An Amperometric Biosensor for Uric Acid Determination Prepared From Uricase Immobilized in Polyaniline-Polypyrrole Film
Abstract
:1. Introduction
2. Result and Discussion
2.1. The working potential
2.2. The effect of pH
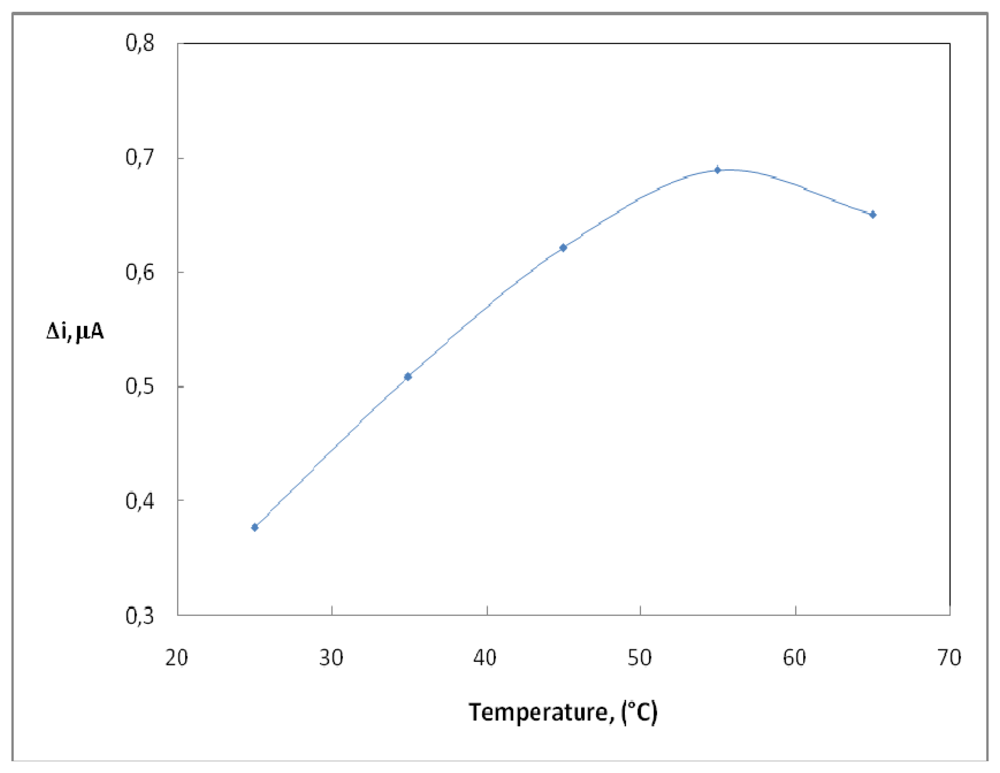
2.3. The effect of Temperature
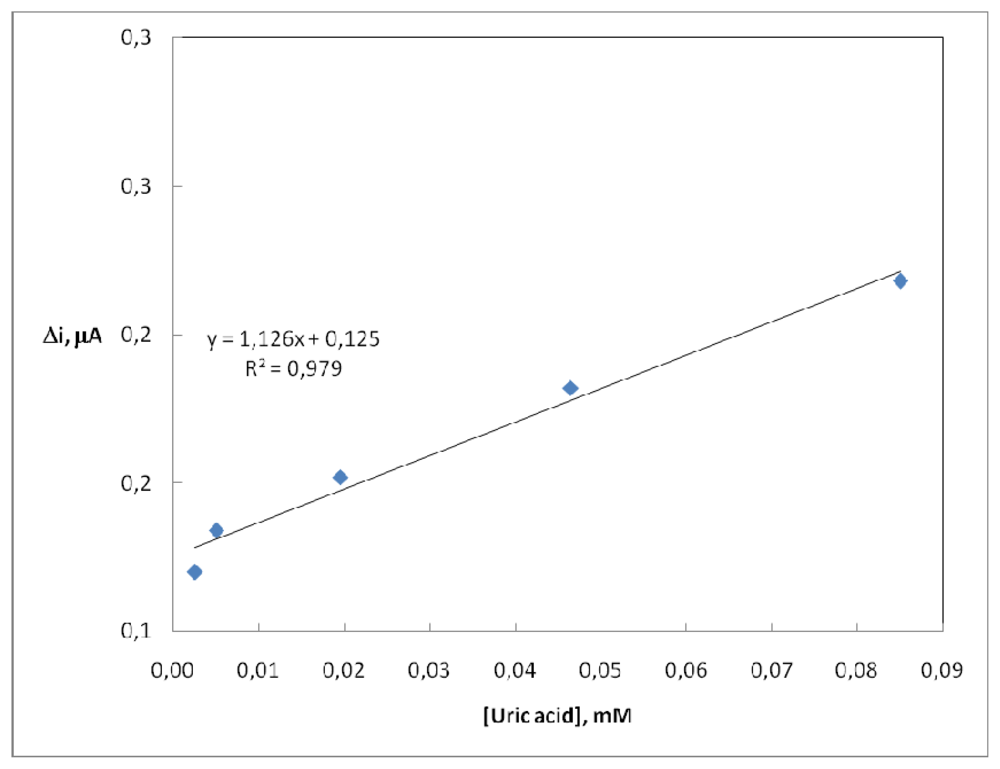
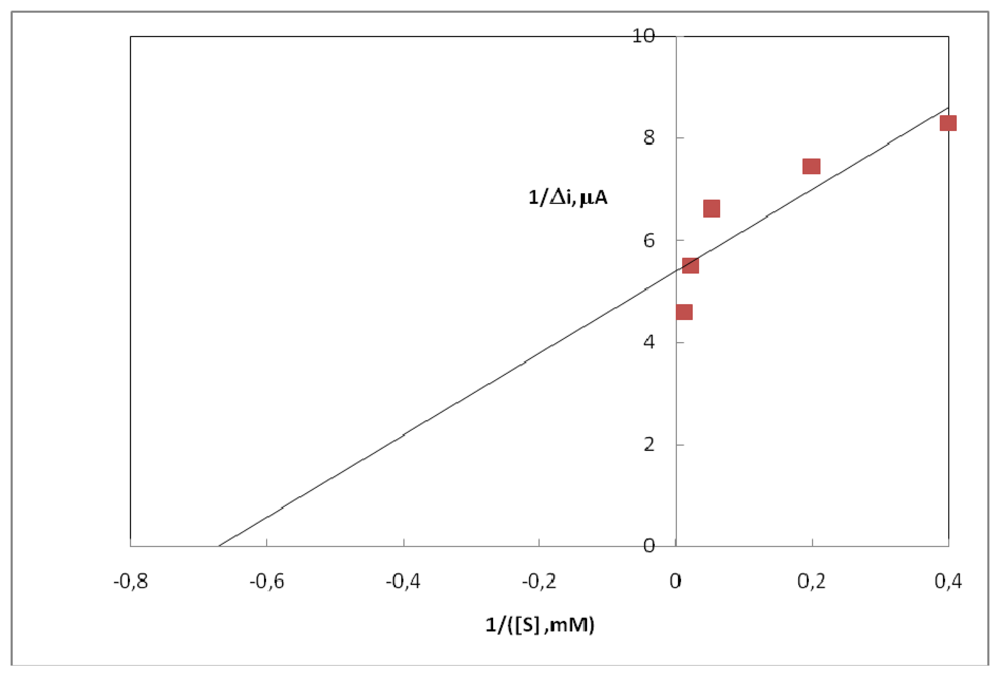
2.4. Substrate Concentration and Calibration Curves
2.5. The Reproducibility of the Enzyme Electrode
2.6. Storage Stability
2.7. Interference Effect
3. Experimental Section
3.1. Equipment and Reagents
3.2. Preparation of Uric Acid Biosensor
3.3. Amperometric Measurements
4. Conclusions
- Is usable in a large concentration range (2.5×0.10-6-8.5×0.10-5 M).
- Has a very low detection limit 1.0×0.10-6 M.
- Has an acceptable response time for a biosensor (70 second).
- Gives highly reproducible results (the electrode lost 4% of the initial amperometric response at the end of the 15th measurement).
- Has a satisfactory storage stabilization (the electrode lost 20% of the initial amperometric response at the end of the 4th week).
- The Km (app) value of uricase enzyme immobilized in polyaniline-polypyrrole film was 1.57 mM.
References
- Yurahi, H.; Tetsuhika, S.; Hajime, I. Cloning sequence analysis and expression in Escherichia coli at the gene encoding a uricase from the yeastlike symbiant of the Brown planthappen. Insect Biochem. Mol. Biol. 2000, 30, 173–188. [Google Scholar]
- Raab, L.S.; Decker, G.L.; Jonas, A.J.; Kaetzel, M.A.; Dedman, J.R. Glucocoticoid regulation of rat liver urate oxidase. J. Cell. Biochem. 1991, 47, 18–30. [Google Scholar]
- Dussossoy, M.I.; Pastor, G.; Baulenc, X. Development of a two site immunoassay measurement urate oxidase (SR 29142) and its use for determination of pharmacokinetic parameters in rats and Baboons. J. Pharm. Sci.-US 1996, 85, 955–959. [Google Scholar]
- Çete, S.; Yaşar, A.; Arslan, F. An amperometric biosensor for Uric acid determination prepared from uricase immobilized in polypyrrole film. Artif. Cell Blood Subs. 2006, 34, 367–380. [Google Scholar]
- Ramanavicius, A. Amperometric biosensor for the determination of creatine. Anal. Bioanal. Chem. 2007, 387, 1899–1906. [Google Scholar]
- Singh, S.; Chaubey, A.; Malhotra, B.D. Amperometric cholesterol oxidase on conducting Polypyrrole films. Anal. Chim. Acta 2004, 502, 229–234. [Google Scholar]
- Erden, P.E.; Pekyardımcı, Ş; Kılıç, E.; Arslan, F. An amperometric enzyme electrode for Creatine determination prepared by the immobilization of Creatinase and Sarcosine Oxidase in poly(vinlyferrocenium). Artif. Cell Blood Subs. 2006, 34, 223–239. [Google Scholar]
- Uchiyama, S.; Sakamoto, H. Immobilization of uricase to gas diffusion carbon felt by elegtropolymerization of aniline and its application as an enzyme reactor for uric acid sensor. Talanta 1997, 44, 1435–1439. [Google Scholar]
- Arslan, F.; Yaşar, A.; Kılıç, E. An amperometric biosensor for xanthine determination prepared from xanthine oxidase immobilized in polypyrrole film. Artif. Cell Blood Subs. 2006, 34, 1–16. [Google Scholar]
- Ramanavicius, A.; Ramanaviciene, A.; Malinauskas, A. Electrochemical sensors based on conducting polymer-polypyrrole. Electrochim. Acta 2006, 5, 6025–6037. [Google Scholar]
- Malinauskas, A.; Garjonyte, R.; Mazeikiene, R.; Jurevicivte, I. Elecrochemical response of ascorbic acid of conducting and electrogenerated polymer modified electrodes for electroanalytical applications:a review. Talanta 2004, 64, 121–129. [Google Scholar]
- Sung, W.J.; Bae, Y.H. Glucose oxidase, lactate oxidase, and galactose oxidase enxyme electrode based on polypyrrole with polyanion/PEG/enzyme conjugate dopant. Sens. Actuat. B-Chem. 2006, 114, 164–169. [Google Scholar]
- Telefoncu, A. Immobilize enzimler. Enzimoloji. Biyokimya Lisans Üstü Yazokulu, Kuşadası 1997, 193–243. [Google Scholar]
- Hu, S.; Liu, C.-C. Development of a hypoxanthine biosensor based on immobilized xanthine oxidase chemically modified electrode. Electroanalysis 1997, 9, 372–377. [Google Scholar]
- Nakamura, N.; Murayama, K.; Kinoshita, T. Immobilization of uricase on protamine bound to glass beads and its application to determination of uric acid. Anal. Bioanal. Chem. 1986, 152, 386–390. [Google Scholar]
- Kawabata, S.; Nakaminami, T.; Ito, S.; Yoneyama, H. Preparation and properties of amperometric uric acid sensors. Sens. Actuat. B-Chem. 1998, 52, 72–77. [Google Scholar]
- Li, J.-P.; Gu, H.-N. A selective Cholesterol Biosensor Based on Composite Film Modified Electrode for Amperometric Detection. J. Chin. Chem. Soc-Taip. 2006, 53, 575–582. [Google Scholar]
- Muhammet, S.M. Kolesterol tayini için biyosensör hazırlanması. Ph.D. Thesis., Gazi Üniversitesi Fen Bilimleri Enstitüsü, 2008; pp. 40–46. [Google Scholar]




| Interfering substances | Concentration | Interference effect of Substances |
|---|---|---|
| Ascorbic acid | 1.0×10- M | - |
| Glucose | 5.0×10- M | - |
| Bilirubin | 1.0×10- M | - |
| Urea | 1.0×10- M | - |
| Paracetamol | 1.0×10- M | 31% |
© 2008 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Arslan, F. An Amperometric Biosensor for Uric Acid Determination Prepared From Uricase Immobilized in Polyaniline-Polypyrrole Film. Sensors 2008, 8, 5492-5500. https://doi.org/10.3390/s8095492
Arslan F. An Amperometric Biosensor for Uric Acid Determination Prepared From Uricase Immobilized in Polyaniline-Polypyrrole Film. Sensors. 2008; 8(9):5492-5500. https://doi.org/10.3390/s8095492
Chicago/Turabian StyleArslan, Fatma. 2008. "An Amperometric Biosensor for Uric Acid Determination Prepared From Uricase Immobilized in Polyaniline-Polypyrrole Film" Sensors 8, no. 9: 5492-5500. https://doi.org/10.3390/s8095492
APA StyleArslan, F. (2008). An Amperometric Biosensor for Uric Acid Determination Prepared From Uricase Immobilized in Polyaniline-Polypyrrole Film. Sensors, 8(9), 5492-5500. https://doi.org/10.3390/s8095492




