2017 FDA Peptide Harvest
Abstract
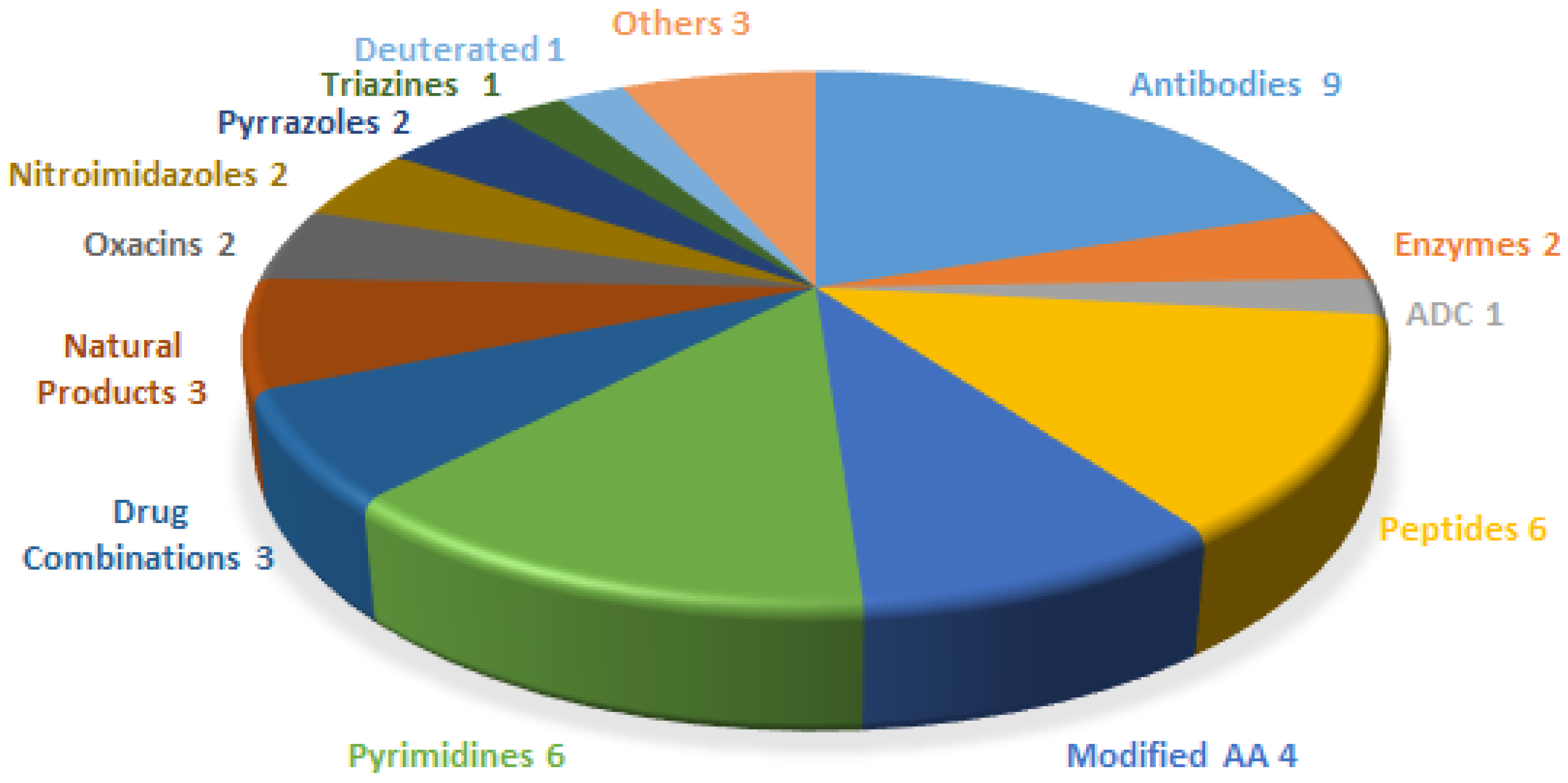
:1. Introduction
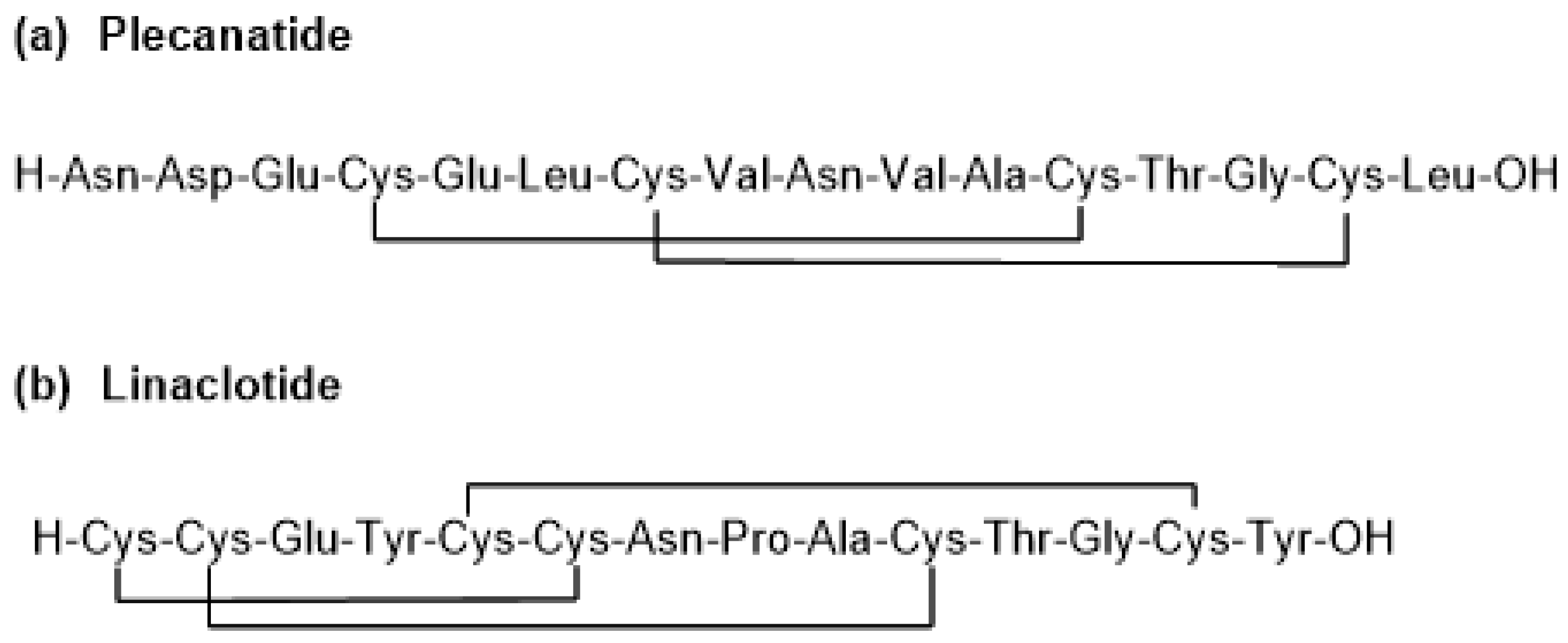
2. Plecanatide (Trulance)
3. Etelcalcetide (Parsabiv)
4. Abaloparatide (Tymlos)
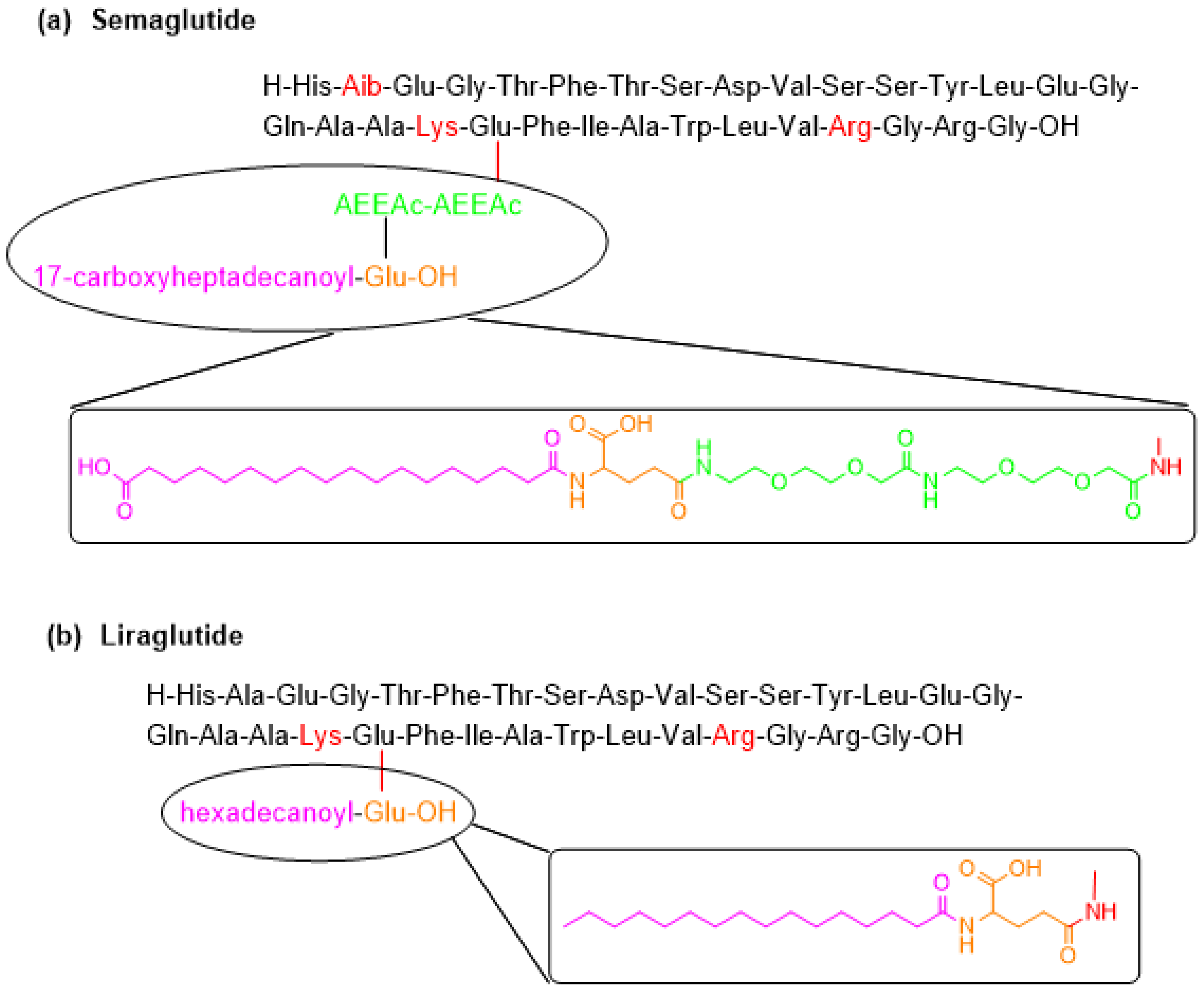
5. Semaglutide (Ozempic)
6. Macimorelin (Macrilen)
7. Angiotensin II (Giapreza)
Author Contributions
Acknowledgments
Conflicts of Interest
References
- De la Torre, B.G.; Albericio, F. The pharmaceutical industry in 2017. An analysis of fda drug approvals from the perspective of molecules. Molecules 2018, 23, 533. [Google Scholar] [CrossRef] [PubMed]
- Mullard, A. 2017 fda drug approvals. Nat. Rev. Drug Discov. 2018, 17, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Al-Salama, Z.T.; Syed, Y.Y. Plecanatide: First global approval. Drugs 2017, 77, 593–598. [Google Scholar] [CrossRef] [PubMed]
- FDA. Plecanatide (Trulance) Approval Letter. 2017. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2017/208745orig1s000ltr.pdf (accessed on 3 May 2018).
- Thomas, R.H.; Luthin, D.R. Current and emerging treatments for irritable bowel syndrome with constipation and chronic idiopathic constipation: Focus on prosecretory agents. Pharmacotherapy 2015, 35, 613–630. [Google Scholar] [CrossRef] [PubMed]
- Góngora-Benítez, M.; Tulla-Puche, J.; Albericio, F. Constella™(eu)-linzess™(USA): The last milestone in the long journey of the peptide linaclotide and its implications for the future of peptide drugs. Future Med. Chem. 2013, 5, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Shailubhai, K.; Comiskey, S.; Foss, J.A.; Feng, R.; Barrow, L.; Comer, G.M.; Jacob, G.S. Plecanatide, an oral guanylate cyclase c agonist acting locally in the gastrointestinal tract, is safe and well-tolerated in single doses. Dig. Dis. Sci. 2013, 58, 2580–2586. [Google Scholar] [CrossRef] [PubMed]
- Brancale, A.; Shailubhai, K.; Ferla, S.; Ricci, A.; Bassetto, M.; Jacob, G.S. Mo1316 structural and dynamic features of plecanatide: Insights from molecular dynamics simulations. Gastroenterology 2016, 150, S695. [Google Scholar] [CrossRef]
- Hamra, F.K.; Forte, L.R.; Eber, S.L.; Pidhorodeckyj, N.V.; Krause, W.J.; Freeman, R.H.; Chinii, D.T.; Tompkinsii, J.A.; Fok, K.F.; Smith, C.E.; et al. Uroguanylin: Structure and activity of a second endogenous peptide that stimulatesintestinal guanylate cyclase. Proc. Natl. Acad. Sci. USA 1993, 90, 10464–10468. [Google Scholar] [CrossRef] [PubMed]
- Gadsby, D.C.; Vergani, P.; Csanady, L. The abc protein turned chloride channel whose failure causes cystic fibrosis. Nature 2006, 440, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, R.; Zhu, X.; Kerr, S.J.; Esmay, J.D.; Louie, S.W.; Edson, K.Z.; Walter, S.; Fitzsimmons, M.; Wagner, M.; Soto, M.; et al. Nonclinical pharmacokinetics, disposition, and drug-drug interaction potential of a novel d-amino acid peptide agonist of the calcium-sensing receptor amg 416 (etelcalcetide). Drug Metab. Dispos. 2016, 44, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Edson, K.Z.; Wu, B.M.; Iyer, A.; Goodman, W.; Skiles, G.L.; Subramanian, R. Determination of etelcalcetide biotransformation and hemodialysis kinetics to guide the timing of its dosing. Kidney Int. Rep. 2016, 1, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, M.; Galassi, A.; Conte, F.; Mangano, M.; Di Lullo, L.; Bellasi, A. Treatment of secondary hyperparathyroidism: The clinical utility of etelcalcetide. Ther. Clin. Risk Manag. 2017, 13, 679–689. [Google Scholar] [CrossRef] [PubMed]
- FDA. Etelcalcetide (Parsabiv) Approval Letter. 2017. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/208325Orig1s000Approv.pdf (accessed on 3 May 2018).
- Baker, D.E. Formulary drug review: Etelcalcetide. Hosp. Pharm. 2017, 52, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Tomlinson, J.E.; Alexander, S.T.; Hensley, K.; Han, C.Y.; Dwyer, D.; Stolina, M.; Dean, C., Jr.; Goodman, W.G.; Richards, W.G.; et al. Etelcalcetide, a novel calcimimetic, prevents vascular calcification in a rat model of renal insufficiency with secondary hyperparathyroidism. Calcif. Tissue Int. 2017, 101, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, L.; Asuncion, F.; Grisanti, M.; Alexander, S.; Hensley, K.; Han, C.Y.; Niu, Q.T.; Dwyer, D.; Villasenor, K.; et al. Etelcalcetide (amg 416), a peptide agonist of the calcium-sensing receptor, preserved cortical bone structure and bone strength in subtotal nephrectomized rats with established secondary hyperparathyroidism. Bone 2017, 105, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.J.; Bell, G.; Pickthorn, K.; Huang, S.; Vick, A.; Hodsman, P.; Peacock, M. Velcalcetide (amg 416), a novel peptide agonist of the calcium-sensing receptor, reduces serum parathyroid hormone and fgf23 levels in healthy male subjects. Nephrol. Dial. Transplant. 2014, 29, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Lavi-Moshayoff, V.; Wasserman, G.; Meir, T.; Silver, J.; Naveh-Many, T. PTH increases fgf23 gene expression and mediates the high-fgf23 levels of experimental kidney failure: A bone parathyroid feedback loop. Am. J. Physiol. Renal Physiol. 2010, 299, F882–F889. [Google Scholar] [CrossRef] [PubMed]
- Block, G.A.; Bushinsky, D.A.; Cheng, S.; Cunningham, J.; Dehmel, B.; Drueke, T.B.; Ketteler, M.; Kewalramani, R.; Martin, K.J.; Moe, S.M.; et al. Effect of etelcalcetide vs cinacalcet on serum parathyroid hormone in patients receiving hemodialysis with secondary hyperparathyroidism: A randomized clinical trial. JAMA 2017, 317, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Eidman, K.E.; Wetmore, J.B. Managing hyperparathyroidism in hemodialysis: Role of etelcalcetide. Int. J. Nephrol. Renovasc. Dis. 2018, 11, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Tella, S.H.; Kommalapati, A.; Correa, R. Profile of abaloparatide and its potential in the treatment of postmenopausal osteoporosis. Cureus 2017, 9, e1300. [Google Scholar] [CrossRef] [PubMed]
- FDA. Abaloparatide (Tymlos) Approval Letter. 2017. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/208743Orig1s000Approv.pdf (accessed on 3 May 2018).
- Yang, L.; Morriello, G.; Pan, Y.; Nargund, R.P.; Barakat, K.; Prendergast, K.; Cheng, K.; Chan, W.W.-S.; Smith, R.G.; Patchett, A.A. Tripeptide growth hormone secretagogues. Bioorg. Med. Chem. Lett. 1998, 8, 759–764. [Google Scholar] [CrossRef]
- FDA. Liraglutide Approval Letter. 2010. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/022341s027lbl.pdf (accessed on 3 May 2018).
- Lau, J.; Bloch, P.; Schaffer, L.; Pettersson, I.; Spetzler, J.; Kofoed, J.; Madsen, K.; Knudsen, L.B.; McGuire, J.; Steensgaard, D.B.; et al. Discovery of the once-weekly glucagon-like peptide-1 (glp-1) analogue semaglutide. J. Med. Chem. 2015, 58, 7370–7380. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.; Helleberg, H.; Roffel, A.; van Lier, J.J.; Bjornsdottir, I.; Pedersen, P.J.; Rowe, E.; Derving Karsbol, J.; Pedersen, M.L. Absorption, metabolism and excretion of the glp-1 analogue semaglutide in humans and nonclinical species. Eur. J. Pharm. Sci. 2017, 104, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Pratley, R.E.; Aroda, V.R.; Lingvay, I.; Lüdemann, J.; Andreassen, C.; Navarria, A.; Viljoen, A. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (sustain 7): A randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol. 2018, 1–12. [Google Scholar] [CrossRef]
- FDA. Semaglutide (Ozempic) Approval Letter. 2017. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2017/209637s000ltr.pdf (accessed on 3 May 2018).
- Jacobsen, L.V.; Flint, A.; Olsen, A.K.; Ingwersen, S.H. Liraglutide in type 2 diabetes mellitus: Clinical pharmacokinetics and pharmacodynamics. Clin. Pharmacokinet. 2016, 55, 657–672. [Google Scholar] [CrossRef] [PubMed]
- Guerlavais, V.; Boeglin, D.; Mousseaux, D.; Oiry, C.; Heitz, A.; Deghenghi, R.; Locatelli, V.; Torsello, A.; Ghé, C.; Catapano, F.; et al. New Active Series of Growth Hormone Secretagogues. J. Med. Chem. 2003, 46, 1196–1203. [Google Scholar] [CrossRef] [PubMed]
- FDA. Macimorelin (Macimorelin) Approval Letter. 2017. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/205598Orig1s000Approv.pdf (accessed on 3 May 2018).
- Garcia, J.M.; Swerdloff, R.; Wang, C.; Kyle, M.; Kipnes, M.; Biller, B.M.; Cook, D.; Yuen, K.C.; Bonert, V.; Dobs, A.; et al. Macimorelin (aezs-130)-stimulated growth hormone (gh) test: Validation of a novel oral stimulation test for the diagnosis of adult gh deficiency. J. Clin. Endocrinol. Metab. 2013, 98, 2422–2429. [Google Scholar] [CrossRef] [PubMed]
- Broglio, F.; Boutignon, F.; Benso, A.; Gottero, C.; Prodam, F.; Arvat, E.; Ghè, C.; Catapano, F.; Torsello, A.; Locatelli, V.; et al. Ep1572: A novel peptido-mimetic gh secretagogue with potent and selective gh-releasing activity in man. J. Endocrinol. Investig. 2002, 25, RC26–RC28. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Hosoda, H.; Matsuo, H.; Kangawa, K. Ghrelin: Discovery of the natural endogenous ligand for the growth hormone secretagogue receptor. Trends Endocrinol. Metab. 2001, 12, 118–126. [Google Scholar] [CrossRef]
- Varamini, P.; Toth, I. Recent advances in oral delivery of peptide hormones. Expert Opin. Drug Deliv. 2016, 13, 507–522. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, F.; Degen, L.; MacLean, C.; Peter, S.; Baselgia, L.; Larsen, F.; Beglinger, C.; Drewe, J. Pharmacokinetics and pharmacodynamic effects of an oral ghrelin agonist in healthy subjects. J. Clin. Endocrinol. Metab. 2007, 92, 1814–1820. [Google Scholar] [CrossRef] [PubMed]
- FDA. Angiotensin II (Giapreza) Aproval Letter. 2017. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/209360Orig1s000Approv.pdf (accessed on 3 May 2018).
- Tigerstedt, R.; Bergman, P.G. Niere und kreislauf. Arch. Physiol. 1898, 8, 223–271. [Google Scholar] [CrossRef]
- Basso, N.; Terragno, N.A. History about the discovery of the renin-angiotensin system. Hypertension 2001, 38, 1246–1249. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, H.; Bumpus, F.M.; Page, I.H. Synthesis of a biologically active octapeptide similar to natural isoleucine angiotonin octapeptide. J. Am. Chem. Soc. 1957, 79, 5697–5703. [Google Scholar] [CrossRef]
- Rittel, W.; Iselin, B.; Kappeler, H.; Riniker, B.; Schwyzer, R. Synthese eines hochwirksamen Hypertensin II-amids (L-Asparaginyl-L-arginyl-L-valyl-L-tyrosyl-L-isoleucyl-L-histidyl-L-prolyl-L-phenylalanin). Helvetica Chim. Acta 1957, 40, 614–624. [Google Scholar] [CrossRef]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Singh, I.; Kaur, P.; Kaur, R. Food and drug administration (fda) approved peptide drugs. Asian J. Res. Biol. Pharm. Sci. 2015, 3, 75–88. [Google Scholar]
- Ghosh, S. Peptide therapeutics market: Forecast and analysis 2015–2025. Oligos Pept. Chim. Oggi Chem. Today 2016, 34, 5–7. [Google Scholar]
- Albericio, F.; Kruger, H.G. Therapeutic peptides. Future Med. Chem. 2012, 4, 1527–1531. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.L.; Dunn, M.K. Therapeutic peptides: Historical perspectives, current development trends, and future directions. Bioorg. Med. Chem. 2017. [Google Scholar] [CrossRef] [PubMed]
- Henninot, A.; Collins, J.C.; Nuss, J.M. The current state of peptide drug discovery: Back to the future? J. Med. Chem. 2018, 61, 1382–1414. [Google Scholar] [CrossRef] [PubMed]
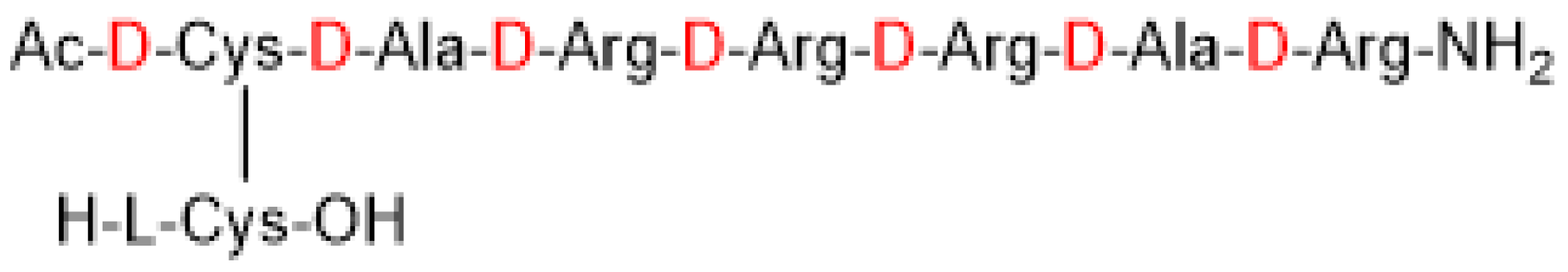

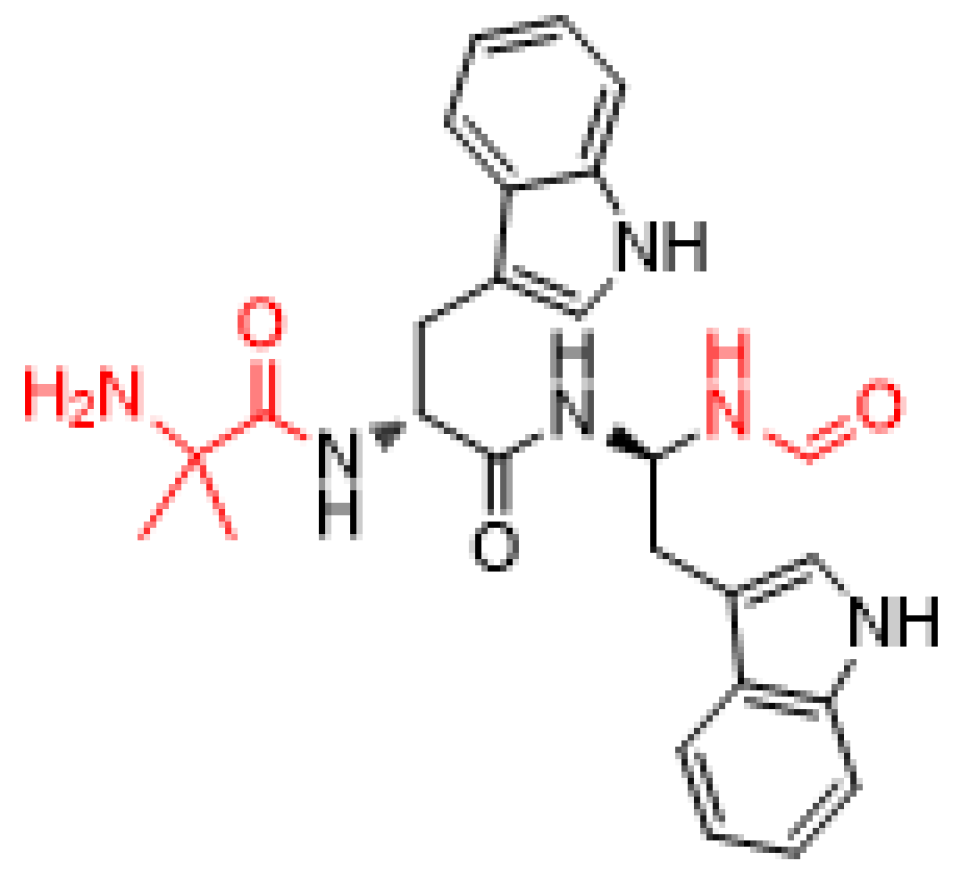
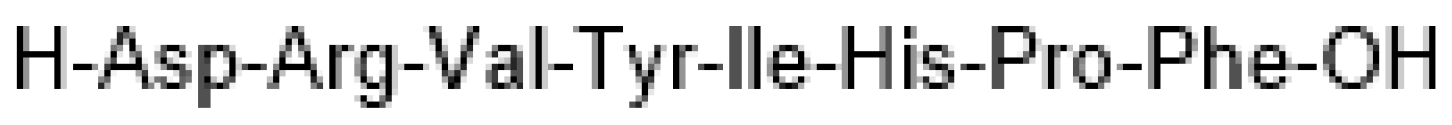
| Generic Name (Trade Name) | Company | Mode of Action | Therapeutic Use | Administration |
|---|---|---|---|---|
| Plecanatide (Trulance) | Synergy Pharmaceuticals, Inc. | Activation of guanylate cyclase-C | Gastrointestinal laxative | Oral |
| Etelcalcetide (Parsabiv) | KAI Pharmaceuticals, Inc. * | Activation of CaSR on parathyroid chief cells | Secondary hyperpara-thyroidism in adult patients with chronic kidney disease on hemodialysis | IV |
| Abaloparatide (Tymlos) | Radius Health, Inc. | Selective activation of the parathyroid hormone 1 receptor | Osteoporosis | SC |
| Semaglutide (Ozempic) | Novo Nordisk, Inc. | Acts as a Glucagon-like Peptide-1 agonist | Treatment of type 2 diabetes mellitus | SC |
| Macimorelin (Macrilen) | Aeterna Zentaris, Inc. | Mimic the endogenous ligand for the secretagogue (Ghrelin) | For the diagnosis of adult growth hormone deficiency | Oral |
| Angiotensin II (Giapreza) | La Jolla Pharm Co. | Acts on the CNS to increase ADH production | Control of blood pressure in adults with sepsis or other critical conditions | IV |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Musaimi, O.; Al Shaer, D.; De la Torre, B.G.; Albericio, F. 2017 FDA Peptide Harvest. Pharmaceuticals 2018, 11, 42. https://doi.org/10.3390/ph11020042
Al Musaimi O, Al Shaer D, De la Torre BG, Albericio F. 2017 FDA Peptide Harvest. Pharmaceuticals. 2018; 11(2):42. https://doi.org/10.3390/ph11020042
Chicago/Turabian StyleAl Musaimi, Othman, Danah Al Shaer, Beatriz G. De la Torre, and Fernando Albericio. 2018. "2017 FDA Peptide Harvest" Pharmaceuticals 11, no. 2: 42. https://doi.org/10.3390/ph11020042
APA StyleAl Musaimi, O., Al Shaer, D., De la Torre, B. G., & Albericio, F. (2018). 2017 FDA Peptide Harvest. Pharmaceuticals, 11(2), 42. https://doi.org/10.3390/ph11020042








