PKC in Regenerative Therapy: New Insights for Old Targets
Abstract
1. Introduction
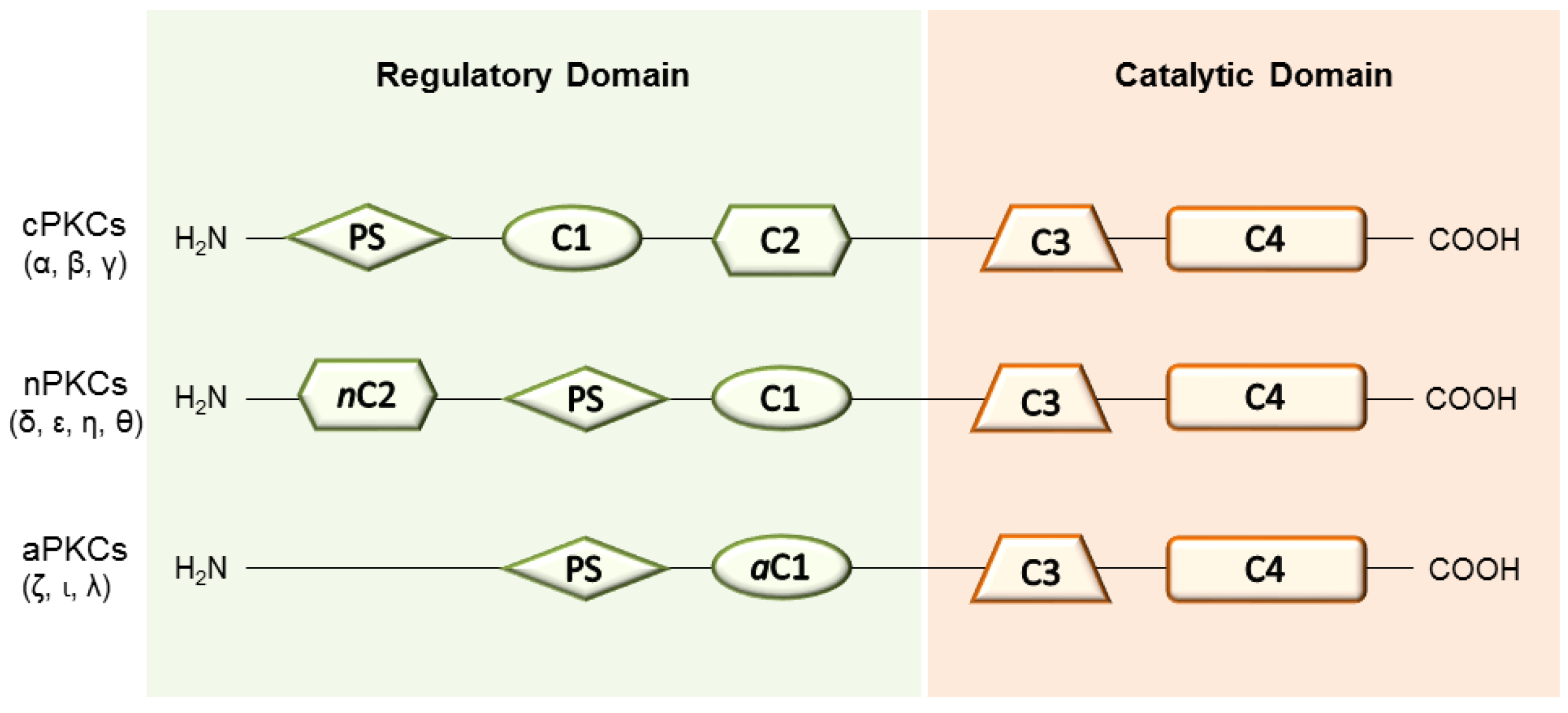
2. PKC Isoenzymes and Their Role in Tissue Regeneration
3. PKC Ligands
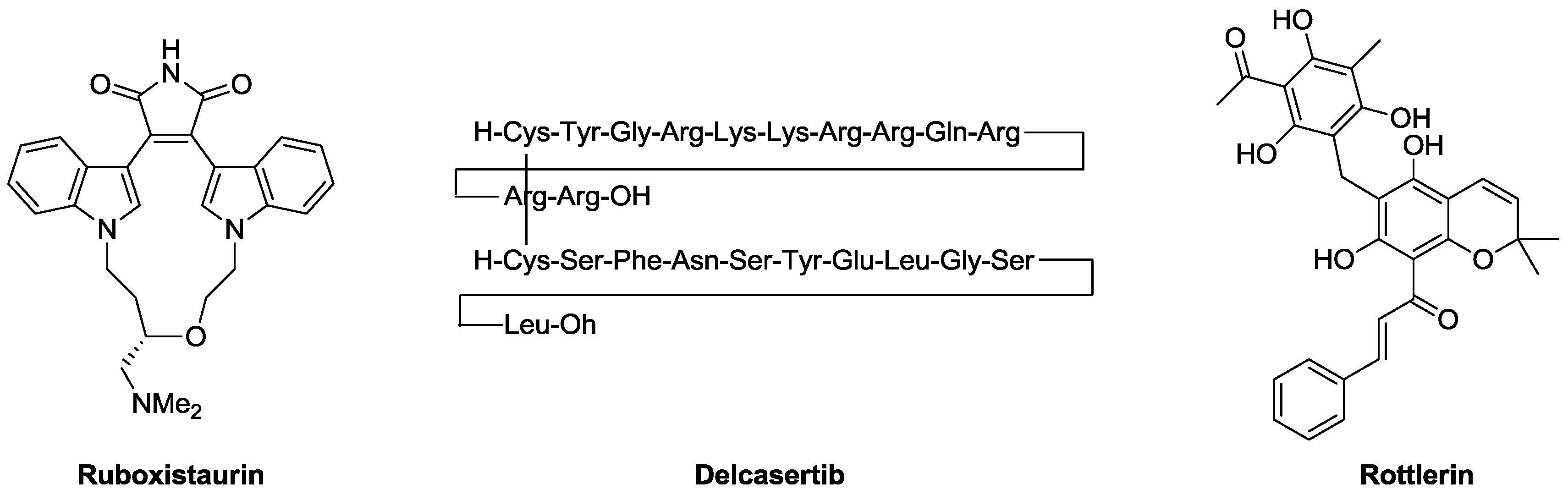
3.1. C3 Domain Ligands
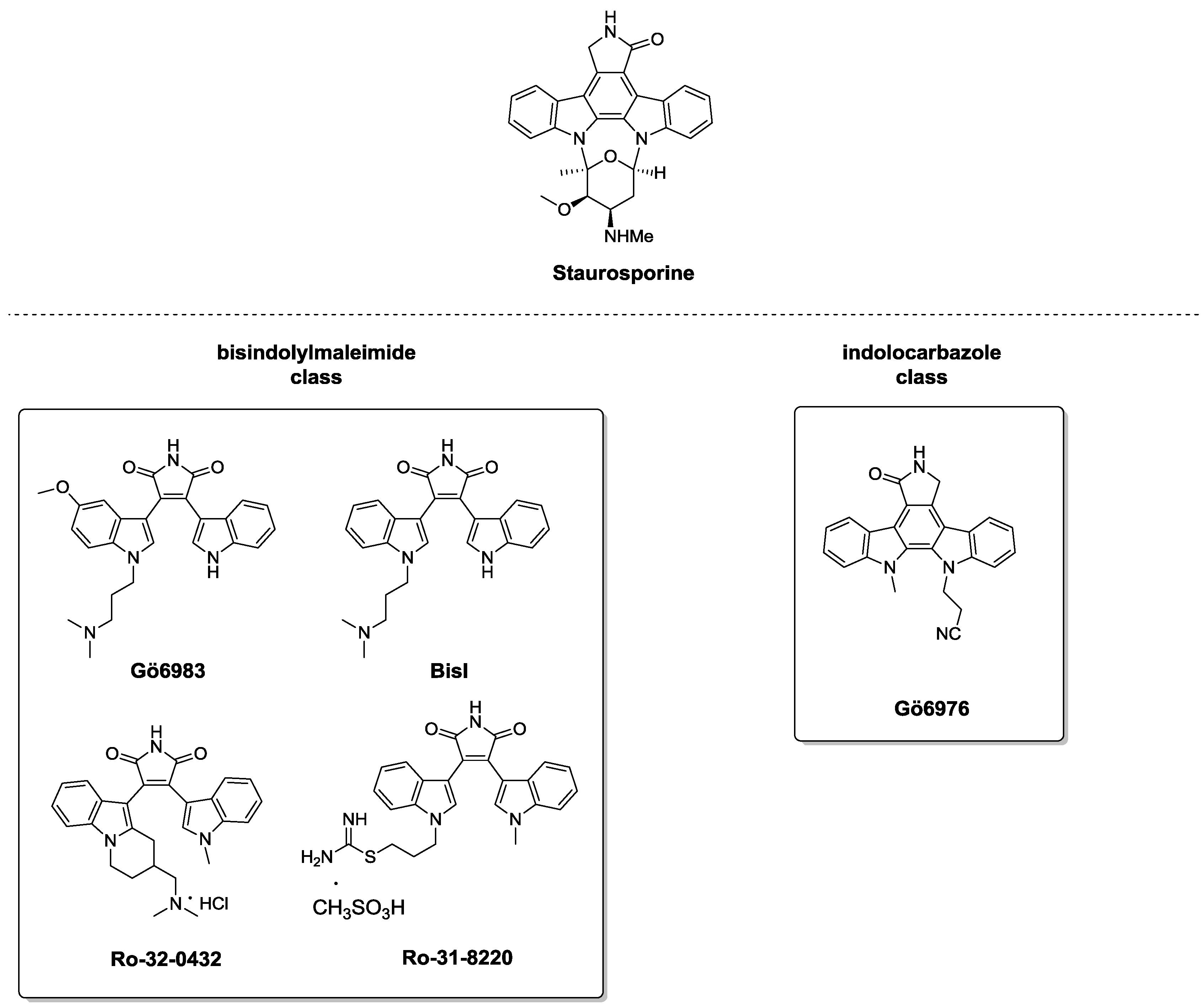
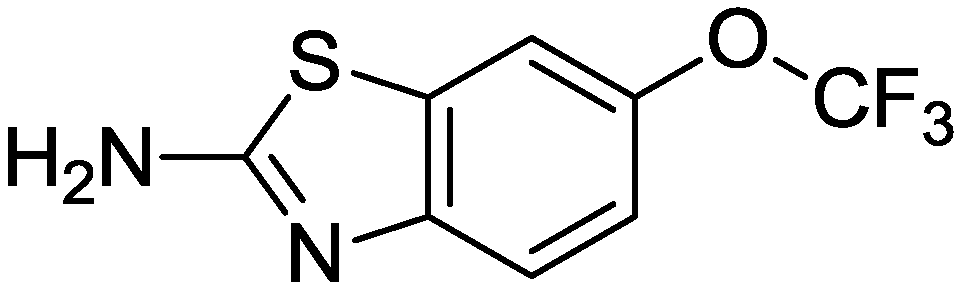
3.2. C1 Domain Ligands
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Mason, C.; Dunnill, P. A brief definition of regenerative medicine. Regen. Med. 2008, 3, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef] [PubMed]
- Pierce, G.F. Inflammation in nonhealing diabetic wounds: The space-time continuum does matter. Am. J. Pathol. 2001, 159, 399–403. [Google Scholar] [CrossRef]
- Edwards, J.; Howley, P.; Cohen, I.K. In vitro inhibition of human neutrophil elastase by oleic acid albumin formulations from derivatized cotton wound dressings. Int. J. Pharm. 2004, 284, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yannas, I.V.; Lee, E.; Orgill, D.P.; Skrabut, E.M.; Murphy, G.F. Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin. Proc. Natl. Acad. Sci. USA 1989, 86, 933–937. [Google Scholar] [CrossRef] [PubMed]
- Richmond, N.A.; Maderal, A.D.; Vivas, A.C. Evidence-based management of common chronic lower extremity ulcers. Dermatol. Ther. 2013, 26, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Geoffrey, C.; Gurtner, G.C.; Chapman, M.A. Regenerative Medicine: Charting a New Course in Wound Healing. Adv. Wound Care 2016, 5, 314–328. [Google Scholar]
- Kemp, P. History of regenerative medicine: Looking backwards to move forwards. Regen. Med. 2006, 1, 653–669. [Google Scholar] [CrossRef] [PubMed]
- Nunan, R.; Harding, K.G.; Martin, P. Clinical challenges of chronic wounds: Searching for an optimal animal model to recapitulate their complexity. Dis. Model Mech. 2014, 7, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Geraldes, P.; King, G.L. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ. Res. 2010, 106, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Takai, Y.; Kishimoto, A.; Inoue, M.; Nishizuka, Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. I. Purification and characterization of an active enzyme from bovine cerebellum. J. Biol. Chem. 1977, 252, 7603–7609. [Google Scholar] [PubMed]
- Shun-ichi, N.; Hirokei, Y.; Nishizuka, Y. Father of protein kinase C. J. Biochem. 2010, 148, 125–130. [Google Scholar]
- Van der Zee, E.A.; Luiten, P.G.; Disterhoft, J.F. Learning-Induced Alterations in Hippocampal PKC-immunoreactivity: A Review and Hypothesis of Its Functional Significance. Prog. Neuropsychopharmacol. Biol. Psychiatry 1997, 21, 531–572. [Google Scholar] [CrossRef]
- Pascale, A.; Noguès, X.; Marighetto, A.; Micheau, J.; Battaini, F.; Govoni, S.; Jaffard, R. Cytosolic hippocampal PKC and aging: Correlation with discrimination performance. NeuroReport 1998, 9, 725–729. [Google Scholar] [CrossRef]
- Newton, A.C. Regulation of the ABC kinases by phosphorylation: Protein kinase C as a paradigm. Chem. Rev. 2001, 101, 2353–2364. [Google Scholar] [CrossRef] [PubMed]
- Battaini, F.; Mochly-Rosen, D. Happy birthday protein kinase C: Past, present and future of a superfamily. Pharmacol. Res. 2007, 55, 461–466. [Google Scholar] [CrossRef] [PubMed]
- House, C.; Kemp, B.E. Protein kinase C contains a pseudosubstrate prototope in its regulatory domain. Science 1987, 238, 1726–1728. [Google Scholar] [CrossRef] [PubMed]
- Newton, A.C. Protein Kinase C: Structure, Function, and Regulation. J. Biol. Chem. 1995, 270, 28495–28498. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, S.F. Structural basis of protein kinase C isoform function. Physiol. Rev. 2008, 88, 1341–1378. [Google Scholar] [CrossRef] [PubMed]
- Newton, A.C. Protein kinase C: Poised to signal. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E395–E402. [Google Scholar] [CrossRef] [PubMed]
- Nishizuka, Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature 1988, 334, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Nishizuka, Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science 1992, 258, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Govoni, S.; Amadio, M.; Battaini, F.; Pascale, A. Senescence of the brain: Focus on cognitive kinases. Curr. Pharm. Des. 2010, 16, 660–671. [Google Scholar] [CrossRef] [PubMed]
- Battaini, F. Protein kinase C isoforms as therapeutic targets in nervous system disease states. Pharmacol. Res. 2001, 44, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, J. Protein kinase C isozymes as potential targets for anticancer therapy. Curr. Cancer Drug Targets 2004, 4, 125–146. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, P.M.; Kedei, N.; Lewin, N.E.; Yang, D.; Czifra, G.; Pu, Y.; Peach, M.L.; Marquez, V.E. Wealth of opportunity—the C1 domain as a target for drug development. Curr. Drug Targets 2008, 9, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Bynagari-Settipalli, Y.S.; Chari, R.; Kilpatrick, L.; Kunapuli, S.P. Protein kinase C—possible therapeutic target to treat cardiovascular diseases. Cardiovasc. Hematol. Disord. Drug Targets 2010, 10, 292–308. [Google Scholar] [CrossRef] [PubMed]
- Mochly-Rosen, D.; Das, K.; Grimes, K.V. Protein kinase C, an elusive therapeutic target? Nat. Rev. Drug Discov. 2012, 11, 937–957. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.H. ABC transporters as multidrug resistance mechanisms and the development of chemosensitizers for their reversal. Cancer Cell Int. 2005, 5, 1–13. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug resistance in cancer: Role of ATP-dependent transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Martiny-Baron, G.; Fabbro, D. Classical PKC isoforms in cancer. Pharmacol. Res. 2007, 55, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Lamark, T.; Perander, M.; Outzen, H.; Kristiansen, K.; Øvertan, A.; Michaelsen, M.; Bjørkøy, G.; Johansen, T. Interaction codes within the family of mammalian Phox and Bem1p domain containing proteins. J. Biol. Chem. 2003, 278, 34568–34581. [Google Scholar] [CrossRef] [PubMed]
- Murray, N.R.; Kalari, K.R.; Fields, A.P. Protein kinase C expression and oncogenic signaling mechanisms in cancer. J. Cell Physiol. 2011, 226, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Antal, C.E.; Hudson, A.M.; Kang, E.; Zanca, C.; Wirth, C.; Stephenson, N.L.; Trotter, E.W.; Gallegos, L.L.; Miller, C.J.; Furnari, F.B.; et al. Cancer-Associated Protein Kinase C Mutations Reveal Kinase’s Role as Tumor Suppressor. Cell 2015, 160, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Newton, A.C.; Brognard, J. Reversing the Paradigm: Protein Kinase C as a Tumor Suppressor. Trends Pharmacol. Sci. 2017, 38, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Kun-Sun, M.; Alkon, D.L. Pharmacology of protein kinase C activators: Cognition-enhancing and antidementic therapeutics. Pharmacol. Ther. 2010, 127, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Pascale, A.; Amadio, M.; Govoni, S.; Battaini, F. The aging brain, a key target for the future: The protein kinase C involvement. Pharmacol. Res. 2007, 55, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Pascale, A.; Amadio, M.; Scampagnini, G.; Lanni, C.; Racchi, M.; Provenzani, A.; Govoni, S.; Alkon, D.L.; Quattrone, A. Neuronal ELAV proteins enhance mRNA stability by a PKCalpha-dependent pathway. PNAS 2005, 102, 12065–12070. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, S.I.; Callender, J.A.; Hooli, B.; Antal, C.E.; Mullin, K.; Sherman, M.A.; Lesné, S.E.; Leitges, M.; Newton, A.C.; Tanzi, R.E.; et al. Gain-of-function mutations in protein kinase Cα (PKCα) may promote synaptic defects in Alzheimer’s disease. Sci. Signal 2016, 9, ra47. [Google Scholar] [CrossRef] [PubMed]
- Amadio, M.; Bucolo, C.; Leggio, G.M.; Drago, F.; Govoni, S.; Pascale, A. The PKCbeta/HuR/VEGF pathway in diabetic retinopathy. Biochem. Pharm. 2010, 80, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Casellini, C.M.; Barlow, P.M.; Rice, A.L.; Casey, M.; Simmons, K.; Pittenger, G.; Bastyr, E.J., 3rd; Wolka, A.M.; Vinik, A.I. A 6-month, randomized, double-masked, placebo-controlled study evaluating the effects of the protein kinase C-beta inhibitor ruboxistaurin on skin microvascular blood flow and other measures of diabetic peripheral neuropathy. Diabetes Care 2007, 30, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.D.; Sheetz, M.J.; Aiello, L.P.; Milton, R.C.; Danis, R.P.; Zhi, X.; Girach, A.; Jimenez, M.C.; Vignati, L. Effect of ruboxistaurin on the visual acuity decline associated with long-standing diabetic macular edema. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Lincoff, A.M.; Roe, M.; Aylward, P.; Galla, J.; Rynkiewicz, A.; Guetta, V.; Zelizko, M.; Kleiman, N.; White, H.; McErlean, E.; et al. Inhibition of delta-protein kinase C by delcasertib as an adjunct to primary percutaneous coronary intervention for acute anterior ST-segment elevation myocardial infarction: Results of the PROTECTION AMI Randomized Controlled Trial. Eur. Heart J. 2014, 35, 2516–2523. [Google Scholar] [CrossRef] [PubMed]
- Joyce, N.C.; Meklir, B. Protein kinase C activation during corneal endothelial wound repair. Investig. Ophthalmol. Vis. Sci. 1992, 33, 1958–1973. [Google Scholar] [PubMed]
- Thomason, H.A.; Cooper, N.H.; Ansell, D.M.; Chiu, M.; Merrit, A.J.; Hardman, M.J.; Garrod, D.R. Direct evidence that PKCα positively regulates wound re-epithelialization: Correlation with changes in desmosomal adhesiveness. J. Pathol. 2012, 227, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Khamaisi, M.; Katagiri, S.; Keenan, H.; Park, K.; Maeda, Y.; Li, Q.; Qi, W.; Thomou, T.; Eschuk, D.; Tellechea, A.; et al. PKCδ inhibition normalizes the wound-healing capacity of diabetic human fibroblasts. J. Clin. Investig. 2016, 126, 837–853. [Google Scholar] [CrossRef] [PubMed]
- Wu-Zhang, A.X.; Newton, A.C. Protein kinase C pharmacology: Refining the toolbox. Biochem. J. 2013, 452, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Darby, I.A.; Laverdet, B.; Bonté, F.; Desmoulière, A. Fibroblasts and myofibroblasts in wound healing. Clin. Cosmet. Investig. Dermatol. 2014, 7, 301–311. [Google Scholar] [PubMed]
- Driskell, R.R.; Lichtenberger, B.M.; Hoste, E.; Kretzschmar, K.; Simons, B.D.; Charalambous, M.; Ferron, S.R.; Herault, Y.; Pavlovic, G.; Ferguson-Smith, A.C.; et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 2013, 504, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Boije, A.G.G.; Talman, V.; Yli-Kauhaluoma, J.; Tuominen, R.K.; Ekokoski, E. Current Status and Future Prospects of C1 Domain Ligands as Drug Candidates. Curr. Top. Med. Chem. 2011, 11, 1370–1392. [Google Scholar] [CrossRef]
- Davis, M.I.; Hunt, J.P.; Herrgard, S.; Ciceri, P.; Wodicka, L.M.; Pallares, G.; Hocker, M.; Treiber, D.K.; Zarrinkar, P.P. Comprehensive analysis of kinase inhibitor selectivity. Nat. Biotechnol. 2011, 29, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Anastassiadis, T.; Deacon, S.W.; Devarajan, K.; Ma, H.; Peterson, J.R. Comprehensive assay of kinase catalytic activity reveals features of kinase inhibitor selectivity. Nat. Biotechnol. 2011, 29, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.E.; Parker, P.J.; Nixon, J.S. Isoenzyme specificity of bisindolylmaleimides, selective inhibitors of protein kinase C. Biochem. J. 1993, 294, 335–337. [Google Scholar] [CrossRef] [PubMed]
- Omura, S.; Iwai, Y.; Hirano, A.; Nakagawa, A.; Awaya, J.; Tsuchya, H.; Takahashi, Y.; Masuma, R. A new alkaloid AM-2282 of streptomyces origin. Taxonomy, fermentation, isolation and preliminary characterization. J. Antibiot. 1977, 30, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Martiny-Baron, G.; Kazanietz, M.G.; Mischak, H.; Blumberg, P.M.; Kochs, G.; Hug, H.; Marme, D.; Schachtele, C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Gö6976. J. Biol. Chem. 1993, 268, 9194–9197. [Google Scholar] [PubMed]
- Gschwendt, M.; Dieterich, S.; Rennecke, J.; Kittstein, W.; Mueller, H.J.; Johannes, F.J. Inhibition of protein kinase Cμ by various inhibitors. Differentiation from protein kinase C isoenzymes. FEBS Lett. 1996, 392, 77–80. [Google Scholar] [CrossRef]
- Alessi, D.R. The protein kinase C inhibitors Ro 318220 and GF 109203X are equally potent inhibitors of MAPKAP kinase-1β (Rsk-2) and p70 S6 kinase. FEBS Lett. 1997, 402, 121–123. [Google Scholar] [CrossRef]
- Davies, S.P.; Reddy, H.; Caivano, M.; Cohen, P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 2000, 351, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Bain, J.; Plater, L.; Elliott, M.; Shpiro, N.; Hastie, C.J.; McLauchlan, H.; Klevernic, I.; Arthur, J.S.; Alessi, D.R.; Cohen, P. The selectivity of protein kinase inhibitors: A further update. Biochem. J. 2007, 408, 297–315. [Google Scholar] [CrossRef] [PubMed]
- Takagi, T.; Naito, Y.; Uchiyama, K.; Mizuhima, K.; Suzuki, T.; Horie, R.; Hirata, I.; Tsuboi, H.; Yoshikawa, T. Carbon monoxide promotes gastric wound healing in mice via the protein kinase C pathway. Free Radic. Res. 2016, 50, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Ryu, J.H.; Zuo, Z.; Yang, S.M.; Chang, H.W.; Do, S.H. Riluzole attenuates excitatory amino acid transporter type 3 activity in Xenopus oocytes via protein kinase C inhibition. Eur. J. Pharmacol. 2013, 713, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Lamanauskas, N.; Nistri, A. Riluzole blocks persistent Na+ and Ca2+ currents and modulates release of glutamate via presynaptic NMDA receptors on neonatal rat hypoglossal motoneurons in vitro. Eur. J. Neurosci. 2008, 27, 2501–2514. [Google Scholar] [CrossRef] [PubMed]
- Noh, K.M.; Hwang, J.Y.; Shin, H.C.; Koh, J.Y. A Novel Neuroprotective Mechanism of Riluzole: Direct Inhibition of Protein Kinase C. Neurobiol. Dis. 2000, 7, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Parang, K.; Cole, P.A. Designing bisubstrate analog inhibitors for protein kinases. Pharmacol. Ther. 2002, 93, 145–157. [Google Scholar] [CrossRef]
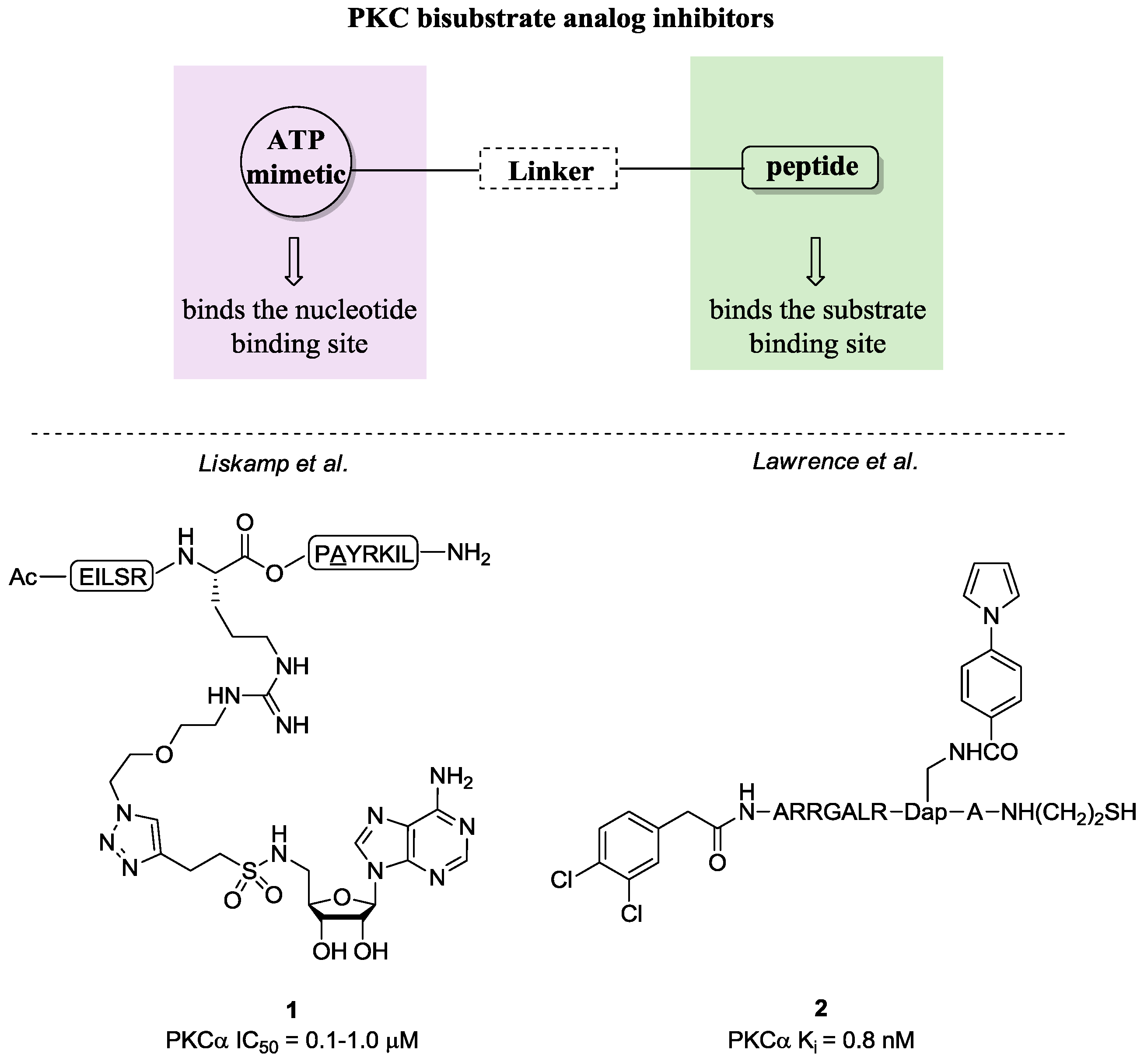
- Poot, A.J.; van Ameijde, J.; Slijper, M.; van den Berg, A.; Hilhorst, R.; Ruijtenbeek, R.; Rijkers, D.T.S; Liskamp, R.M.J. Development of selective bisubstrate-based inhibitors against protein kinase C (PKC) isozymes by using dynamic peptide microarrays. Chem. Biol. Chem. 2009, 10, 2042–2051. [Google Scholar] [CrossRef] [PubMed]
- Van Wandelen, L.T.M.; van Ameijde, J.; Mady, A.S.A.; Wammes, A.E.M.; Bode, A.; Poot, A.J.; Ruijtenbeek, R.; Liskamp, R.M.J. Directed modulation of protein kinase C isozyme selectivity with bisubstrate-based inhibitors. Chem. Med. Chem. 2012, 7, 2113–2121. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Nandy, S.K.; Lawrence, D.S. A highly potent and selective PKC alpha inhibitor generated via combinatorial modification of a peptide scaffold. J. Am. Chem. Soc. 2004, 126, 3394–3395. [Google Scholar] [CrossRef] [PubMed]
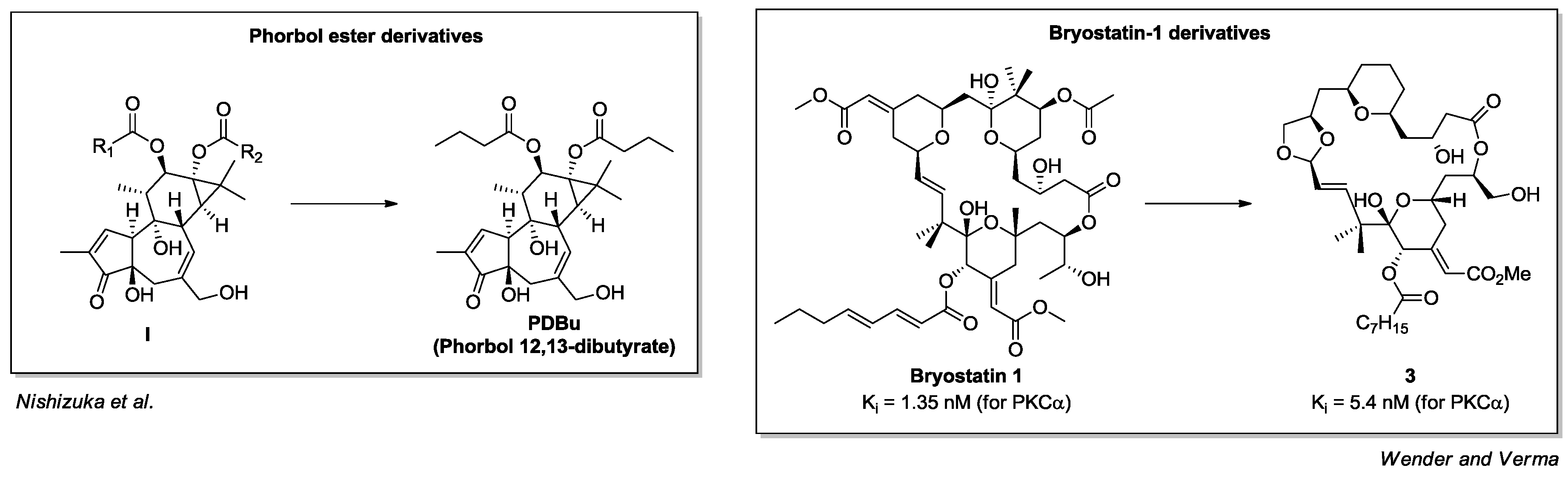
- Castagna, M.; Takai, Y.; Kaibuchi, K.; Sano, K.; Kikkawa, U.; Nishizuka, Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J. Biol. Chem. 1982, 257, 7847–7851. [Google Scholar] [PubMed]
- Kortmansky, J.; Schwartz, G.K. Bryostatin-1: A novel PKC inhibitor in clinical development. Cancer Investig. 2003, 21, 924–936. [Google Scholar] [CrossRef] [PubMed]
- Driedger, P.E.; Blumberg, P.M. Specific binding of phorbol ester tumor promoters. Proc. Natl. Acad. Sci. USA 1980, 77, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Pettit, G.R.; Herald, C.L.; Doubek, D.L.; Herald, D.L.; Arnold, E.; Clardy, J. Isolation and structure of bryostatin 1. J. Am. Chem. Soc. 1982, 104, 6846–6848. [Google Scholar] [CrossRef]
- Wender, P.A.; Hinkle, K.W.; Koehler, M.F.; Lippa, B. The rational design of potential chemotherapeutic agents: Synthesis of bryostatin analogues. Med. Res. Rev. 1999, 19, 388–407. [Google Scholar] [CrossRef]
- Keck, G.E.; Kraft, M.B.; Truong, A.P.; Li, W.; Sanchez, C.C.; Kedei, N.; Lewin, N.E.; Blumberg, P.M. Convergent assembly of highly potent analogues of bryostatin 1 via pyran annulation: Bryostatin look-alikes that mimic phorbol ester function. J. Am. Chem. Soc. 2008, 130, 6660–6661. [Google Scholar] [CrossRef] [PubMed]
- Wender, P.A.; Verma, V.A. Design, synthesis, and biological evaluation of a potent, PKC selective, B-ring analog of bryostatin. Org. Lett. 2006, 8, 1893–1896. [Google Scholar] [CrossRef] [PubMed]
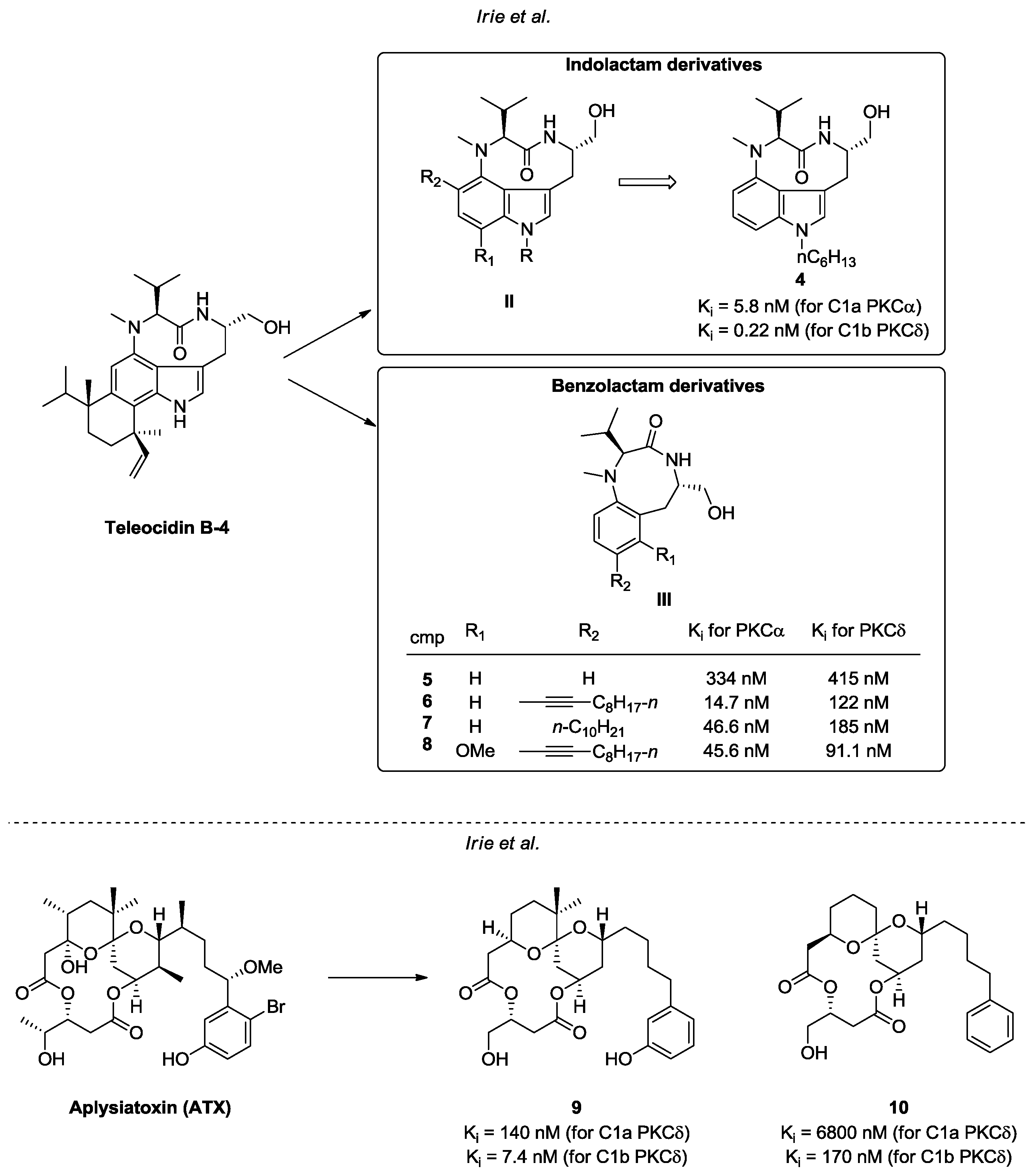
- Irie, K.; Yanagita, R.C. Synthesis and biological activities of simplified analogs of the natural PKC ligands, bryostatin-1 and aplysiatoxin. Chem. Rec. 2014, 14, 251–267. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, K.; Weinstein, I.B.; Horowitz, A.; Fujiki, H.; Matsushima, T.; Sugimura, T. Similarity of teleocidin B and phorbol ester tumour promoters in effects on membrane receptors. Nature 1981, 290, 411–413. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, A.D.; Fujiki, H.; Weinstein, I.B. Comparative effects of aplysiatoxin, debromoaplysiatoxin, and teleocidin on receptor binding and phospholipid metabolism. Cancer Res. 1983, 43, 1529–1535. [Google Scholar] [PubMed]
- Fujiki, H.; Mori, M.; Nakayasu, M.; Terada, M.; Sugimura, T.; Moore, R.E. Indole alkaloids: Dihydroteleocidin B, teleocidin, and lyngbyatoxin A as members of a new class of tumor promoters. Proc. Natl. Acad. Sci. USA 1981, 78, 3872–3876. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, H.; Suganuma, M.; Nakayasu, M.; Hoshino, H.; Moore, R.E.; Sugimura, T. The third class of new tumor promoters, polyacetates (debromoaplysiatoxin and aplysiatoxin), can differentiate biological actions relevant to tumor promoters. Gann 1982, 73, 495–497. [Google Scholar] [PubMed]
- Irie, K.; Nakagawa, Y.; Ohigashi, H. Toward the Development of New Medicinal Leads with Selectivity for Protein Kinase C Isozymes. Chem. Rec. 2005, 5, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Yanagita, R.C.; Torii, K.; Nakagawa, Y.; Irie, K. Binding selectivity of 1- or 12- substituted indolactam derivatives for protein kinase C isozymes. Heterocycles 2007, 73, 289–302. [Google Scholar]
- Ma, D.; Tang, G.; Kozikowski, A.P. Synthesis of 7-Substituted Benzolactam-V8s and Their Selectivity for Protein Kinase C Isozymes. Org. Lett. 2002, 4, 2377–2380. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Zhang, T.; Wang, G.; Kozikowski, A.P.; Lewinc, N.E.; Blumbergc, P.M. Synthesis of 7,8-Disubstituted Benzolactam-V8 and Its Binding to Protein Kinase C. Bioorg. Med. Chem. Lett. 2001, 11, 99–101. [Google Scholar] [CrossRef]
- Irie, K.; Kikumori, M.; Kamachi, H.; Tanaka, K.; Murakami, A.; Yanagita, R.C.; Tokuda, H.; Suzuki, N.; Nagai, H.; Suenaga, K.; et al. Synthesis and structure–activity studies of simplified analogues of aplysiatoxin with antiproliferative activity like bryostatin-1. Pure Appl. Chem. 2012, 84, 1341–1351. [Google Scholar] [CrossRef]
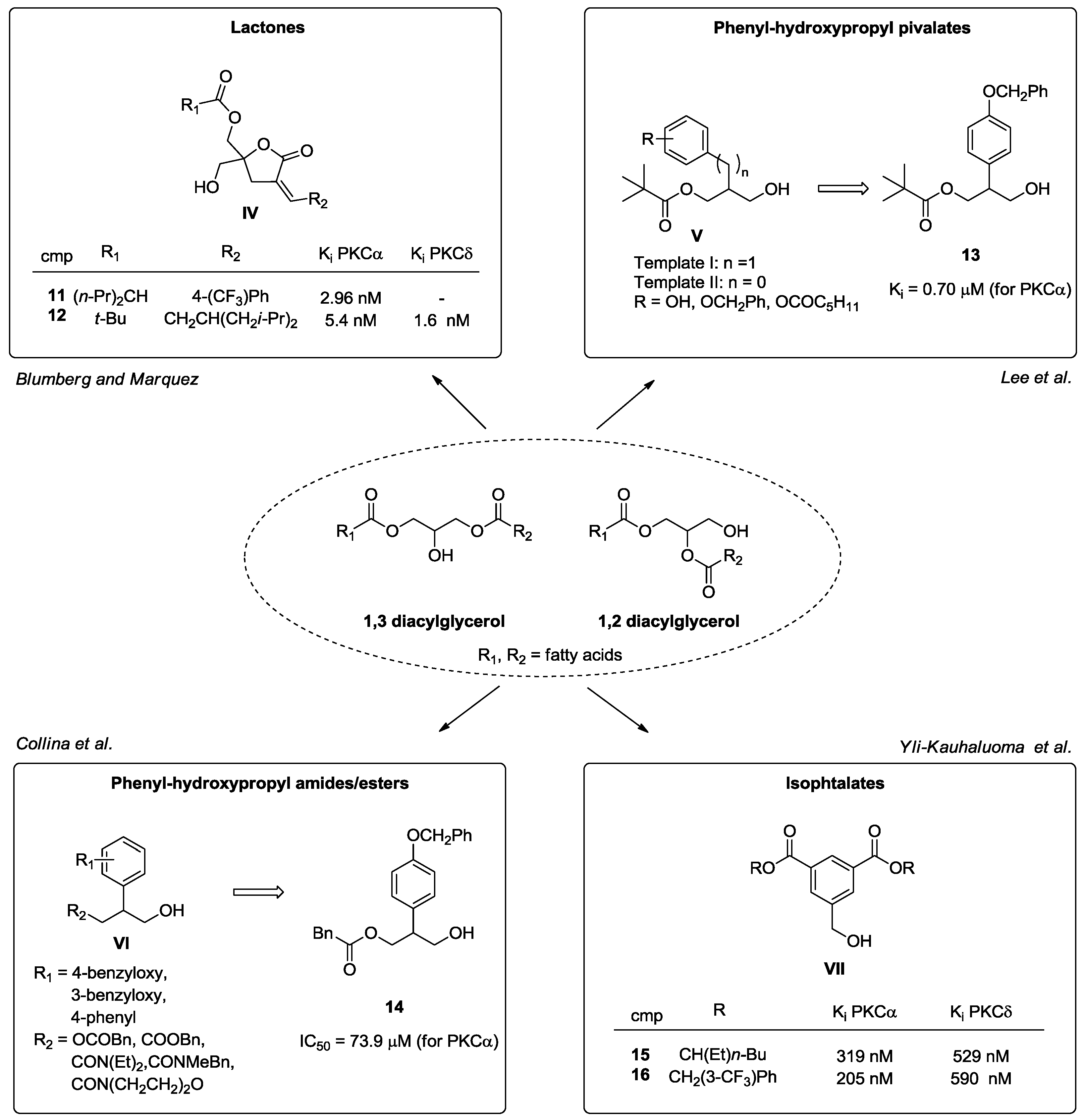
- Marquez, V.E.; Blumberg, P.M. Synthetic diacylglycerols (DAG) and DAG-lactones as activators of protein kinase C (PK-C). Acc. Chem. Res. 2003, 36, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Siddiqui, M.A.; Sigano, D.M.; Krajewski, K.; Lewin, N.E.; Pu, Y.; Blumberg, P.M.; Lee, J.; Marquez, V.E. Conformationally constrained analogues of diacylglycerol. 24. Asymmetric synthesis of a chiral (R)-DAG-lactone template as a versatile precursor for highly functionalized DAG-lactones. Org. Lett. 2004, 6, 2413–2416. [Google Scholar] [CrossRef] [PubMed]
- El Kazzouli, S.; Lewin, N.E.; Blumberg, P.M.; Marquez, V.E. Conformationally constrained analogues of diacylglycerol. 30. An investigation of diacylglycerol-lactones contained heteroaryl groups reveals compounds with high selectivity for Ras guanyl nucleotide-releasing proteins. J. Med. Chem. 2008, 51, 5371–5386. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.; Sigano, D.M.; Kelley, J.A.; Lai, C.C.; Lewin, N.E.; Kedei, N.; Peach, M.L.; Lee, J.; Abeyweera, T.P.; Rotenberg, S.A.; et al. Conformationally Constrained Analogues of Diacylglycerol. 29. Cells Sort Diacylglycerol-Lactone Chemical Zip Codes to Produce Diverse and Selective Biological Activities. J. Med. Chem. 2008, 51, 5198–5220. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, J.H.; Kim, S.Y.; Perry, N.A.; Lewin, N.E.; Ayres, J.A.; Blumberg, P.M. 2-Benzyl and 2-phenyl-3-hydroxypropyl pivalates as protein kinase C ligands. Bioorg. Med. Chem. 2006, 14, 2022–2031. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.; Talman, V.; Gennäs, G.B.A.; Marra, A.; Picconi, P.; Nasti, R.; Serra, M.; Ann, J.; Amadio, M.; Pascale, A.; et al. Beyond the affinity for protein kinase C: Exploring 2-phenyl-3-hydroxypropyl pivalate analogues as C1 domain-targeting ligands. Med. Chem. Commun. 2015, 6, 547–554. [Google Scholar] [CrossRef]
- Boije, A.G.G.; Talman, V.; Aitio, O.; Ekokoski, E.; Finel, M.; Tuominen, R.K.; Yli-Kauhaluoma, J. Design, Synthesis, and Biological Activity of Isophthalic Acid Derivatives Targeted to the C1 Domain of Protein Kinase C. J. Med. Chem. 2009, 52, 3969–3981. [Google Scholar]
- Talmana, V.; Amadio, M.; Osera, C.; Sorvari, S.; Gennäs, G.B.A.; Yli-Kauhaluoma, J.; Rossi, D.; Govoni, S.; Collina, S.; Ekokoski, E.; et al. The C1 domain-targeted isophthalate derivative HMI-1b11 promotes neurite outgrowth and GAP-43 expression through PKC activation in SH-SY5Y cells. Pharmacol. Res. 2013, 73, 44–54. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rui, M.; Nasti, R.; Bignardi, E.; Della Volpe, S.; Rossino, G.; Rossi, D.; Collina, S. PKC in Regenerative Therapy: New Insights for Old Targets. Pharmaceuticals 2017, 10, 46. https://doi.org/10.3390/ph10020046
Rui M, Nasti R, Bignardi E, Della Volpe S, Rossino G, Rossi D, Collina S. PKC in Regenerative Therapy: New Insights for Old Targets. Pharmaceuticals. 2017; 10(2):46. https://doi.org/10.3390/ph10020046
Chicago/Turabian StyleRui, Marta, Rita Nasti, Emanuele Bignardi, Serena Della Volpe, Giacomo Rossino, Daniela Rossi, and Simona Collina. 2017. "PKC in Regenerative Therapy: New Insights for Old Targets" Pharmaceuticals 10, no. 2: 46. https://doi.org/10.3390/ph10020046
APA StyleRui, M., Nasti, R., Bignardi, E., Della Volpe, S., Rossino, G., Rossi, D., & Collina, S. (2017). PKC in Regenerative Therapy: New Insights for Old Targets. Pharmaceuticals, 10(2), 46. https://doi.org/10.3390/ph10020046







