Recent Findings Concerning PAMAM Dendrimer Conjugates with Cyclodextrins as Carriers of DNA and RNA
Abstract
:1. Introduction
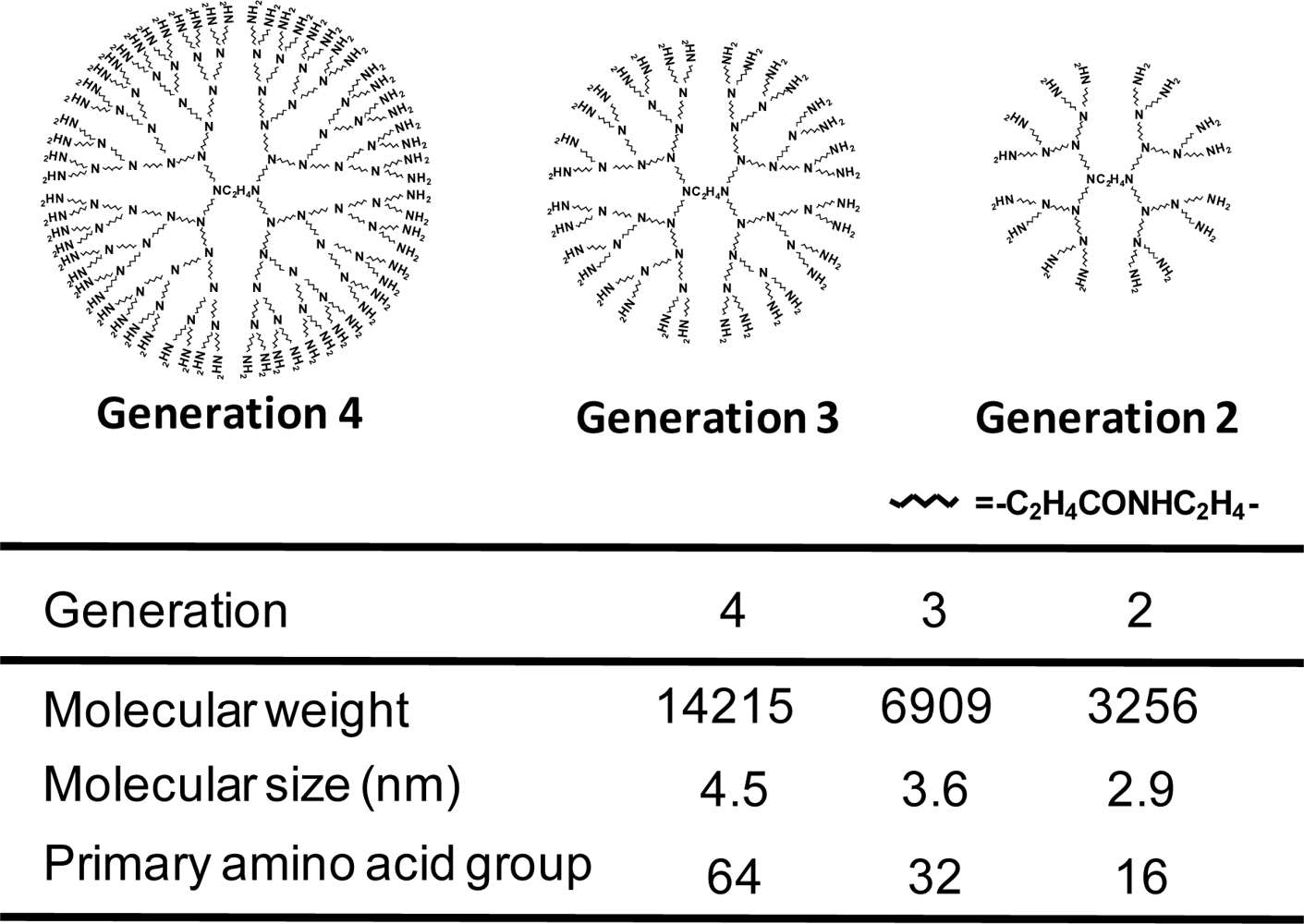
2. Polyamidoamine (PAMAM) Starburst™ Dendrimers (Dendrimers) as DNA, shRNA and siRNA Carriers
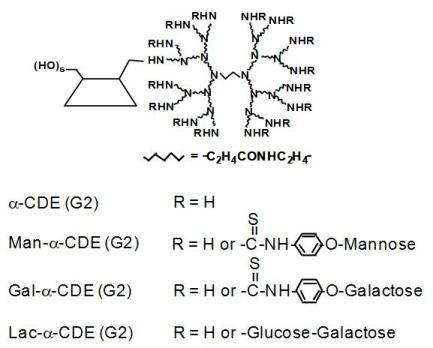
3. α-Cyclodextrin Conjugates with Dendrimers (α-CDE) as DNA, shRNA and siRNA Carriers
4. Sugar-appended α-CDEs as DNA Carriers
5. Folate-appended α-CDEs as DNA Carriers
6. Conclusions
Acknowledgments
References and Notes
- Pfeifer, A.; Verma, I.M. Gene therapy: promises and problems. Annu. Rev. Genomics Hum. Genet 2001, 2, 177–211. [Google Scholar]
- Lowenstein, P.R. Clinical trials in gene therapy: ethics of informed consent and the future of experimental medicine. Curr. Opin. Mol. Ther 2008, 10, 428–430. [Google Scholar]
- Fischer, A.; Cavazzana-Calvo, M. Gene therapy of inherited diseases. Lancet 2008, 371, 2044–2047. [Google Scholar]
- Raty, J.K.; Lesch, H.P.; Wirth, T.; Yla-Herttuala, S. Improving safety of gene therapy. Curr. Drug Saf 2008, 3, 46–53. [Google Scholar]
- Shen, Y. Advances in the development of siRNA-based therapeutics for cancer. IDrugs 2008, 11, 572–578. [Google Scholar]
- Ghildiyal, M.; Zamore, P.D. Small silencing RNAs: an expanding universe. Nat. Rev. Genet 2009, 10, 94–108. [Google Scholar]
- Castanotto, D.; Rossi, J.J. The promises and pitfalls of RNA-interference-based therapeutics. Nature 2009, 457, 426–433. [Google Scholar]
- Rao, D.D.; Vorhies, J.S.; Senzer, N.; Nemunaitis, J. siRNA vs. shRNA: similarities and differences. Adv. Drug Deliv. Rev 2009, 61, 746–759. [Google Scholar]
- Blau, H.M.; Springer, M.L. Gene therapy--a novel form of drug delivery. N. Engl. J. Med 1995, 333, 1204–1207. [Google Scholar]
- Afione, S.A.; Conrad, C.K.; Flotte, T.R. Gene therapy vectors as drug delivery systems. Clin. Pharmacokinet 1995, 28, 181–189. [Google Scholar]
- Grimm, D. Small silencing RNAs: state-of-the-art. Adv. Drug Deliv. Rev 2009, 61, 672–703. [Google Scholar]
- Takeda, K. Delivery of magic bullets: on the still rocky road to gene therapy. Br. J. Pharmacol 2009, 157, 151–152. [Google Scholar]
- Li, S.D.; Huang, L. Gene therapy progress and prospects: non-viral gene therapy by systemic delivery. Gene Ther 2006, 13, 1313–1319. [Google Scholar]
- Akhtar, S. Non-viral cancer gene therapy: beyond delivery. Gene Ther 2006, 13, 739–740. [Google Scholar]
- Rettig, G.R.; Rice, K.G. Non-viral gene delivery: from the needle to the nucleus. Expert Opin. Biol. Ther 2007, 7, 799–808. [Google Scholar]
- Cristiano, R.J.; Xu, B.; Nguyen, D.; Schumacher, G.; Kataoka, M.; Spitz, F.R.; Roth, J.A. Viral and nonviral gene delivery vectors for cancer gene therapy. Cancer Detect. Prev 1998, 22, 445–454. [Google Scholar]
- Ma, H.; Diamond, S.L. Nonviral gene therapy and its delivery systems. Curr. Pharm. Biotechnol 2001, 2, 1–17. [Google Scholar]
- Smaglik, P. Tighter watch urged on adenoviral vectors.with proposal to report all ‘adverse events’. Nature 1999, 402, 707. [Google Scholar]
- Yi, Y.; Hahm, S.H.; Lee, K.H. Retroviral gene therapy: safety issues and possible solutions. Curr. Gene Ther 2005, 5, 25–35. [Google Scholar]
- Boyce, N. Trial halted after gene shows up in semen. Nature 2001, 414, 677. [Google Scholar]
- Hacein-Bey-Abina, S.; Von Kalle, C.; Schmidt, M.; McCormack, M.P.; Wulffraat, N.; Leboulch, P.; Lim, A.; Osborne, C.S.; Pawliuk, R.; Morillon, E.; Sorensen, R.; Forster, A.; Fraser, P.; Cohen, J.I.; de Saint Basile, G.; Alexander, I.; Wintergerst, U.; Frebourg, T.; Aurias, A.; Stoppa-Lyonnet, D.; Romana, S.; Radford-Weiss, I.; Gross, F.; Valensi, F.; Delabesse, E.; Macintyre, E.; Sigaux, F.; Soulier, J.; Leiva, L.E.; Wissler, M.; Prinz, C.; Rabbitts, T.H.; Le Deist, F.; Fischer, A.; Cavazzana-Calvo, M. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 2003, 302, 415–419. [Google Scholar]
- Thomas, M.; Klibanov, A.M. Non-viral gene therapy: polycation-mediated DNA delivery. Appl. Microbiol. Biotechnol 2003, 62, 27–34. [Google Scholar]
- Eliyahu, H.; Barenholz, Y.; Domb, A.J. Polymers for DNA delivery. Molecules 2005, 10, 34–64. [Google Scholar]
- Park, J.S.; Akiyama, Y.; Yamasaki, Y.; Kataoka, K. Preparation and characterization of polyion complex micelles with a novel thermosensitive poly(2-isopropyl-2-oxazoline) shell via the complexation of oppositely charged block ionomers. Langmuir 2007, 23, 138–146. [Google Scholar]
- Akagi, D.; Oba, M.; Koyama, H.; Nishiyama, N.; Fukushima, S.; Miyata, T.; Nagawa, H.; Kataoka, K. Biocompatible micellar nanovectors achieve efficient gene transfer to vascular lesions without cytotoxicity and thrombus formation. Gene Ther 2007, 14, 1029–1038. [Google Scholar]
- Gao, K.; Huang, L. Nonviral Methods for siRNA Delivery. Mol. Pharm 2009, 6, 651–658. [Google Scholar]
- Reischl, D.; Zimmer, A. Drug delivery of siRNA therapeutics: potentials and limits of nanosystems. Nanomedicine 2009, 5, 8–20. [Google Scholar]
- Tomalia, D.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roek, J.; Ryder, J.; Smith, P. A new class of polymers: starburt-dendritic macromolecules. Polymer J 1985, 17, 117–132. [Google Scholar]
- Tomalia, D.A. Dendrimer Research. Science 1991, 252, 1231. [Google Scholar]
- Esfand, R.; Tomalia, D.A. Poly(amidoamine) (PAMAM) dendrimers: from biomimicry to drug delivery and biomedical applications. Drug Discov. Today 2001, 6, 427–436. [Google Scholar]
- Fant, K.; Esbjorner, E.K.; Lincoln, P.; Norden, B. DNA condensation by PAMAM dendrimers: self-assembly characteristics and effect on transcription. Biochemistry 2008, 47, 1732–1740. [Google Scholar]
- Braun, C.S.; Vetro, J.A.; Tomalia, D.A.; Koe, G.S.; Koe, J.G.; Middaugh, C.R. Structure/function relationships of polyamidoamine/DNA dendrimers as gene delivery vehicles. J. Pharm. Sci 2005, 94, 423–436. [Google Scholar]
- Dutta, T.; Jain, N.K.; McMillan, N.A.; Parekh, H.S. Dendrimer Nanocarriers as Versatile Vectors in Gene Delivery. Nanomedicine 2009. Epub ahead of print.. [Google Scholar]
- Hui, Z.; He, Z.G.; Zheng, L.; Li, G.Y.; Shen, S.R.; Li, X.L. Studies on polyamidoamine dendrimers as efficient gene delivery vector. J. Biomater. Appl 2008, 22, 527–544. [Google Scholar]
- Klajnert, B.; Bryszewska, M. Dendrimers: properties and applications. Acta. Biochim. Pol 2001, 48, 199–208. [Google Scholar]
- Boussif, O.; Lezoualc’h, F.; Zanta, M.A.; Mergny, M.D.; Scherman, D.; Demeneix, B.; Behr, J.P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc. Natl. Acad. Sci. USA 1995, 92, 7297–7301. [Google Scholar]
- Kukowska-Latallo, J.F.; Bielinska, A.U.; Johnson, J.; Spindler, R.; Tomalia, D.A.; Baker, J.R., Jr. Efficient transfer of genetic material into mammalian cells using Starburst polyamidoamine dendrimers. Proc. Natl. Acad. Sci. USA 1996, 93, 4897–4902. [Google Scholar]
- Haensler, J.; Szoka, F.C., Jr. Polyamidoamine cascade polymers mediate efficient transfection of cells in culture. Bioconjug. Chem 1993, 4, 372–379. [Google Scholar]
- Morgan, D.M.; Larvin, V.L.; Pearson, J.D. Biochemical characterisation of polycation-induced cytotoxicity to human vascular endothelial cells. J. Cell Sci 1989, 94 Pt 3, 553–559. [Google Scholar]
- Tang, M.X.; Redemann, C.T.; Szoka, F.C., Jr. In vitro gene delivery by degraded polyamidoamine dendrimers. Bioconjug. Chem 1996, 7, 703–714. [Google Scholar]
- Hudde, T.; Rayner, S.A.; Comer, R.M.; Weber, M.; Isaacs, J.D.; Waldmann, H.; Larkin, D.F.; George, A.J. Activated polyamidoamine dendrimers, a non-viral vector for gene transfer to the corneal endothelium. Gene Ther 1999, 6, 939–943. [Google Scholar]
- Chauhan, A.S.; Diwan, P.V.; Jain, N.K.; Tomalia, D.A. Unexpected in vivo anti-inflammatory activity observed for simple, surface functionalized poly(amidoamine) dendrimers. Biomacromolecules 2009, 10, 1195–1202. [Google Scholar]
- Kuo, J.H.; Jan, M.S.; Chiu, H.W. Mechanism of cell death induced by cationic dendrimers in RAW 264.7 murine macrophage-like cells. J. Pharm. Pharmacol 2005, 57, 489–495. [Google Scholar]
- Szejtli, J. Medicinal applications of cyclodextrins. Med. Res. Rev 1994, 14, 353–386. [Google Scholar]
- Uekama, K. Pharmaceutical application of cyclodextrins as multi-functional drug carriers. Yakugaku Zasshi 2004, 124, 909–935. [Google Scholar]
- Davis, M.E.; Brewster, M.E. Cyclodextrin-based pharmaceutics: past, present and future. Nat. Rev. Drug Discov 2004, 3, 1023–1035. [Google Scholar]
- Uekama, K.; Hirayama, F.; Irie, T. Cyclodextrin Drug Carrier Systems. Chem. Rev 1998, 98, 2045–2076. [Google Scholar]
- Szente, L.; Szejtli, J. Highly soluble cyclodextrin derivatives: chemistry, properties, and trends in development. Adv. Drug Deliv. Rev 1999, 36, 17–28. [Google Scholar]
- Irie, T.; Otagiri, M.; Sunada, M.; Uekama, K.; Ohtani, Y.; Yamada, Y.; Sugiyama, Y. Cyclodextrin-induced hemolysis and shape changes of human erythrocytes in vitro. J. Pharmacobiodyn 1982, 5, 741–744. [Google Scholar]
- Fauvelle, F.; Debouzy, J.C.; Crouzy, S.; Goschl, M.; Chapron, Y. Mechanism of α-cyclodextrin-induced hemolysis. 1. The two-step extraction of phosphatidylinositol from the membrane. J. Pharm. Sci 1997, 86, 935–943. [Google Scholar]
- Ohtani, Y.; Irie, T.; Uekama, K.; Fukunaga, K.; Pitha, J. Differential effects of α-, β- and γ-cyclodextrins on human erythrocytes. Eur. J. Biochem 1989, 186, 17–22. [Google Scholar]
- Irie, T.; Uekama, K. Pharmaceutical applications of cyclodextrins. III. Toxicological issues and safety evaluation. J. Pharm. Sci 1997, 86, 147–162. [Google Scholar]
- Uekama, K.; Otagiri, M. Cyclodextrins in drug carrier systems. Crit. Rev. Ther. Drug Carrier Syst 1987, 3, 1–40. [Google Scholar]
- Motoyama, K.; Toyodome, H.; Onodera, R.; Irie, T.; Hirayama, F.; Uekama, K.; Arima, H. Involvement of lipid rafts of rabbit red blood cells in morphological changes induced by methylated beta-cyclodextrins. Biol. Pharm. Bull 2009, 32, 700–705. [Google Scholar]
- Motoyama, K.; Kameyama, K.; Onodera, R.; Araki, N.; Hirayama, F.; Uekama, K.; Arima, H. Involvement of PI3K-Akt-Bad pathway in apoptosis induced by 2,6-di-O-methyl-β-cyclodextrin, not 2,6-di-O-methyl-α-cyclodextrin, through cholesterol depletion from lipid rafts on plasma membranes in cells. Eur. J. Pharm. Sci 2009, in press.. [Google Scholar]
- Arima, H. Polyfection as nonviral gene transfer method - design of novel nonviral vector using α-cyclodextrin. Yakugaku Zasshi 2004, 124, 451–464. [Google Scholar]
- Aachmann, F.L.; Aune, T.E. Use of cyclodextrin and its derivatives for increased transformation efficiency of competent bacterial cells. Appl. Microbiol. Biotechnol 2009, 83, 589–596. [Google Scholar]
- Gonzalez, H.; Hwang, S.J.; Davis, M.E. New class of polymers for the delivery of macromolecular therapeutics. Bioconjug. Chem 1999, 10, 1068–1074. [Google Scholar]
- Hwang, S.J.; Bellocq, N.C.; Davis, M.E. Effects of structure of β-cyclodextrin-containing polymers on gene delivery. Bioconjug. Chem 2001, 12, 280–290. [Google Scholar]
- Pun, S.H.; Tack, F.; Bellocq, N.C.; Cheng, J.; Grubbs, B.H.; Jensen, G.S.; Davis, M.E.; Brewster, M.; Janicot, M.; Janssens, B.; Floren, W.; Bakker, A. Targeted delivery of RNA-cleaving DNA enzyme (DNAzyme) to tumor tissue by transferrin-modified, cyclodextrin-based particles. Cancer Biol. Ther 2004, 3, 641–650. [Google Scholar]
- Bartlett, D.W.; Davis, M.E. Impact of tumor-specific targeting and dosing schedule on tumor growth inhibition after intravenous administration of siRNA-containing nanoparticles. Biotechnol. Bioeng 2008, 99, 975–985. [Google Scholar]
- Yang, C.; Wang, X.; Li, H.; Goh, S.H.; Li, J. Synthesis and characterization of polyrotaxanes consisting of cationic alpha-cyclodextrins threaded on poly(ethylene oxide)-ran-(propylene oxide) as gene carriers. Biomacromolecules 2007, 8, 3365–3374. [Google Scholar]
- Zhang, W.; Chen, Z.; Song, X.; Si, J.; Tang, G. Low generation polypropylenimine dendrimer graft β-cyclodextrin: an efficient vector for gene delivery system. Technol. Cancer Res. Treat 2008, 7, 103–108. [Google Scholar]
- Huang, H.; Tang, G.; Wang, Q.; Li, D.; Shen, F.; Zhou, J.; Yu, H. Two novel non-viral gene delivery vectors: low molecular weight polyethylenimine cross-linked by (2-hydroxypropyl)-β-cyclodextrin or (2-hydroxypropyl)-γ-cyclodextrin. Chem. Commun. (Camb.) 2006, 2382–2384. [Google Scholar]
- Tang, G.P.; Guo, H.Y.; Alexis, F.; Wang, X.; Zeng, S.; Lim, T.M.; Ding, J.; Yang, Y.Y.; Wang, S. Low molecular weight polyethylenimines linked by β-cyclodextrin for gene transfer into the nervous system. J. Gene Med 2006, 8, 736–744. [Google Scholar]
- Ooya, T.; Choi, H.S.; Yamashita, A.; Yui, N.; Sugaya, Y.; Kano, A.; Maruyama, A.; Akita, H.; Ito, R.; Kogure, K.; Harashima, H. Biocleavable polyrotaxane-plasmid DNA polyplex for enhanced gene delivery. J. Am. Chem. Soc 2006, 128, 3852–3853. [Google Scholar]
- Yang, C.; Li, H.; Wang, X.; Li, J. Cationic supramolecules consisting of oligoethylenimine-grafted α-cyclodextrins threaded on poly(ethylene oxide) for gene delivery. J. Biomed. Mater. Res. A 2008, 89A, 13–23. [Google Scholar]
- Teijeiro-Osorio, D.; Remunan-Lopez, C.; Alonso, M.J. Chitosan/cyclodextrin nanoparticles can efficiently transfect the airway epithelium in vitro. Eur. J. Pharm. Biopharm 2009, 71, 257–263. [Google Scholar]
- Arima, H.; Kihara, F.; Hirayama, F.; Uekama, K. Enhancement of gene expression by polyamidoamine dendrimer conjugates with α-, β-, and γ-cyclodextrins. Bioconjug. Chem 2001, 12, 476–484. [Google Scholar]
- Kihara, F.; Arima, H.; Tsutsumi, T.; Hirayama, F.; Uekama, K. Effects of structure of polyamidoamine dendrimer on gene transfer efficiency of the dendrimer conjugate with α-cyclodextrin. Bioconjug. Chem 2002, 13, 1211–1219. [Google Scholar]
- Kihara, F.; Arima, H.; Tsutsumi, T.; Hirayama, F.; Uekama, K. In vitro and in vivo gene transfer by an optimized α-cyclodextrin conjugate with polyamidoamine dendrimer. Bioconjug. Chem 2003, 14, 342–350. [Google Scholar]
- Arima, H. Recent findings of dendrimers and their conjugates as non-viral vectors. Recent Res. Devel. Bioconj. Chem 2005, 2, 109–126. [Google Scholar]
- Tsutsumi, T.; Hirayama, F.; Uekama, K.; Arima, H. Evaluation of polyamidoamine dendrimer/α-cyclodextrin conjugate (generation 3, G3) as a novel carrier for small interfering RNA (siRNA). J. Control Release 2007, 119, 349–359. [Google Scholar]
- Tsutsumi, T.; Hirayama, F.; Uekama, K.; Arima, H. Potential use of polyamidoamine dendrimer/α-cyclodextrin conjugate (generation 3, G3) as a novel carrier for short hairpin RNA-expressing plasmid DNA. J. Pharm. Sci 2008, 97, 3022–3034. [Google Scholar]
- Arima, H.; Motoyama, K.; Hirayama, F.; Uekama, K. Recent Findings of Polyamidoamine Dendrimer Conjugates with Cyclodextrins as DNA and Small-interferring RNA Carriers; The Society of Cyclodextrins: Japan, Tokyo, 2008. [Google Scholar]
- Roche, A.C.; Fajac, I.; Grosse, S.; Frison, N.; Rondanino, C.; Mayer, R.; Monsigny, M. Glycofection: facilitated gene transfer by cationic glycopolymers. Cell Mol. Life Sci 2003, 60, 288–297. [Google Scholar]
- Fajac, I.; Briand, P.; Monsigny, M. Gene therapy of cystic fibrosis: the glycofection approach. Glycoconj. J 2001, 18, 723–729. [Google Scholar]
- Monsigny, M.; Rondanino, C.; Duverger, E.; Fajac, I.; Roche, A.C. Glyco-dependent nuclear import of glycoproteins, glycoplexes and glycosylated plasmids. Biochim. Biophys. Acta 2004, 1673, 94–103. [Google Scholar]
- Diebold, S.S.; Kursa, M.; Wagner, E.; Cotten, M.; Zenke, M. Mannose polyethylenimine conjugates for targeted DNA delivery into dendritic cells. J. Biol. Chem 1999, 274, 19087–19094. [Google Scholar]
- Zanta, M.A.; Boussif, O.; Adib, A.; Behr, J.P. In vitro gene delivery to hepatocytes with galactosylated polyethylenimine. Bioconjug. Chem 1997, 8, 839–844. [Google Scholar]
- Arima, H.; Chihara, Y.; Arizono, M.; Yamashita, S.; Wada, K.; Hirayama, F.; Uekama, K. Enhancement of gene transfer activity mediated by mannosylated dendrimer/α-cyclodextrin conjugate (generation 3, G3). J. Control Release 2006, 116, 64–74. [Google Scholar]
- Wada, K.; Arima, H.; Tsutsumi, T.; Hirayama, F.; Uekama, K. Enhancing effects of galactosylated dendrimer/α-cyclodextrin conjugates on gene transfer efficiency. Biol. Pharm. Bull 2005, 28, 500–505. [Google Scholar]
- Wada, K.; Arima, H.; Tsutsumi, T.; Chihara, Y.; Hattori, K.; Hirayama, F.; Uekama, K. Improvement of gene delivery mediated by mannosylated dendrimer/α-cyclodextrin conjugates. J. Control Release 2005, 104, 397–413. [Google Scholar]
- Jackman, A.L.; Theti, D.S.; Gibbs, D.D. Antifolates targeted specifically to the folate receptor. Adv. Drug Deliv. Rev 2004, 56, 1111–1125. [Google Scholar]
- Konda, S.D.; Aref, M.; Wang, S.; Brechbiel, M.; Wiener, E.C. Specific targeting of folate-dendrimer MRI contrast agents to the high affinity folate receptor expressed in ovarian tumor xenografts. Magma 2001, 12, 104–113. [Google Scholar]
- Shukla, S.; Wu, G.; Chatterjee, M.; Yang, W.; Sekido, M.; Diop, L.A.; Muller, R.; Sudimack, J.J.; Lee, R.J.; Barth, R.F.; Tjarks, W. Synthesis and biological evaluation of folate receptor-targeted boronated PAMAM dendrimers as potential agents for neutron capture therapy. Bioconjug. Chem 2003, 14, 158–167. [Google Scholar]
- Singh, P.; Gupta, U.; Asthana, A.; Jain, N.K. Folate and Folate-PEG-PAMAM dendrimers: synthesis, characterization, and targeted anticancer drug delivery potential in tumor bearing mice. Bioconjug. Chem 2008, 19, 2239–2252. [Google Scholar]





© 2009 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Arima, H.; Motoyama, K. Recent Findings Concerning PAMAM Dendrimer Conjugates with Cyclodextrins as Carriers of DNA and RNA. Sensors 2009, 9, 6346-6361. https://doi.org/10.3390/s90806346
Arima H, Motoyama K. Recent Findings Concerning PAMAM Dendrimer Conjugates with Cyclodextrins as Carriers of DNA and RNA. Sensors. 2009; 9(8):6346-6361. https://doi.org/10.3390/s90806346
Chicago/Turabian StyleArima, Hidetoshi, and Keiichi Motoyama. 2009. "Recent Findings Concerning PAMAM Dendrimer Conjugates with Cyclodextrins as Carriers of DNA and RNA" Sensors 9, no. 8: 6346-6361. https://doi.org/10.3390/s90806346
APA StyleArima, H., & Motoyama, K. (2009). Recent Findings Concerning PAMAM Dendrimer Conjugates with Cyclodextrins as Carriers of DNA and RNA. Sensors, 9(8), 6346-6361. https://doi.org/10.3390/s90806346