An Approach for Measuring the Sorptive Behavior of Odorants Using a Multifunction Thermal Desorber Unit: Preliminary Tests on Reduced Sulfur Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1 The basics of the Experimental Design
2.2 Details of Experiment Types I and II
2.3 Analytical set-up for RSC quantification
- Flow rate (mL min−1): Air(1) =Air(2)= 10; H2=11.5; Carrier gas: N2= 1.2 (at 20 psi)
- Detector temperature of PFPD: 220°C; Flow path temp: 80 °C
- Cold trap: -15 (low end) ∼ 300°C (high end) with holding time of 5 min.
- Outlet split: 5.0 mL min−1 (5:1 split ratio)
3. Results and discussion
3.1 The effect of changes in RSC desorption condition - Experiment type I
3.2 Influences of initial adsorption condition changes on the RSC desorption pattern – Experiment type 2
4. Conclusions
Acknowledgments
References
- Burbank, H.M.; Qian, M.C. Volatile sulfur compounds in Cheddar cheese determined by headspace solid-phase microextraction and gas chromatograph-pulsed flame photometric detection. J. Chromatogr. A 2005, 1066, 149–157. [Google Scholar]
- Lillo, M.P.; Latrille, E.; Casaubon, G.; Agosin, E.; Bordeu, E.; Martin, N. Comparison between odour and aroma profiles of Chilean Pisco spirit. Food Qual. Pref. 2005, 16, 59–70. [Google Scholar]
- Willig, S.; Lacorn, M.; Claus, R. Development of a rapid and accurate method for the determination of key compounds of pig odor. J. Chromatogr. A 2003, 1038, 11–18. [Google Scholar]
- Hallier, A.; Courcoux, P.; Serot, T.; Prost, C. New gas chromatography-olfactometric investigative method, and its application to cooked Silurus glanis (European catfish) odor characterization. J. Chromatogr. A 2004, 1056, 201–208. [Google Scholar]
- van Ruth, S. M. Evaluation of two gas chromatography-olfactory methods: the detection frequency and perceived intensity method. J. Chromatogr. A 2004, 1054, 33–37. [Google Scholar]
- Cometto-Muniz, J.E.; Cain, W.S.; Abraham, M.H. Odor detection of single chemicals and binary mixtures. Behav. Brain Res. 2005, 156, 115–123. [Google Scholar]
- Baek, S.O.; Jenkins, R.A. Characterization of trace organic compounds associated with aged and diluted sidestream tobacco smoke in a controlled atmosphere – volatile organic compounds and polycyclic aromatic hydrocarbons. Atmos. Environ. 2004, 38, 6583–6599. [Google Scholar]
- Kim, K.-H.; Choi, Y.-J.; Yang, H.S. The adsorption/desorption behavior of odorous compounds on clothing materials: a case study on reduced sulfur compounds. Korean J. Atmos. Environ. 2006, 22, 249–257. [Google Scholar]
- Muezzinoglu, A. A study of volatile organic sulfur emissions causing urban odors. Chemosphere 2003, 51, 245–252. [Google Scholar]
- Ohmori, S.; Ohno, Y.; Makino, T.; Kashihara, T. Application of an electronic nose system for evaluation of unpleasant odor in coated tablets. Eur. J. Pharmaceut. Biopharmaceut. 2005, 59, 289–297. [Google Scholar]
- Draper, D.; Rutt, P. Operational experience with management of odours in Thames Water. Second CIWEM National Conference on Odour Control in Sewage Treatment, London, 30 April 1988.
- Schultz, T. J.; van Harreveld, A. P. International moves towards standardisation of odour measurement using olfactometry. Water Sci. Tech. 1996, 34, 541–547. [Google Scholar]
- Henshaw, P.; Nicell, J.; Sikdar, A. Parameters for the assessment of odour impacts on communities. Atmos. Environ. 2006, 40, 1016–1029. [Google Scholar]
- Kim, K.-H. Some insights into the gas chromatographic determination of reduced sulfur compounds (RSC) in air. Environ. Sci. Technol. 2005, 39, 6765–6769. [Google Scholar]
- Kim, K.-H.; Choi, Y.-J.; Choi, G.-H.; Song, H. N.; Yang, H.-S.; Oh, J. M. The effects of sampling materials selection in the collection of reduced sulfur compounds in air. Talanta 2006, 68, 1713–1719. [Google Scholar]
- Kim, K.-H.; Im, M.-S.; Jeon, E.-C.; Koo, Y.-S.; Youn, Y.-H. An on-line analysis of reduced sulfur gases in the ambient air surrounding a large industrial complex. Atmos. Environ. 2007, 41, 3829–3840. [Google Scholar]




| A. RSC adsorption conditions for experiment type I | ||||||
|---|---|---|---|---|---|---|
| ||||||
| Compound | H2S | CH3SH | DMS | DMDS | ||
| Amount (ng) | 13,980 | 19,740 | 25,500 | 38,650 | ||
| B. Desorption conditions of RSC for experiment type I | ||||||
| A. Experiment type IA: Desorption at fixed flushing flow rate (5 or 40 ml min−1) | B. Experiment type IB: Desorption at fixed flushing duration (10 or 20 ml min−1) | |||||
| Exp No. | Flow rate (mL min−1) | Duration (min) | Exp No. | Flow rate (mL min−1) | Duration (min) | |
| 1 | 5 | 9 | 5 | |||
| 2 | 5 | 10 | 10 | 10 | 10 | |
| 3 | 20 | 11 | 20 | |||
| 4 | 30 | 12 | 40 | |||
| 5 | 5 | 13 | 5 | |||
| 6 | 40 | 10 | 14 | 10 | 20 | |
| 7 | 20 | 15 | 20 | |||
| 8 | 30 | 16 | 40 | |||
| Exp No. | LD time | H2S | CH3SH | DMS | DMDS |
|---|---|---|---|---|---|
| A. Total amount of RSC supplied (ng) | |||||
| 1 | 0.17 | 238 | 336 | 433 | 657 |
| 2 | 0.5 | 699 | 987 | 1275 | 1933 |
| 3 | 1 | 1398 | 1974 | 2550 | 3865 |
| 4 | 2 | 2797 | 3948 | 5100 | 7731 |
| 5 | 5 | 6992 | 9871 | 12750 | 19330 |
| 6 | 10 | 13980 | 19740 | 25500 | 38650 |
| 7 | 20 | 27970 | 39480 | 51000 | 77310 |
| 8 | 30 | 41950 | 59220 | 76500 | 115960 |
| 9 | 40 | 55940 | 78970 | 101990 | 154620 |
| B. Total amount of RSC deloaded (ng g−1) | |||||
| 1 | 0.17 | - | 111 | 251 | 455 |
| 2 | 0.5 | - | 112 | 257 | 211 |
| 3 | 1 | 2.7 | 130 | 273 | 321 |
| 4 | 2 | 5.2 | 99 | 235 | 400 |
| 5 | 5 | 6.1 | 86 | 232 | 441 |
| 6 | 10 | 8.6 | 107 | 260 | 437 |
| 7 | 20 | 10.8 | 122 | 262 | 477 |
| 8 | 30 | 16.5 | 118 | 245 | 432 |
| 9 | 40 | 18.8 | 155 | 277 | 504 |
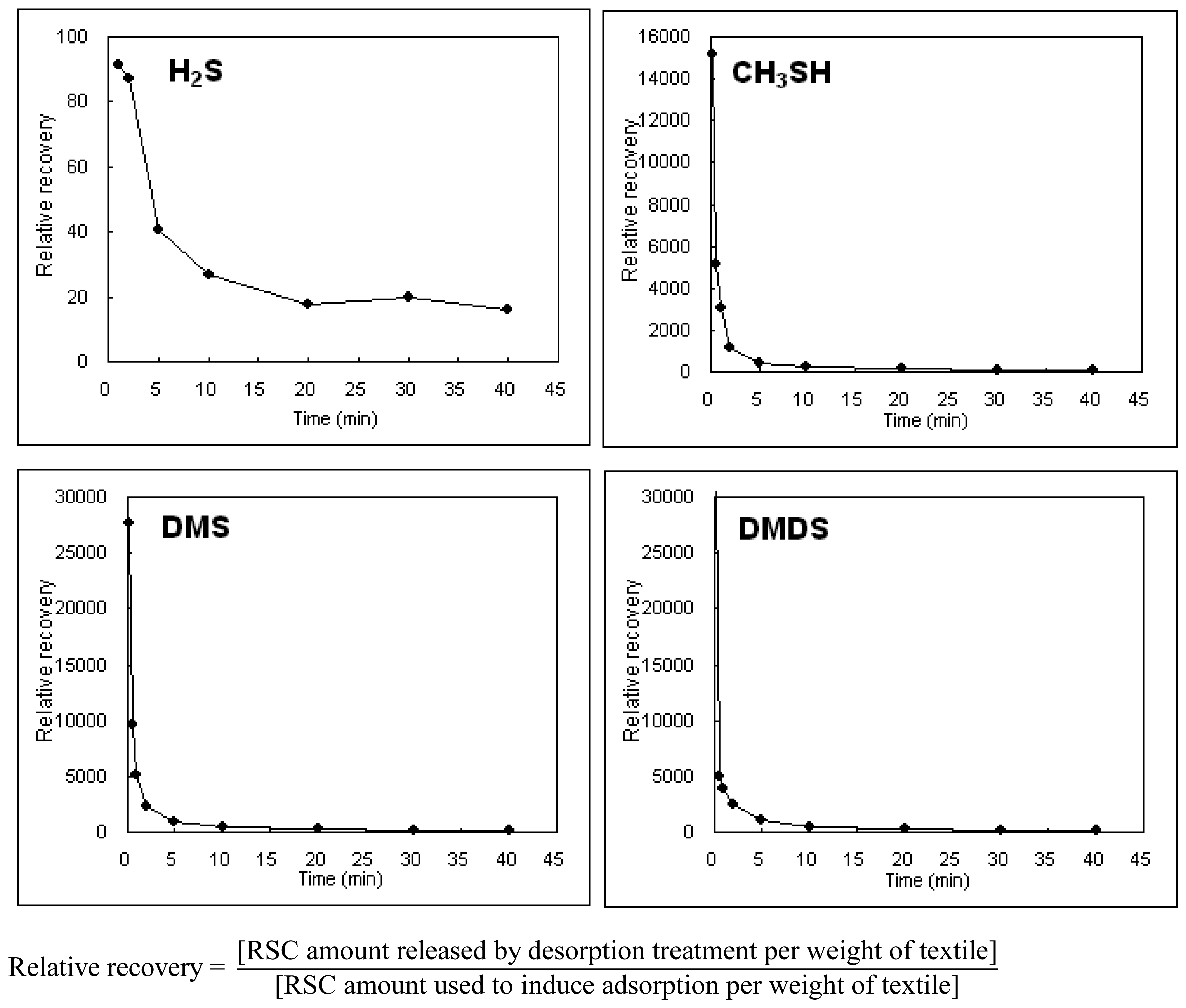
| C. Relative recovery (ppm) | |||||
| 1 | 0.17 | - | 15160 | 26620 | 31850 |
| 2 | 0.5 | - | 5178 | 9178 | 4974 |
| 3 | 1 | 91.6 | 3082 | 5000 | 3875 |
| 4 | 2 | 87.2 | 1189 | 2178 | 2444 |
| 5 | 5 | 40.8 | 406 | 851 | 1066 |
| 6 | 10 | 26.9 | 236 | 446 | 494 |
| 7 | 20 | 17.8 | 143 | 237 | 285 |
| 8 | 30 | 19.6 | 99 | 159 | 185 |
| 9 | 40 | 16.0 | 94 | 130 | 156 |
© 2008 by MDPI Reproduction is permitted for noncommercial purposes.
Share and Cite
Kim, K.-H.; Choi, Y.-J.; Yang, H.-S.; Joo, S.-W. An Approach for Measuring the Sorptive Behavior of Odorants Using a Multifunction Thermal Desorber Unit: Preliminary Tests on Reduced Sulfur Compounds. Sensors 2008, 8, 1858-1871. https://doi.org/10.3390/s8031858
Kim K-H, Choi Y-J, Yang H-S, Joo S-W. An Approach for Measuring the Sorptive Behavior of Odorants Using a Multifunction Thermal Desorber Unit: Preliminary Tests on Reduced Sulfur Compounds. Sensors. 2008; 8(3):1858-1871. https://doi.org/10.3390/s8031858
Chicago/Turabian StyleKim, Ki-Hyun, Ye-Jin Choi, Hye-Soon Yang, and Sang-Woo Joo. 2008. "An Approach for Measuring the Sorptive Behavior of Odorants Using a Multifunction Thermal Desorber Unit: Preliminary Tests on Reduced Sulfur Compounds" Sensors 8, no. 3: 1858-1871. https://doi.org/10.3390/s8031858
APA StyleKim, K.-H., Choi, Y.-J., Yang, H.-S., & Joo, S.-W. (2008). An Approach for Measuring the Sorptive Behavior of Odorants Using a Multifunction Thermal Desorber Unit: Preliminary Tests on Reduced Sulfur Compounds. Sensors, 8(3), 1858-1871. https://doi.org/10.3390/s8031858




