Enhanced Photoelectrochemical Detection of Bioaffinity Reactions by Vertically Oriented Au Nanobranches Complexed with a Biotinylated Polythiophene Derivative
Abstract
:1. Introduction
2. Results and Discussion
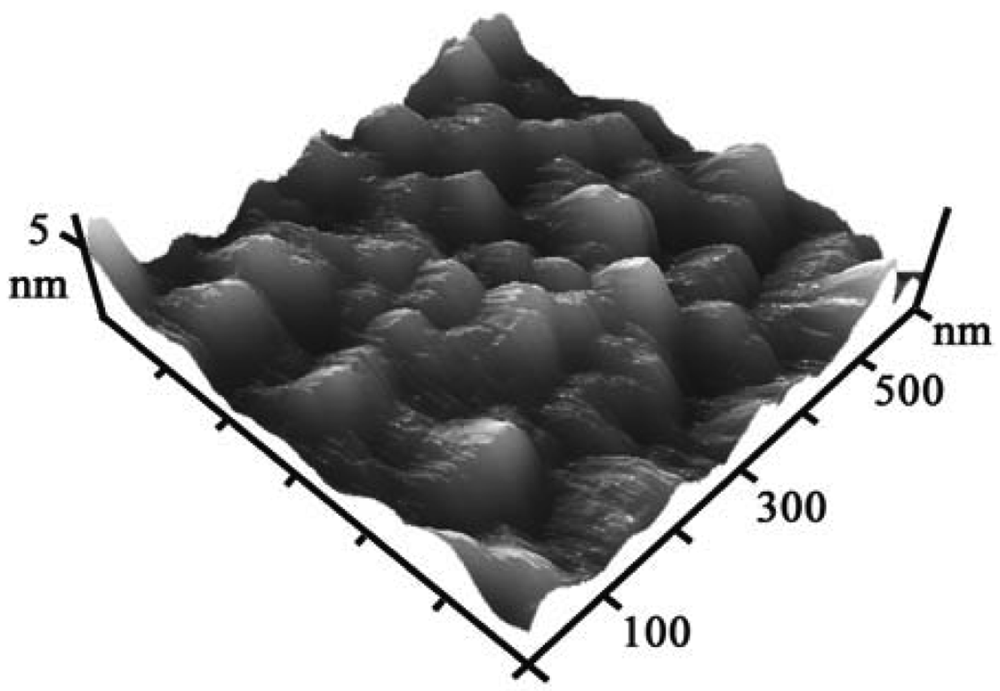
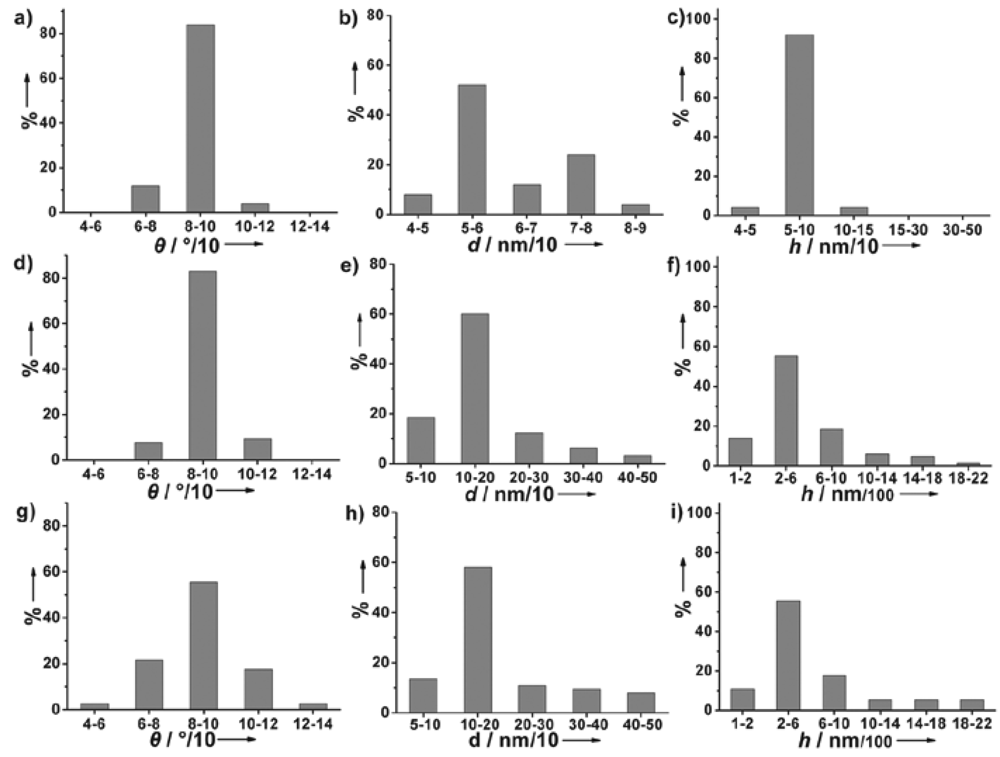
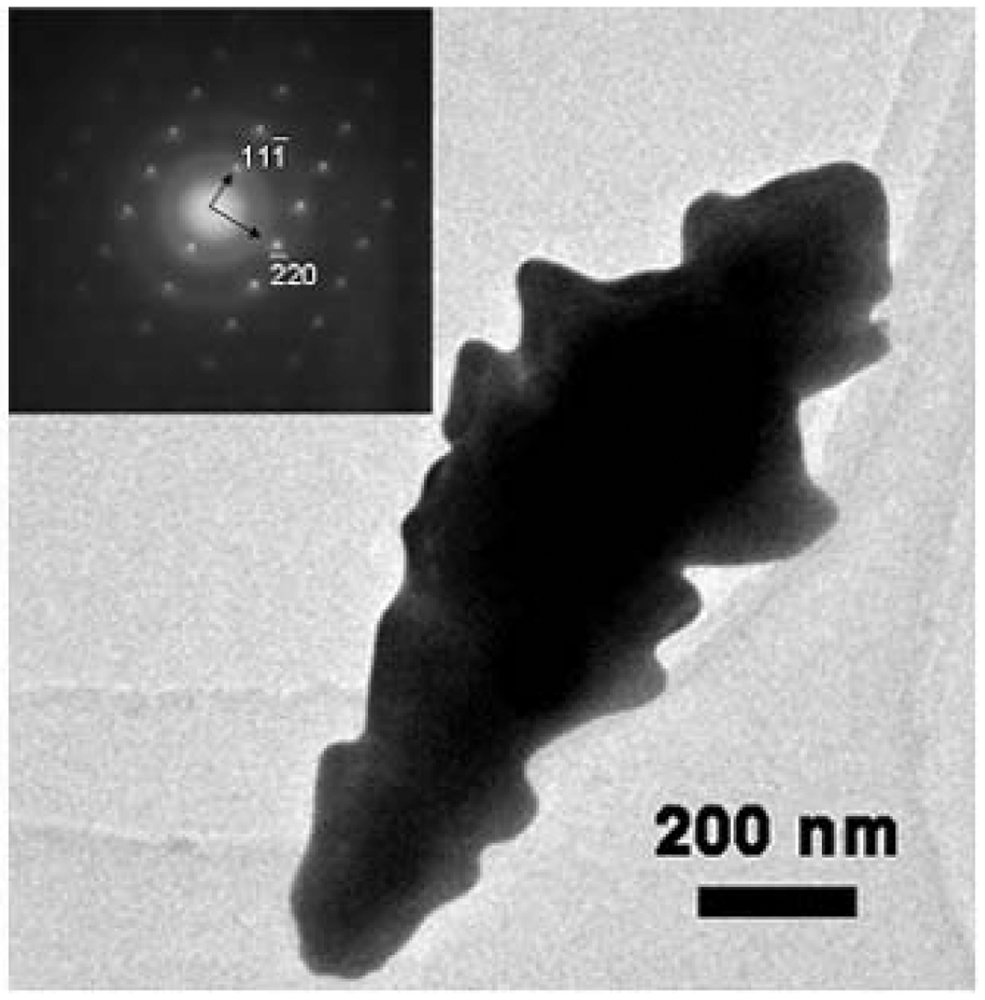
2.1. Characterization of nanostructured Au electrodes
2.2. Photocurrent Enhancement
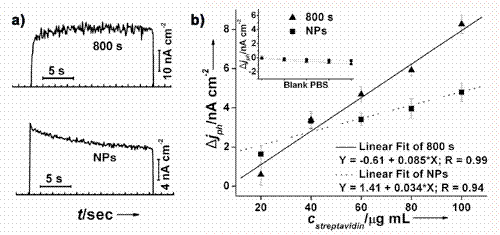
2.3. Photoelectrochemical detection of bioaffinity reaction
3. Experimental Section
3.1. Synthesis of the PTBL polymer
3-(4′-Biotinbutylacylamine)ethylthiophene(3)
Polythiophene copolymer containing biotin and lipid moieties (PTBL, 5)
3.2. Characterization of nanostructured Au electrodes
3.3. Characterization of nanostructured Au electrodes
3.4. Characterization of nanostructured Au electrodes
4. Conclusions
Acknowledgments
References and Notes
- Grätzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar]
- Hayden, O.; Agarwal, R.; Lieber, C.M. Nanoscale avalanche photodiodes for highly sensitive and spatially resolved photon detection. Nat. Mater. 2006, 5, 352–356. [Google Scholar]
- Afzaal, M.; O'Brien, P. Recent developments in II–VI and III–VI semiconductors and their applications in solar cells. J. Mater. Chem. 2006, 16, 1597–1602. [Google Scholar]
- O'Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar]
- Huynh, W.U.; Dittmer, J.J.; Alivisatos, A.P. Hybrid nanorod-polymer solar cells. Science 2002, 295, 2425–2427. [Google Scholar]
- Shipway, A.N.; Katz, E; Willner, I. Nanoparticle arrays on surfaces for electronic, optical, and sensor applications. ChemPhysChem 2000, 1, 18–52. [Google Scholar]
- Katz, E.; Willner, I. Integrated nanoparticle-biomolecule hybrid systems: Synthesis, properties, and applications. Angew. Chem. Int. Ed. 2004, 43, 6042–6108. [Google Scholar]
- Adachi, M.; Murata, Y.; Takao, J.; Jiu, J.; Sakamoto, M.; Wang, F. Highly efficient dye-sensitized solar cells with a titania thin-film electrode composed of a network structure of single-crystal-like TiO2 nanowires made by the “oriented attachment” mechanism. J. Am. Chem. Soc. 2004, 126, 14943–14949. [Google Scholar]
- Liu, D.; Kamat, P. Photoelectrochemical behavior of thin CdSe and coupled TiO2/CdSe semiconductor films. J. Phys. Chem. 1993, 97, 10769–10773. [Google Scholar]
- Robel, I.; Subramanian, V.; Kuno, M.; Kamat, P.V. Quantum dot solar cells. Harvesting light energy with CdSe nanocrystals molecularly linked to mesoscopic TiO2 films. J. Am. Chem. Soc. 2006, 128, 2385–2393. [Google Scholar]
- Yu, P.; Zhu, K.; Norman, A.G.; Ferrere, S.; Frank, A.J.; Nozik, A.J. Nanocrystalline TiO2 solar cells sensitized with InAs quantum dots. J. Phys. Chem. B 2006, 110, 25451–25454. [Google Scholar]
- Vogel, R.; Pohl, K.; Weller, H. Sensitization of highly porous, polycrystalline TiO2 electrodes by quantum sized CdS. Chem. Phys. Lett. 1990, 174, 241–246. [Google Scholar]
- Papageorgiou, N.; Barbé, C.; Grätzel, M. Morphology and adsorbate dependence of ionic transport in dye sensitized mesoporous TiO2 films. J. Phys. Chem. B 1998, 102, 4156–4164. [Google Scholar]
- Hore, S.; Nitz, P.; Vetter, C.; Prahl, C.; Niggemann, M.; Kern, R. Scattering spherical voids in nanocrystalline TiO2-enhancement of efficiency in dye-sensitized solar cells. Chem. Comm. 2005, 15, 2011–2013. [Google Scholar]
- Heng, L.; Zhai, J.; Zhao, Y.; Xu, J.; Sheng, X.; Jiang, L. Enhancement of photocurrent generation by honeycomb structures in organic thin films. ChemPhysChem 2006, 7, 2520–2525. [Google Scholar]
- Zhao, Y.; Sheng, X.; Zhai, J.; Jiang, L.; Yang, C.; Sun, Z.; Li, Y.; Zhu, D. TiO2 porous electrodes with hierarchical branched inner channels for charge transport in viscous electrolytes. ChemPhysChem 2007, 8, 856–861. [Google Scholar]
- Zhao, Y.; Zhai, J.; Tan, S.; Wang, L.; Jiang, L.; Zhu, D. TiO2 micro/nano-composite structured electrodes for quasi-solid-state dye-sensitized solar cells. Nanotechnology 2006, 17, 2090–2097. [Google Scholar]
- Leschkies, K.S.; Divakar, R.; Basu, J.; Enache-Pommer, E.; Boercher, J.E.; Carter, C.B.; Kortshagen, U.R.; Norris, D.J.; Aydil, E.S. Photosensitization of ZnO nanowires with CdSe quantum dots for photovoltaic devices. Nano Lett. 2007, 7, 1793–1798. [Google Scholar]
- Law, M.; Greene, L.E.; Johnson, J.C.; Saykally, R.; Yang, P. Nanowire dye-sensitized solar cells. Nat. Mater. 2005, 4, 455–459. [Google Scholar]
- Liang, Y.; Zhen, C.; Zou, D.; Xu, D. Preparation of free-standing nanowire arrays on conductive substrates. J. Am. Chem. Soc. 2004, 126, 16338–16339. [Google Scholar]
- Jeong-Mi, M.; Wei, A. Uniform gold nanorod arrays from polyethylenimine-coated alumina templates. J. Phys. Chem. B 2005, 109, 23336–23341. [Google Scholar]
- Lassalle, N.; Vieil, E.; Correia, J.P.; Abrantes, L.M. Study of DNA hybridization on polypyrrole grafted with oligonucleotides by photocurrent spectroscopy. Biosensors Bioelectron. 2001, 16, 295–303. [Google Scholar]
- Haddour, N.; Chauvin, J.; Gondran, C.; Cosnier, S. Photoelectrochemical immunosensor for label-free detection and quantification of anti-cholera toxin antibody. J. Am. Chem. Soc. 2006, 128, 9693–9698. [Google Scholar]
- Liang, M.; Liu, S.; Wei, M.; Guo, L. Photoelectrochemical oxidation of DNA by ruthenium tris(bipyridine) on a tin oxide nanoparticle electrode. Anal. Chem. 2006, 78, 621–623. [Google Scholar]
- Liang, M.; Jia, S.; Zhu, S.; Guo, L. Photoelectrochemical sensor for the rapid detection of in situ DNA damage induced by enzyme-catalyzed fenton reaction. Environ. Sci. Technol. 2008, 42, 635–639. [Google Scholar]
- Dong, D.; Zheng, D.; Wang, F.; Yang, X.; Wang, N.; Li, Y.; Guo, L.; Cheng, J. Quantitative photoelectrochemical detection of biological affinity reaction: biotin-avidin interaction. Anal. Chem. 2004, 76, 499–501. [Google Scholar]
- Pardo-Yissar, V.; Katz, E.; Wasserman, J.; Willner, I. Acetylcholine esterase-labeled CdS nanoparticles on electrodes: Photoelectrochemical sensing of the enzyme inhibitors. J. Am. Chem. Soc. 2003, 125, 622–623. [Google Scholar]
- Freeman, R.; Gill, R.; Beissenhirtz, M.; Willner, I. Self-assembly of semiconductor quantum-dots on electrodes for photoelectrochemical biosensing. Photochem. Photobiol. Sci. 2007, 6, 416–422. [Google Scholar]
- Stoll, C.; Gehring, C.; Schubert, K.; Zanella, M.; Parak, W.J.; Lisdat, F. Photoelectrochemical signal chain based on quantum dots on gold-Sensitive to superoxide radicals in solution. Biosensors Bioelectron. 2008, 24, 260–265. [Google Scholar]
- Lu, W.; Jin, Y.; Wang, G.; Chen, D.; Li, J. Enhanced photoelectrochemical method for linear DNA hybridization detection using Au-nanopaticle labeled DNA as probe onto titanium dioxide electrode. Biosensors Bioelectron. 2008, 23, 1534–1539. [Google Scholar]
- de la Garza, L; Saponjic, Z.V.; Rajh, T; Dimitrijevic, N.M. Photoelectroactivity of a hybrid system constructed by immobilization of avidin onto biotinylated TiO2 electrodes. Chem. Mater. 2006, 18, 2682–2688. [Google Scholar]
- Wang, G.; Wang, Q.; Lu, W.; Li, J. Photoelectrochemical study on charge transfer properties of TiO2-B nanowires with an application as humidity sensors. J. Phys. Chem. B 2006, 110, 22029–22034. [Google Scholar]
- Yu, X.; Wang, Z.Q.; Jiang, Y.G.; Zhang, X. Surface gradient material: from superhydrophobicity to superhydrophilicity. Langmuir 2006, 22, 4483–4486. [Google Scholar]
- Walsh, F.C.; Herron, M.E. Electrocrystallization and electrochemical control of crystal-growth-fundamental considereations and electroderations and electrodeposition of metals. J. Phys. D: Appl. Phys. 1991, 24, 217–225. [Google Scholar]
- Grier, D.; Ben-Jacob, E.; Clarke, R.; Sander, L.M. Morphology and microstructure in electrochemical deposition of zinc. Phys. Rev. Lett. 1986, 56, 1264–1267. [Google Scholar]
- Sirringhaus, H.; Tessler, N.; Friend, R.H. Integrated optoelectronic devices based on conjugated polymers. Science 1998, 280, 1741–1744. [Google Scholar]
- Bulović, V.; Forrest, S.R. Study of localized and extended excitons in 3,4,9,10-perylenetetracarboxylic dianhydride (PTCDA) II. Photocurrent response at low electric fields. Chem. Phys. 1996, 210, 13–25. [Google Scholar]
- Onoda, M.; Tada, K. Photovoltaic effects of p–n heterojunction device. Curr. Appl. Phys. 2003, 3, 141–147. [Google Scholar]








© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhou, H.; Tang, Y.; Zhai, J.; Wang, S.; Tang, Z.; Jiang, L. Enhanced Photoelectrochemical Detection of Bioaffinity Reactions by Vertically Oriented Au Nanobranches Complexed with a Biotinylated Polythiophene Derivative. Sensors 2009, 9, 1094-1107. https://doi.org/10.3390/s90201094
Zhou H, Tang Y, Zhai J, Wang S, Tang Z, Jiang L. Enhanced Photoelectrochemical Detection of Bioaffinity Reactions by Vertically Oriented Au Nanobranches Complexed with a Biotinylated Polythiophene Derivative. Sensors. 2009; 9(2):1094-1107. https://doi.org/10.3390/s90201094
Chicago/Turabian StyleZhou, Huiqiong, Yanli Tang, Jin Zhai, Shu Wang, Zhiyong Tang, and Lei Jiang. 2009. "Enhanced Photoelectrochemical Detection of Bioaffinity Reactions by Vertically Oriented Au Nanobranches Complexed with a Biotinylated Polythiophene Derivative" Sensors 9, no. 2: 1094-1107. https://doi.org/10.3390/s90201094
APA StyleZhou, H., Tang, Y., Zhai, J., Wang, S., Tang, Z., & Jiang, L. (2009). Enhanced Photoelectrochemical Detection of Bioaffinity Reactions by Vertically Oriented Au Nanobranches Complexed with a Biotinylated Polythiophene Derivative. Sensors, 9(2), 1094-1107. https://doi.org/10.3390/s90201094