Amperometric Hydrogen Peroxide Biosensor Based on Immobilization of Hemoglobin on a Glassy Carbon Electrode Modified with Fe3O4/Chitosan Core-Shell Microspheres
Abstract
:1. Introduction
2. Experimental Section
2.1. Apparatus
2.2. Materials
2.3. Synthesis of Fe3O4 Nanoparticles [37]
2.4. Preparation of Magnetic Microspheres (Fe3O4/CS) [31]
2.5. Configuration of Fe3O4/CS-Hb-Fe3O4/CS-GCE
3. Results and Discussion
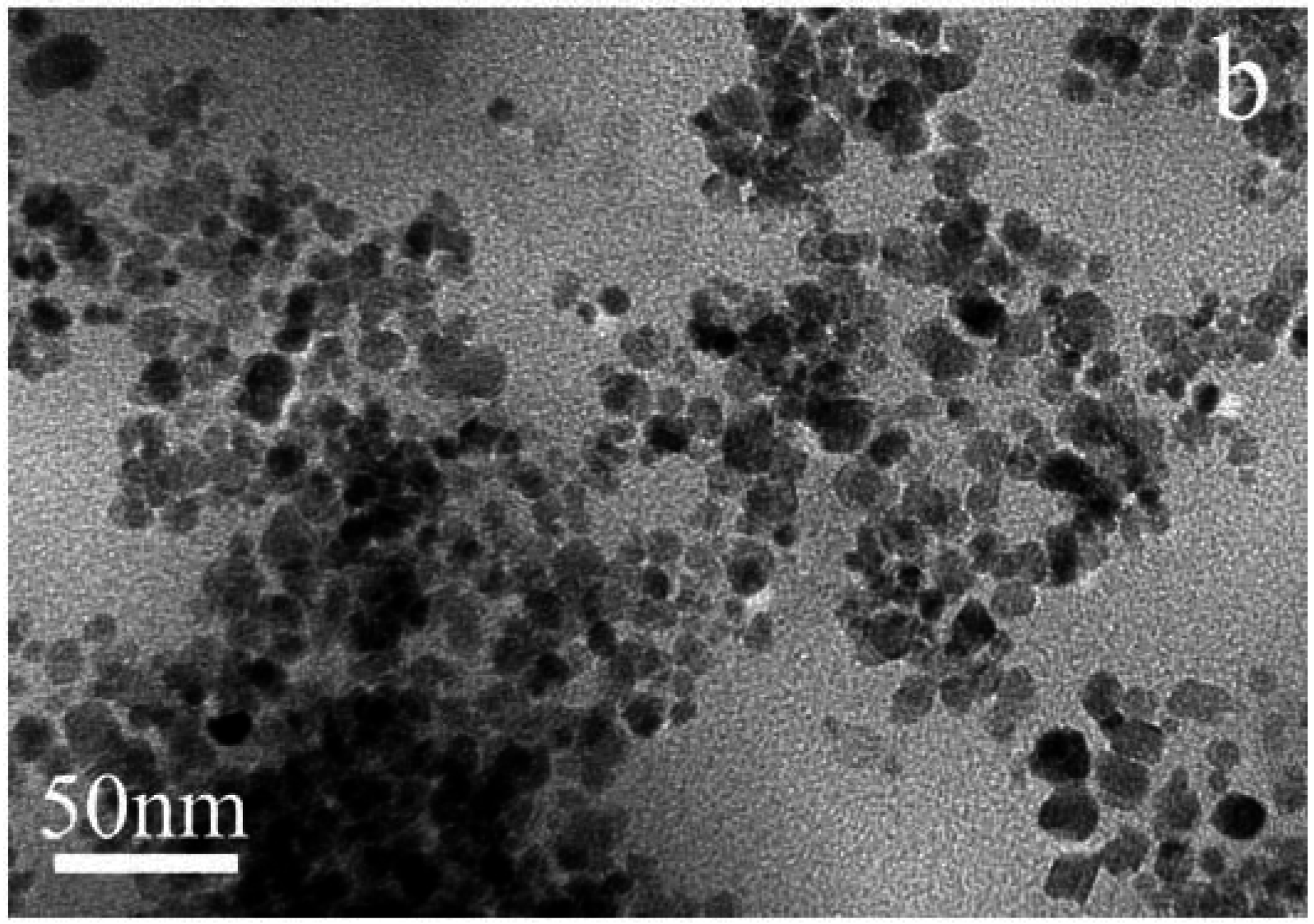
3.1. TEM Characterization of Fe3O4 Nanoparticles and Magnetic Microspheres
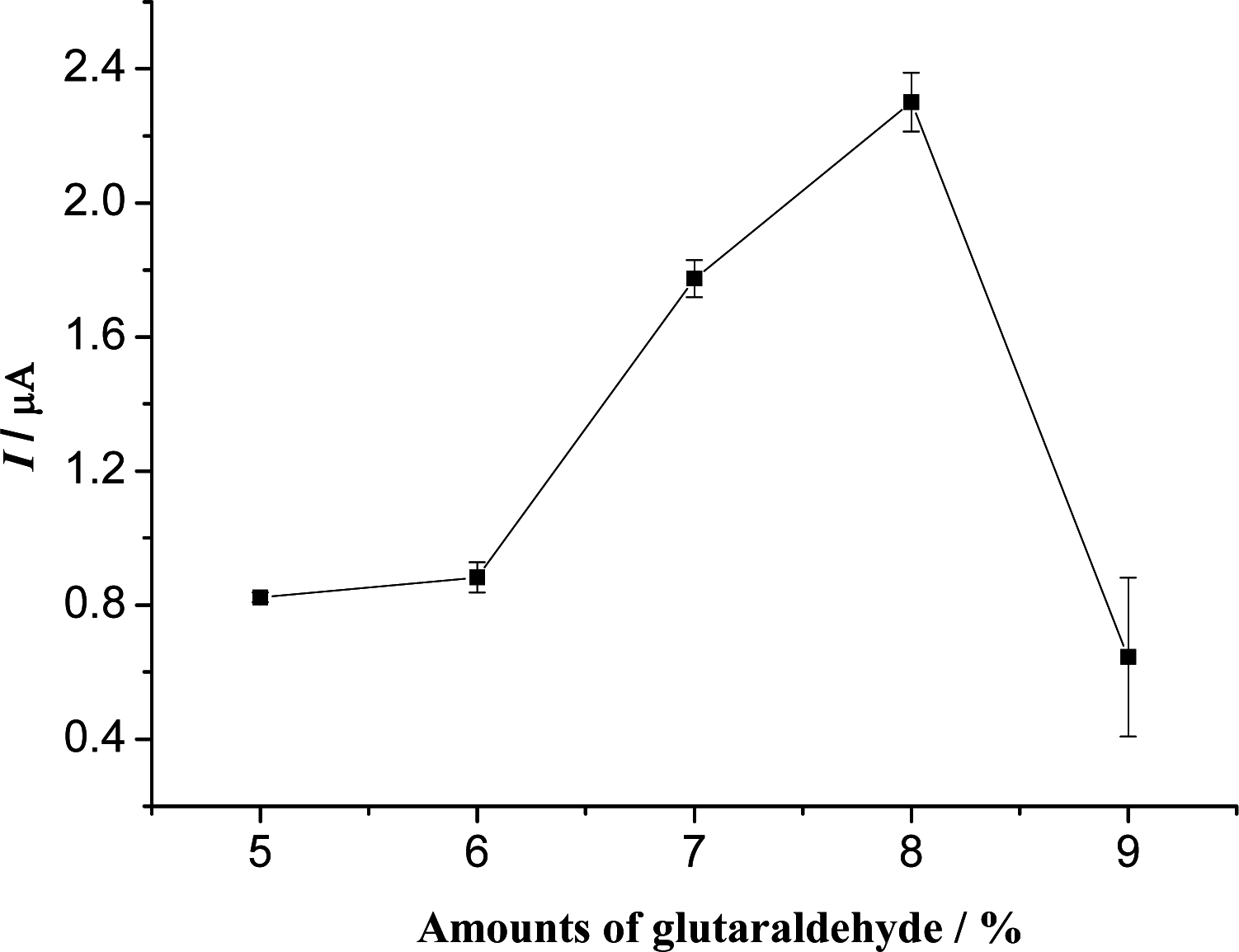
3.2. Optimization of Experimental Variables
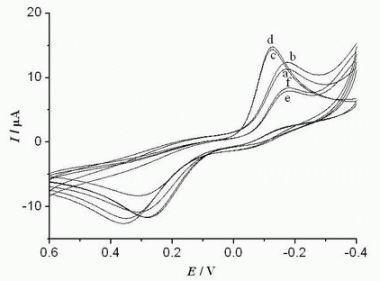
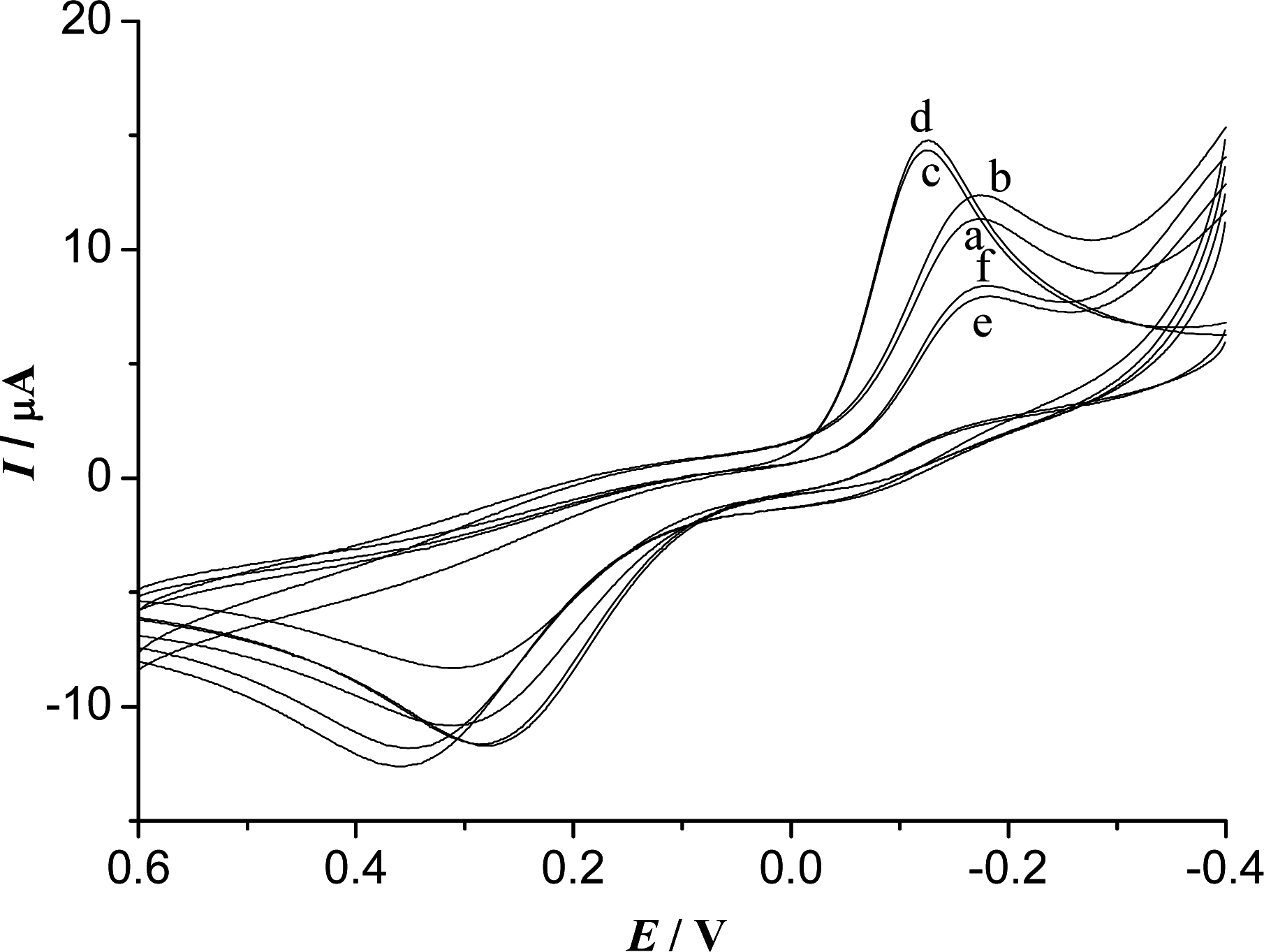
3.3. Electrochemical Characteristics of the Biosensor
3.4. Electrochemical Impedance Spectroscopy Characterization
3.5. Amperometric Response of the H2O2 Biosensor
3.6. Interference and Selectivity
3.7. Biosensor Repeatability and Stability
3.8. Analytical Application of the Biosensor
4. Conclusions
Acknowledgments
References and Notes
- Forzani, E.S.; Rivas, G.A.; Solis, V.M. Amperometric determination of dopamine on an enzymatically modified carbon paste electrode. J. Electroanal. Chem 1995, 382, 33–40. [Google Scholar]
- Kulys, J.; Gorton, L.; Domingues, E.; Emnéus, J.; Jarskog, H. Electrochemical characterization of carbon pastes modified with proteins and polycations. J. Electroanal. Chem 1994, 372, 49–55. [Google Scholar]
- Kulys, J.; Wang, L.Z.; Maksimoviene, A. L-Lactate oxidase electrode based on methylene green and carbon paste. Anal. Chim. Acta 1993, 274, 53–58. [Google Scholar]
- Lei, C.X.; Hu, S.Q.; Shen, G.L.; Yu, R.Q. Immobilization of horseradish peroxidase to a nano-Au monolayer modified chitosan-entrapped carbon paste electrode for the detection of hydrogen peroxide. Talanta 2003, 59, 981–988. [Google Scholar]
- Tripathi, V.S.; Kandimalla, V.B.; Ju, H.X. Amperometric biosensor for hydrogen peroxide based on ferrocene-bovine serum albumin and multiwall carbon nanotube modified ormosil composite. Biosens. Bioelectron 2006, 21, 1529–1535. [Google Scholar]
- Zhang, Z.; Chouchane, S.; Magliozzo, R.S.; Rusting, J.F. Direct voltammetry and catalysis with mycobacterium tuberculosis catalase-peroxidase, peroxidases and catalase in lipid films. Anal. Chem 2002, 74, 163–170. [Google Scholar]
- Wade, R.S.; Castro, C.E. Oxidation of heme proteins by alkyl halides. J. Am. Chem. Soc 1973, 95, 231–234. [Google Scholar]
- Schlereth, D.; Mantele, W. Redox-Induced conformational changes in myoglobin and hemoglobin: Electrochemistry and ultraviolet-visible and Fourier transform infrared difference spectroscopy at surface-modified gold electrodes in an ultra-Thin-Layer spectroelectrochemical cell. Biochemistry 1992, 31, 7494–7502. [Google Scholar]
- Ye, J.; Baldwin, R.P. Catalytic reduction of myoglobin and hemoglobin at chemically modified electrodes containing methylene blue. Anal. Chem 1988, 60, 2263–2268. [Google Scholar]
- Reed, D.E.; Hawkridge, F.M. Direct electron transfer reactions of cytochrome C at silver electrodes. Anal. Chem 1987, 59, 2334–2339. [Google Scholar]
- Fan, C.H.; Wang, H.Y.; Sun, S.; Zhu, D.X.; Wagner, G.; Li, G.X. Electron-transfer reactivity and enzymatic activity of hemoglobin in a SP sephadex membrane. Anal. Chem 2001, 73, 2850–2854. [Google Scholar]
- Sun, H.; Hu, N.F.; Ma, H.Y. Direct electrochemistry of hemoglobin in polyacrylamide hydrogel films on pyrolytic graphite electrodes. Electroanalysis 2000, 12, 1064–1070. [Google Scholar]
- Liu, S.Q.; Dai, Z.H.; Chen, H.Y.; Ju, H.X. Immobilization of hemoglobin on zirconium dioxide nanoparticles for preparation of a novel hydrogen peroxide biosensor. Biosens. Bioelectron 2004, 19, 963–969. [Google Scholar]
- Li, Q.W.; Luo, G.A.; Feng, J. Direct electron transfer for heme proteins assembled on nanocrystalline TiO2 film. Electroanalysis 2001, 13, 359–363. [Google Scholar]
- Kafi, A.K.M.; Lee, D.Y.; Park, S.H.; Kwon, Y.S. A hydrogen peroxide biosensor based on peroxidase activity of hemoglobin in polymeric film. J. Nanosci. Nanotechnol 2007, 7, 4005–4008. [Google Scholar]
- Lu, Z.Q.; Huang, Q.D.; Rusling, J.F. Films of hemoglobin and didodecyldimethylammonium bromide with enhanced electron transfer rates. J. Electroanal. Chem 1997, 423, 59–66. [Google Scholar]
- Kumar, S.A.; Lo, P.H.; Chen, S.M. Electrochemical analysis of H2O2 and nitrite using copper nanoparticles/poly(o-phenylenediamine) film modified glassy carbon electrode. J. Electrochem. Soc 2009, 156, E118–E123. [Google Scholar]
- Lo, P.H.; Kumar, S.A.; Chen, S.M. Amperometric determination of H2O2 at nano-TiO2/DNA/thionin nanocomposite modified electrode. Colloids Surf. B 2008, 66, 266–273. [Google Scholar]
- Kumar, S.A.; Lo, P.H.; Chen, S.M. Electrochemical synthesis and characterization of TiO2 nanoparticles and their use as a platform for flavin adenine dinucleotide immobilization and efficient electrocatalysis. Nanotechnology 2008, 19, 255501:1–255501:7. [Google Scholar]
- Vázquez, M.; Luna, C.; Morales, M.P.; Sanz, R.; Serna, C.J.; Mijangos, C. Magnetic nanoparticles: synthesis, ordering and properties. Phys. B 2004, 354, 71–79. [Google Scholar]
- Lu, A.H.; Salabas, E.L.; Schüth, F. Magnetic nanoparticles: synthesis protection, functionalization and application. Angew. Chem. Int. Ed 2007, 46, 1222–1244. [Google Scholar]
- Osaka, T.; Matsunaga, T.; Nakanishi, T.; Arakaki, A.; Niwa, D.; Iida, H. Synthesis of magnetic nanoparticles and their application to bioassays. Anal. Bioanal. Chem 2006, 384, 593–600. [Google Scholar]
- Pankhurst, Q.A.; Connolly, J.; Jones, S.K.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D: Appl. Phys 2003, 36, R167–R181. [Google Scholar]
- Koneraacká, M.; Kopcansky, P.; Antalik, M.; Timko, M.; Ramchand, C.N.; Lobo, D.; Mehta, R.V.; Upadhyay, R.V. Immobilization of proteins and enzymes to fine magnetic particles. J. Magn. Magn. Mater 1999, 201, 427–430. [Google Scholar]
- Chan, H.T.; Do, Y.Y.; Huang, P.L.; Chien, P.L.; Chan, T.S.; Liu, R.S.; Huang, C.Y.; Yang, S.Y.; Horng, H.E. Preparation and properties of bio-compatible magnetic Fe3O4 nanoparticles. Magn. Magn. Mater 2006, 304, 415–417. [Google Scholar]
- Gong, J.M.; Lin, X.Q. Facilitated electron transfer of hemoglobin embedded in nanosized Fe3O4 matrix based on paraffin impregnated graphite electrode and electrochemical catalysis for trichloroacetic acid. Microchem. J 2003, 75, 51–57. [Google Scholar]
- Fan, A.P.; Lau, C.W.; Lu, J.Z. Magnetic based-based chemiluminescent metal immunoassay with a colloidal gold label. Anal. Chem 2005, 77, 3238–3242. [Google Scholar]
- Kouassi, G.K.; Irudayaraj, J. Magnetic and gold-coated magnetic nanoparticles as a DNA sensor. Anal. Chem 2006, 78, 3234–3241. [Google Scholar]
- Cho, Y.J.; Song, H.; Lee, K.; Kim, K.; Kwak, K.; Kim, S.; Park, J.T. The first observation of four-electron reduction in [60]fullerene-metal cluster self-assembled monolayers(SAMs). Chem. Commun 2002, 2, 2966–2967. [Google Scholar]
- Qiu, J.D.; Peng, H.P.; Liang, R.P. Ferrocene-modified Fe3O4@SiO2 magnetic nanoparticles as building block for construction of reagentless enzyme-based biosensosr. Electrochem. Commun 2007, 9, 2734–2738. [Google Scholar]
- Lai, G.S.; Zhang, H.L.; Han, D.Y. A novel hydrogen peroxide biosensor based on hemoglobin immobilized on magnetic chitosan microspheres modified electrode. Sens. Actuat. B 2008, 129, 497–503. [Google Scholar]
- Lu, G.H.; Yao, X.; Wu, X.G.; Zhang, T. Determination of the total iron by chitosan-modified glassy carbon electrode. Microchem. J 2001, 69, 81–87. [Google Scholar]
- Ye, X.Z.; Yang, Q.H.; Wang, Y.; Li, N.Q. Electrochemical behaviour of gold, sliver, platinum and palladium on the glass carbon electrode modified by chitosan and its application. Talanta 1998, 47, 1099–1106. [Google Scholar]
- Wang, G.; Xu, J.J.; Chen, H.Y.; Lu, Z.H. Amperometric hydrogen peroxide biosensor with sol-gel/chitosan network-like film as immobilization matrix. Biosens. Bioelectron 2003, 18, 335–343. [Google Scholar]
- Tan, X.C.; Tian, Y.X.; Cai, P.X.; Zou, X.Y. Glucose biosensor based on glucose oxidase immobilized in sol-gel chitosan/silica hybrid composite film on Prussian blue modified glass carbon electrode. Anal. Bioanal. Chem 2005, 381, 500–507. [Google Scholar]
- Tan, X.C.; Li, M.J.; Cai, P.X.; Luo, L.J.; Zou, X.Y. An amperometric cholesterol biosensor based on multiwalled carbon nanotubes and organically modified sol-gel/chitosan hybrid composite film. Anal. Biochem 2005, 337, 111–120. [Google Scholar]
- Reddy, K.R.; Lee, K.P.; Gopalan, A.I. Self-assembly approach for the synthesis of electro-magnetic functionalized Fe3O4/polyaniline nanocomposites: Effect of dopant on the properties. Colloids Surf. A 2008, 320, 49–56. [Google Scholar]
- Xiao, Y.; Ju, H.X.; Chen, H.Y. Hydrogen peroxide sensor based on horseradish peroxidase-labeled Au colloids immobilized on gold electrode surface by cysteamine monolayer. Anal. Chim. Acta 1999, 391, 73–82. [Google Scholar]
- Zhang, H.L.; Zou, X.Z.; Han, D.Y. Hydrogen peroxide sensor based on hemoglobin immobilized on glassy carbon electrode with SiO2 nanoparticles/Chitosan film as immobilization matrix. Anal. Lett 2007, 40, 661–676. [Google Scholar]
- Kharitonov, A.B.; Alfonta, L.; Katz, E.; Willner, I. Probing of bioaffinity interactions at interfaces using impedance spectroscopy and chronopotentiometry. J. Electroanal. Chem 2000, 487, 133–141. [Google Scholar]
- Xiong, H.Y.; Chen, T.; Zhang, X.H.; Wang, S.F. High performance and stability of a hemoglobin-biosensor based on an ionic liquid as nonaqueous media for hydrogen peroxide monitoring. Electrochem. Commun 2007, 9, 2671–2675. [Google Scholar]
- Kamin, R.A.; Willson, G.S. Rotating ring-disk enzyme electrode for biocatalysis kinetic studies and characterization of the immobilized enzyme layer. Anal. Chem 1980, 52, 1198–1205. [Google Scholar]
- Shan, D.; Wang, S.X.; Xue, H.G.; Cosnier, S. Direct electrochemistry and electrocatalysis of hemoglobin entrapped in composite matrix based on chitosan and CaCO3 nanoparticles. Electrochem. Commun 2007, 9, 529–534. [Google Scholar]
- Wang, Q.L.; Lu, G.X.; Yang, B.J. Direct electrochemistry and electrocatalysis of hemoglobin immobilized on carbon paste electrode by silica sol–gel film. Biosens. Bioelectron 2004, 19, 1269–1275. [Google Scholar]
- Lei, C.X.; Hu, S.Q.; Gao, N.; Shen, G.L.; Yu, R.Q. An amperometric hydrogen peroxide biosensor based on immobilizing horseradish peroxidase to a nano-Au monolayer supported by sol–gel derived carbon ceramic electrode. Bioelectrochemistry 2004, 65, 33–39. [Google Scholar]






| Possible interferences | Current ratioa |
|---|---|
| Glucose | 1.02 |
| l-Cystine | 0.99 |
| l-Lysine | 1.01 |
| l-Glycine | 1.01 |
| Uric acid | 1.06 |
| Ascorbic acid | 0.37 |
| Sample number | Added H2O2 (mM) | Founda H2O2 by the present biosensor (mM) | Recovery by the resent biosensor (%) | Founda H2O2 by the titration method (mM) | Recovery by the titration method (%) |
|---|---|---|---|---|---|
| 1 | 0.2 | 0.846 | 100.5 | 0.837 | 96.0 |
| 2 | 0.6 | 1.291 | 107.7 | 1.283 | 106.3 |
| 3 | 1.2 | 1.822 | 98.1 | 1.850 | 100.4 |
© 2009 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tan, X.-C.; Zhang, J.-L.; Tan, S.-W.; Zhao, D.-D.; Huang, Z.-W.; Mi, Y.; Huang, Z.-Y. Amperometric Hydrogen Peroxide Biosensor Based on Immobilization of Hemoglobin on a Glassy Carbon Electrode Modified with Fe3O4/Chitosan Core-Shell Microspheres. Sensors 2009, 9, 6185-6199. https://doi.org/10.3390/s90806185
Tan X-C, Zhang J-L, Tan S-W, Zhao D-D, Huang Z-W, Mi Y, Huang Z-Y. Amperometric Hydrogen Peroxide Biosensor Based on Immobilization of Hemoglobin on a Glassy Carbon Electrode Modified with Fe3O4/Chitosan Core-Shell Microspheres. Sensors. 2009; 9(8):6185-6199. https://doi.org/10.3390/s90806185
Chicago/Turabian StyleTan, Xue-Cai, Jin-Lei Zhang, Sheng-Wei Tan, Dan-Dan Zhao, Zen-Wei Huang, Yan Mi, and Zai-Yin Huang. 2009. "Amperometric Hydrogen Peroxide Biosensor Based on Immobilization of Hemoglobin on a Glassy Carbon Electrode Modified with Fe3O4/Chitosan Core-Shell Microspheres" Sensors 9, no. 8: 6185-6199. https://doi.org/10.3390/s90806185
APA StyleTan, X.-C., Zhang, J.-L., Tan, S.-W., Zhao, D.-D., Huang, Z.-W., Mi, Y., & Huang, Z.-Y. (2009). Amperometric Hydrogen Peroxide Biosensor Based on Immobilization of Hemoglobin on a Glassy Carbon Electrode Modified with Fe3O4/Chitosan Core-Shell Microspheres. Sensors, 9(8), 6185-6199. https://doi.org/10.3390/s90806185