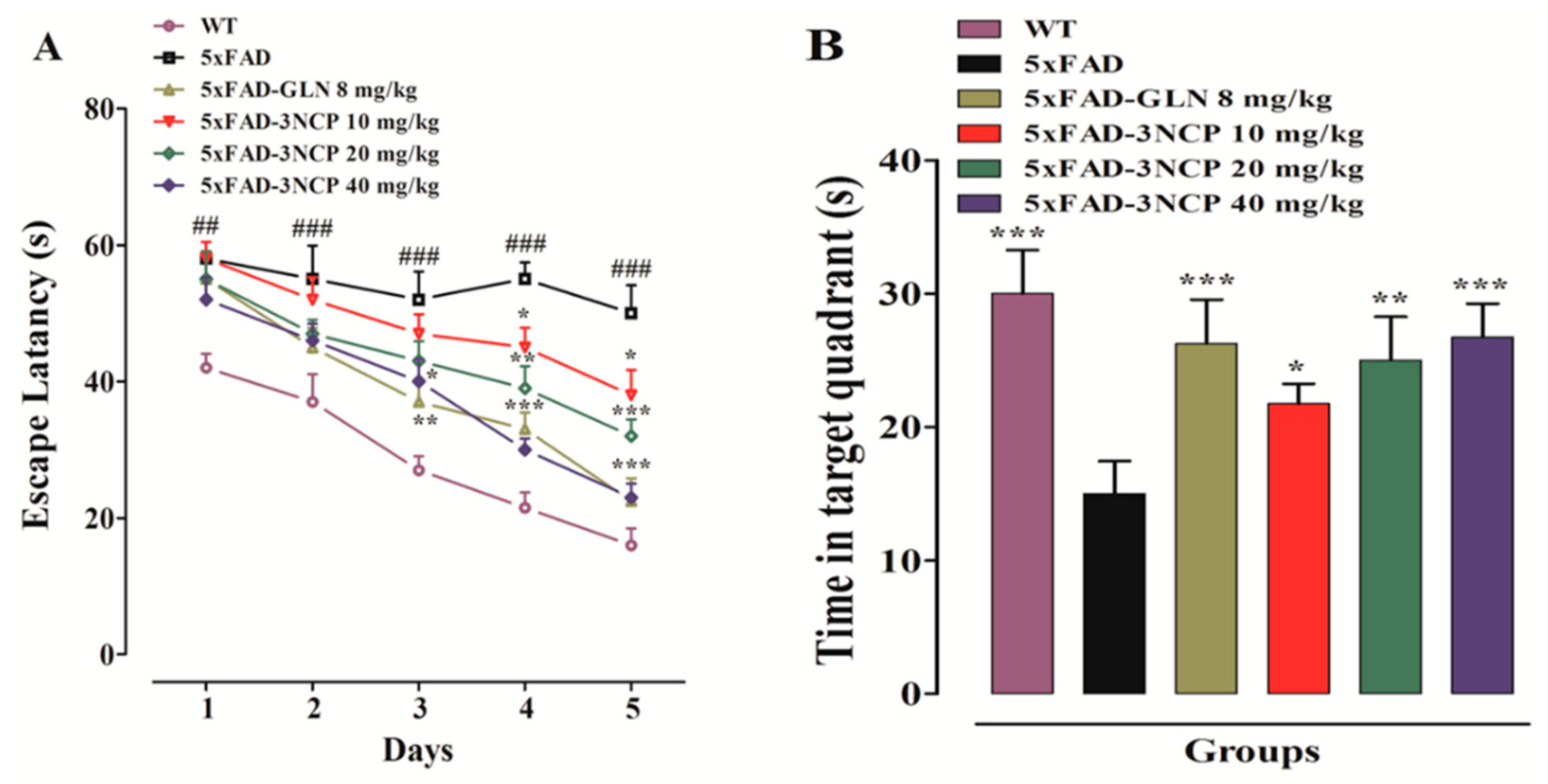
2.1.3. Effect of Cyclopentanone Derivative “3NCP” in the Morris Water Maze Test
2.1.4. Effect of Cyclopentanone Derivative “3NCP”in the Y-Maze Test
2.2. In Vitro Cholinesterases Inhibition by Cyclopentanone Derivative “3NCP”
2.3. Ex Vivo Cholinesterases
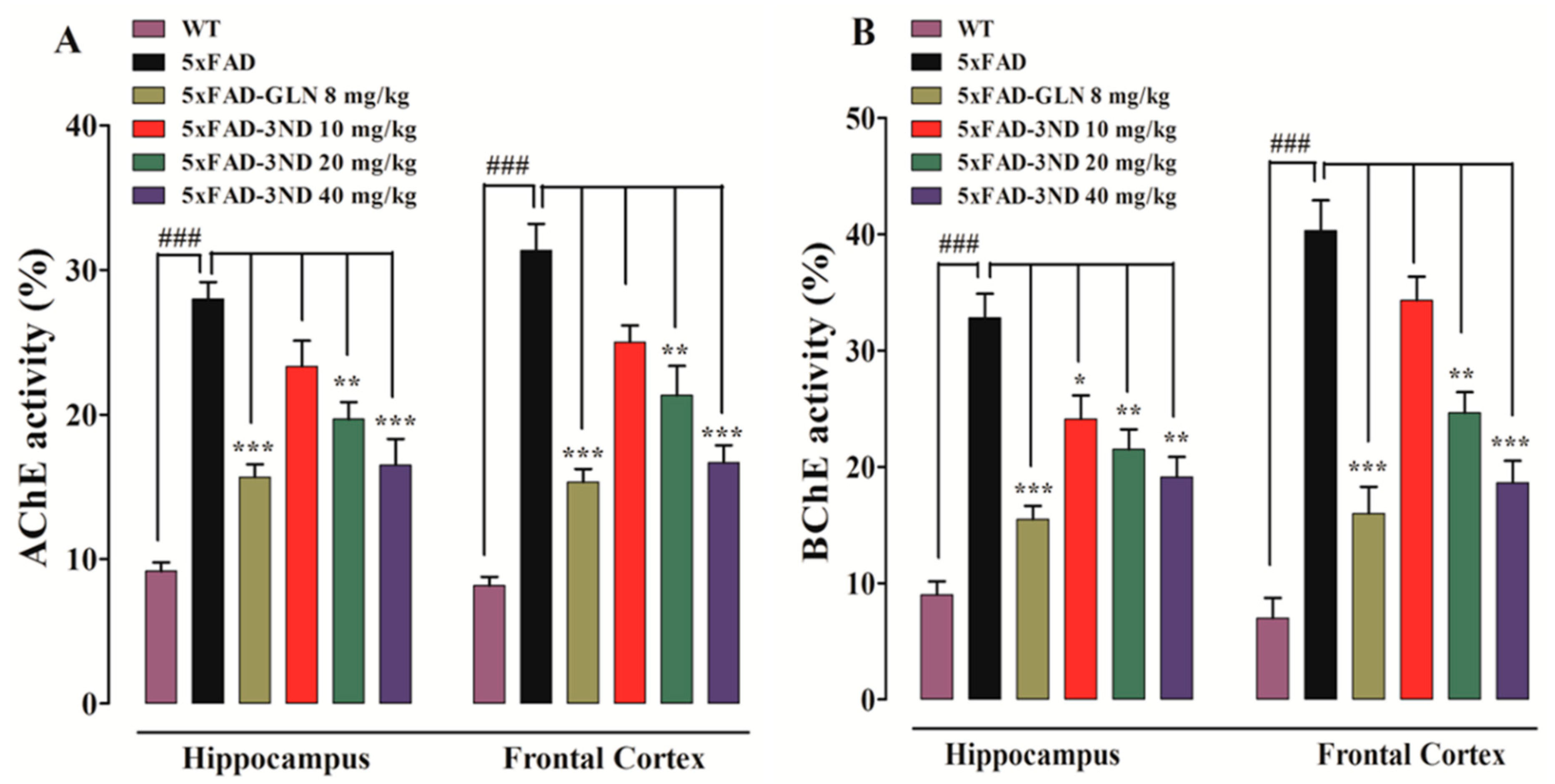
2.3.1. Effect of Cyclopentanone Derivative 3NCP on Cortical and Hippocampal AChE, BChE Activities
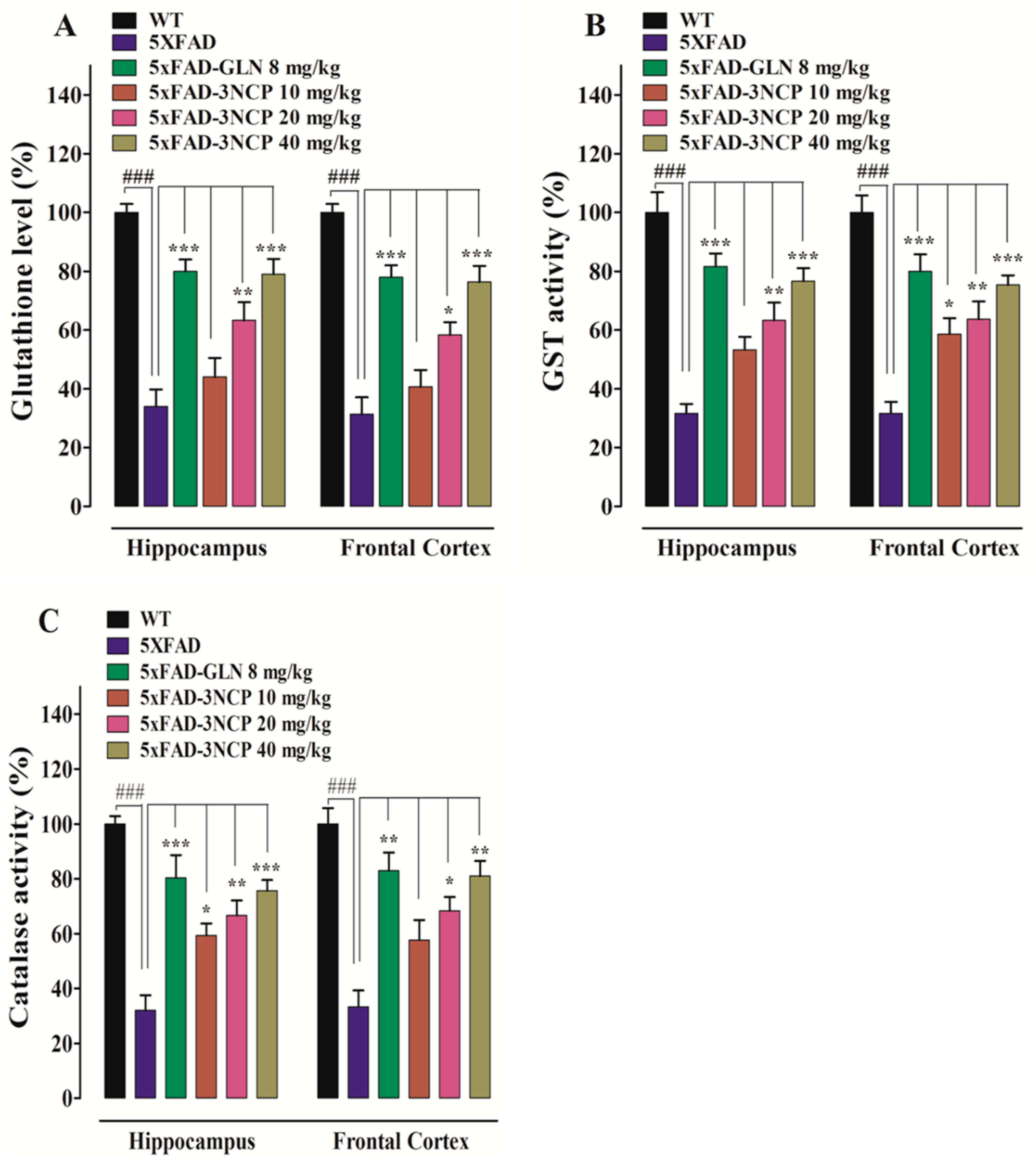
2.3.2. Effect of Cyclopentanone Derivative 3NCP on GSH Level, GST, and Catalase Activities
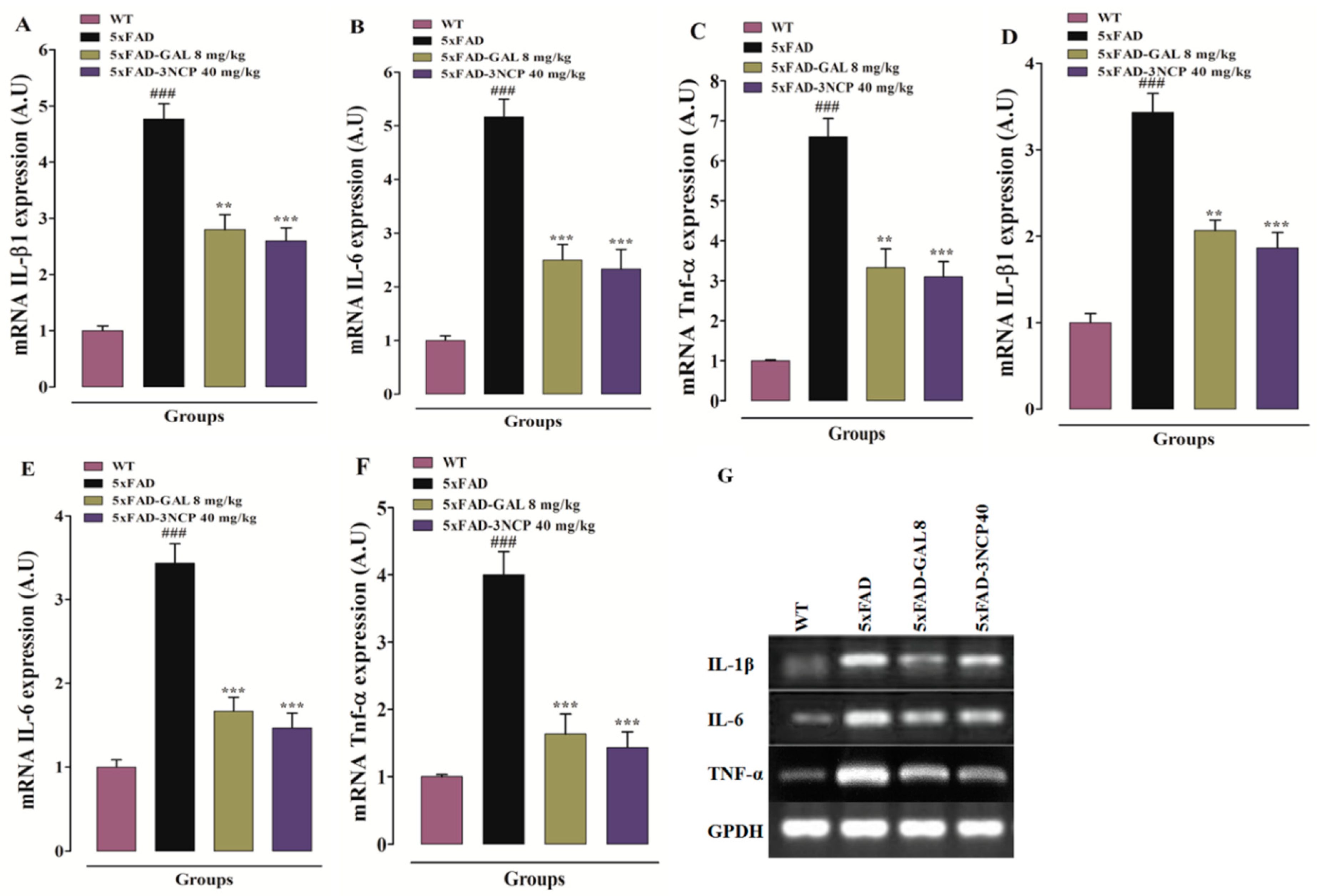
2.3.3. Effect of Cyclopentanone Derivative 3NCP on Cytokines (IL-1β, IL-6, TNF-α) in HC and FC Tissues
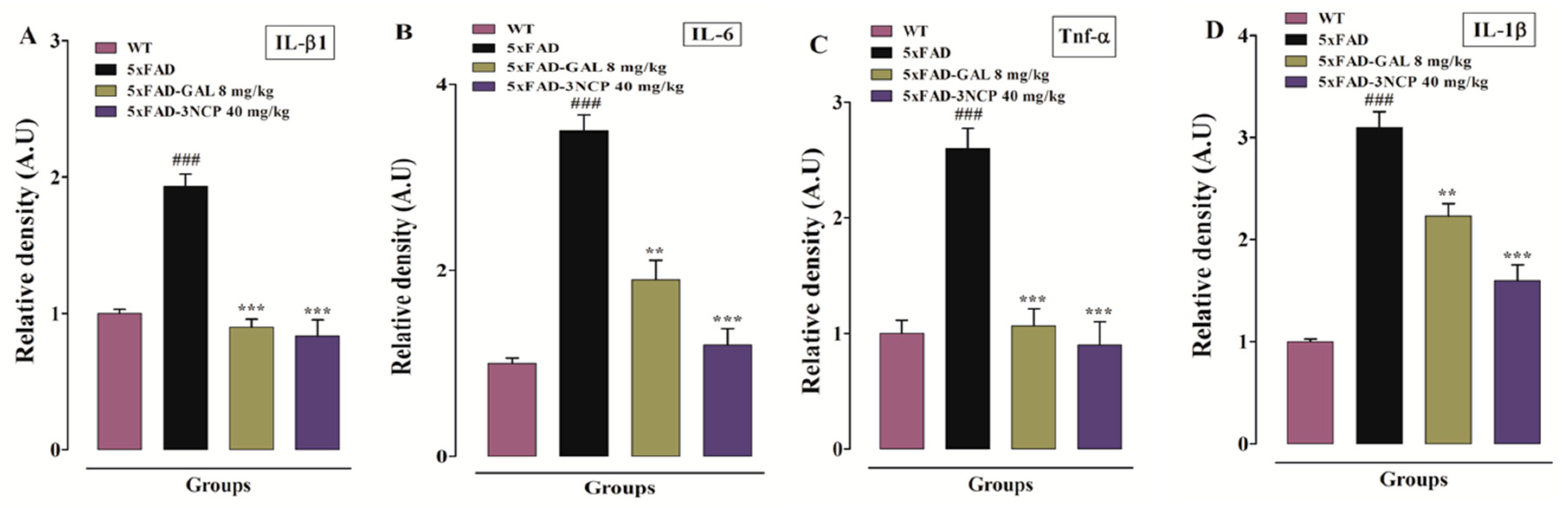
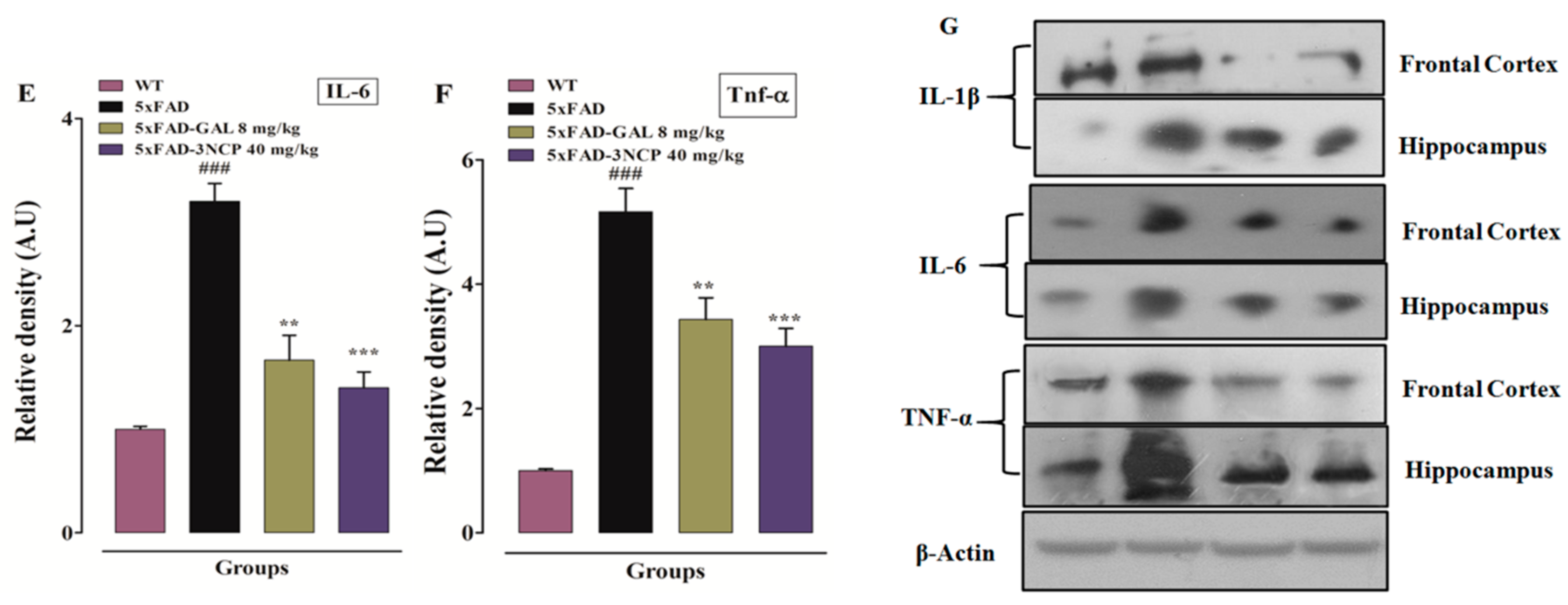
2.3.4. Effect of Cyclopentanone Derivative 3NCP on Cytokines (IL-1β, IL-6, TNF-α) Protein Expression in FC and HC Tissues
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. In Vivo Activities
4.3.1. Genotyping of Transgenic Mice
4.3.2. Behavioral Activities
Animal Grouping and Route of Drug Administration
- Group I: WT mice
- Group II: 5xFAD mice
- Group III: 5xFAD-GLN (8 mg/kg, i.p)
- Group IV: 5xFAD-3NCP (10 mg/kg i.p)
- Group V: 5xFAD-3NCP (20 mg/kg i.p)
- Group VI: 5xFAD-3NCP (40 mg/kg i.p)
Open Field Test
Rotarod Test
Morris Water Maze
Y Maze Test
4.4. In Vitro Assays
Acetylcholinesterase and Butyrylcholinesterase Inhibition Assays
4.5. Ex Vivo Assays
4.5.1. Assessment of Inhibition of Cholinesterases (AChE and BChE) in FC and HC
4.5.2. Glutathione (GSH), Glutathione S-Transferase (GST), and Catalase Assay
4.5.3. RT-PCR
4.5.4. Western Blot Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maczurek, A.; Hager, K.; Kenklies, M.; Sharman, M.; Martins, R.; Engel, J.; Carlson, D.A.; Münch, G. Lipoic acid as an anti-inflammatory and neuroprotective treatment for Alzheimer’s disease. Adv. Drug Deliv. Rev. 2008, 60, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, A.; Munder, T.; Schreyer, S.; Klein, C.; Rasińska, J.; Winter, Y.; Steiner, B. Behavioral and psychological symptoms of dementia (BPSD) and impaired cognition reflect unsuccessful neuronal compensation in the pre-plaque stage and serve as early markers for Alzheimer’s disease in the APP23 mouse model. Behav. Brain Res. 2018, 347, 300–313. [Google Scholar] [CrossRef]
- Zheng, H.; Zhou, Q.; Du, Y.; Li, C.; Xu, P.; Lin, L.; Xiao, J.; Gao, H. The hypothalamus as the primary brain region of metabolic abnormalities in APP/PS1 transgenic mouse model of Alzheimer’s disease. BBA Mol. Basis Dis. 2018, 1864, 263–273. [Google Scholar] [CrossRef]
- Shal, B.; Ding, W.; Ali, H.; Kim, Y.S.; Khan, S. Anti-neuroinflammatory Potential of Natural Products in Attenuation of Alzheimer’s Disease. Front. Pharmacol. 2018, 9, 548. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Ullah, F.; Ayaz, M.; Sadiq, A.; Shah, M.R.; Jan, M.S.; Ullah, F. Anticholinesterase and antioxidant potentials of NoneamicranthaBioss. &Reut along with GC-MS analysis. BMC Complement. Altern. Med. 2017, 17, 499. [Google Scholar]
- Öztürk, M.; Kolak, U.; Topçu, G.; Öksüz, S.; Choudhary, M.I. Antioxidant and anticholinesterase active constituents from Micromeriacilicica by radical-scavenging activity-guided fractionation. Food Chem. 2011, 126, 31–38. [Google Scholar] [CrossRef]
- Stuchbury, G.; Münch, G. Alzheimer’s associated inflammation, potential drug targets and future therapies. J. Neural. Transm. 2005, 112, 429–453. [Google Scholar] [CrossRef]
- Martono, S. Benzylidene cyclopentanone derivatives as inhibitors of rat liver glutathione s-transferase activities. Indones. J. Chem. 2010, 5, 71–75. [Google Scholar] [CrossRef]
- Hoan, D.Q.; Chi, N.Q.; Hien, N. Preparing and evaluating the antioxidant of an acid derivative from a monocarbonylcurcumin analog of cyclopentanone. TạpchíKhoahọc 2016, 12, 60–65. [Google Scholar]
- KunhannaSarojini, B.; GovindarajuDarshan Raj, C.; Kyathegowda Ramakrishna, M.; Ramesh, S.R.; RudreshBharath, B.; Manjunatha, H. In Silico Studies of (2E, 5E)-2, 5-bis (3-methoxy-4-hydroxy-benzylidene) Cyclopentanone on Proteins AChE and BChE Involved in Alzheimers disease and Ameliorative Effects on Paraquat Induced Oxidative Stress Markers in Drosophila melanogaster. Lett. Drug Des. Discov. 2011, 8, 260–267. [Google Scholar] [CrossRef]
- Ahmed, T.; Khan, A.-U.; Abbass, M.; Rodrigues Filho, E.; Din, Z.U.; Khan, A. Synthesis, characterization, molecular docking, analgesic, antiplatelet and anticoagulant effects of dibenzylidene ketone derivatives. Chem. Cent. J. 2018, 12, 134. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Gao, J.-l.; Xu, H.; Zheng, L.-Y.; Huang, W.-B.; Liu, Q.-W.; Zhang, S.-Q. Proline-based dipeptides as efficient organocatalysts for asymmetric aldol reactions in brine. Tetrahedron Asymmetry 2011, 22, 1074–1080. [Google Scholar] [CrossRef]
- Secci, D.; Carradori, S.; Petzer, A.; Guglielmi, P.; D’Ascenzio, M.; Chimenti, P.; Bagetta, D.; Alcaro, S.; Zengin, G.; Petzer, J.P. 4-(3-Nitrophenyl) thiazol-2-ylhydrazone derivatives as antioxidants and selective hMAO-B inhibitors: Synthesis, biological activity and computational analysis. J. Enzyme Inhib. Med. Chem. 2019, 34, 597–612. [Google Scholar] [CrossRef] [PubMed]
- Benabdesselam, S.; Izza, H.; Lanez, T.; Guechi, E. Synthesis, antioxidant and antibacterial activities of 3-nitrophenyl ferrocene. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Dubai, UAE, 24–26 November 2017; p. 012007. [Google Scholar]
- Shruthi, C.D.; Shetty, V.R.; Suresh, G.S. Indian Journal of Advances in Chemical Science. Indian J. Adv. Chem. Sci. 2018, 6, 182–186. [Google Scholar]
- Brinkerhoff, R.C.; Santa-Helena, E.; do Amaral, P.C.; Cabrera, D.D.C.; Ongaratto, R.F.; de Oliveira, P.M.; D’Oca, C.D.R.M.; Gonçalves, C.A.N.; Nery, L.E.M.; D’Oca, M.G.M. Evaluation of the antioxidant activities of fatty polyhydroquinolines synthesized by Hantzsch multicomponent reactions. RSC Adv. 2019, 9, 24688–24698. [Google Scholar] [CrossRef]
- De Resende, M.F.; Lino, C.I.; de Souza-Fagundes, E.M.; Rettore, J.V.P.; de Oliveira, R.B.; Labanca, R.A. Assessment of anti-diabetic activity of a novel hydrazine-thiazole derivative: In vitro and in vivo method. Braz. J. Pharm. Sci. 2019, 55. [Google Scholar] [CrossRef]
- Henderson, S.T. Ketone bodies as a therapeutic for Alzheimer’s disease. Neurotherapeutics 2008, 5, 470–480. [Google Scholar] [CrossRef]
- Gany, S.A.; Tan, S.C.; Gan, S.Y. Antioxidative, anticholinesterase and anti-neuroinflammatory properties of Malaysian brown and green seaweeds. IJIME 2015, 8, 1269–1275. [Google Scholar]
- Das, A.; Shanker, G.; Nath, C.; Pal, R.; Singh, S.; Singh, H.K. A comparative study in rodents of standardized extracts of Bacopamonniera and Ginkgo biloba: Anticholinesterase and cognitive enhancing activities. Pharmacol. Biochem. Behav. 2002, 73, 893–900. [Google Scholar] [CrossRef]
- Deng, G.; Wu, C.; Rong, X.; Li, S.; Ju, Z.; Wang, Y.; Ma, C.; Ding, W.; Guan, H.; Cheng, X. Ameliorative effect of deoxyvasicine on scopolamine-induced cognitive dysfunction by restoration of cholinergic function in mice. Phytomedicine 2019, 63, 153007. [Google Scholar] [CrossRef]
- Li, J.; Li, W.; Jiang, Z.-G.; Ghanbari, H.A. Oxidative stress and neurodegenerative disorders. Int. J. Mol. Sci. 2013, 14, 24438–24475. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, A.; Jaafar, H.Z.; Rahmat, A. Antioxidant activities, total phenolics and flavonoids content in two varieties of Malaysia young ginger (Zingiber officinale Roscoe). Molecules 2010, 15, 4324–4333. [Google Scholar] [CrossRef] [PubMed]
- Madamanchi, N.R.; Vendrov, A.; Runge, M.S. Oxidative stress and vascular disease. Arter. Thromb. Vasc. Biol. 2005, 25, 29–38. [Google Scholar] [CrossRef]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef]
- Mates, J. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology 2000, 153, 83–104. [Google Scholar] [CrossRef]
- Su, F.; Bai, F.; Zhang, Z. Inflammatory Cytokines and Alzheimer’s Disease: A Review from the Perspective of Genetic Polymorphisms. Neurosci. Bull. 2016, 32, 469–480. [Google Scholar] [CrossRef]
- Jung, H.W.; Yoon, C.-H.; Park, K.M.; Han, H.S.; Park, Y.-K. Hexane fraction of ZingiberisRhizomaCrudus extract inhibits the production of nitric oxide and proinflammatory cytokines in LPS-stimulated BV2 microglial cells via the NF-kappaB pathway. Food Chem. Toxicol. 2009, 47, 1190–1197. [Google Scholar] [CrossRef]
- Wong, A.; Lüth, H.J.; Deuther-Conrad, W.; Dukic-Stefanovic, S.; Gasic-Milenkovic, J.; Arendt, T.; Münch, G. Advanced glycationendproducts co-localize with inducible nitric oxide synthase in Alzheimer’s disease. Brain Res. 2001, 920, 32–40. [Google Scholar] [CrossRef]
- Griffin, W.S.T.; Sheng, J.G.; Roberts, G.W.; Mrak, R.E. Interleukin-1 expression in different plaque types in Alzheimer’s disease: Significance in plaque evolution. J. Neuropathol. Exp. Neurol. 1995, 54, 276–281. [Google Scholar] [CrossRef]
- van Gijsel-Bonnello, M.; Baranger, K.; Benech, P.; Rivera, S.; Khrestchatisky, M.; de Reggi, M.; Gharib, B. Metabolic changes and inflammation in cultured astrocytes from the 5xFAD mouse model of Alzheimer’s disease: Alleviation by pantethine. PLoS ONE 2017, 12, e0175369. [Google Scholar] [CrossRef]
- Yang, H.; Xie, J.; Mu, W.; Ruan, X.; Zhang, J.; Yao, L.; Diao, Z.; Wu, M.; Li, Y.; Ren, W. Tea polyphenols protect learning and memory in sleep-deprived mice by promoting AMPA receptor internalization. Neuroreport 2020, 31, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-Y.; Tan, M.-S.; Yu, J.-T.; Tan, L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl. Med. 2015, 3, 136. [Google Scholar] [PubMed]
- Pan, M.-H.; Lin-Shiau, S.-Y.; Lin, J.-K. Comparative studies on the suppression of nitric oxide synthase by curcumin and its hydrogenated metabolites through down-regulation of IκB kinase and NFκB activation in macrophages. Biochem. Pharmacol. 2000, 60, 1665–1676. [Google Scholar] [CrossRef]
- Wagner, J.M.; Sichler, M.E.; Schleicher, E.M.; Franke, T.N.; Irwin, C.; Löw, M.J.; Beindorff, N.; Bouter, C.; Bayer, T.A.; Bouter, Y. Analysis of motor function in the Tg4-42 mouse model of Alzheimer’s disease. Front. Behav. Neurosci. 2019, 13, 107. [Google Scholar] [CrossRef]
- Hui, J.; Feng, G.; Zheng, C.; Jin, H.; Jia, N. Maternal separation exacerbates Alzheimer’s disease-like behavioral and pathological changes in adult APPswe/PS1dE9 mice. Behav. Brain Res. 2017, 318, 18–23. [Google Scholar] [CrossRef]
- Brooks, S.P.; Dunnett, S.B. Tests to assess motor phenotype in mice: A user’s guide. Nat. Rev. Neurosci. 2009, 10, 519–529. [Google Scholar] [CrossRef]
- Einat, H.; Yuan, P.; Manji, H.K. Increased anxiety-like behaviors and mitochondrial dysfunction in mice with targeted mutation of the Bcl-2 gene: Further support for the involvement of mitochondrial function in anxiety disorders. Behav. Brain Res. 2005, 165, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Sturman, O.; Germain, P.-L.; Bohacek, J. Exploratory rearing: A context-and stress-sensitive behavior recorded in the open-field test. Stress 2018, 21, 443–452. [Google Scholar] [CrossRef]
- Beaufour, C.C.; Le Bihan, C.; Hamon, M.; Thiébot, M.-H. Extracellular dopamine in the rat prefrontal cortex during reward-, punishment-and novelty-associated behaviour. Effects of diazepam. Pharmacol. Biochem. Behav. 2001, 69, 133–142. [Google Scholar] [CrossRef]
- Nitz, D.A. Tracking route progression in the posterior parietal cortex. Neuron 2006, 49, 747–756. [Google Scholar] [CrossRef]
- Fan, J.; Li, D.; Chen, H.S.; Huang, J.G.; Xu, J.F.; Zhu, W.W.; Chen, J.G.; Wang, F. Metformin produces anxiolytic-like effects in rats by facilitating GABAA receptor trafficking to membrane. Br. J. Pharmacol. 2019, 176, 297–316. [Google Scholar] [CrossRef] [PubMed]
- Sestakova, N.; Puzserova, A.; Kluknavsky, M.; Bernatova, I. Determination of motor activity and anxiety-related behaviour in rodents: Methodological aspects and role of nitric oxide. Interdiscip. Toxicol. 2013, 6, 126–135. [Google Scholar] [CrossRef]
- Bartolomé, I.; Llidó, A.; Darbra, S.; Pallarès, M. Early postnatal neuroactive steroid manipulation differentially affects recognition memory and passive avoidance performance in male rats. Behav. Brain Res. 2020, 394, 112833. [Google Scholar]
- Carter, R.J.; Morton, J.; Dunnett, S.B. Motor coordination and balance in rodents. Curr. Protoc. Neurosci. 2001, 15, 8.12.1–8.12.14. [Google Scholar] [CrossRef] [PubMed]
- Wenk, G.L. Assessment of spatial memory using the radial arm maze and Morris water maze. Curr. Protoc. Neurosci. 2004, 26, 8.5A.1–8.5A.12. [Google Scholar] [CrossRef]
- Vorhees, C.V.; Williams, M.T. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006, 1, 848–858. [Google Scholar] [CrossRef]
- Ali, T.; Kim, M.O. Melatonin ameliorates amyloid beta-induced memory deficits, tau hyperphosphorylation and neurodegeneration via PI 3/Akt/GS k3β pathway in the mouse hippocampus. J. Pineal. Res. 2015, 59, 47–59. [Google Scholar] [CrossRef]
- Kraeuter, A.-K.; Guest, P.C.; Sarnyai, Z. The Y-Maze for assessment of spatial working and reference memory in mice. In Pre-Clinical Models; Springer: Berlin/Heidelberg, Germany, 2019; pp. 105–111. [Google Scholar]
- Oakley, H.; Cole, S.L.; Logan, S.; Maus, E.; Shao, P.; Craft, J.; Guillozet-Bongaarts, A.; Ohno, M.; Disterhoft, J.; Van Eldik, L. Intraneuronal β-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: Potential factors in amyloid plaque formation. J. Neurosci. 2006, 26, 10129–10140. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres Jr, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Semmler, A.; Frisch, C.; Debeir, T.; Ramanathan, M.; Okulla, T.; Klockgether, T.; Heneka, M.T. Long-term cognitive impairment, neuronal loss and reduced cortical cholinergic innervation after recovery from sepsis in a rodent model. Exp. Neurol. 2007, 204, 733–740. [Google Scholar] [CrossRef]
- Gao, J.; Wang, L.; Gao, C.; Arakawa, H.; Perry, G.; Wang, X. TDP-43 inhibitory peptide alleviates neurodegeneration and memory loss in an APP transgenic mouse model for Alzheimer’s disease. BBA Mol. Basis Dis. 2020, 1866, 165580. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Kosaraju, J.; Zhou, W.; Tam, K.Y. SLOH, a carbazole-based fluorophore, mitigates neuropathology and behavioral impairment in the triple-transgenic mouse model of Alzheimer’s disease. Neuropharmacology 2018, 131, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Naveed, M.; Khan, S.Z.; Zeeshan, S.; Khan, A.; Shal, B.; Atiq, A.; Ali, H.; Ullah, R.; Khan, S. A new cationic palladium (II) dithiocarbamate exhibits anti-inflammatory, analgesic, and antipyretic activities through inhibition of inflammatory mediators in in vivo models. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019, 392, 961–977. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.; Ullah, M.Z.; Khan, A.U.; Afridi, R.; Rasheed, H.; Khan, A.; Ali, H.; Kim, Y.S.; Khan, S. Antihyperalgesic properties of honokiol in inflammatory pain models by targeting of NF-κB and Nrf2 signaling. Front. Pharmacol. 2018, 9, 140. [Google Scholar] [CrossRef]
- Shah, S.; Yoon, G.; Chung, S.; Abid, M.; Kim, T.; Lee, H.; Kim, M. Novel osmotin inhibits SREBP2 via the AdipoR1/AMPK/SIRT1 pathway to improve Alzheimer’s disease neuropathological deficits. Mol. Psychiatry 2017, 22, 407–416. [Google Scholar] [CrossRef]


| Samples | Conc. (µg/mL) | Acetylcholinesterase (AChE) | Buterylcholinesterase (BChE) | ||
|---|---|---|---|---|---|
| Inhibition (%) | IC50 µg/mL | Inhibition (%) | IC50 µg/mL | ||
| 3NCP | 07.8 15.6 | 16.17 ± 1.53 25.0 ± 5.0 * | 16.17 | 66.0 ± 2.65 224.0 ± 5.29 | 20.51 |
| 31.25 | 45.7 ± 4.04 | 44.3 ± 6.03 | |||
| 62.5 | 57.0 ± 3.0 | 55.0 ± 4.36 * | |||
| 125 | 65.3 ± 4.16 | 65.8 ± 1.89 | |||
| 250 | 72.3 ± 3.06 | 71.0 ± 5.57 | |||
| 500 | 77.0 ± 3.0 | 76.0 ± 1.73 | |||
| 1000 | 84.9 ± 5.0 | 86.3 ± 5.51 | |||
| Galantamine | 07.8 15.6 | 11.3 ± 1.6 36.0 ± 2.0 | 13.12 | 16.0 ± 2.0 31.7 ± 5.77 | 14.43 |
| 31.25 | 54.0 ± 3.0 | 57.0 ± 2.0 | |||
| 62.5 | 62.0 ± 2.0 | 70.0 ± 5.0 | |||
| 125 | 71.0 ± 2.2 | 75.0 ± 5.0 | |||
| 250 | 77.0 ± 3.0 | 80.0 ± 4.5 | |||
| 500 | 84.7 ± 2.0 | 88.0 ± 4.0 | |||
| 1000 | 88.7 ± 4.15 | 91.3 ± 2.31 | |||
| Primer | Sequence (5′-3′) |
|---|---|
| Internal positive control | CTAGGCCACAGAATTGAAAGATCT |
| GTAGGTGGAAATTCTAGCATCATCC | |
| Transgene | AGGACTGACCACTCGACCAG |
| CGGGGGTCTAGTTCTGCAT | |
| IL-1β | AGAAGCTTCCACCAATACTC |
| AGCACCTAGTTGTAAGGAAG | |
| IL-6 | GCCCTTCAGGAACAGCTATGA |
| TGTCAACAACATCAGTCCCAAGA | |
| TNF-α | CTTCTCCTTCCTGATCGTGG |
| GCTGGTTATCTCTCAGCTCCA | |
| GAPDH | TGCACCACCAACTGCTTAGC |
| GGCATGGACTGTGGTCATGAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, R.; Ali, G.; Ahmad, N.; Akram, M.; Kumari, G.; Amin, M.U.; Umar, M.N. Attenuation of Spatial Memory in 5xFAD Mice by Halting Cholinesterases, Oxidative Stress and Neuroinflammation Using a Cyclopentanone Derivative. Pharmaceuticals 2020, 13, 318. https://doi.org/10.3390/ph13100318
Ullah R, Ali G, Ahmad N, Akram M, Kumari G, Amin MU, Umar MN. Attenuation of Spatial Memory in 5xFAD Mice by Halting Cholinesterases, Oxidative Stress and Neuroinflammation Using a Cyclopentanone Derivative. Pharmaceuticals. 2020; 13(10):318. https://doi.org/10.3390/ph13100318
Chicago/Turabian StyleUllah, Rahim, Gowhar Ali, Nisar Ahmad, Muhammad Akram, Geeta Kumari, Muhammad Usman Amin, and Muhammad Naveed Umar. 2020. "Attenuation of Spatial Memory in 5xFAD Mice by Halting Cholinesterases, Oxidative Stress and Neuroinflammation Using a Cyclopentanone Derivative" Pharmaceuticals 13, no. 10: 318. https://doi.org/10.3390/ph13100318
APA StyleUllah, R., Ali, G., Ahmad, N., Akram, M., Kumari, G., Amin, M. U., & Umar, M. N. (2020). Attenuation of Spatial Memory in 5xFAD Mice by Halting Cholinesterases, Oxidative Stress and Neuroinflammation Using a Cyclopentanone Derivative. Pharmaceuticals, 13(10), 318. https://doi.org/10.3390/ph13100318







