Antitumor Mechanism of the Essential Oils from Two Succulent Plants in Multidrug Resistance Leukemia Cell
Abstract
1. Introduction
2. Results
2.1. Chemical Composition
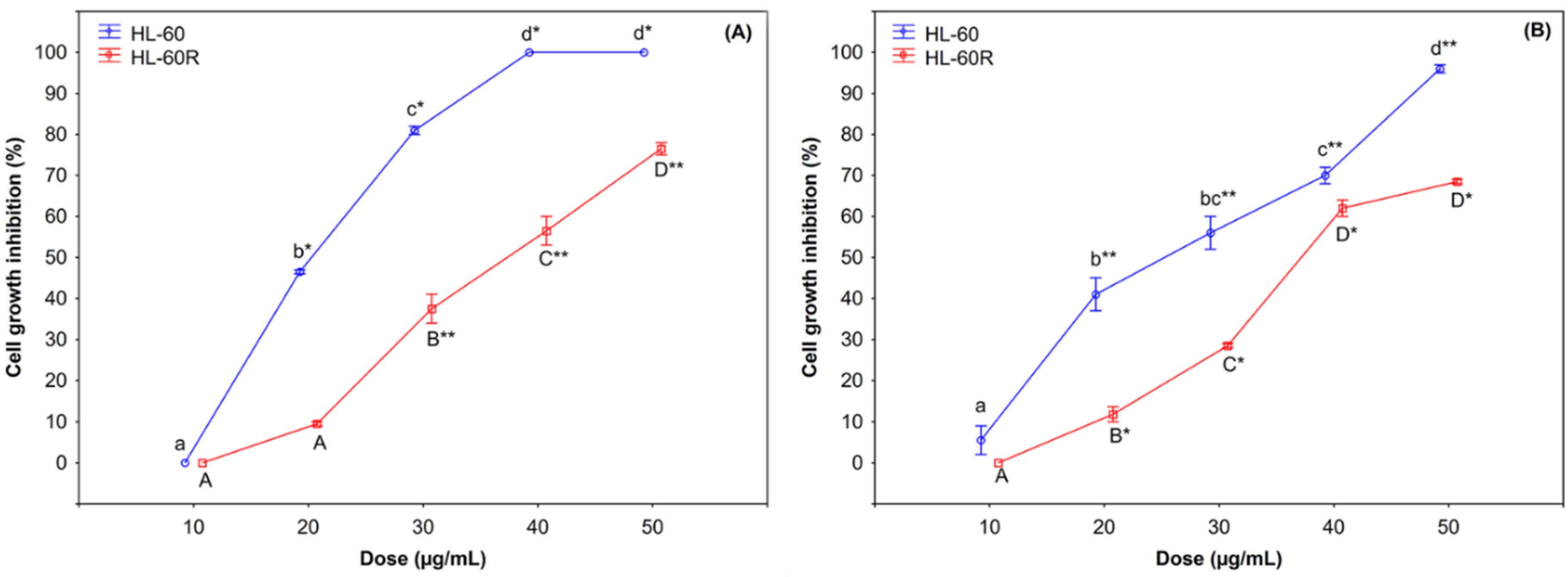
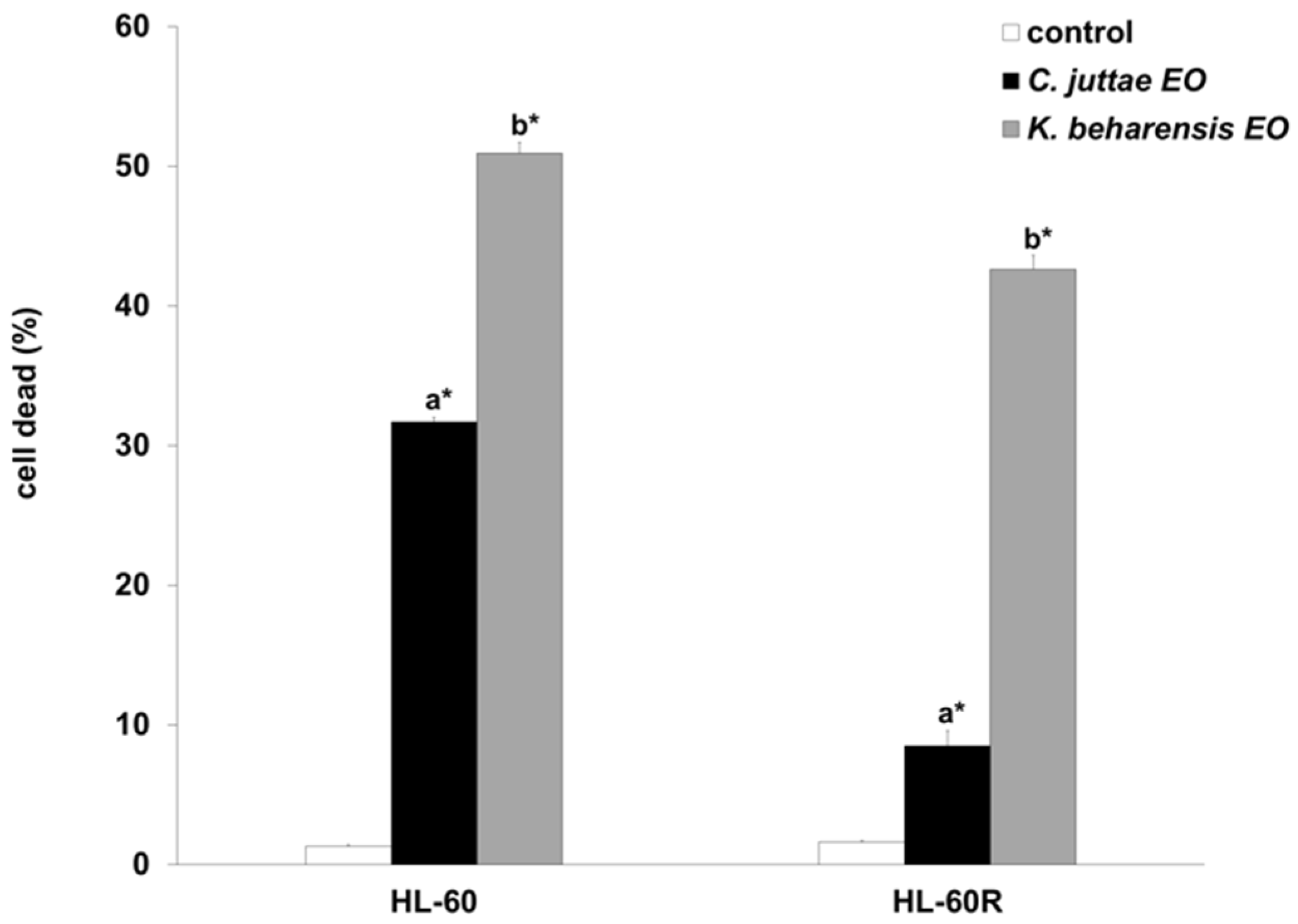
2.2. Cytotoxic Effects of C. juttae and K. beharensis Essential Oils
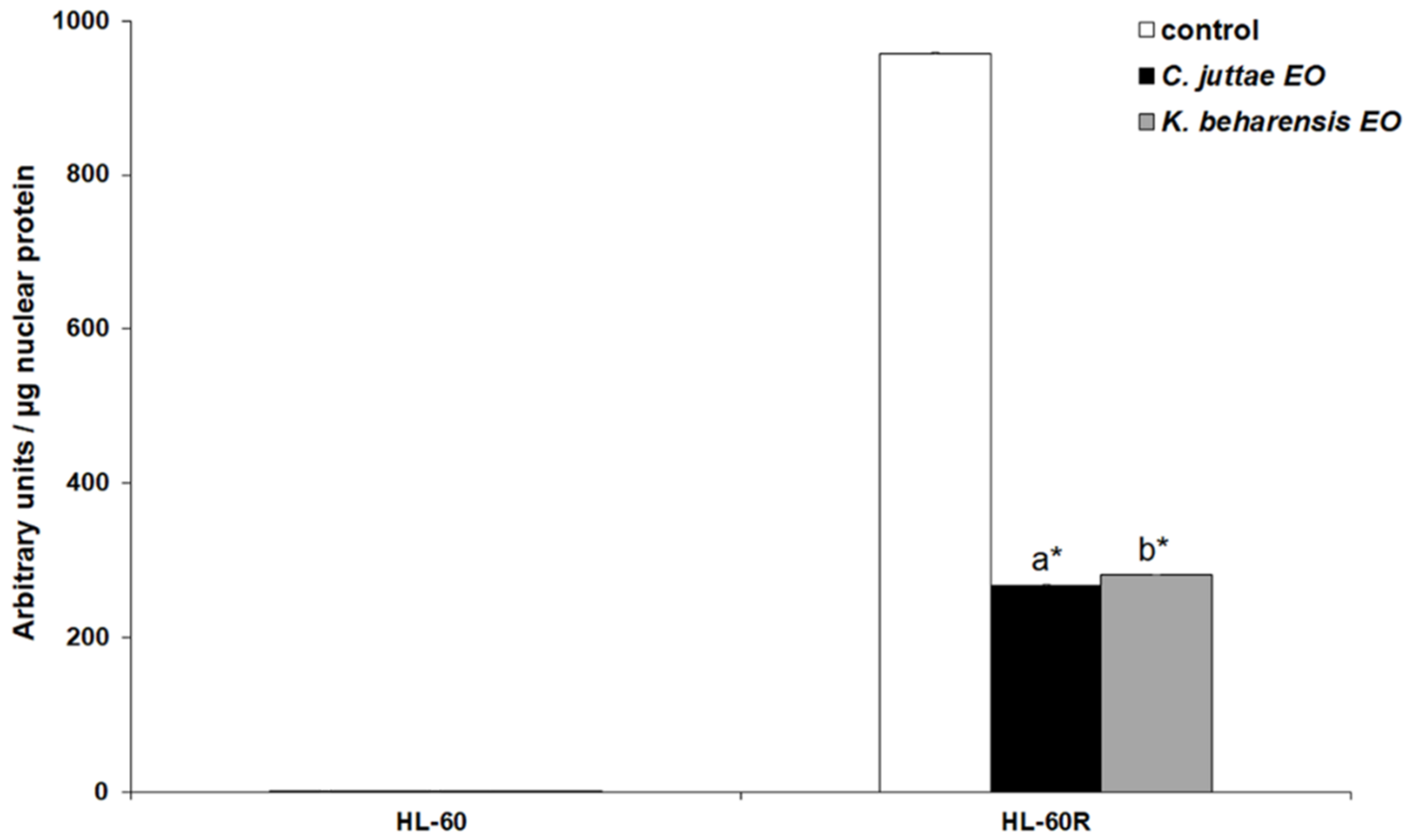
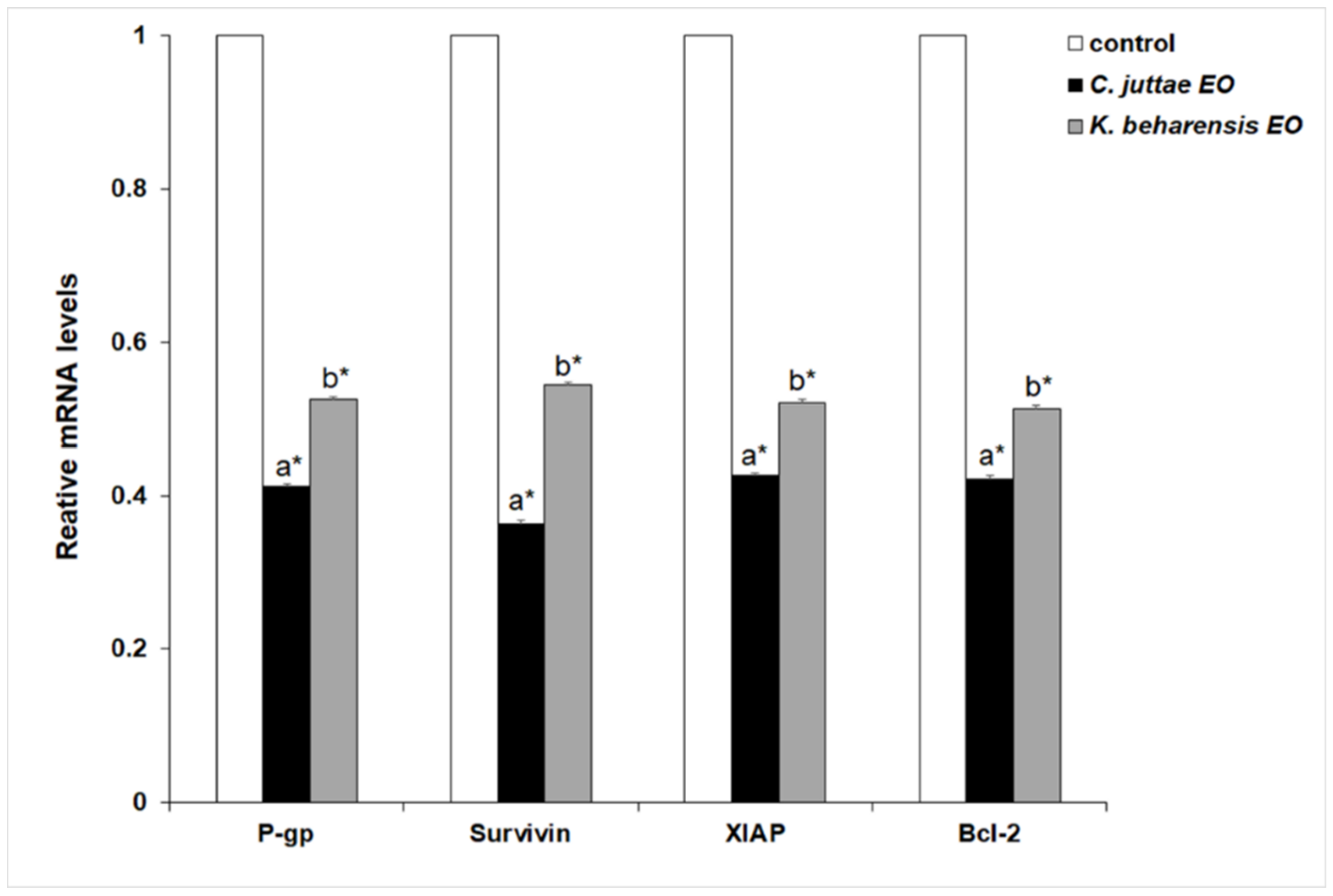
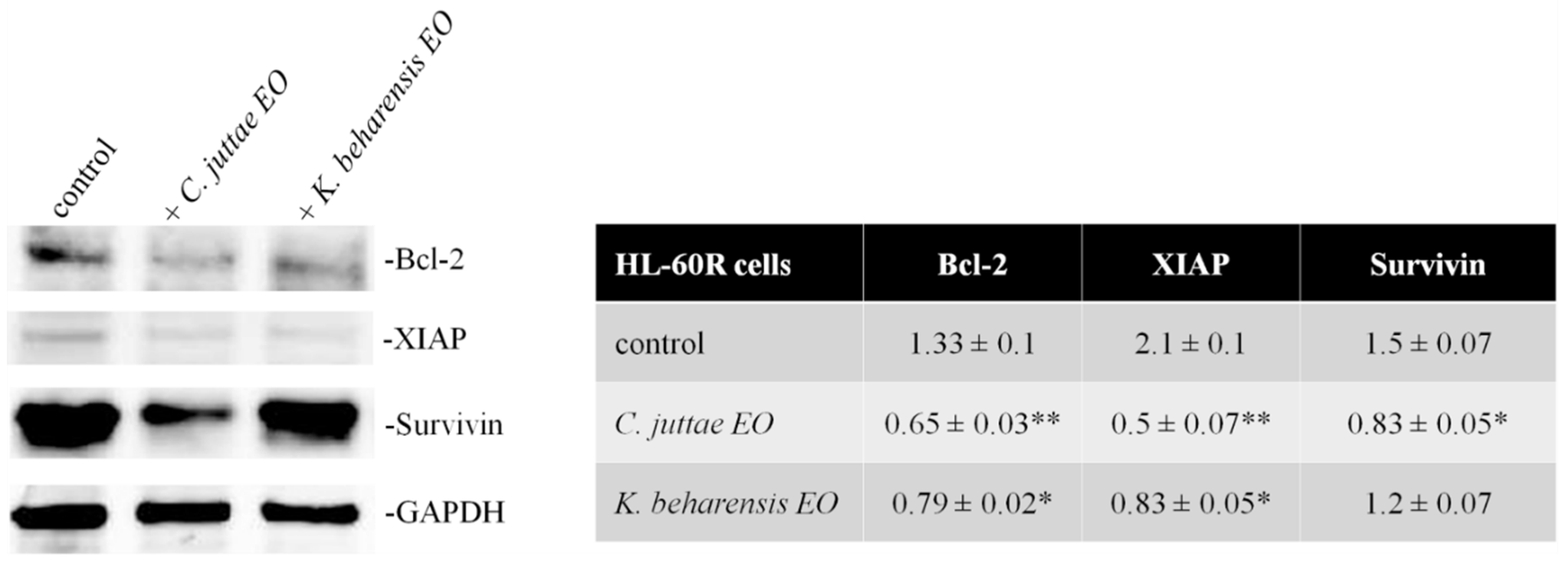
2.3. Effects of Essential Oils on NF-κB (p65 subunit) Pathway in HL-60/HL-60R Cells
3. Discussion
4. Material and Methods
4.1. Plant Species
4.2. Plant Material
4.3. Essential Oil Extraction
4.4. Gas Chromatography-Mass Spectrometry
4.5. Identification of Compounds
4.6. Cell Lines and Culture Conditions
4.7. Cell Growth Inhibition Assays
4.8. Evaluation of Cell Death by Flow Cytometry
4.9. NF-κB Activation
4.10. Extraction of Cellular RNA and Reverse Transcription-Quantitative PCR (RT-qPCR)
4.11. Western Blot Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Szakacs, G.; Paterson, J.K.; Ludwig, J.A.; Booth-Genthe, C.; Gottesman, M.M. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 2006, 5, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Choudhuri, S.; Klaassen, C.D. Structure, function, expression, genomic organization, and single nucleotide polymorphisms of human ABCB1 (MDR1), ABCC (MRP), and ABCG2 (BCRP) efflux transporters. Int. J. Toxicol. 2006, 25, 231–259. [Google Scholar] [CrossRef] [PubMed]
- Staud, F.; Pavek, P. Breast cancer resistance protein (BCRP/ABCG2). Int. J. Biochem. Cell. Biol. 2005, 37, 720–772. [Google Scholar] [CrossRef] [PubMed]
- Gillet, J.P.; Gottesman, M.M. Mechanisms of multidrug resistance in cancer. In Multi-Drug Resistance in Cancer. Methods in Molecular Biology (Methods and Protocols); Zhou, J., Ed.; Humana Press: New York, NY, USA, 2010; Volume 596, pp. 47–76. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Li, L.; Kong, R.; Pan, S.; Ji, L.; Liu, H.; Chen, H.; Sun, B. Hyperoside induces apoptosis and inhibits growth in pancreatic cancer via Bcl-2 family and NF-kappaB signaling pathway both in vitro and in vivo. Tumour Biol. 2016, 37, 7345–7355. [Google Scholar] [CrossRef] [PubMed]
- Darwish, N.H.E.; Sudha, T.; Godugu, K.; Bharali, D.J.; Elbaz, O.; El-Ghaffar, H.A.A.; Azmy, E.; Anber, N.; Mousa, S.A. Novel targeted nano-parthenolide molecule against NF-kB in Acute Myeloid Leukemia. Molecules 2019, 24, 2103. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ching, Y.Q.; Chng, W.J. Aberrant nuclear factor-kappa B activity in acute myeloid leukemia: From molecular pathogenesis to therapeutic target. Oncotarget 2015, 6, 5490–5500. [Google Scholar] [CrossRef] [PubMed]
- Griessinger, E.; Frelin, C.; Cuburu, N.; Imbert, V.; Dageville, C.; Hummelsberger, M.; Sirvent, N.; Dreano, M.; Peyron, J.F. Preclinical targeting of NF-kB and FLT3 pathways in AML cells. Leukemia 2008, 22, 1466–1469. [Google Scholar] [CrossRef]
- Braun, T.; Carvalho, G.; Fabre, C.; Grosjean, J.; Fenaux, P.; Kroemer, G. Targeting NF-kB in hematologic malignancies. Cell. Death Differ. 2006, 13, 748–758. [Google Scholar] [CrossRef]
- Poma, P.; Labbozzetta, M.; Notarbartolo, M.; Bruno, M.; Maggio, A.; Rosselli, S.; Sajeva, M.; Zito, P. Chemical composition, in vitro antitumor and pro-oxidant activities of Glandora rosmarinifolia (Boraginaceae) essential oil. PLoS ONE 2018, 13, e0196947. [Google Scholar] [CrossRef]
- Zito, P.; Labbozzetta, M.; Notarbartolo, M.; Sajeva, M.; Poma, P. Essential oil of Cyphostemma juttae (Vitaceae): Chemical composition and antitumor mechanism in triple negative breast cancer cells. PLoS ONE 2019, 14, e0214594. [Google Scholar] [CrossRef]
- Poma, P.; Labbozzetta, M.; Zito, P.; Alduina, R.; Ramarosandratana, A.V.; Bruno, M.; Rosselli, S.; Sajeva, M.; Notarbartolo, M. Essential oil composition of Alluaudia procera and in vitro biological activity on two drug-resistant models. Molecules 2019, 24, 2871. [Google Scholar] [CrossRef]
- Allorge-Boiteau, L. Madagascar centre de spéciation et d’origine du genre Kalanchoe (Crassulaceae). In Biogéographie de Madagascar; Lourenço, W.R., Ed.; Editions de L’ORSTOM: Paris, France, 1996; pp. 137–145. [Google Scholar]
- Mackenzie, K.K.; Lütken, H.; Coelho, L.L.; Kaaber, M.D.; Hegelund, J.N.; Müller, R. Kalanchoë. In Ornamental Crops, Handbook of Plant Breeding; Van Huylenbroeck, J., Ed.; Springer: Cham, Switzerland, 2018; Volume 11, pp. 453–479. [Google Scholar]
- Descoings, B. Le genre Kalanchoe, structure et définition. In Le journal de Botanique; Le Strat, F., Ed.; Société Botanique de France: Paris, France, 2006; Volume 33, pp. 3–28. [Google Scholar]
- Akulova-Barlow, Z. Kalanchoe . Cact. Succ. J. 2009, 81, 268–276. [Google Scholar] [CrossRef]
- Yamagishi, T.; Haruna, M.; Yan, X.Z.; Chang, J.J.; Lee, K.H. Antitumor agents, Bryophyllin B, a novel potent cytotoxic bufadienolide from Bryophyllum pinnatum. J. Nat. Prod. 1989, 52, 1071–1079. [Google Scholar] [CrossRef]
- Shirobokov, V.P.; Evtushenko, A.I.; Lapchik, V.F.; Shirobokov, D.N.; Suptel, E.A. Antiviral activity of representatives of the family Crassulaceae. Antibiotiki 1981, 26, 897–900. [Google Scholar]
- Milad, R.; El-Ahmady, S.; Singab, A.N. Genus Kalanchoe (Crassulaceae): A review of its ethnomedicinal, botanical, chemical and pharmacological properties. Eur. J. Med. Plants 2014, 4, 86. [Google Scholar] [CrossRef]
- Kluge, M.; Razanoelisoa, B.; Ravelomanana, D.; Brulfert, J. In situ studies of crassulacean acid metabolism in Kalanchoe beharensis Drake Del Castillo, a plant of the semi-arid southern region of Madagascar. New Phytol. 1992, 120, 323–334. [Google Scholar] [CrossRef]
- Walker, C.C. Two shrubby Madagascan kalanchoes. N.Z. Cactus Succul. J. 2019, 72, 5–9. [Google Scholar]
- Nagasaki, M.S. Transmission of Environmental and Conservation Knowledge in Andohahela National Park. Independent Study Project (ISP) Collection 485. 2005. Available online: https://digitalcollections.sit.edu/isp_collection/485 (accessed on 25 August 2019).
- Andriamparany, J.N.; Brinkmann, K.; Jeannoda, V.; Buerkert, A. Effects of socio-economic household characteristics on traditional knowledge and usage of wild yams and medicinal plants in the Mahafaly region of south-western Madagascar. J. Ethnobiol. Ethnomed. 2014, 10, 82. [Google Scholar] [CrossRef]
- Simmen, B.; Hladik, A.; Ramasiarisoa, P. Food intake and dietary overlap in native Lemur catta and Propithecus verreauxi and introduced Eulemur fulvus at Berenty, Southern Madagascar. Int. J. Primatol. 2003, 24, 949–968. [Google Scholar] [CrossRef]
- Gould, L.; Constabel, P.; Mellway, R.; Rambeloarivony, H. Condensed tannin intake in spiny-forest-dwelling Lemur catta, at Berenty Reserve, Madagascar, during reproductive periods. Folia Primatol. 2009, 80, 249–263. [Google Scholar] [CrossRef]
- Ghaly, N.S.; Mina, S.A.; Abdel-Aziz, N.F.; Sammour, E.A. Insecticidal activity of the main flavonoids from the leaves of Kalanchoe beharensis and Kalanchoe longiflora. J. Nat. Prod. 2014, 7, 196–202. [Google Scholar]
- Teklehaymanot, T.; Giday, M. Ethnobotanical study of medicinal plants used by people in Zegie Peninsula, Northwestern Ethiopia. J. Ethnobiol. Ethnomed. 2007, 3, 12. [Google Scholar] [CrossRef]
- Opoku, A.R.; Geheeb-Keller, M.; Lin, J.; Terblanche, S.E.; Hutchings, A.; Chuturgoon, A.; Pillay, D. Preliminary screening of some traditional Zulu medicinal plants for antineoplastic activities versus the HepG2 cell line. Phytother. Res. 2000, 14, 534–537. [Google Scholar] [CrossRef]
- Notarbartolo, M.; Cervello, M.; Poma, P.; Dusonchet, L.; Meli, M.; D’Alessandro, N. Expression of the IAPs in multidrug resistant tumor cells. Oncol. Rep. 2004, 11, 133–136. [Google Scholar] [CrossRef]
- Notarbartolo, M.; Cervello, M.; Dusonchet, L.; Cusimano, A.; D’Alessandro, N. Resistance to diverse apoptotic triggers in multidrug resistant HL60 cells and its possible relationship to the expression of P-glycoprotein, Fas and of the novel anti-apoptosis factors IAP (inhibitory of apoptosis proteins). Cancer Lett. 2002, 180, 91–101. [Google Scholar] [CrossRef]
- Hrdinka, M.; Yabal, M. Inhibitor of apoptosis proteins in human health and disease. Genes Immun. 2019, 1. [Google Scholar] [CrossRef]
- Knudsen, J.T.; Eriksson, R.; Gershenzon, J.; Stahl, B. Diversity and distribution of floral scent. Bot. Rev. 2006, 72, 1–120. [Google Scholar] [CrossRef]
- Islam, M.T.; Ali, E.S.; Uddin, S.J.; Shaw, S.; Islam, M.A.; Ahmed, M.I.; Chandra Shill, M.; Karmakar, U.K.; Yarla, N.S.; Khan, I.N.; et al. Phytol: A review of biomedical activities. Food Chem. Toxicol. 2018, 121, 82–94. [Google Scholar] [CrossRef]
- Dudareva, N.; Negre, F.; Dinesh, A.; Nagegowda, D.A.; Orlova, I. Plant volatiles: Recent advances and future perspectives. Crit. Rev. Plant Sci. 2006, 25, 417–440. [Google Scholar] [CrossRef]
- Wu, D.; Wu, P.; Zhao, L.; Huang, L.; Zhang, Z.; Zhao, S.; Huang, J. NF-kappaB expression and outcomes in solid tumors: A systematic review and meta-analysis. Medicine (Baltimore) 2015, 94, e1687. [Google Scholar] [CrossRef]
- Rathore, R.; McCallum, J.E.; Varghese, E.; Florea, A.M.; Busselberg, D. Overcoming chemotherapy drug resistance by targeting inhibitors of apoptosis proteins (IAPs). Apoptosis 2017, 22, 898–919. [Google Scholar] [CrossRef]
- Fontana, G.; Bruno, M.; Notarbartolo, M.; Labbozzetta, M.; Poma, P.; Spinella, A.; Rosselli, S. Cytotoxicity of oleanolic and ursolic acid derivatives toward hepatocellular carcinoma and evaluation of NF-κB involvement. Bioorg. Chem. 2019, 103054. [Google Scholar] [CrossRef]
- Notarbartolo, M.; Labbozzetta, M.; Pojero, F.; D’Alessandro, N.; Poma, P. Potential therapeutic applications of MDA-9/Syntenin-NF-ΚB-RKIP loop in human liver carcinoma. Curr. Mol. Med. 2018, 18, 630–639. [Google Scholar] [CrossRef]
- Poma, P.; Labbozzetta, M.; D’Alessandro, N.; Notarbartolo, M. NF-κB is a potential molecular drug target in triple-negative breast cancers. OMICS 2017, 21, 225–231. [Google Scholar] [CrossRef]
- Shen, M.Y.; Wang, Y.; Cui, S.Y.; Wu, X.L.; Guo, Y.; Xu, R.R. MicroRNA-125a regulates proliferation and apoptosis of acute myeloid leukemia through targeting NF-κB pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 3594–3601. [Google Scholar] [CrossRef]
- Czop, M.; Bogucka-Kocka, A.; Kubrak, T.; Knap-Czop, K.; Makuch-Kocka, A.; Galkowski, D.; Wawer, J.; Kocki, T.; Kocki, J. Imaging flow cytometric analysis of stilbene-dependent apoptosis in drug resistant human leukemic cell lines. Molecules 2019, 24, 1896. [Google Scholar] [CrossRef]
- Pan, L.; Li, Y.; Zhang, H.Y.; Zheng, Y.; Liu, X.L.; Hu, Z.; Wang, Y.; Wang, J.; Cai, Y.H.; Liu, Q.; et al. DHX15 is associated with poor prognosis in acute myeloid leukemia (AML) and regulates cell apoptosis via the NF-kB signaling pathway. Oncotarget 2017, 8, 89643–89654. [Google Scholar] [CrossRef]
- Zhou, J.; Lu, X.; Tan, T.Z.; Chung, W.J. X-linked inhibitor of apoptosis inhibition sensitizes acute myeloid leukemia cell response to TRAIL and chemotherapy through potentiated induction of proapoptotic machinery. Mol. Oncol. 2018, 12, 33–47. [Google Scholar] [CrossRef]
- Regnault-Roger, C.; Vincent, C.; Arnason, J.T. Essential oils in insect control: Low-risk products in a high-stakes world. Annu. Rev. Entomol. 2012, 57, 405–424. [Google Scholar] [CrossRef]
- Gilmore, T.D.; Wolenski, F.S. NF-κB: Where did it come from and why? Immunol. Rev. 2012, 246, 14–35. [Google Scholar] [CrossRef]
- Silverman, N.; Maniatis, T. NF-κB signaling pathways in mammalian and insect innate immunity. Genes Dev. 2001, 15, 2321–2342. [Google Scholar] [CrossRef]
- Zito, P.; Sajeva, M.; Bruno, M.; Rosselli, S.; Maggio, A.; Senatore, F. Essential oils composition of Periploca laevigata Aiton subsp. angustifolia (Labill.) Markgraf (Apocynaceae–Periplocoideae). Nat. Prod. Res. 2013, 27, 255–265. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured Publishing: Carol Stream, IL, USA, 2007. [Google Scholar]
- El-Sayed, A.M. The Pherobase: Database of Insect Pheromones and Semiochemicals. Available online: http://www.pherobase.com/ (accessed on 10 June 2019).

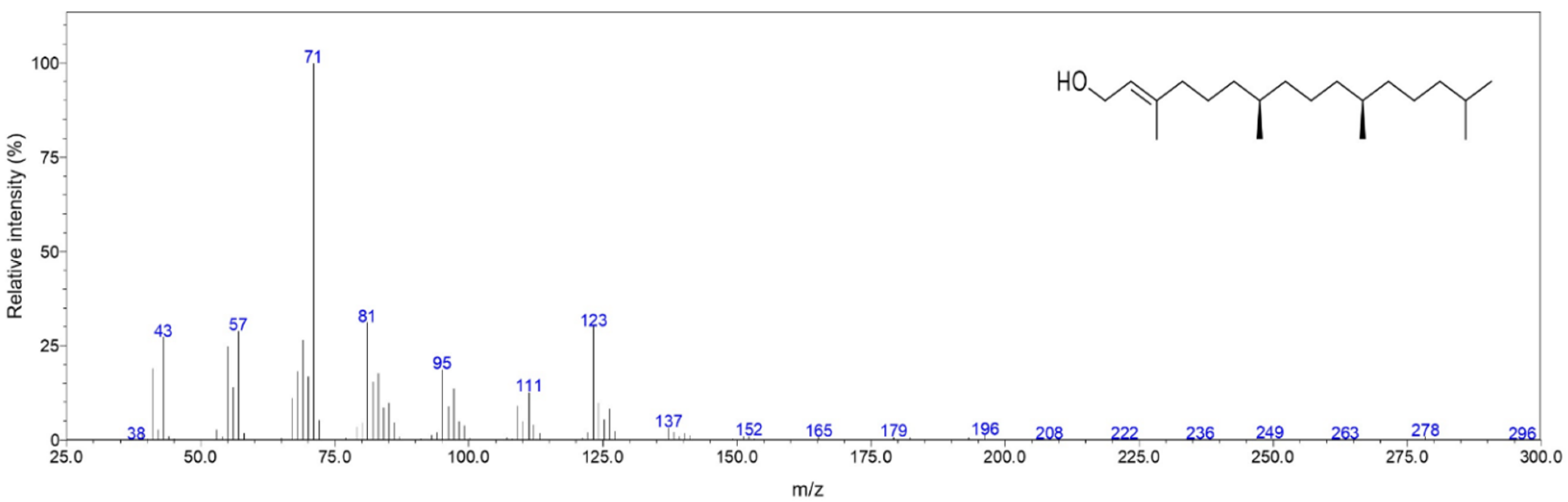
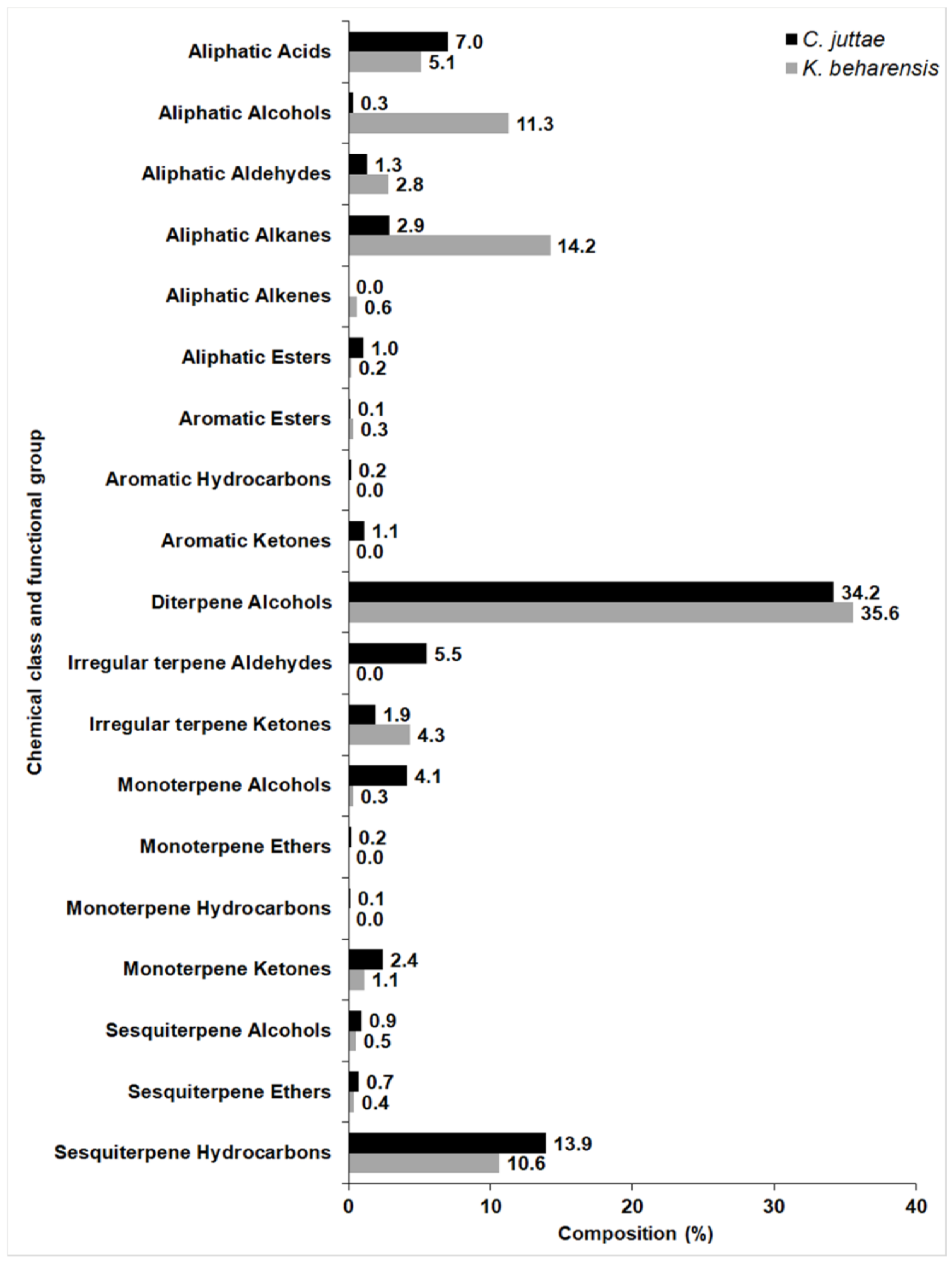

| RI a | Ident b | Compound | Relative Amount (%) | MSS c (%) |
|---|---|---|---|---|
| Aliphatic Acids | ||||
| 2720 | RI, MS | Tetradecanoic Acid | 5.1 | 94 |
| Aliphatic Alcohols | ||||
| 1354 | RI, MS | Hexanol | 0.2 | 95 |
| 1407 | RI, MS | (E)-2-Hexen-1-ol | 0.1 | 88 |
| 1452 | RI, MS | 1-Octen-3-ol | 1.3 | 95 |
| 1558 | RI, MS | Octanol | 1.5 | 97 |
| 1616 | RI, MS | (E)-2-Octen-1-ol | 1.0 | 91 |
| 1661 | RI, MS | Nonanol | 0.1 | 88 |
| 1761 | MS | (Z)-9-Tetradecen-1-ol | 0.2 | 87 |
| 1763 | RI, MS | Decanol | 0.1 | 87 |
| 1968 | RI, MS | Dodecanol | 0.2 | 88 |
| 2174 | RI, MS | Tetradecanol | 0.3 | 83 |
| 2585 | RI, MS | Octadecanol | 0.4 | 90 |
| 2793 | MS | Eicosanol | 0.8 | 91 |
| 2816 | MS | (Z,Z,Z)-9,12,15-Octadecatrien-1-ol | 5.1 | 85 |
| Aliphatic Aldehydes | ||||
| 1050 | RI, MS | Hexanal | 0.5 | 92 |
| 1218 | RI, MS | (E)-2-Hexenal | 0.7 | 95 |
| 1389 | RI, MS | Nonanal | 0.4 | 96 |
| 1494 | RI, MS | Decanal | 0.6 | 95 |
| 2023 | RI, MS | Pentadecanal | 0.5 | 90 |
| 2129 | RI, MS | Hexadecanal | 0.1 | 89 |
| Aliphatic Alkanes* | ||||
| 1400 | RI, MS, Co-GC | Tetradecane | 0.1 | standard |
| 1700 | RI, MS, Co-GC | Heptadecane | 0.04 | standard |
| 1900 | RI, MS, Co-GC | Nonadecane | 0.2 | standard |
| 2200 | RI, MS, Co-GC | Docosane | 0.4 | standard |
| 2300 | RI, MS, Co-GC | Tricosane | 2.4 | standard |
| 2500 | RI, MS, Co-GC | Pentacosane | 3.6 | standard |
| 2600 | RI, MS, Co-GC | Hexacosane | 0.7 | standard |
| 2700 | RI, MS, Co-GC | Heptacosane | 5.0 | standard |
| 2900 | RI, MS, Co-GC | Nonacosane | 1.8 | standard |
| Aliphatic Alkenes | ||||
| 1862 | MS | (Z,Z,Z)-3,6,9-Tetradecatriene | 0.6 | 87 |
| Aliphatic Esters | ||||
| 1607 | RI, MS | Hexyl hexanoate | 0.1 | 86 |
| 2213 | RI, MS | Methyl palmitate | 0.1 | 88 |
| Aromatic Esters | ||||
| 2164 | MS | 2-Ethylhexyl benzoate | 0.1 | 93 |
| 2286 | MS | 2-Ethylhexyl salicylate | 0.2 | 80 |
| Diterpene Alcohols | ||||
| 2296 | RI, MS | Isophytol | 0.6 | 88 |
| 2615 | RI, MS | Phytol | 35.0 | 96 |
| Irregular terpene Ketones | ||||
| 1807 | RI, MS | (E)-β-Damascenone | 0.2 | 83 |
| 2123 | RI, MS | Hexahydrofarnesyl acetone | 4.1 | 94 |
| Monoterpene Alcohols | ||||
| 1789 | RI, MS | α-Campholenol | 0.1 | 84 |
| 2216 | RI, MS | Carvacrol | 0.2 | 91 |
| Monoterpene Ketones | ||||
| 1630 | RI, MS | Pulegone | 0.5 | 95 |
| 1908 | RI, MS | Piperitenone | 0.6 | 86 |
| Sesquiterpene Alcohols | ||||
| 1552 | RI, MS | (Z)-Sesquisabinene hydrate | 0.2 | 86 |
| 2042 | RI, MS | (E)-Nerolidol | 0.1 | 83 |
| 2308 | MS | 6-epi-Shyobunol | 0.2 | 83 |
| Sesquiterpene Ethers | ||||
| 1956 | RI, MS | Caryophyllene oxyde | 0.4 | 92 |
| Sesquiterpene Hydrocarbons | ||||
| 1470 | RI, MS | α-Copaene | 0.2 | 94 |
| 1572 | RI, MS | β-Caryophyllene | 4.5 | 97 |
| 1644 | RI, MS | α-Humulene | 0.1 | 85 |
| 1665 | MS | (E)-β-Bergamotene | 0.04 | 83 |
| 1696 | RI, MS | γ-Humulene | 0.1 | 85 |
| 1736 | RI, MS | δ-Cadinene | 0.1 | 85 |
| 1926 | RI, MS | Neophytadiene | 2.8 | 94 |
| 1955 | MS | Neophytadiene isomer # | 1.1 | 95 |
| 1982 | MS | Neophytadiene isomer # | 1.4 | 95 |
| 2236 | MS | Neophytadiene isomer # | 0.3 | 86 |
| HL-60 | HL-60R | |
|---|---|---|
| IC50 | IC50 | |
| C. juttae EO | 22.0 ± 0.3 μg/mL | 36 ± 1.2 μg/mL |
| K. beharensis EO | 25.0 ± 0.6 μg/mL | 36.5 ± 0.3 μg/mL |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poma, P.; Labbozzetta, M.; McCubrey, J.A.; Ramarosandratana, A.V.; Sajeva, M.; Zito, P.; Notarbartolo, M. Antitumor Mechanism of the Essential Oils from Two Succulent Plants in Multidrug Resistance Leukemia Cell. Pharmaceuticals 2019, 12, 124. https://doi.org/10.3390/ph12030124
Poma P, Labbozzetta M, McCubrey JA, Ramarosandratana AV, Sajeva M, Zito P, Notarbartolo M. Antitumor Mechanism of the Essential Oils from Two Succulent Plants in Multidrug Resistance Leukemia Cell. Pharmaceuticals. 2019; 12(3):124. https://doi.org/10.3390/ph12030124
Chicago/Turabian StylePoma, Paola, Manuela Labbozzetta, James A. McCubrey, Aro Vonjy Ramarosandratana, Maurizio Sajeva, Pietro Zito, and Monica Notarbartolo. 2019. "Antitumor Mechanism of the Essential Oils from Two Succulent Plants in Multidrug Resistance Leukemia Cell" Pharmaceuticals 12, no. 3: 124. https://doi.org/10.3390/ph12030124
APA StylePoma, P., Labbozzetta, M., McCubrey, J. A., Ramarosandratana, A. V., Sajeva, M., Zito, P., & Notarbartolo, M. (2019). Antitumor Mechanism of the Essential Oils from Two Succulent Plants in Multidrug Resistance Leukemia Cell. Pharmaceuticals, 12(3), 124. https://doi.org/10.3390/ph12030124







