Convenient Preparation of 18F-Labeled Peptide Probes for Potential Claudin-4 PET Imaging
Abstract
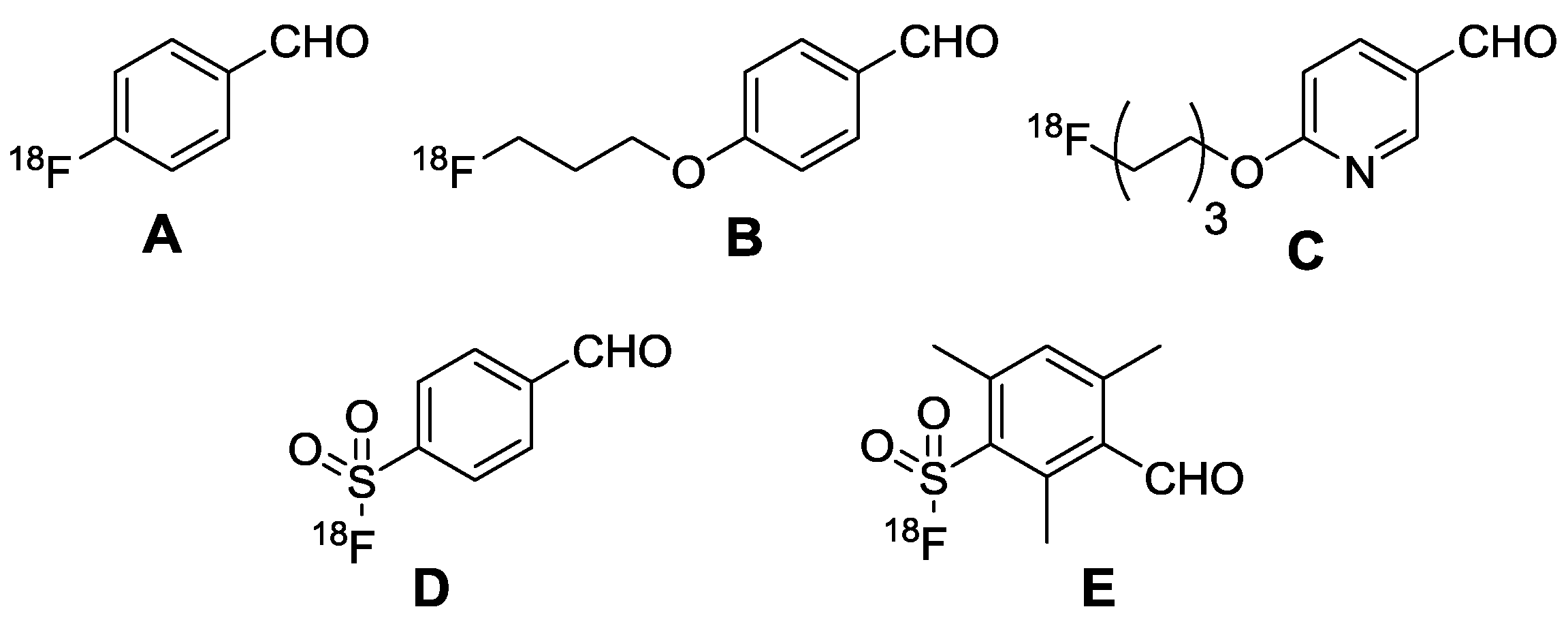
1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of 5-FDR-Peptides
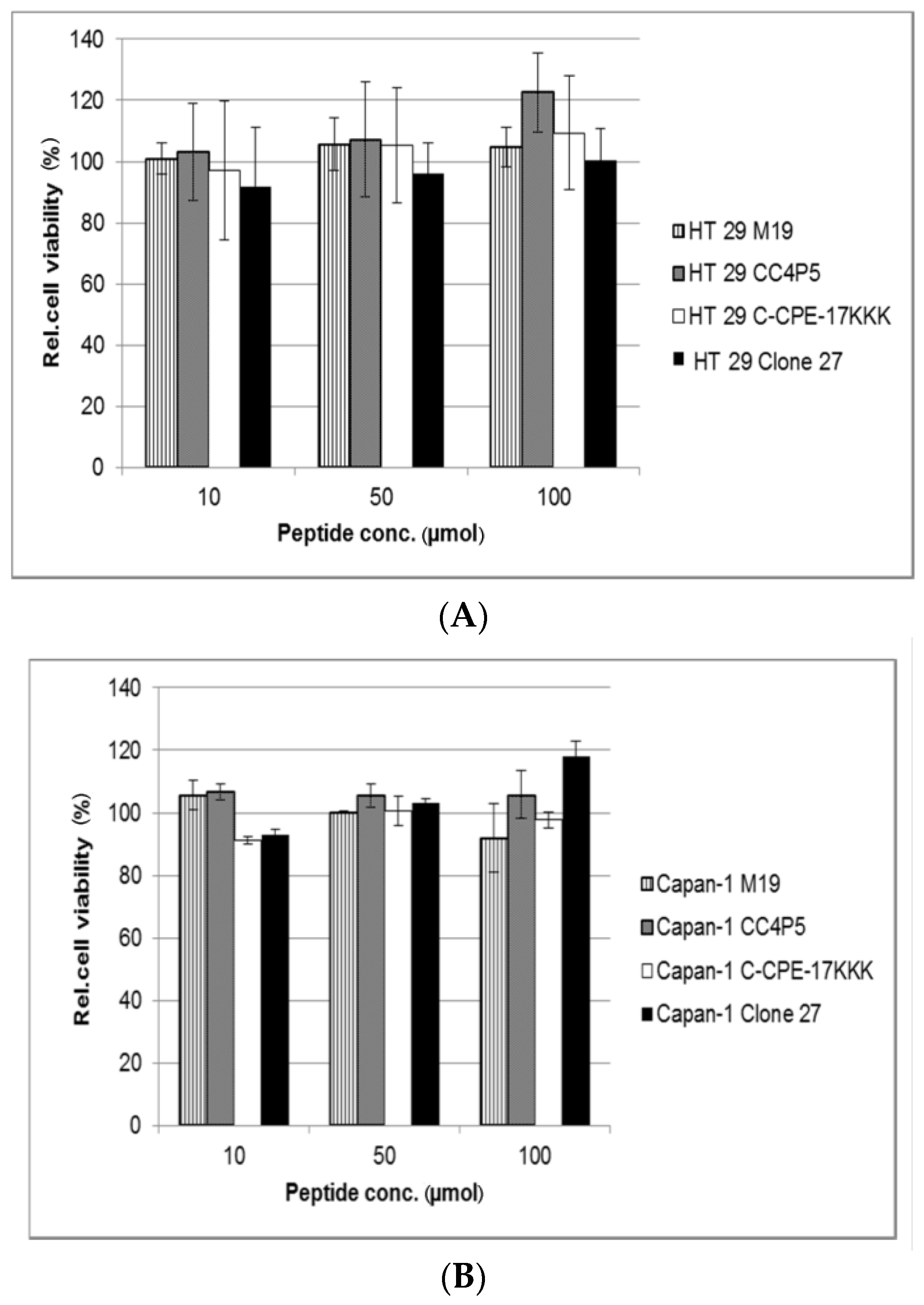
2.2. Preliminary Biological Evaluation
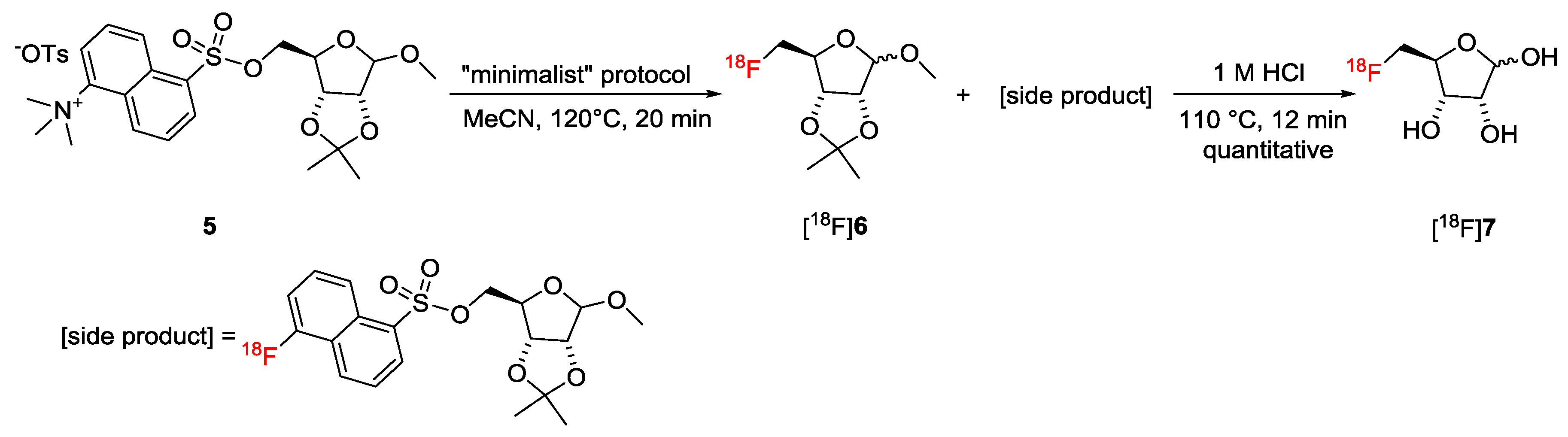
2.3. Optimization of the Preparation of 5-[18F]FDR Peptide Conjugates
3. Materials and Methods
3.1. Materials
3.2. Peptide Synthesis
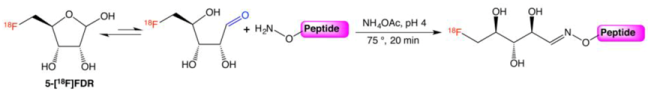
3.3. FDR Coupling
3.4. CD Spectroscopy
3.5. Cell Culture
3.6. Cytotoxicity Assay
3.7. Synthesis of the Precursor 5
3.8. Radiochemistry
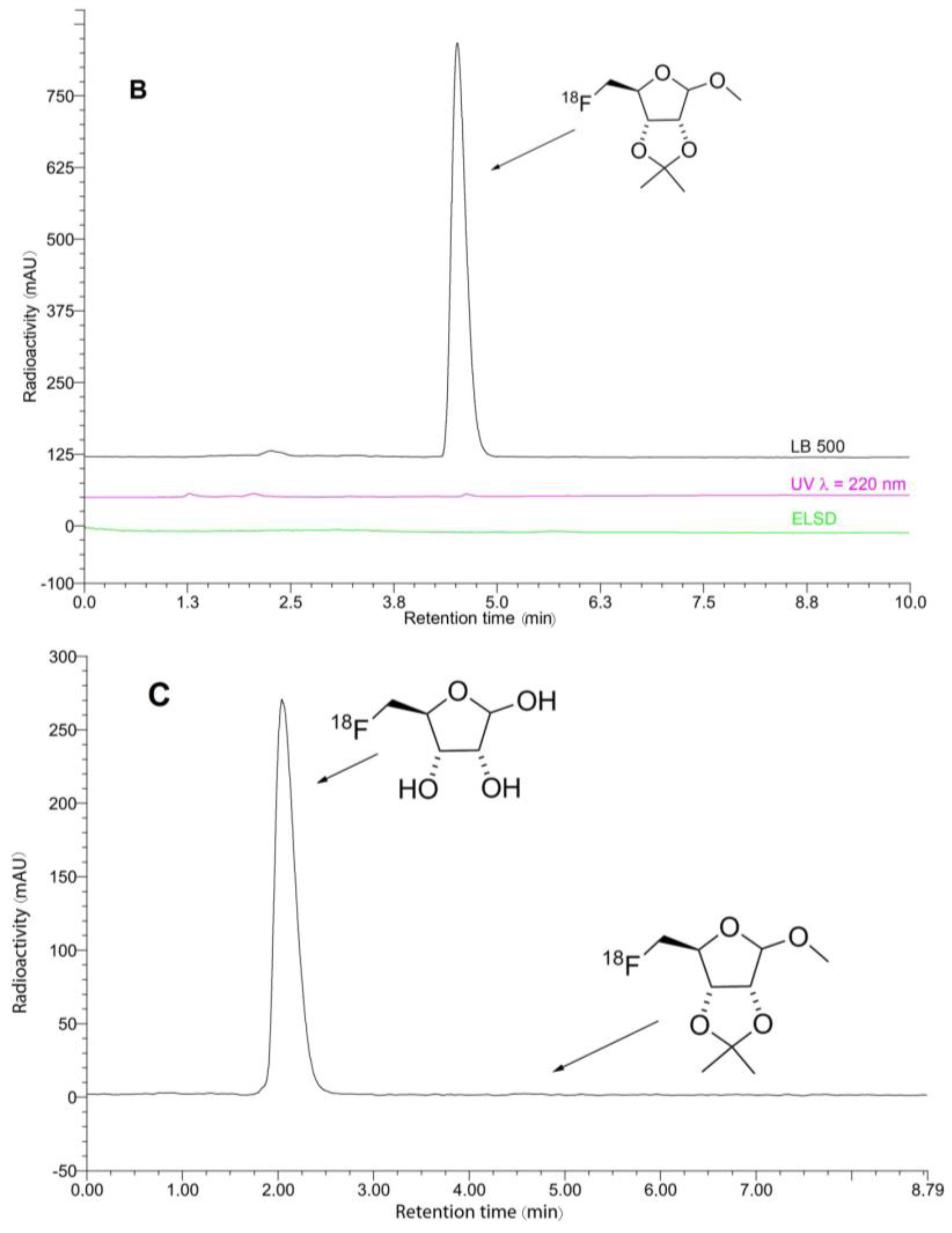
3.8.1. Preparation of 5-[18F]Fluoro-5-Deoxy-d-Ribofuranoside (5-[18F]FDR, [18F]7)
3.8.2. Conjugation of 5-[18F]FDR to Aminooxy Functionalized Peptides
3.8.3. Molar Activity Calculation
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Brunner, T.B.; Seufferlein, T. Pancreatic cancer chemoradiotherapy. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Lee, J.M. Imaging diagnosis of pancreatic cancer: A state-of-the-art review. World J. Gastroenterol. 2014, 20, 7864–7877. [Google Scholar] [CrossRef] [PubMed]
- Kauhanen, S.P.; Komar, G.; Seppänen, M.P.; Dean, K.I.; Minn, H.R.; Kajander, S.A.; Rinta-Kiikka, I.; Alanen, K.; Borra, R.J.; Puolakkainen, P.A.; et al. A prospective diagnostic accuracy study of 18F-fluorodeoxyglucose positron emission tomography/computed tomography, multidetector row computed tomography, and magnetic resonance imaging in primary diagnosis and staging of pancreatic cancer. Ann. Surg. 2009, 250, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Li, X.G.; Helariutta, K.; Roivainen, A.; Jalkanen, S.; Knuuti, J.; Airaksinen, A.J. Using 5-deoxy-5-[18F]fluororibose to glycosylate peptides for positron emission tomography. Nat. Protoc. 2014, 9, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Hermanson, G.T. Bioconjugate Techniques, 2nd ed.; Elsevier: San Diego, CA, USA, 2008. [Google Scholar]
- Pretze, M.; Pietzsch, D.; Mamat, C. Recent trends in bioorthogonal click-radiolabeling reactions using fluorine-18. Molecules 2013, 18, 8618–8665. [Google Scholar] [CrossRef] [PubMed]
- Li, X.G.; Autio, A.; Ahtinen, H.; Helariutta, K.; Liljenbäck, H.; Jalkanen, S.; roivainen, A.; Airaksinen, A.J. Translating the concept of peptide labeling with 5-deoxy-5-[18F]fluororibose into preclinical practice: 18F-labeling of Siglec-9 peptide for PET imaging of inflammation. Chem. Commun. 2013, 49, 3682–3684. [Google Scholar] [CrossRef] [PubMed]
- Li, X.G.; Haaparanta, M.; Solin, O. Oxime formation for fluorine-18 labeling of peptides and proteins for positron emission tomography (PET) imaging: A review. J. Fluor. Chem. 2012, 143, 49–56. [Google Scholar] [CrossRef]
- Richarz, R.; Krapf, P.; Zarrad, F.; Urusova, E.A.; Neumaier, B.; Zlatopolskiy, B.D. Neither azeotropic drying, nor base nor other additives: A minimalist approach to 18F-labeling. Org. Biomol. Chem. 2014, 12, 8094–8099. [Google Scholar] [CrossRef] [PubMed]
- Li, X.G.; Dall’Angelo, S.; Schweiger, L.F.; Zanda, M.; O’Hagan, D. [18F]-5-Fluoro-5-deoxyribose, an efficient peptide bioconjugation ligand for positron emission tomography (PET) imaging. Chem. Commun. 2012, 48, 5247–5249. [Google Scholar] [CrossRef] [PubMed]
- Krause, G.; Winkler, L.; Mueller, S.L.; Haseloff, R.F.; Piontek, J.; Blasig, I.E. Structure and function of claudins. Biochim. Biophys. Acta 2008, 1778, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Morita, K.; Furuse, M.; Fujimoto, K.; Tsukita, S. Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc. Natl. Acad. Sci. USA 1999, 96, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Saito, Y.; Kondoh, M.; Matsushita, K.; Krug, S.M.; Suzuki, H.; Tirofumi, H.; Li, X.; Aoyama, H.; Matsuhisa, K.; et al. Creation and biochemical analysis of a broad-specific claudin binder. Biomaterials 2012, 33, 3464–3474. [Google Scholar] [CrossRef] [PubMed]
- Furuse, M.; Tsukita, S. Claudins in occluding junctions of humans and flies. Trends Cell Biol. 2006, 16, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Morin, P.J. Claudin proteins in human cancer: Promising new targets for diagnosis and therapy. Cancer Res. 2005, 65, 9603–9606. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.S.; Morgado-Diaz, J.A. Claudins: Multifunctional players in epithelial tight junctions and their role in cancer. Cell. Mol. Life Sci. 2007, 64, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Michl, P.; Barth, C.; Buchholz, M.; Lerch, M.M.; Rolke, M.; Holzmann, K.H.; Menke, A.; Fensterer, H.; Giehl, K.; Lohr, M.; et al. Claudin-4 expression decreases invasiveness and metastatic potential of pancreatic cancer. Cancer Res. 2003, 63, 6265–6271. [Google Scholar] [PubMed]
- Kominsky, S.L.; Argani, P.; Korz, D.; Evron, E.; Raman, V.; Garrett, E.; Rein, A.; Sauter, G.; Kallioniemi, O.-P.; Sukumar, S. Loss of the tight junction protein claudin-7 correlates with histological grade in both ductal carcinoma in situ and invasive ductal carcinoma of the breast. Oncogene 2003, 22, 2021–2033. [Google Scholar] [CrossRef] [PubMed]
- Kominsky, S.L.; Vali, M.; Korz, D.; Gabig, T.G.; Weitzman, S.A.; Argani, P.; Sukumar, S. Clostridium perfringens enterotoxin elicits rapid and specific cytolysis of breast carcinoma cells mediated through tight junction proteins claudin 3 and 4. Am. J. Pathol. 2004, 164, 1627–1633. [Google Scholar] [CrossRef]
- Long, H.; Crean, C.D.; Lee, W.-H.; Cummings, O.W.; Gabig, T.G. Expression of Clostridium perfringens enterotoxin receptors claudin-3 and claudin-4 in prostate cancer epithelium. Cancer Res. 2001, 61, 7878–7881. [Google Scholar] [PubMed]
- Nichols, L.S.; Ashfaq, R.; Iacobuzio-Donahue, C.A. Claudin 4 protein expression in primary and metastatic pancreatic cancer—Support for use as a therapeutic target. Am. J. Clin. Pathol. 2004, 121, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Soini, Y.; Tommola, S.; Helin, H.; Martikainen, P. Claudins 1, 3, 4 and 5 in gastric carcinoma, loss of claudin expression associates with the diffuse subtype. Virchows Arch. 2006, 448, 52–58. [Google Scholar] [CrossRef] [PubMed]
- McClane, B.A. Clostridium-Perfringens Enterotoxin Acts by Producing Small-Molecule Permeability Alterations in Plasma-Membranes. Toxicology 1994, 87, 43–67. [Google Scholar] [CrossRef]
- Sonoda, N.; Furuse, M.; Sasaki, H.; Yonemura, S.; Katahira, J.; Horiguchi, Y.; Tsukita, S. Clostridium perfringens enterotoxin fragment removes specific claudins from tight junction strands: Evidence for direct involvement of claudins in tight junction barrier. J. Cell Biol. 1999, 147, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Katahira, J.; Horiguchi, Y.; Sonoda, N.; Furuse, M.; Tsukita, S. Clostridium perfringens enterotoxin binds to the second extracellular loop of claudin-3, a tight junction integral membrane protein. FEBS Lett. 2000, 476, 258–261. [Google Scholar] [CrossRef]
- Ling, J.; Liao, H.; Ling, J.; Clark, R.; Wong, M.S.; Lo, D.D. Structural Constraints for the Binding of Short Peptides to Claudin-4 Revealed by Surface Plasmon Resonance. J. Biol. Chem. 2008, 283, 30585–30595. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.A.; Bardeesy, N.; Anbazhagan, R.; Gurumurthy, S.; Berger, J.; Alencar, H.; DePinho, R.A.; Mahmood, U.; Weissleder, R. Targeted nanoparticles for imaging incipient pancreatic ductal adenocarcinoma. PLoS Med. 2008, 5, e85. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.B.; Knight, J.C.; Mosley, M.J.; Kersemans, V.; Koustoulidou, S.; Allen, D.; Kinchesh, P.; Smart, S.; Cornelissen, B. Imaging of claudin-4 in pancreatic ductal adenocarcinoma using a radiolabelled anti-claudin-4 monoclonal antibody. Mol. Imaging Biol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, X.; Ma, X.; Zhu, H.; Loft, M.; Liu, H.; Cheng, Z. Development of PET probes for molecular imaging of tight junction membrane proteins, claudin-4, in ovarian cancer. J. Nucl. Med. 2016, 57, 530. [Google Scholar]
- Neesse, A.; Hahnenkamp, A.; Griesmann, H.; Buchholz, M.; Hahn, S.A.; Maghnouj, A.; Fendrich, V.; Ring, J.; Sipos, B.; Tuveson, D.A.; et al. Claudin-4-targeted optical imaging detects pancreatic cancer and its precursor lesions. Gut 2013, 62, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Zischler, J.; Krapf, P.; Richarz, R.; Zlatopolskiy, B.D.; Neumaier, B. Automated synthesis of 4-[F-18]fluoroanisole, [F-18]DAA1106 and 4-[F-18]FPhe using Cu-mediated radiofluorination under “minimalist” conditions. Appl. Radiat. Isot. 2016, 115, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Radiochemical conversion (RCC) refers to the amount of [18F]fluoride which is transformed into the desired 18F-labeled compound; it was determined by radio-HPLC. Radiochemical yield(RCY) refers to the decay corrected isolated yield of the radiochemically and chemically pure radiolabeled compound.
- Dirksen, A.; Hackeng, T.M.; Dawson, P.E. Nucleophilic catalysis of oxime ligation. Angew. Chem. Int. Ed. Engl. 2006, 45, 7581–7584. [Google Scholar] [CrossRef] [PubMed]
- Rayo, J.; Amara, N.; Krief, P.; Meijler, M.M. Live Cell Labeling of Native Intracellular Bacterial Receptors Using Aniline-Catalyzed Oxime Ligation. J. Am. Chem. Soc. 2011, 133, 7469–7475. [Google Scholar] [CrossRef] [PubMed]
- Kohler, J.J. Aniline: A Catalyst for Sialic Acid Detection. Chembiochem 2009, 10, 2147–2150. [Google Scholar] [CrossRef] [PubMed]
- Mezo, G.; Szabó, I.; Kertész, I.; Hegedüs, R.; Orbán, E.; Leurs, U.; Szilvia Bösze, S.; Halmos, G.; Manea, M. Efficient synthesis of an (aminooxy) acetylated-somatostatin derivative using (aminooxy) acetic acid as a ‘carbonyl capture’ reagent. J. Pept. Sci. 2011, 17, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Dall’Angelo, S.; Qingzhi Zhang, Q.; Fleming, I.N.; Piras, M.; Schweiger, L.F.; O’Hagan, D.; Zanda, M. Efficient bioconjugation of 5-fluoro-5-deoxy-ribose (FDR) to RGD peptides for positron emission tomography (PET) imaging of alpha(v)beta(3) integrin receptor. Org. Biomol. Chem. 2013, 11, 4551–4558. [Google Scholar] [CrossRef] [PubMed]
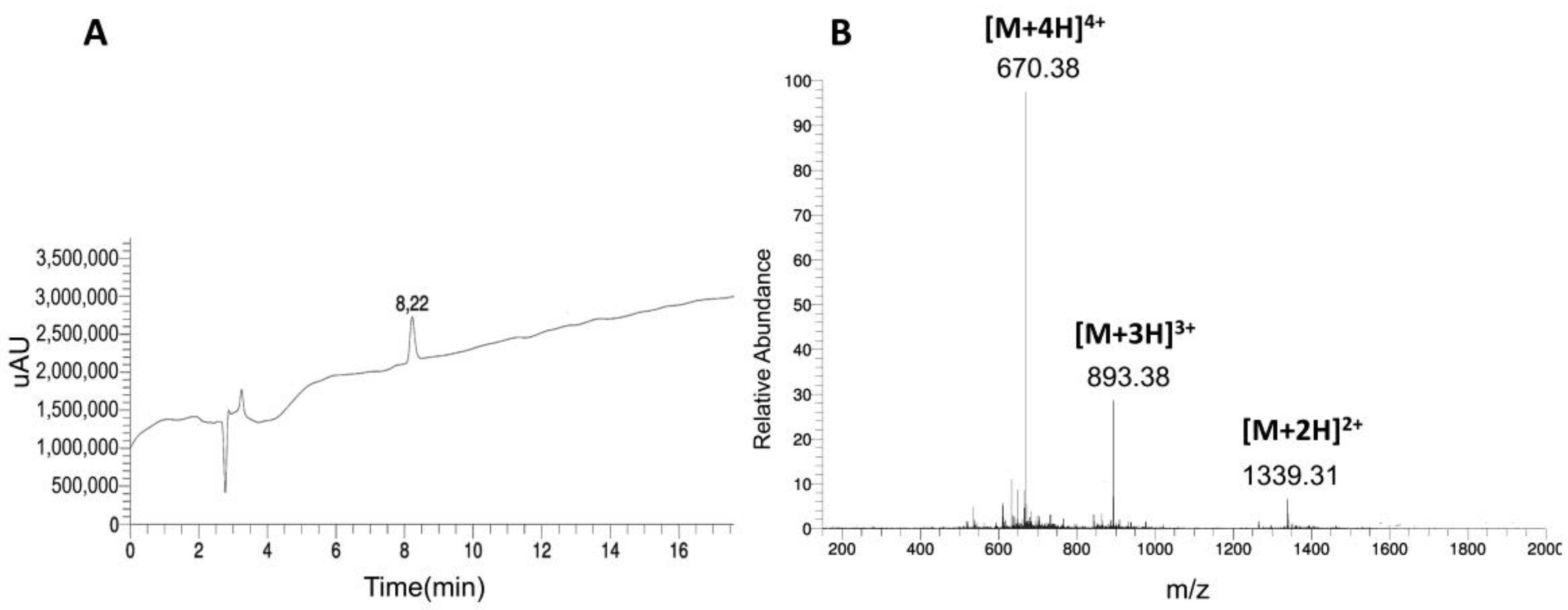


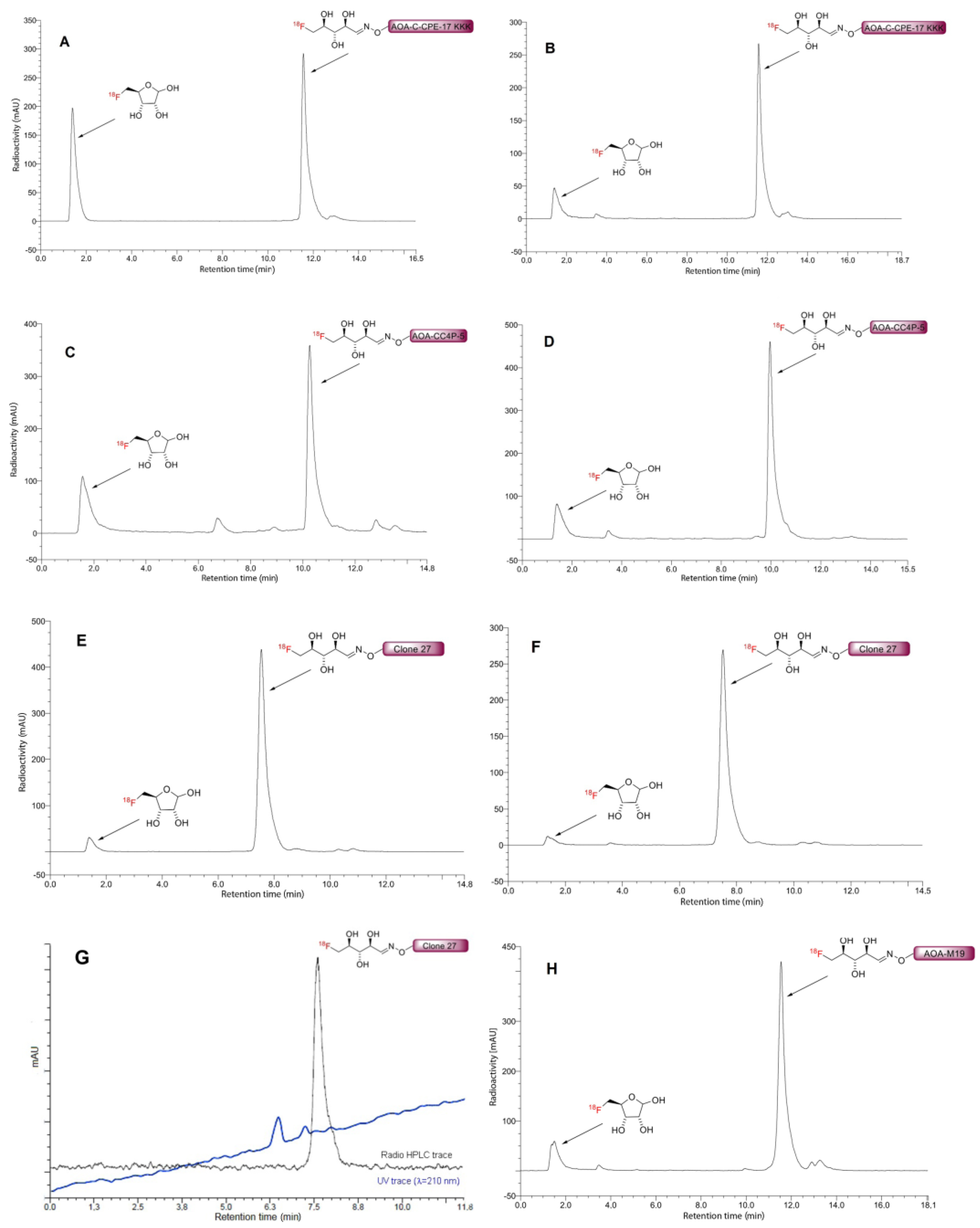
| Compound Number | Peptide | Sequence | MWcalc. [Da] | MWexp [Da] | RT [min] | Purity (%) |
|---|---|---|---|---|---|---|
| 1 | 5-FDR-C-CPE-17-KKK | 5-FDR = AOA-βA-NSSYSGNYPYSILFQKFKKK | 2676.85 | 2677.17 | 8.2 | 99 |
| 2 | 5-FDR-M19 | 5-FDR = AOA-βA-NAPYRGHYPYHILFQKF | 2428.29 | 2428.89 | 8.0 | 99 |
| 3 | 5-FDR-CC4P-5 | 5-FDR = AOA-βA-SPWSEPAYTLAP | 1595.39 | 1596.12 | 9.7 | 84 |
| 4 | 5-FDR-Clone 27 | 5-FDR = AOA-βA-KTLLPTP | 1046.41 | 1046.45 | 7.8 | 88 |
| Conjugate | Peptide Precursor | Amount Used (µmol) | RCC b (%) |
|---|---|---|---|
| [18F]8 | AOA-C-CPE-17KKK | 1.0 | 59 |
| [18F]9 | AOA-M19 | 1.6 | 46 |
| [18F]10 | AOA-CC4P-5 | 1.6 | 60 |
| [18F]11 | AOA-Clone 27 | 1.0 | 91 |

| Conjugate | Peptide Precursor | Amount Used [µmol] | RCC b [%] |
|---|---|---|---|
| [18F]8 | AOA-C-CPE-17KKK | 1.0 | 76 |
| [18F]9 | AOA-M19 | 1.6 | 79 |
| [18F]10 | AOA-CC4P-5 | 1.6 | 77 |
| [18F]11 | AOA-Clone 27 | 1.0 | 93 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feni, L.; Omrane, M.A.; Fischer, M.; Zlatopolskiy, B.D.; Neumaier, B.; Neundorf, I. Convenient Preparation of 18F-Labeled Peptide Probes for Potential Claudin-4 PET Imaging. Pharmaceuticals 2017, 10, 99. https://doi.org/10.3390/ph10040099
Feni L, Omrane MA, Fischer M, Zlatopolskiy BD, Neumaier B, Neundorf I. Convenient Preparation of 18F-Labeled Peptide Probes for Potential Claudin-4 PET Imaging. Pharmaceuticals. 2017; 10(4):99. https://doi.org/10.3390/ph10040099
Chicago/Turabian StyleFeni, Lucia, M. Aymen Omrane, Moritz Fischer, Boris D. Zlatopolskiy, Bernd Neumaier, and Ines Neundorf. 2017. "Convenient Preparation of 18F-Labeled Peptide Probes for Potential Claudin-4 PET Imaging" Pharmaceuticals 10, no. 4: 99. https://doi.org/10.3390/ph10040099
APA StyleFeni, L., Omrane, M. A., Fischer, M., Zlatopolskiy, B. D., Neumaier, B., & Neundorf, I. (2017). Convenient Preparation of 18F-Labeled Peptide Probes for Potential Claudin-4 PET Imaging. Pharmaceuticals, 10(4), 99. https://doi.org/10.3390/ph10040099






