A Portable Luminometer with a Disposable Electrochemiluminescent Biosensor for Lactate Determination
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Materials
2.2. Apparatus and Software
2.3. Disposable Biosensor Preparation
2.4. Procedure for Lactate Determination
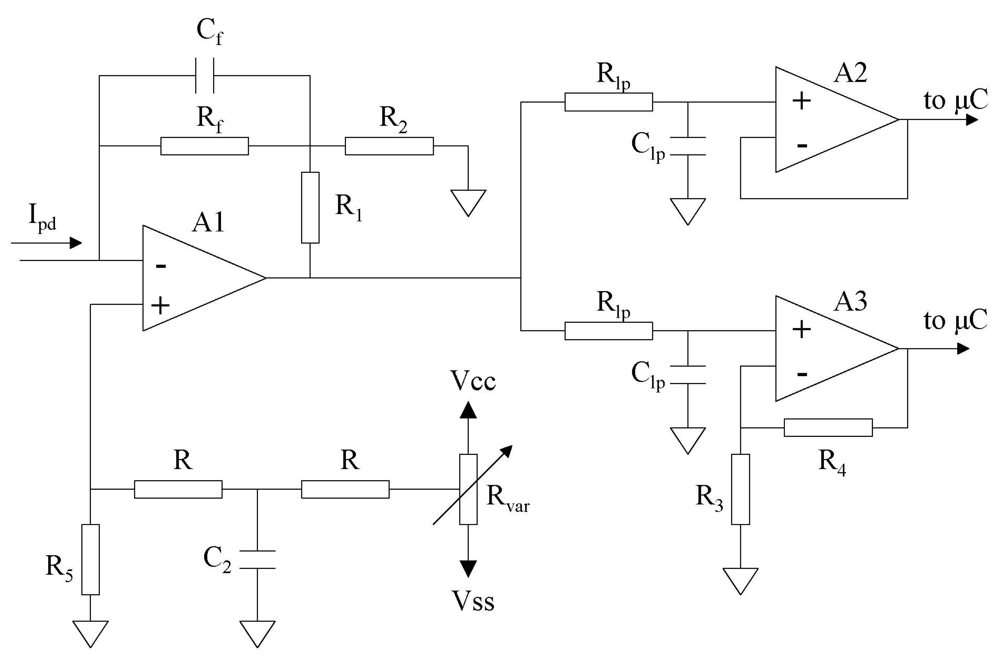
2.5. Measurement Electronics Description
Built-in Potentiostat
Photocurrent conditioning circuit
3. Results and Discussion
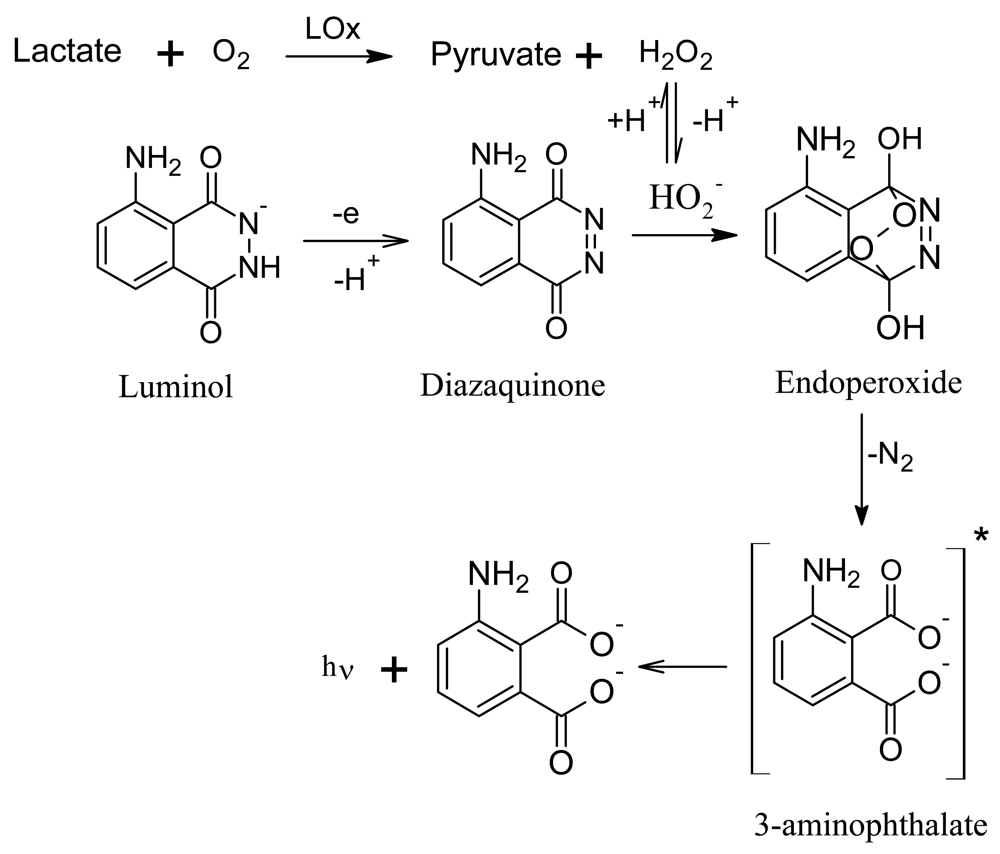
3.1. Lactate Biosensor
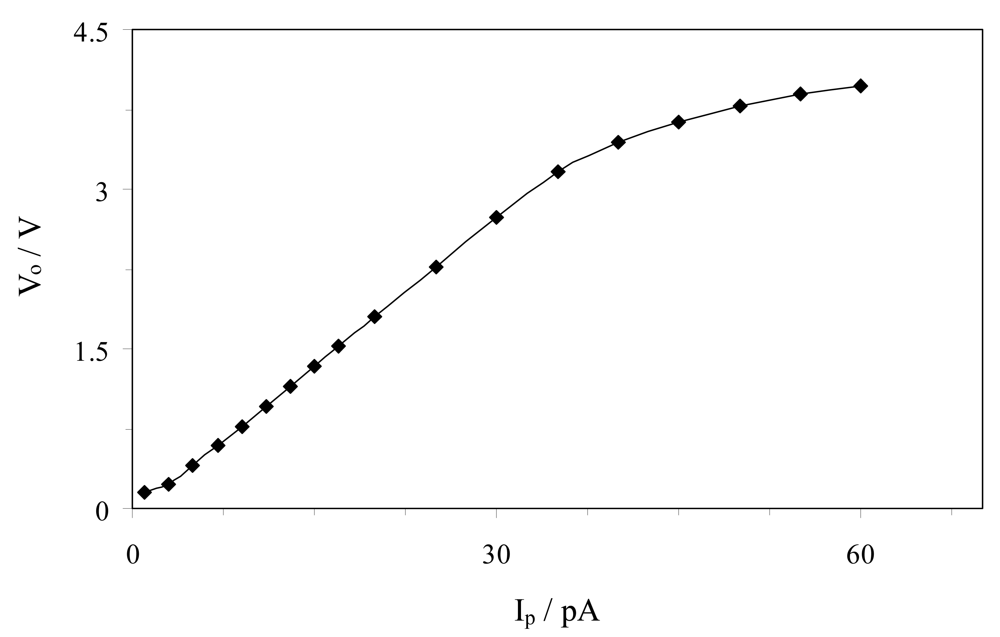
3.2. I/V Converter Calibration
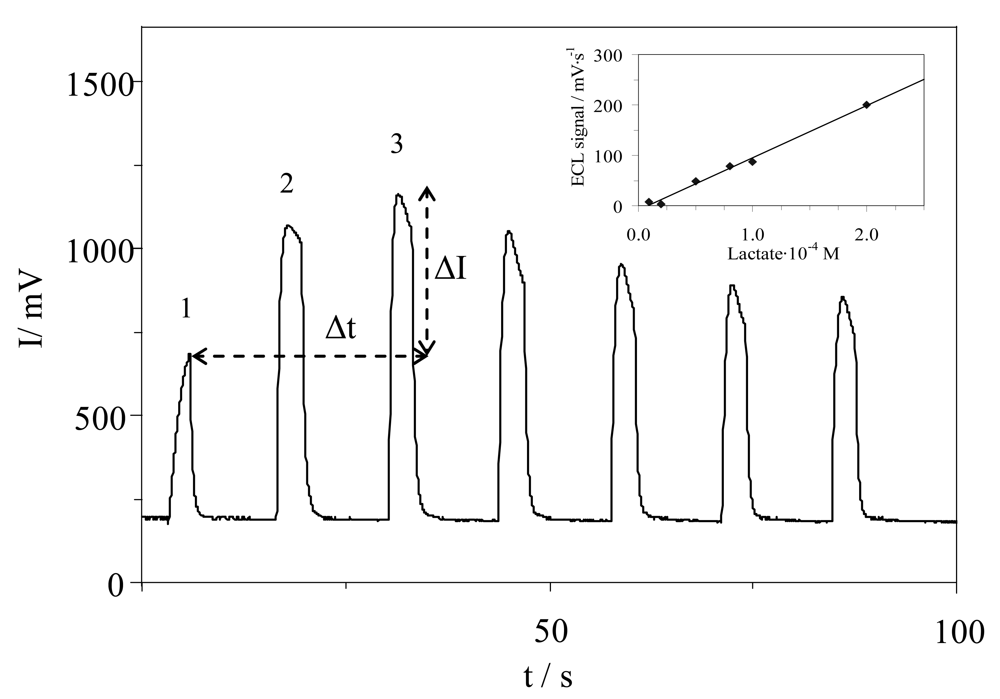
3.3. Measurement Conditions and ECL Analytical Signal
- The analytical signal increases when the waiting time increases up to 2 min, remaining constant onwards (Figure 7A). This could be attributed to the limiting effect of H2O2 at low waiting times. At higher waiting times, the signal is constant due to the compensation by the higher H2O2 concentration and the limiting effect of luminol present. 3 min was selected as the waiting time to ensure the independence of this factor.
- The higher pulse time the lower signal was, as shown in Figure 7B, due to the increase in H2O2 consumption with the time pulse, which results in a lower ECL increasing (ΔIECL) between pulses. Thus we chose 1 s as the pulse time to avoid the observed decrease. (C) On the other hand, the use of a higher time between pulses means an increase in H2O2 production and hence in the signal because of the enzymatic kinetic of this biosensor (Figure 7C). Then, 10 s was chosen as optimal because the ECL signal becomes stable at values of more than 5 s.
- The sample volume placed in the receptacle of the biosensor was studied, showing that the ECL signal decreases one order of magnitude on average when the volume increases from 20 to 30 μL, then increasing slowly for higher volumes up to 45 μL, the maximum capacity of the receptacle (Figure 7D). Volumes of less than 25 μL do not cover the whole cell and the applied potential can experience some fluctuations that result in the apparently higher signals along with high irreproducibility. Consequently, we selected 35 μL as the sample volume because of its good precision (7.2% RSD).
3.4. Calibration
3.5. Technical Specifications and the Lifetime of the Biosensor
4. Conclusions
Acknowledgments
References and Notes
- Friedl, C. Analysis: the promise of lactic acid monitoring in ambulatory individuals. Diabetes Tech. Therapeut. 2004, 6, 402–404. [Google Scholar]
- Suman, C.; Kumar, A. Recent advances in lactate estimation and lactate sensors for diagnosis of diseases. Sens. Trans. J. 2008, 96, 18–31. [Google Scholar]
- Yoshioka, T. Enzyme-based electrochemical biosensor. Bio. Ind. 2002, 19, 26–32. [Google Scholar]
- Nikolaus, N.; Strehlitz, B. Amperometric lactate biosensors and their application in (sports) medicine, for life quality and wellbeing. Microchim. Acta 2008, 160, 15–55. [Google Scholar]
- Yoda, K.; Shimazaki, K.; Ueda, Y. Analysis of glycolysis relevant compounds in saliva by microbiosensors. Ann. NY Acad. Sci. 1998, 864, 600–604. [Google Scholar]
- Gerard, M.; Chaubey, A.; Malhotra, B.D. Application of conducting polymers to biosensors. Biosens. Bioelectron. 2002, 17, 345–359. [Google Scholar]
- Cui, X.; Li, C.M.; Zang, J.; Yu, S. Highly sensitive lactate biosensor by engineering chitosan/PVI-Os/CNT/LOD network nanocomposite. Biosens. Bioelectron. 2007, 22, 3288–3292. [Google Scholar]
- Medizin. Overview: Lactate Analysers. Available online: http://medizin.li/lactate/ (accessed on September 22, 2009).
- Marquette, C.A.; Degiuli, A.; Blum, L.J. Fiberoptic biosensors based on chemiluminescent reactions. Appl. Biochem. Biotechnol. 2000, 89, 107–115. [Google Scholar]
- Girotti, S.; Ghini, S.; Carrea, G.; Bovara, R.; Roda, A.; Budini, R. Bioluminescent flow sensor for D(+) lactate. Anal. Chim. Acta 1991, 255, 259–268. [Google Scholar]
- Nakamura, H.; Murakami, Y.; Yokoyama, K.; Tamiya, E.; Karube, I.; Suda, M.; Uchiyama, S. A compactly integrated flow cell with a chemiluminescent fia system for determining lactate concentration in serum. Anal. Chem. 2001, 73, 373–378. [Google Scholar]
- Hemmi, A.; Yagiuda, K.; Funazaki, N.; Ito, S.; Asano, Y.; Imato, T.; Hayashi, K.; Karube, I. Development of a chemiluminescence detector with photodiode detection for flow-injection analysis and its application to L-lactate analysis. Anal. Chim. Acta 1995, 316, 323–327. [Google Scholar]
- Richter, M.M. Electrochemiluminescence (ECL). Chem. Rev. 2004, 104, 3003–3036. [Google Scholar]
- Marquette, C.A.; Blum, L.J. Self-containing reactant biochips for the electrochemiluminescent determination of glucose, lactate and choline. Sens. Actuat. B 2003, B90, 112–117. [Google Scholar]
- Gautron, J.; Dalbera, J.P.; Lemasson, P. Electroluminescence of the electrolyte/n-zinc selenide junction under anodic polarization. Surf. Sci. 1980, 99, 300–308. [Google Scholar]
- Zheng, X.; Zhang, Z.; Li, B. Flow injection chemiluminescence determination of captopril with in situ electrogenerated Mn3+ as the oxidant. Electroanalysis 2001, 13, 1046–1050. [Google Scholar]
- Almuaibed, A.M.; Townshend, A. Flow injection amperometric and chemiluminescence individual and simultaneous determination of lysine and glucose with immobilized lysine oxidase and glucose oxidase. Anal. Chim. Acta. 1997, 338, 149–154. [Google Scholar]
- Nie, L.H.; Zhao, H.C.; Wang, X.; Yi, L.; Lu, Y.; Jin, L.P.; Ma, H.M. Determination of lomefloxacin by terbium sensitized chemiluminescence method. Anal. Bioanal. Chem. 2002, 374, 1187–1190. [Google Scholar]
- Zhou, H.; Zhang, Z.; He, D.; Hu, Y.; Huang, Y.; Chen, D. Flow chemiluminescence sensor for determination of clenbuterol based on molecularly imprinted polymer. Anal. Chim. Acta 2004, 523, 237–242. [Google Scholar]
- Oyama, M.; Mitani, M.; Washida, M.; Masuda, T.; Okazaki, S. Two-dimensional CCD detection of electrogenerated chemiluminescence (ECL) on an electrode surface. ECL reactions involving microcrystals of the perylene dimer cation radical salt. J. Electroanal. Chem. 1999, 473, 166–172. [Google Scholar]
- Momeni, N.; Ramanathan, K.; Larsson, P.O.; Danielsson, B.; Bengmark, S.; Khayyami, M. CCD-camera based capillary chemiluminescent detection of retinol binding protein. Anal. Chim. Acta 1999, 387, 21–27. [Google Scholar]
- Hofmann, O.; Miller, P.; Sullivan, P.; Jones, T.S.; de Mello, J.C.; Bradley, D.D.C.; de Mello, A.J. Thin-film organic photodiodes as integrated detectors for microscale chemiluminescence assays. Sens. Actuat. B 2005, B106, 878–884. [Google Scholar]
- Ho, W.J.; Chen, J.S.; Ker, M.D.; Wu, T.K.; Wu, C.Y.; Yang, Y.S.; Li, Y.K.; Yuan, C.J. Fabrication of a miniature CMOS-based optical biosensor. Biosens. Bioelectron. 2007, 22, 3008–3013. [Google Scholar]
- Pittet, P.; Lu, G.N.; Galvan, J.M.; Ferrigno, R.; Blum, L.J.; Leca-Bouvier, B.D. PCB technology-based electrochemiluminescence microfluidic device for low-cost portable analytical systems. IEEE Sens. J. 2008, 8, 565–571. [Google Scholar]
- Ballesta-Claver, J.; Valencia-Miron, M.C.; Capitan-Vallvey, L.F. Disposable electrochemiluminescent biosensor for lactate determination in saliva. Analyst 2009, 134, 1423–1432. [Google Scholar]
- Hemmi, A.; Yagiuda, K.; Funazaki, N.; Ito, S.; Asano, Y.; Imato, T.; Hayashi, K.; Karube, I. Development of a chemiluminescence detector with photodiode detection for flow-injection analysis and its application to L-lactate analysis. Anal. Chim. Acta 1995, 316, 323–327. [Google Scholar]
- Okamoto, T.; Tanaka, K.I.; Goto, H.; Lin, J.M.; Yamada, M.; Asano, Y. Luminol immobilized anion-exchange resin as an indicator phase for a chemiluminescence oxygen gas sensor. Anal. Commun. 1999, 36, 181–183. [Google Scholar]
- Fidler, J.C.; Penrose, W.R.; Bobis, J.P. A potentiostat based on a voltage-controlled current source for use with amperometric gas sensors. IEEE Trans. Instrum. Meas. 1992, 41, 308–310. [Google Scholar]
- Graeme, J.G. Photodiode amplifiers: OP AMP solutions; McGraw-Hill: New York NY, USA, 1996. [Google Scholar]
- Sakura, S. Electrochemiluminescence of hydrogen peroxide-luminol at a carbon electrode. Anal. Chim. Acta. 1992, 262, 49–57. [Google Scholar]
- Fahnrich, K.A.; Pravda, M.; Guilbault, G.G. Recent applications of electrogenerated chemiluminescence in chemical analysis. Talanta 2001, 54, 531–559. [Google Scholar]
- Salgado, G.; Navarrete, J.; Bustos, C.; Sanchez, C.; Ugarte, R. Quimioluminiscencia electrogenerada del luminol usando electrodos de bajo costo. Quim. Nova 2006, 29, 381–384. [Google Scholar]
- Haapakka, K.E.; Kankare, J.J. The mechanism of the electrogenerated chemiluminescence of luminol in aqueous alkaline solution. Anal. Chim. Acta 1982, 138, 263–275. [Google Scholar]
- Corgier, B.P.; Marquette, C.A.; Blum, L.J. Screen-printed electrode microarray for electrochemiluminescent measurements. Anal. Chim. Acta 2005, 538, 1–7. [Google Scholar]
- Zhu, L.; Li, Y.; Tian, F.; Xu, B.; Zhu, G. Electrochemiluminescent determination of glucose with a sol-gel derived ceramic-carbon composite electrode as a renewable optical fiber biosensor. Sens. Actuat. B 2002, 84, 265–270. [Google Scholar]
- Miller, N.J.; Miller, J.C. Estadística y Quimiometría para Química Analítica; Prentice Hall: Madrid, Spain, 2002; p. 125. [Google Scholar]



| Parameter | Initial rate estimation | ||
|---|---|---|---|
| vECL1-2 | vECL1-3 | vECL2-3 | |
| Linear range / (M) | 2 × 10−5 – 2 × 10−4 | 8 × 10−6 – 2 × 10−4 | 8 × 10−6 – 2 × 10−4 |
| Intercept (b) / (V/s) | -0.5 | 0.5 | -2.5 |
| Slope (a) / (V·s−1·M−1) | 155.8 | 195.4 | 137.6 |
| r2 | 0.938 | 0.998 | 0.925 |
| Detection limit / (M) | 3.2 × 10−6 | 2.4 × 10−6 | 1.8 × 10−5 |
| RSD lactate (%) 2 × 10−4 M | 11.1% | 10.3% | 11.0% |
| Parameter | Photodiode (This work) | PMT [25] | CCD camera [34] |
|---|---|---|---|
| Linear equation | vECL = a· [lactate] + b | log vECL = a·log[lactate] + b | IECL = a· [lactate] + b |
| Linear range / (M) | 8 × 10−6 – 2 × 10−4 | 10−5 – 5 × 10−4 | 3 × 10−7 – 10−4 |
| r2 | 0.998 | 0.996 | 0.972 |
| Detection limit / (M) | 2.4 × 10−6 | 5.0 × 10−6 | 3.0 × 10−7 |
| RSD lactate (%) 2 × 10−4 M | 10.3% | 3.3% | 4.4% |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Martínez-Olmos, A.; Ballesta-Claver, J.; Palma, A.J.; Valencia-Mirón, M.d.C.; Capitán-Vallvey, L.F. A Portable Luminometer with a Disposable Electrochemiluminescent Biosensor for Lactate Determination. Sensors 2009, 9, 7694-7710. https://doi.org/10.3390/s91007694
Martínez-Olmos A, Ballesta-Claver J, Palma AJ, Valencia-Mirón MdC, Capitán-Vallvey LF. A Portable Luminometer with a Disposable Electrochemiluminescent Biosensor for Lactate Determination. Sensors. 2009; 9(10):7694-7710. https://doi.org/10.3390/s91007694
Chicago/Turabian StyleMartínez-Olmos, Antonio, Julio Ballesta-Claver, Alberto J. Palma, Maria del Carmen Valencia-Mirón, and Luis Fermin Capitán-Vallvey. 2009. "A Portable Luminometer with a Disposable Electrochemiluminescent Biosensor for Lactate Determination" Sensors 9, no. 10: 7694-7710. https://doi.org/10.3390/s91007694
APA StyleMartínez-Olmos, A., Ballesta-Claver, J., Palma, A. J., Valencia-Mirón, M. d. C., & Capitán-Vallvey, L. F. (2009). A Portable Luminometer with a Disposable Electrochemiluminescent Biosensor for Lactate Determination. Sensors, 9(10), 7694-7710. https://doi.org/10.3390/s91007694







