Role of Dietary Flavonoids in Iron Homeostasis
Abstract
1. Biological Importance of Iron
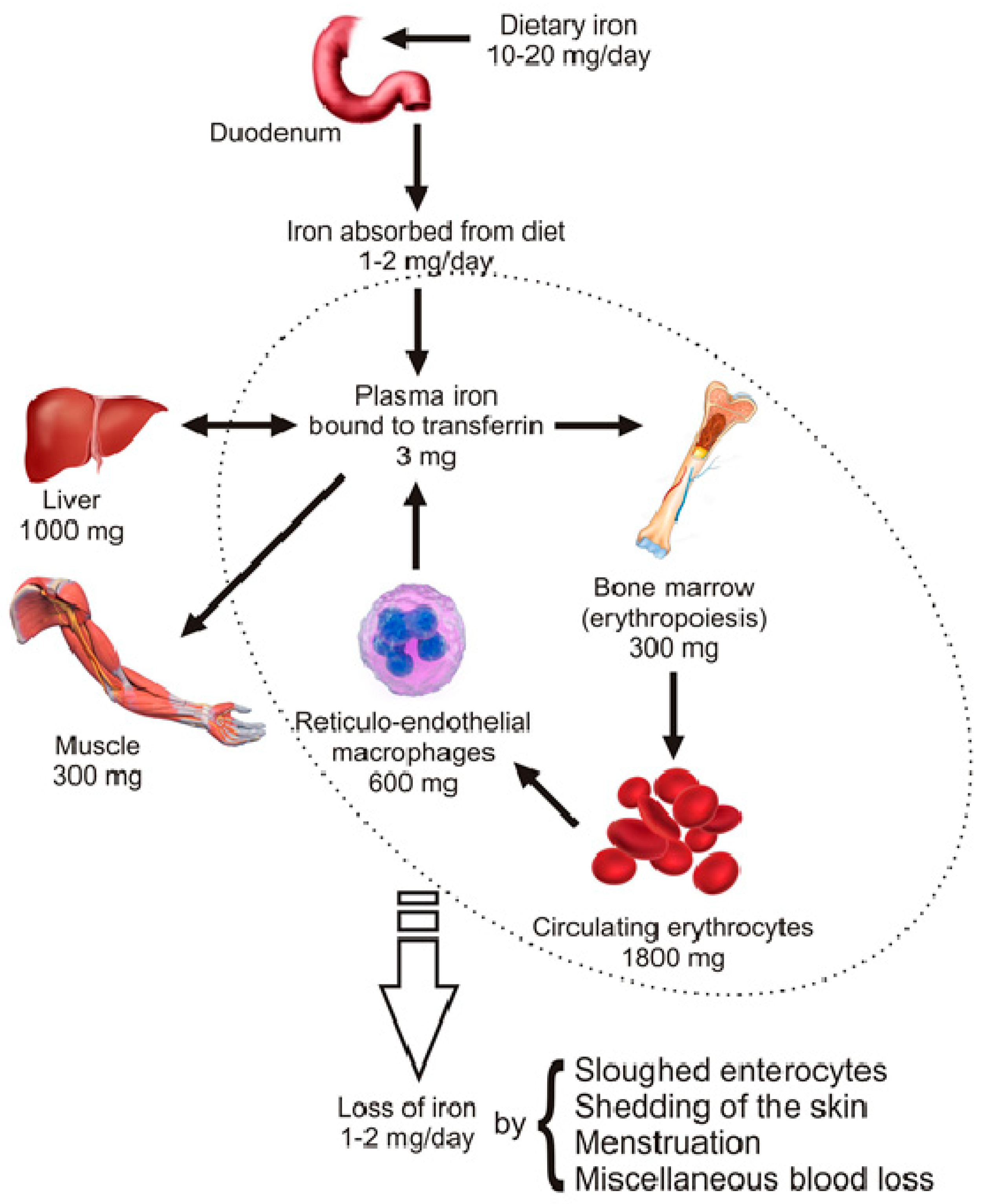
2. Distribution and Homeostasis of Body Iron
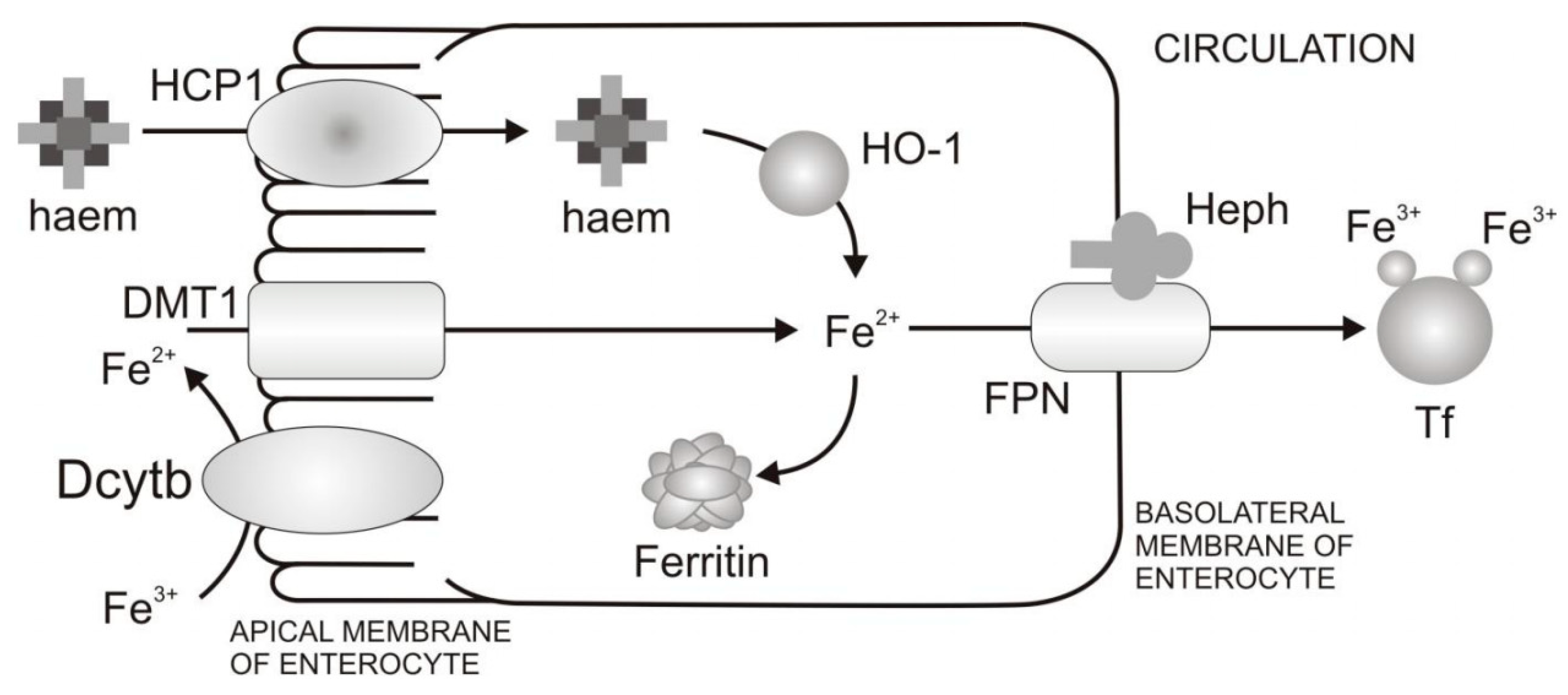
Mechanism of Dietary Iron Uptake
- Reduction of Fe3+ and uptake of Fe2+ from the diet through the apical membrane of enterocytes. In the diet iron is mainly present as Fe3+. However, the absorption of Fe2+ is more efficient than Fe3+. In order to increase Fe3+ bioavailability, Fe3+ firstly needs to be reduced. Duodenal cytohrome b (Dcytb) is an iron-regulated ferric reductase, highly expressed on the apical membrane of duodenal enterocytes [18]. After being reduced by Dcytb, Fe2+ is transported across the apical membrane by the divalent metal transporter 1 (DMT1) [19].
- Intracellular processing of iron and iron transport to the basolateral membrane of enterocytes. Even though mechanisms of intracellular iron transport are not fully elucidated, it is assumed that poly r(C)-binding proteins (PCBPs) play important roles in this transport. Namely, PCBP1 is identified as an iron chaperone for ferritin, the main iron storage protein in the cell, while PCBP2 is assumed to transfer of iron from DMT1 to the cytosol and later to iron efflux transporter ferroportin (FPN). In addition, NCOA4 was identified as autophagic receptor for ferritin, which during iron deficiency in cell leads to ferritin autophagy and iron liberation [20]. In general, the fate of absorbed iron is closely related to the body’s demands for iron. If there is a need for more iron, then iron is exported from the cell via the basolateral membrane of enterocytes which is followed by iron binding to Tf and transport to peripheral tissues that require iron. If there is no need for additional iron in the body, iron is stored in the cell in the form of ferritin, and returned to the lumen at a time when the villus enterocytes die [8].
- Transfer of iron through the basolateral membrane to the circulation. The mechanism of Fe2+ transport through the basolateral membrane includes synchronized activity of two proteins, FPN [21,22,23] and transmembrane copper-dependent ferroxidase, hephaestin (Heph) [24,25]. Before entering circulation, Fe2+ firstly needs to be oxidized to the Fe3+ state, which is catalysed by hephaestin, the intestinal ferroxidase. Fe3+ then binds to the serum glycoprotein Tf [26], the key iron transporting protein in the serum and extracellular fluids.
3. Bioavailability of Iron
3.1. Anaemias
3.2. Dietary Inhibitors of Iron Absorption
3.3. Dietary Enhancers of Iron Absorption
3.4. Ways to Prevent Anaemia
4. Plant Polyphenols
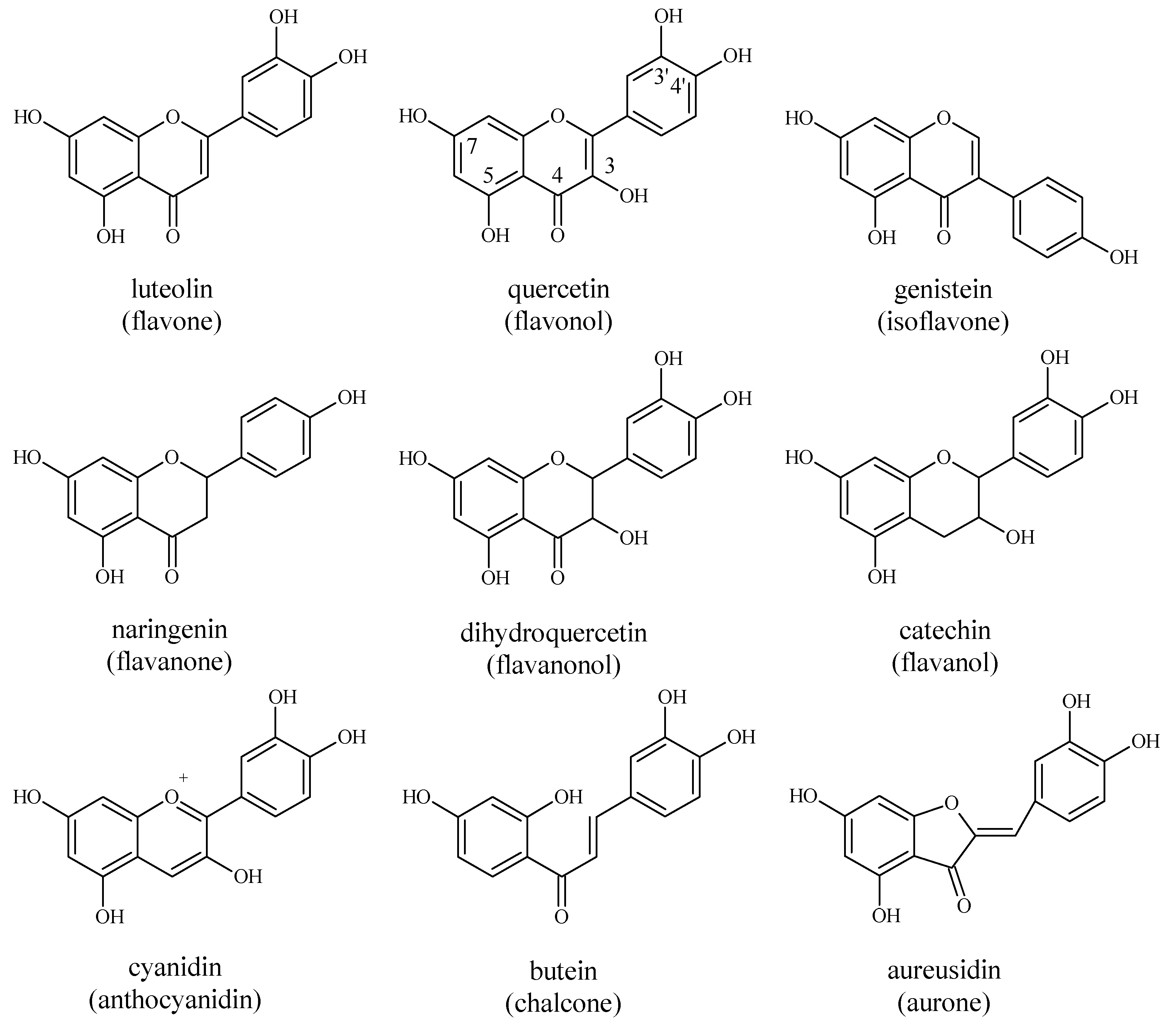
4.1. Flavonoids
4.2. Absorption and Metabolism of Flavonoids in Humans
4.3. Occurrence and Intake of Dietary Flavonoids
4.4. Links between Flavonoids and Iron Homeostasis
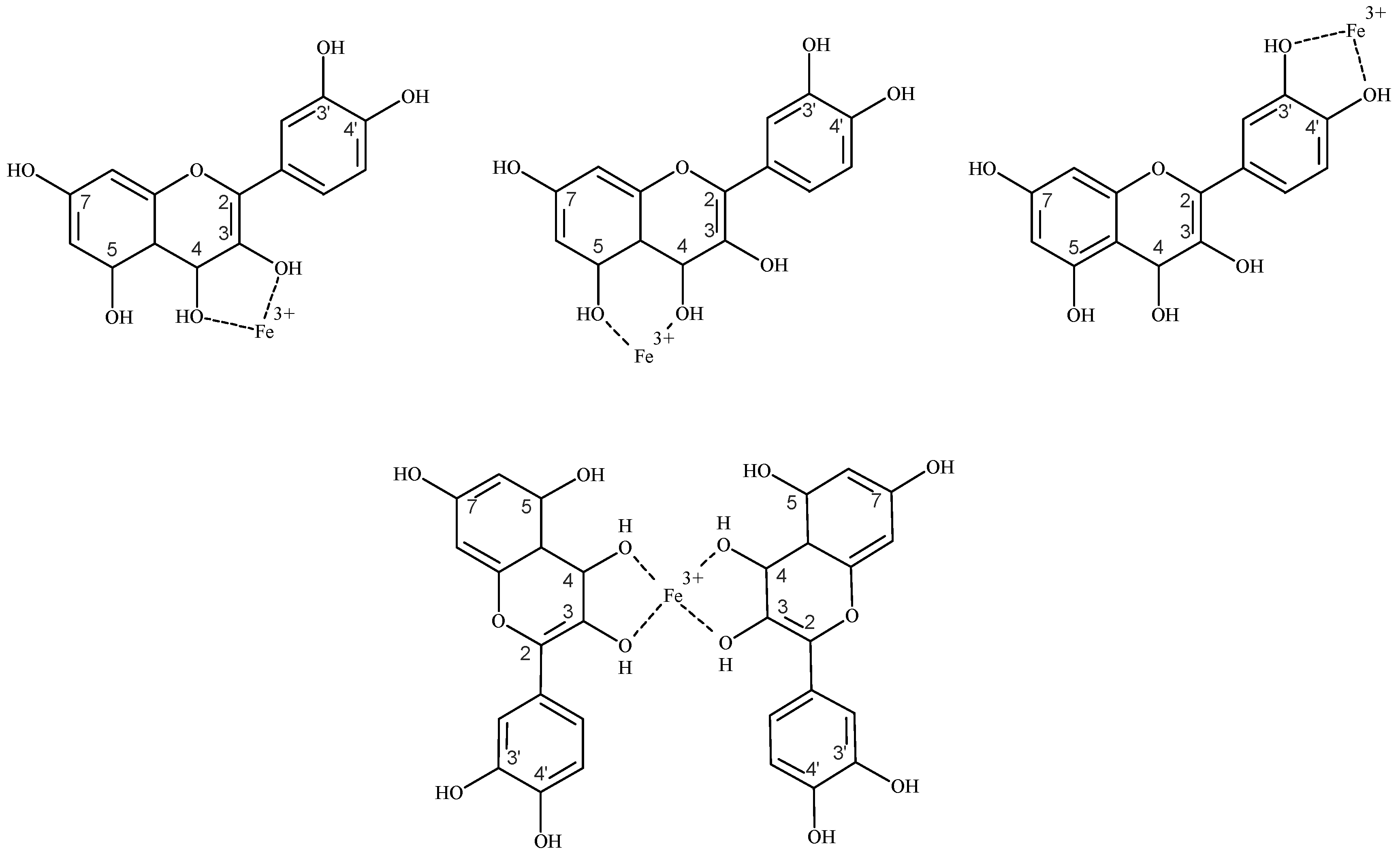
4.4.1. Flavonoids as Iron Chelators
4.4.2. Flavonoids as Regulators of Systemic Iron Metabolism
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Crichton, R.R. Inorganic Biochemistry of Iron Metabolism: From Molecular Mechanisms to Clinical Consequences, 2nd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2001. [Google Scholar]
- Lieu, P.T.; Heiskala, M.; Peterson, P.A.; Yang, Y. The roles of iron in health and disease. Mol. Asp. Med. 2001, 22, 1–87. [Google Scholar] [CrossRef]
- Yehuda, S.; Mostofsky, D.I. Iron Deficiency and Overload: From Basic Biology to Clinical Medicine; Humana Press: New York, NY, USA, 2010. [Google Scholar]
- Ying-Wu, L.; Jiangyun, W. Structure and function of heme proteins in non–native states: A mini–review. J. Inorg. Biochem. 2013, 129, 162–171. [Google Scholar]
- Lill, R.; Muhlenhoff, U. Iron-sulfur protein biogenesis in eukaryotes: Components and mechanisms. Annu. Rev. Cell Dev. Biol. 2006, 22, 457–486. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Iron Deficiency Anaemia: Assessment, Prevention, and Control. A Guide for Programme Managers; WHO Press: Geneva, Switzerland, 2001. [Google Scholar]
- Dunn, L.L.; Suryo Rahmanto, Y.; Richardson, D.R. Iron uptake and metabolism in the new millennium. Trends Cell Biol. 2007, 17, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Sharp, P.; Srai, S.K. Molecular mechanisms involved in intestinal iron absorption. World J. Gastroenterol. 2007, 13, 4716–4724. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.C.; Almeida, J.I.; Lima, I.S.; Kapitão, A.S.; Gozzelino, R. Iron Metabolism and the Inflammatory Response. IUBMB Life 2017, 69, 442–450. [Google Scholar] [CrossRef]
- Wang, L.; Cherayil, B.J. Ironing out the wrinkles in host defense: Interactions between iron homeostasis and innate immunity. J. Innate Immun. 2009, 1, 455–464. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 4th ed.; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Koppenol, W.H. The Haber-Weiss cycle—70 years later. Redox Rep. 2001, 6, 229–234. [Google Scholar] [CrossRef]
- Hentze, M.W.; Muckenthaler, M.U.; Andrews, N.C. Balancing acts: Molecular control of mammalian iron metabolism. Cell 2004, 117, 285–297. [Google Scholar] [CrossRef]
- Andrews, N.C. Disorders of iron metabolism. N. Engl. J. Med. 1999, 341, 1986–1995. [Google Scholar] [CrossRef]
- Papanikolaoua, G.; Pantopoulos, K. Iron metabolism and toxicity. Toxicol. Appl. Pharmacol. 2005, 202, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, C.; Delaby, C. Recycling iron in normal and pathological states. Semin. Hematol. 2009, 46, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Srai, S.K.; Bomford, A.; McArdle, H.J. Iron transport across cell membranes: Molecular understanding of duodenal and placental iron uptake. Best Pract. Res. Clin. Haematol. 2002, 15, 243–259. [Google Scholar] [CrossRef] [PubMed]
- McKie, A.T.; Barrow, D.; Latunde-Dada, G.O.; Rolfs, A.; Sager, G.; Mudaly, E.; Mudaly, M.; Richardson, C.; Barlow, D.; Bomford, A.; et al. An iron–regulated ferric reductase associated with the absorption of dietary iron. Science 2001, 291, 1755–1759. [Google Scholar] [CrossRef] [PubMed]
- Gunshin, H.; Mackenzie, B.; Berger, U.V.; Gunshin, Y.; Romero, M.F.; Boron, W.F.; Nussberger, S.; Gollan, J.L.; Hediger, M.A. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 1997, 388, 482–488. [Google Scholar] [CrossRef]
- Philpott, C.C.; Ryu, M.S.; Frey, A.; Patel, S. Cytosolic iron chaperones: Proteins delivering iron cofactors in the cytosol of mammalian cells. J. Biol. Chem. 2017, 292, 12764–12771. [Google Scholar] [CrossRef]
- Abboud, S.; Haile, D.J. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J. Biol. Chem. 2000, 275, 19906–19912. [Google Scholar] [CrossRef]
- Donovan, A.; Brownlie, A.; Zhou, Y.; Shepard, J.; Pratt, S.J.; Moynihan, J.; Paw, B.H.; Drejer, A.; Barut, B.; Zapata, A.; et al. Positional cloning of zebrafish ferroportin 1 identifies a conserved vertebrate iron exporter. Nature 2000, 403, 776–781. [Google Scholar] [CrossRef]
- McKie, A.T.; Marciani, P.; Rolfs, A.; Brennan, K.; Wehr, K.; Barrow, D.; Miret, S.; Bomford, A.; Peters, T.J.; Farzaneh, F.; et al. A novel duodenal iron–regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol. Cell 2000, 5, 299–309. [Google Scholar] [CrossRef]
- Vulpe, C.D.; Kuo, Y.M.; Murphy, T.L.; Cowley, L.; Askwith, C.; Libina, N.; Gitschier, J.; Anderson, G.J. Hephaestin, a ceruloplasmin homologue implicated in intestinal iron transport, is defective in the sla mouse. Nat. Genet. 1999, 21, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Attieh, Z.K.; Su, T.; Syed, B.A.; Gao, H.; Alaeddine, R.M.; Fox, T.C.; Usta, J.; Naylor, C.E.; Evans, R.W.; et al. Hephaestin is a ferroxidase that maintains partial activity in sex-linked anemia mice. Blood 2004, 103, 3933–3939. [Google Scholar] [CrossRef] [PubMed]
- MacGillivray, R.T.; Mendez, E.; Shewale, J.G.; Sinha, S.K.; Lineback-Zins, J.; Brew, K. The Primary Structure of Human Serum Transferrin; The structures of seven cyanogen bromide fragments and the assembly of the complete structure. J. Biol. Chem. 1983, 258, 3543–3553. [Google Scholar] [PubMed]
- Shayeghi, M.; Latunde-Dada, G.O.; Oakhill, J.S.; Laftah, A.H.; Takeuchi, K.; Halliday, N.; Khan, Y.; Warley, A.; McCann, F.E.; Hider, R.C.; et al. Identification of an intestinal heme transporter. Cell 2005, 122, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Nakai, Y.; Ueda, S.; Kamigaso, S.; Ohta, K.-Y.; Hatakeyama, M.; Hayashi, Y.; Otagiri, M.; Yuasa, H. Functional characterization of PCFT/HCP1 as the molecular entity of the carrier-mediated intestinal folate transport system in the rat model. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G660–G668. [Google Scholar] [CrossRef] [PubMed]
- Duffy, S.P.; Shing, J.; Saraon, P.; Berger, L.C.; Eiden, M.V.; Wilde, A.; Tailor, C.S. The Fowler syndrome-associated protein FLVCR2 is an importer of heme. Mol. Cell. Biol. 2010, 30, 5318–5324. [Google Scholar] [CrossRef] [PubMed]
- Tenhunen, R.; Marver, H.S.; Schmid, R. Microsomal heme oxygenase. Characterization of the enzyme. J. Biol. Chem. 1969, 244, 6388–6394. [Google Scholar] [PubMed]
- Hurrell, R.; Egli, I. Iron bioavailability and dietary reference values. Am. J. Clin. Nutr. 2010, 91, 1461S–1467S. [Google Scholar] [CrossRef]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar]
- DeMaeyer, E.M.; Dallman, P.; Gurney, J.M.; Hallberg, L.; Sood, S.K.; Srikantia, S.G. Preventing and Controlling Iron Deficiency Anaemia Through Primary Health Care: A Guide for Health Administrators and Programme Managers; World Health Organization: Geneva, Switzerland, 1989. [Google Scholar]
- World Health Organization. Assessing the Iron Status of Populations: Including Literature Reviews, 2nd ed.; WHO Press: Geneva, Switzerland, 2007. [Google Scholar]
- World Health Organization. Worldwide Prevalence of Anaemia 1993–2005; WHO Press: Geneva, Switzerland, 2008. [Google Scholar]
- Franchini, M.; Montagnana, M.; Lippi, G. Hepcidin and iron metabolism: From laboratory to clinical implications. Clin. Chim. Acta 2010, 411, 1565–1569. [Google Scholar] [CrossRef]
- World Health Organization. The World Health Report 2002. Reducing Risks, Promoting Healthy Life; WHO Press: Geneva, Switzerland, 2002. [Google Scholar]
- World Health Organization. The Global Prevalence of Anaemia in 2011; WHO Document Production Services: Geneva, Switzerland, 2017. [Google Scholar]
- USDA. USDA Food Composition Databases. Available online: https://ndb.nal.usda.gov/ndb/search/list?home=true (accessed on 4 July 2019).
- Siegenberg, D.; Baynes, R.D.; Bothwell, T.H.; Macfarlane, B.J.; Lamparelli, R.D.; Car, N.G.; MacPhail, P.; Schmidt, U.; Tal, A.; Mayet, F. Ascorbic acid prevents the dose-dependent inhibitory effects of polyphenols and phytates on nonheme-iron absorption. Am. J. Clin. Nutr. 1991, 53, 537–541. [Google Scholar] [CrossRef]
- Hurrell, R.F.; Reddy, M.B.; Juillerat, M.A.; Cook, J.D. Degradation of phytic acid in cereal porridges improves iron absorption by human subjects. Am. J. Clin. Nutr. 2003, 77, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, R.F. Phytic acid degradation as a means of improving iron absorption. Int. J. Vitam. Nutr. Res. 2004, 74, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Schlemmer, U.; Frølich, W.; Prieto, R.M.; Grases, F. Phytate in foods and significance for humans: Food sources, intake, processing, bioavailability, protective role and analysis. Mol. Nutr. Food Res. 2009, 53, S330–S375. [Google Scholar] [CrossRef] [PubMed]
- Hallberg, L.; Rossander-Hulten, L.; Brune, M.; Gleerup, A. Calcium and iron absorption: Mechanism of action and nutritional importance. Eur. J. Clin. Nutr. 1992, 46, 317–327. [Google Scholar] [PubMed]
- Roughead, Z.K.F.; Zito, C.A.; Hunt, J.R. Inhibitory effects of dietary calcium on the initial uptake and subsequent retention of heme and nonheme iron in humans: Comparisons using an intestinal lavage method. Am. J. Clin. Nutr. 2005, 82, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, R.F.; Lynch, S.R.; Trinidad, T.P.; Dassenko, S.A.; Cook, J.D. Iron absorption in humans: Bovine serum albumin compared with beef muscle and egg white. Am. J. Clin. Nutr. 1988, 47, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, R.F.; Lynch, S.R.; Trinidad, T.P.; Dassenko, S.A.; Cook, J.D. Iron absorption in humans as influenced by bovine milk proteins. Am. J. Clin. Nutr. 1989, 49, 546–552. [Google Scholar] [CrossRef]
- Cook, J.D.; Monsen, E.R. Food iron absorption in human subjects III. Comparison of the effect of animal proteins on nonheme iron absorption. Am. J. Clin. Nutr. 1976, 29, 859–867. [Google Scholar] [CrossRef]
- Lynch, S.R.; Dassenko, S.A.; Cook, J.D.; Juillerat, M.A.; Hurrell, R.F. Inhibitory effect of a soybean-protein-related moiety on iron absorption in humans. Am. J. Clin. Nutr. 1994, 60, 567–572. [Google Scholar] [CrossRef]
- Gillooly, M.; Bothwell, T.H.; Torrance, J.D.; MacPhail, A.P.; Derman, D.P.; Bezwoda, W.R.; Mills, W.; Charlton, R.W. The effects of organic acids, phytates and polyphenols on the absorption of iron from vegetables. Br. J. Nutr. 1983, 49, 331–3423. [Google Scholar] [CrossRef]
- Ballot, D.; Baynes, R.D.; Bothwell, T.H.; Gillooly, M.; MacFarlane, B.J.; MacPhail, A.P.; Lyons, G.; Derman, D.P.; Bezwoda, W.R.; Torrance, J.D.; et al. The effects of fruit juices and fruits on the absorption of iron from a rice meal. Br. J. Nutr. 1987, 57, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Hallberg, L.; Brune, M.; Rossander, L. Iron absorption in man: Ascorbic acid and dose-dependent inhibition by phytate. Am. J. Clin. Nutr. 1989, 49, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Frei, B. Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am. J. Clin. Nutr. 1999, 69, 1086–1107. [Google Scholar] [CrossRef] [PubMed]
- Teucher, B.; Olivares, M.; Cori, H. Enhancers of iron absorption: Ascorbic acid and other organic acids. Int. J. Vitam. Nutr. Res. 2004, 74, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.D.; Reddy, M.B. Effect of ascorbic acid intake on nonheme-iron absorption from a complete diet. Am. J. Clin. Nutr. 2001, 73, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, R.F.; Reddy, M.B.; Juillerat, M.; Cook, J.D. Meat protein fractions enhance nonheme iron absorption in humans. J. Nutr. 2006, 136, 2808–2812. [Google Scholar] [CrossRef] [PubMed]
- Storcksdieck, S.; Bonsmann, G.; Hurrell, R.F. Iron-binding properties, amino acid composition, and structure of muscle tissue peptides from in vitro digestion of different meat sources. J. Food Sci. 2007, 72, S19–S29. [Google Scholar] [CrossRef] [PubMed]
- Armah, C.N.; Sharp, P.; Mellon, F.A.; Pariagh, S.; Lund, E.K.; Dainty, J.R.; Teucher, B.; Fairweather-Tait, S.J. L-alpha-glycerophosphocholine contributes to meat’s enhancement of nonheme iron absorption. J. Nutr. 2008, 138, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Yip, R.; Ramakrishnan, U. Experiences and Challenges in Developing Countries. J. Nutr. 2002, 132, 827S–830S. [Google Scholar] [CrossRef]
- Lynch, S.R. The impact of iron fortification on nutritional anaemia. Best Pract. Res. Clin. Haematol. 2005, 18, 333–346. [Google Scholar] [CrossRef]
- Baltussen, R.; Knai, C.; Sharan, M. Iron Fortification and Iron Supplementation are Cost-Effective Interventions to Reduce Iron Deficiency in Four Subregions of the World. J. Nutr. 2004, 134, 2678–2684. [Google Scholar] [CrossRef] [PubMed]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Robards, K. Strategies for the determination of bioactive phenols in plants, fruit and vegetables. J. Chromatogr. A 2003, 1000, 657–691. [Google Scholar] [CrossRef]
- Lattanzio, V.; Lattanzio, V.M.T.; Cardinali, A. Role of phenolics in the resistance mechanisms of plants against fungal pathogents and insect. In Phytochemistry: Advances in Research; Imperato, F., Ed.; Research Signpost: Trivandrum, Kerala, India, 2006. [Google Scholar]
- Bradshaw, H.D.; Schemske, D.W. Allele substitution at a flower colour locus produces a pollinator shift in monkeyflowers. Nature 2003, 426, 176–178. [Google Scholar] [CrossRef]
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R. Polyphenols in health and disease. Practice and mechanisms of benefits. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 757–778. [Google Scholar]
- Havsteen, B.H. The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar] [CrossRef]
- Rietveld, A.; Wiseman, S. Antioxidant effects of tea: Evidence from human clinical trials. J. Nutr. 2003, 133, 3285S–3292S. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Marzocchella, L.; Fantini, M.; Benvenuto, M.; Masuelli, L.; Tresoldi, I.; Modesti, A.; Bei, R. Dietary flavonoids: Molecular mechanisms of action as anti- inflammatory agents. Recent Pat. Inflamm. Allergy Drug Discov. 2011, 5, 200–220. [Google Scholar] [CrossRef]
- Williamson, G.; Manach, C. Bioavailability and bioefficacy of polyphenols in humans II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005, 81, S243–S255. [Google Scholar] [CrossRef]
- Hooper, L.; Kroon, P.A.; Rimm, E.B.; Cohn, J.S.; Harvey, I.; Le Cornu, K.A.; Ryder, J.J.; Hall, W.L.; Cassidy, A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2008, 88, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Egert, S.; Rimbach, G. Which sources of flavonoids: Complex diets or dietary supplements? Adv. Nutr. 2011, 2, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Mitchell, A.E. Pharmacokinetics of Quercetin Absorption from Apples and Onions in Healthy Humans. J. Agric. Food Chem. 2012, 60, 3874–3881. [Google Scholar] [CrossRef]
- Walle, T.; Otake, Y.; Walle, U.K.; Wilson, F.A. Quercetin glucosides are completely hydrolyzed in ileostomy patients before absorption. J. Nutr. 2000, 130, 2658–2661. [Google Scholar] [CrossRef]
- Wolffram, S.; Blöck, M.; Ader, P. Quercetin-3-glucoside is transported by the glucose carrier SGLT1 across the brush border membrane of rat small intestine. J. Nutr. 2002, 132, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Day, A.J.; Gee, J.M.; DuPont, M.S.; Johnson, I.T.; Williamson, G. Absorption of quercetin-3-glucoside and quercetin-4′-glucoside in the rat small intestine: The role of lactase phlorizin hydrolase and the sodium-dependent glucose transporter. Biochem. Pharmacol. 2003, 65, 1199–1206. [Google Scholar] [CrossRef]
- Ziberna, L.; Fornasaro, S.; Čvorović, J.; Tramer, F.; Passamonti, S. Bioavailability of flavonoids: The role of cell membrane transporters. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 489–511. [Google Scholar]
- Strobel, P.; Allard, C.; Perez-Acle, T.; Calderon, R.; Aldunate, R.; Leighton, F. Myricetin, quercetin and catechin-gallate inhibit glucose uptake in isolated rat adipocytes. Biochem. J. 2005, 386, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.; Bijsman, M.N.; van Gameren, Y.; Cnossen, E.P.; de Vries, J.H.; Katan, M.B. The sugar moiety is a major determinant of the absorption of dietary flavonoid glycosides in man. Free Radic. Res. 1999, 31, 569–573. [Google Scholar] [CrossRef]
- Morand, C.; Manach, C.; Crespy, V.; Remesy, C. Quercetin 3-O-beta-glucoside is better absorbed than other quercetin forms and is not present in rat plasma. Free Radic. Res. 2000, 33, 667–676. [Google Scholar] [CrossRef]
- Olthof, M.R.; Hollman, P.C.H.; Buijsman, M.N.C.P.; Amelsvoort, J.M.M.; Katan, M.B. Chlorogenic acid, quercetin-3-rutinoside and black tea polyphenols are extensively metabolized in humans. J. Nutr. 2003, 133, 1806–1814. [Google Scholar] [CrossRef]
- Mullen, W.; Edwards, C.A.; Crozier, A. Absorption, excretion and metabolite profiling of methyl-, glucuronyl-, glucosyl- and sulpho-conjugates of quercetin in human plasma and urine after ingestion of onions. Br. J. Nutr. 2006, 96, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, H.; Heiss, C.; Spencer, J.P.; Keen, C.L.; Lupton, J.R.; Schmitz, H.H. Recommending flavanols and procyanidins for cardiovascular health: Current knowledge and future needs. Mol. Asp. Med. 2010, 31, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.K.; Rotunno, M.; Lubin, J.H.; Wacholder, S.; Consonni, D.; Pesatori, A.C.; Bertazzi, P.A.; Chanock, S.J.; Burdette, L.; Goldstein, A.M.; et al. Dietary quercetin, quercetin-gene interaction, metabolic gene expression in lung tissue and lung cancer risk. Carcinogenesis 2010, 31, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Ekström, A.M.; Serafini, M.; Nyrén, O.; Wolk, A.; Bosetti, C.; Bellocco, R. Dietary quercetin intake and risk of gastric cancer: Results from a population-based study in Sweden. Ann. Oncol. 2011, 22, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Beecher, G.R. Overview of Dietary Flavonoids: Nomenclature, Occurrence and Intake. J. Nutr. 2003, 133, 3248S–3254S. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, S.; Haytowitz, D.B.; Holden, J.M. USDA Database for the Flavonoid Content of Selected Foods; U.S. Department of Agriculture: Beltsville, MD, USA, 2014.
- Disler, P.B.; Lynch, S.R.; Charlton, R.W.; Torrance, J.D.; Bothwell, T.H.; Walker, R.B.; Mayet, F. The effect of tea on iron absorption. Gut 1975, 16, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Rossander, L.; Hallberg, L.; Bjom-Rasmussen, E. Absorption of iron from breakfast meals. Am. J. Clin. Nutr. 1979, 32, 2484–2489. [Google Scholar] [CrossRef]
- Petry, N. Polyphenols and low iron bioavailability. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 311–322. [Google Scholar]
- Lesjak, M.; Hoque, R.; Balesaria, S.; Skinner, V.; Debnam, E.S.; Srai, S.K.; Sharp, P.A. Quercetin inhibits intestinal iron absorption and ferroportin transporter expression in vivo and in vitro. PLoS ONE 2014, 9, e102900. [Google Scholar] [CrossRef]
- Vlachodimitropoulou, E.; Naftalin, R.J.; Sharp, P.A. Quercetin is a substrate for the transmembrane oxidoreductase Dcytb. Free Radic. Biol. Med. 2010, 48, 1366–1369. [Google Scholar] [CrossRef]
- Poggiali, E.; Cassinerio, E.; Zanaboni, L.; Cappellini, M.D. An update on iron chelation therapy. Blood Transfus. 2012, 10, 411–422. [Google Scholar]
- Baccan, M.M.; Chiarelli-Neto, O.; Pereira, R.M.S.; Espósito, B.P. Quercetin as a shuttle for labile iron. J. Inorg. Biochem. 2012, 107, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Bayele, H.K.; Balesaria, S.; Srai, S.K.S. Phytoestrogens modulate hepcidin expression by Nrf2: Implications for dietary control of iron absorption. Free Radic. Biol. Med. 2015, 89, 1192–1202. [Google Scholar] [CrossRef] [PubMed]
- Granado-Serrano, A.B.; Martín, M.A.; Bravo, L.; Goya, L.; Ramos, S. Quercetin modulates Nrf2 and glutathione-related defenses in HepG2 cells: Involvement of p38. Chem. Biol. Interact. 2012, 195, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Vanhees, K.; Godschalk, R.W.; Sanders, A.; van Doorn, S.B.V.W.; van Schooten, F.J. Maternal quercetin intake during pregnancy results in an adapted iron homeostasis at adulthood. Toxicology 2011, 290, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Mladěnka, P.; Macáková, K.; Filipský, T.; Zatloukalová, L.; Jahodář, L.; Bovicelli, P.; Silvestri, I.P.; Hrdina, R.; Saso, L. In vitro analysis of iron chelating activity of flavonoids. J. Inorg. Biochem. 2011, 105, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Ham, S.; Bradke, D.; Ma, Q.; Han, O. Ascorbic acid offsets the inhibitory effect of bioactive dietary polyphenolic compounds on transepithelial iron transport in Caco-2 intestinal cells. J. Nutr. 2011, 141, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Lesjak, M.; Balesaria, S.; Skinner, V.; Debnam, E.S.; Srai, S.K.S. Quercetin inhibits intestinal non-haem iron absorption by regulating iron metabolism genes in the tissues. Eur. J. Nutr. 2019, 58, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Leopoldini, M.; Russo, N.; Chiodo, S.; Toscano, M. Iron chelation by the powerful antioxidant flavonoid quercetin. J. Agric. Food Chem. 2006, 54, 6343–6351. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Meng, S.; Lekka, C.E.; Kaxiras, E. Complexation of Flavonoids with Iron: Structure and Optical Signatures. J. Phys. Chem. B 2008, 112, 1845–1850. [Google Scholar] [CrossRef] [PubMed]
- Perron, N.R.; Brumaghim, J.L. A Review of the Antioxidant Mechanisms of Polyphenol Compounds Related to Iron Binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef]
- Escudero, L.B.; Fusari, C.M.; Altamirano, J.C.; Camargo, A.B.; Wuilloud, R.G. Stability of Iron-Quercetin Complexes in Synthetic Wine under in vitro Digestion Conditions. J. Food Sci. 2014, 79, C1933–C1938. [Google Scholar] [CrossRef] [PubMed]
- Vlachodimitropoulou, E.; Sharp, P.A.; Naftalin, R.J. Quercetin-iron chelates are transported via glucose transporters. Free Radic. Biol. Med. 2011, 50, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, P.; Afzal-Ahmed, I.; Naftalin, R.J. Docking studies show that D-glucose and quercetin slide through the transporter GLUT1. J. Biol. Chem. 2006, 281, 5797–5803. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Ham, S.; Shigenaga, M.K.; Han, O. The inhibiting bioactive dietary polyphenolic compounds reduce nonheme iron transport across human intestinal cell monolayers. J. Nutr. 2008, 138, 1647–1651. [Google Scholar] [CrossRef] [PubMed]
- Muckenthaler, M.U.; Galy, B.; Hentze, M.W. Systemic iron homeostasis and the iron-responsive element/iron-regulatory protein (IRE/IRP) regulatory network. Annu. Rev. Nutr. 2008, 28, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Hart, J.J.; Tako, E.; Kochian, L.V.; Glahn, R.P. Identification of black bean (Phaseolus vulgaris L.) polyphenols that inhibit and promote iron uptake by Caco-2 cells. J. Agric. Food Chem. 2015, 63, 5950–5956. [Google Scholar] [CrossRef] [PubMed]
- Hart, J.J.; Tako, E.; Glahn, R.P. Characterization of polyphenol effects on inhibition and promotion of iron uptake by Caco-2 cells. J. Agric. Food Chem. 2017, 65, 3285–3294. [Google Scholar] [CrossRef]
- Mu, M.; An, P.; Wu, Q.; Shen, X.; Shao, D.; Wang, H.; Zhang, Y.; Zhang, S.; Yao, H.; Min, J.; et al. The dietary flavonoid myricetin regulates iron homeostasis by suppressing hepcidin expression. J. Nutr. Biochem. 2016, 30, 53–61. [Google Scholar] [CrossRef]
- Zhen, A.W.; Nguyen, N.H.; Gibert, Y.; Motola, S.; Buckett, P.; Wessling-Resnick, M.; Fraenkel, E.; Fraenkel, P.G. The small molecule, genistein, increases hepcidin expression in human hepatocytes. Hepatology 2013, 58, 1315–1325. [Google Scholar] [CrossRef]
- Patchen, B.; Koppe, T.; Cheng, A.; Seo, Y.A.; Wessling-Resnick, M.; Fraenkel, P.G. Dietary supplementation with ipriflavone decreases hepatic iron stores in wild type mice. Blood Cells Mol. Dis 2016, 60, 36–43. [Google Scholar] [CrossRef]
- Grillo, A.S.; SantaMaria, A.M.; Kafina, M.D.; Cioffi, A.G.; Huston, N.C.; Han, M.; Seo, Y.A.; Yien, Y.Y.; Nardone, C.; Menon, A.V.; et al. Restored iron transport by a small molecule promotes absorption and hemoglobinization in animals. Science 2017, 356, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, J.; Guo, W.; Liu, X.; Liu, S.; Yin, H. Icariin regulates systemic iron metabolism by increasing hepatic hepcidin expression through Stat3 and Smad1/5/8 signaling. Int. J. Mol. Med. 2016, 37, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
| Age/State | Absorbed Iron in Duodenum a (mg/day) |
|---|---|
| 4–12 months | 0.96 |
| 13–24 months | 0.61 |
| 2–5 years | 0.70 |
| 6–11 years | 1.17 |
| 12–16 years (girls) | 2.02 |
| 12–16 years (boys) | 1.82 |
| Adult males | 1.14 |
| Women during lactation | 1.31 |
| Women during menstruating period | 2.38 |
| Women during postmenopausal period | 0.96 |
| Women 1st trimester of pregnancy | 0.8 |
| Women 2nd & 3rd trimester of pregnancy | 6.3 |
| Food | mg Iron/100 g Food |
|---|---|
| sources of non–haem iron | |
| red bean | 6.69 |
| parsley | 6.20 |
| wheat flour, whole-grain | 3.71 |
| corn flour, whole-grain, yellow | 2.38 |
| garlic | 1.70 |
| lettuce | 0.86 |
| potato | 0.81 |
| orange | 0.80 |
| red cabbage | 0.80 |
| broccoli | 0.73 |
| blackberry | 0.62 |
| kiwi | 0.54 |
| red pepper | 0.43 |
| cauliflower | 0.42 |
| strawberry | 0.41 |
| apricot | 0.39 |
| fig | 0.37 |
| carrot | 0.30 |
| cucumber | 0.28 |
| blueberry | 0.28 |
| banana | 0.26 |
| watermelon | 0.24 |
| eggplant | 0.23 |
| red onion | 0.21 |
| apple | 0.12 |
| sources of haem iron | |
| goose, liver | 30.53 |
| oyster | 3.86 |
| beef meat | 1.69 |
| lamb meat | 1.55 |
| turkey meat | 1.09 |
| chicken meat | 0.82 |
| Food | g Phytate/100 g Food | mg Iron/100 g Food |
|---|---|---|
| soybean seed | 1.0–2.22 | 15.7 |
| sesame seed | 1.44–5.36 | 14.5 |
| bean | 0.61–2.38 | 9.0 |
| lentil | 0.27–1.51 | 7.4 |
| flax seed | 2.15–3.69 | 7.2 |
| indian walnut | 0.19–4.98 | 6.7 |
| sunflower seeds | 3.9–4.3 | 6.0 |
| wheat seed | 0.39–1.35 | 5.3 |
| oats | 0.42–1.16 | 4.7 |
| pea | 0.22–1.22 | 4.7 |
| hazelnut | 0.23–0.92 | 4.7 |
| peanut | 0.17–4.47 | 4.5 |
| chickpeas | 0.28–1.60 | 4.3 |
| rice | 0.06–1.08 | 4.0 |
| pistachio nuts | 0.29–2.83 | 3.9 |
| almond nuts | 0.35–9.42 | 3.7 |
| corn | 0.72–2.22 | 3.0 |
| walnut | 0.20–6.69 | 2.9 |
| rye seed | 0.54–1.46 | 2.6 |
| Food | mg Flavonoid/100 g | mg Iron/100 g |
|---|---|---|
| parsley | 233.16 | 6.20 |
| garlic | 3.61 | 1.70 |
| lettuce | 4.63 | 0.86 |
| red cabbage | 210.67 | 0.80 |
| broccoli | 11.96 | 0.73 |
| red pepper | 0.86 | 0.43 |
| cauliflower | 1.02 | 0.42 |
| strawberry | 13.35 | 0.41 |
| fig | 8.07 | 0.37 |
| carrot | 0.60 | 0.30 |
| blueberry | 180.82 | 0.28 |
| cucumber | 0.17 | 0.28 |
| tomato | 5.95 | 0.27 |
| banana | 13.69 | 0.26 |
| cranberry | 132.08 | 0.23 |
| eggplant | 85.73 | 0.23 |
| red onion | 56.61 | 0.21 |
| apple | 15.15 | 0.12 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lesjak, M.; K. S. Srai, S. Role of Dietary Flavonoids in Iron Homeostasis. Pharmaceuticals 2019, 12, 119. https://doi.org/10.3390/ph12030119
Lesjak M, K. S. Srai S. Role of Dietary Flavonoids in Iron Homeostasis. Pharmaceuticals. 2019; 12(3):119. https://doi.org/10.3390/ph12030119
Chicago/Turabian StyleLesjak, Marija, and Surjit K. S. Srai. 2019. "Role of Dietary Flavonoids in Iron Homeostasis" Pharmaceuticals 12, no. 3: 119. https://doi.org/10.3390/ph12030119
APA StyleLesjak, M., & K. S. Srai, S. (2019). Role of Dietary Flavonoids in Iron Homeostasis. Pharmaceuticals, 12(3), 119. https://doi.org/10.3390/ph12030119





