Polarographic Electrode Measures of Cerebral Tissue Oxygenation: Implications for Functional Brain Imaging
Abstract
:1. Introduction
2. Brain imaging techniques rely on neurovascular and neurometabolic coupling
3. Does oxygen consumption (CMRO2) increase following neural activation?
4. Optical techniques ‘shed-light’ on the stimulus-evoked hemodynamic response function
5. Relationship between the hemodynamic response function and the BOLD fMRI signal
6. Measurements of brain tissue oxygenation with polarographic electrodes
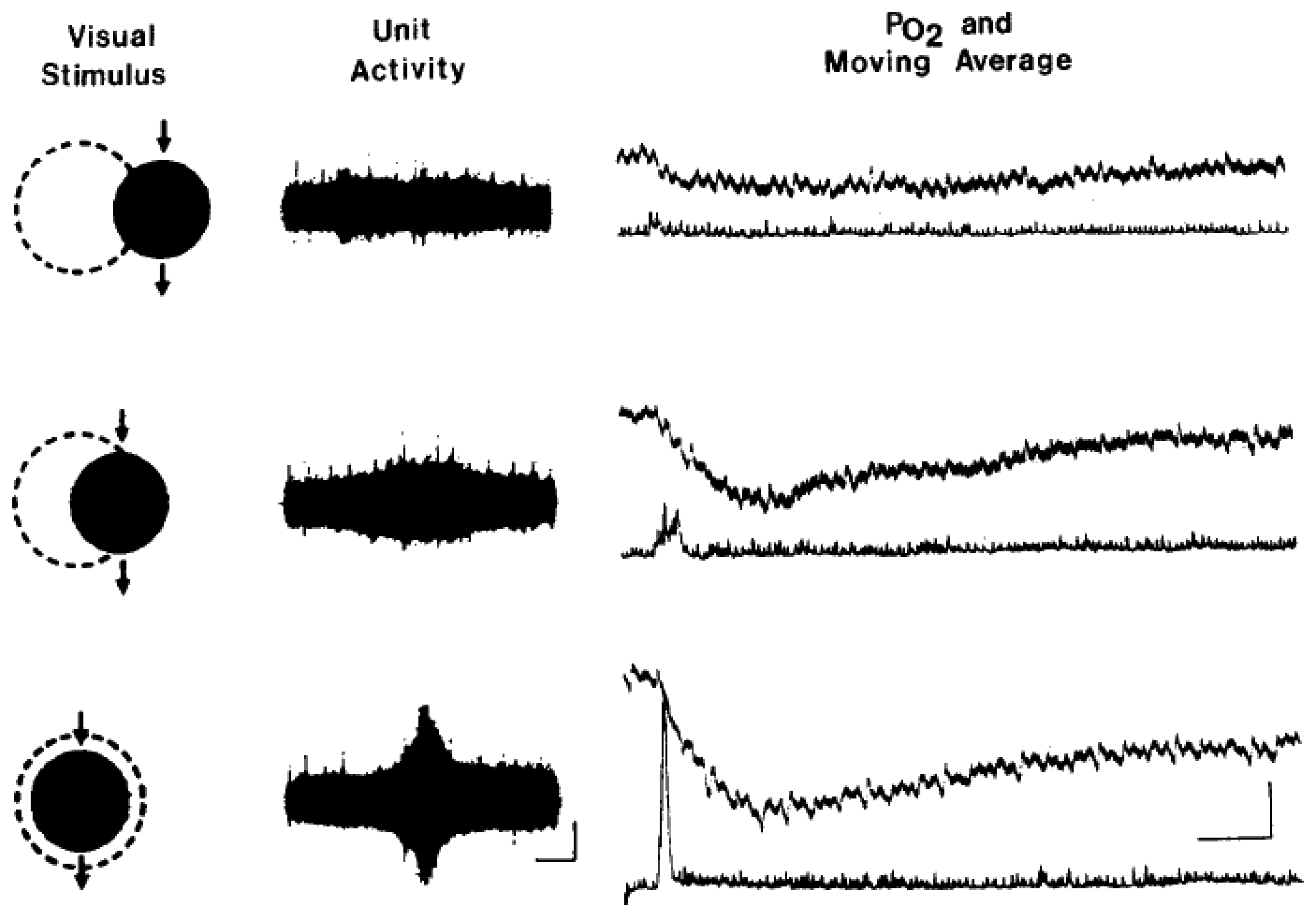
7. Measurement of tissue oxygenation: stimulation induces varying types of brain oxygen responses
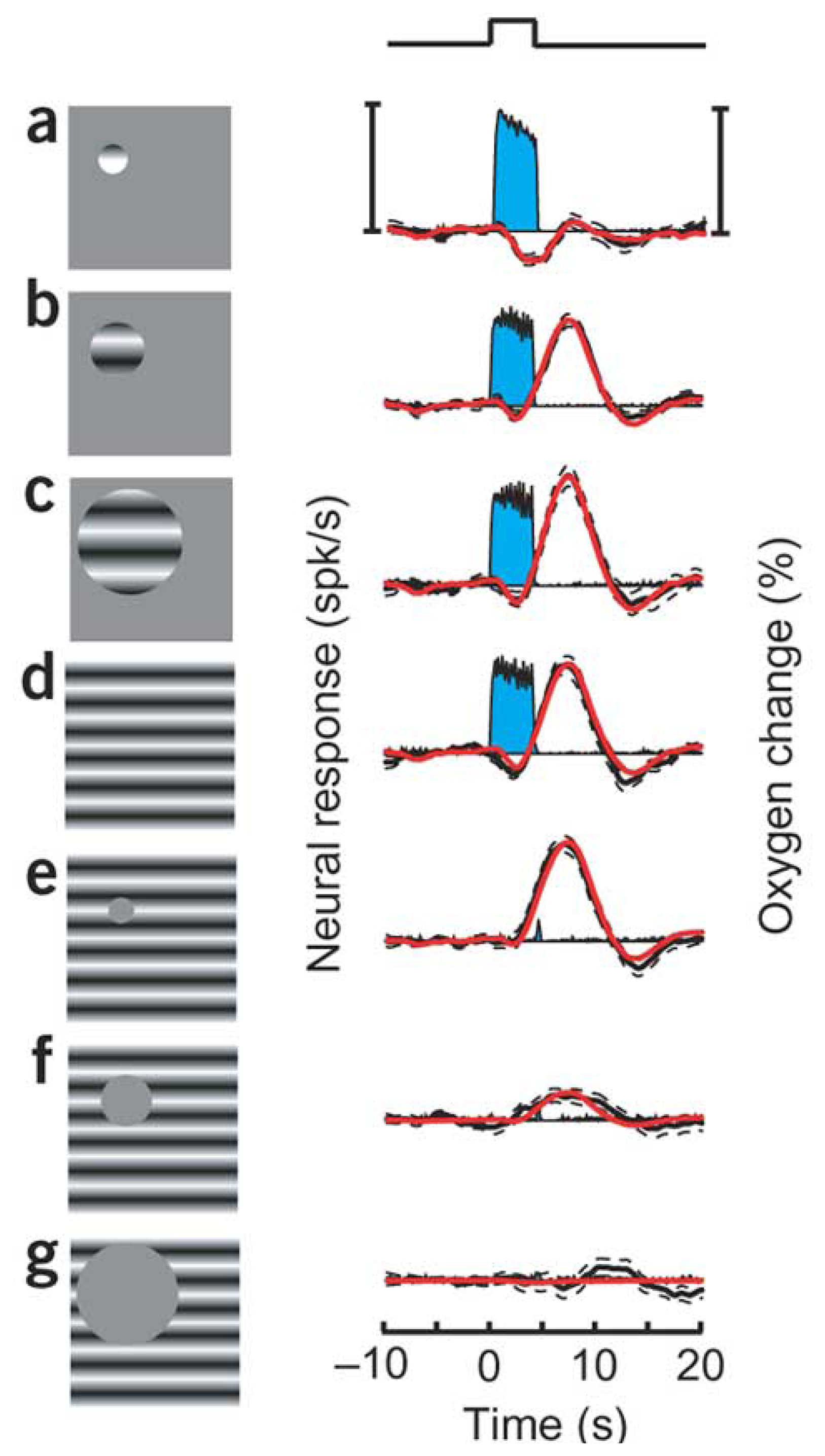
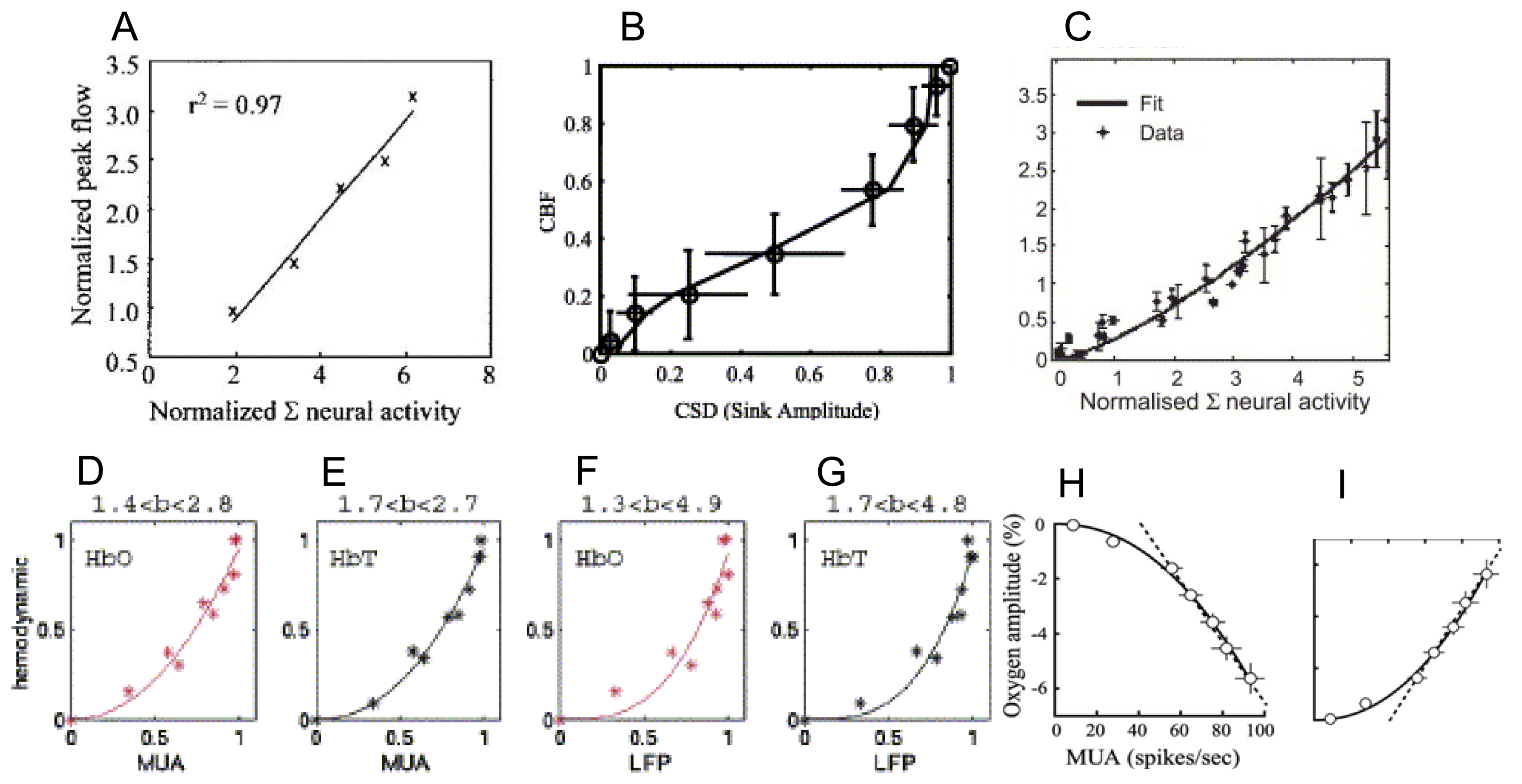
9. How do the magnitudes of neurometabolic signals relate to those of the underlying neural activity?
10. How do the temporal dynamics of neurometabolic signals relate to those of the underlying neural activity?
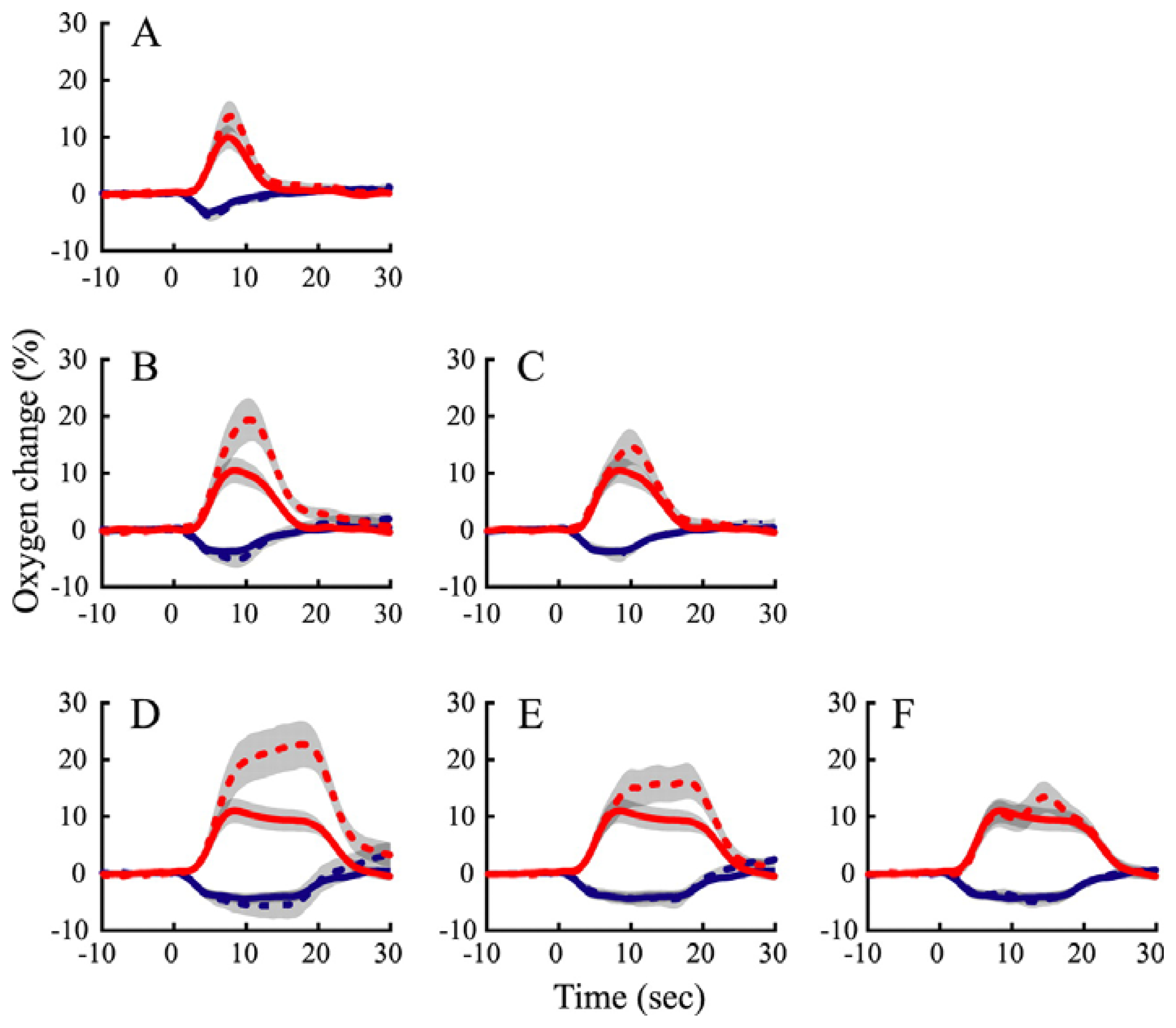
12. Neurovascular coupling and tissue oxygenation across the cortical laminae
13. Summary
Acknowledgments
References and Notes
- Clark, L.C.; Misrahy, G.; Fox, R.P. Chronically implanted polarographic electrodes. J. Appl. Physiol. 1958, 13, 85–91. [Google Scholar]
- Clark, L.C.; Wolf, R.; Granger, D.; Taylor, Z. Continuous recording of blood oxygen tensions by polarography. J. Appl. Physiol. 1953, 6, 189–193. [Google Scholar]
- Gijsbers, K.J.; Melzack, R. Oxygen tension changes evoked in the brain by visual stimulation. Science 1967, 156, 1392–1393. [Google Scholar]
- Sick, T.J.; Kreisman, N.R. Local tissue oxygen tension as an index of changes in oxidative metabolism in the bullfrog optic tectum. Brain Res. 1979, 169, 575–579. [Google Scholar]
- Travis, R.P., Jr.; Clark, L.C., Jr. Changes in evoked brain oxygen during sensory stimulation and conditioning. Electroencephalogr. Clin. Neurophysiol. 1965, 19, 484–491. [Google Scholar]
- Li, B.; Freeman, R.D. High-resolution neurometabolic coupling in the lateral geniculate nucleus. J. Neurosci. 2007, 27, 10223–10229. [Google Scholar]
- Thompson, J.K.; Peterson, M.R.; Freeman, R.D. Single-neuron activity and tissue oxygenation in the cerebral cortex. Science 2003, 299, 1070–1072. [Google Scholar]
- Thompson, J.K.; Peterson, M.R.; Freeman, R.D. High-resolution neurometabolic coupling revealed by focal activation of visual neurons. Nat. Neurosci. 2004, 7, 919–920. [Google Scholar]
- Thompson, J.K.; Peterson, M.R.; Freeman, R.D. Separate spatial scales determine neural activity-dependent changes in tissue oxygen within central visual pathways. J. Neurosci. 2005, 25, 9046–9058. [Google Scholar]
- Raichle, M.E.; Herscovitch, P.; Mintun, M.A.; Martin, W.R.; Powers, W. Dynamic measurements of local blood flow and metabolism in the study of higher cortical function in humans with positron emission tomography. Ann. Neurol. 1984, 15, S48–S49. [Google Scholar]
- Kwong, K.K.; Belliveau, J.W.; Chesler, D.A.; Goldberg, I.E.; Weisskoff, R.M.; Poncelet, B.P.; Kennedy, D.N.; Hoppel, B.E.; Cohen, M.S.; Turner, R. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc. Natl. Acad. Sci. USA 1992, 89, 5675–5679. [Google Scholar]
- Ogawa, S.; Tank, D.W.; Menon, R.; Ellermann, J.M.; Kim, S.G.; Merkle, H.; Ugurbil, K. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc. Natl. Acad. Sci. USA 1992, 89, 5951–5955. [Google Scholar]
- Iadecola, C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat. Rev. Neurosci. 2004, 5, 347–360. [Google Scholar]
- Zacchigna, S.; Lambrechts, D.; Carmeliet, P. Neurovascular signalling defects in neurodegeneration. Nat. Rev. Neurosci. 2008, 9, 169–181. [Google Scholar]
- Rolfe, D.F.; Brown, G.C. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol. Rev. 1997, 77, 731–758. [Google Scholar]
- Mintun, M.A.; Raichle, M.E.; Martin, W.R.; Herscovitch, P. Brain oxygen utilization measured with O-15 radiotracers and positron emission tomography. J. Nucl. Med. 1984, 25, 177–187. [Google Scholar]
- Kim, S.G.; Richter, W.; Ugurbil, K. Limitations of temporal resolution in functional MRI. Magn. Reson. Med. 1997, 37, 631–636. [Google Scholar]
- Thulborn, K.R.; Waterton, J.C.; Matthews, P.M.; Radda, G.K. Oxygenation dependence of the transverse relaxation time of water protons in whole blood at high field. Biochim. Biophys. Acta. 1982, 714, 265–270. [Google Scholar]
- Buxton, R.B. Introduction to Functional Magnetic Resonance Imaging: Principles and Techniques; Cambridge University Press: New York, USA, 2001. [Google Scholar]
- Hoge, R.D.; Atkinson, J.; Gill, B.; Crelier, G.R.; Marrett, S.; Pike, G.B. Investigation of BOLD signal dependence on cerebral blood flow and oxygen consumption: the deoxyhemoglobin dilution model. Magn. Reson. Med. 1999, 42, 849–863. [Google Scholar]
- Fox, P.T.; Raichle, M.E. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc. Natl. Acad. Sci. U S A 1986, 83, 1140–1144. [Google Scholar]
- Raichle, M.E.; Grubb, R.L., Jr.; Gado, M.H.; Eichling, J.O.; Ter-Pogossian, M.M. Correlation between regional cerebral blood flow and oxidative metabolism. In vivo studies in man. Arch. Neurol. 1976, 33, 523–526. [Google Scholar]
- Roland, P.E.; Eriksson, L.; Stone-Elander, S.; Widen, L. Does mental activity change the oxidative metabolism of the brain? J. Neurosci. 1987, 7, 2373–2389. [Google Scholar]
- Fox, P.T.; Raichle, M.E.; Mintun, M.A.; Dence, C. Nonoxidative glucose consumption during focal physiologic neural activity. Science 1988, 241, 462–464. [Google Scholar]
- Vafaee, M.S.; Gjedde, A. Model of blood-brain transfer of oxygen explains nonlinear flow-metabolism coupling during stimulation of visual cortex. J. Cereb. Blood Flow Metab. 2000, 20, 747–754. [Google Scholar]
- Vafaee, M.S.; Marrett, S.; Meyer, E.; Evans, A.C.; Gjedde, A. Increased oxygen consumption in human visual cortex: response to visual stimulation. Acta. Neurol. Scand. 1998, 98, 85–89. [Google Scholar]
- Vafaee, M.S.; Meyer, E.; Marrett, S.; Paus, T.; Evans, A.C.; Gjedde, A. Frequency-dependent changes in cerebral metabolic rate of oxygen during activation of human visual cortex. J. Cereb. Blood Flow Metab. 1999, 19, 272–277. [Google Scholar]
- Calamante, F.; Thomas, D.L.; Pell, G.S.; Wiersma, J.; Turner, R. Measuring cerebral blood flow using magnetic resonance imaging techniques. J. Cereb. Blood Flow Metab. 1999, 19, 701–735. [Google Scholar]
- Davis, T.L.; Kwong, K.K.; Weisskoff, R.M.; Rosen, B.R. Calibrated functional MRI: mapping the dynamics of oxidative metabolism. Proc. Natl. Acad. Sci. USA 1998, 95, 1834–1839. [Google Scholar]
- Kim, S.G.; Rostrup, E.; Larsson, H.B.; Ogawa, S.; Paulson, O.B. Determination of relative CMRO2 from CBF and BOLD changes: significant increase of oxygen consumption rate during visual stimulation. Magn. Reson. Med. 1999, 41, 1152–1161. [Google Scholar]
- Hoge, R.D.; Atkinson, J.; Gill, B.; Crelier, G.R.; Marrett, S.; Pike, G.B. Linear coupling between cerebral blood flow and oxygen consumption in activated human cortex. Proc. Natl. Acad. Sci. USA 1999, 96, 9403–9408. [Google Scholar]
- Cohen, L.B. Changes in neuron structure during action potential propagation and synaptic transmission. Physiol. Rev. 1973, 53, 373–418. [Google Scholar]
- Grinvald, A.; Lieke, E.; Frostig, R.D.; Gilbert, C.D.; Wiesel, T.N. Functional architecture of cortex revealed by optical imaging of intrinsic signals. Nature 1986, 324, 361–364. [Google Scholar]
- Horecker, B.L. the absorption spectra of hemoglobin and itsderivatives in the visible and nearinfra-red regions. J. Biol. Chem. 1943, 148, 173–183. [Google Scholar]
- Frostig, R.D.; Lieke, E.E.; Ts'o, D.Y.; Grinvald, A. Cortical functional architecture and local coupling between neuronal activity and the microcirculation revealed by in vivo high-resolution optical imaging of intrinsic signals. Proc. Natl. Acad. Sci. USA 1990, 87, 6082–6086. [Google Scholar]
- Sheth, S.A.; Nemoto, M.; Guiou, M.; Walker, M.; Pouratian, N.; Hageman, N.; Toga, A.W. Columnar specificity of microvascular oxygenation and volume responses: implications for functional brain mapping. J. Neurosci. 2004, 24, 634–641. [Google Scholar]
- Malonek, D.; Grinvald, A. Interactions between electrical activity and cortical microcirculation revealed by imaging spectroscopy: implications for functional brain mapping. Science 1996, 272, 551–554. [Google Scholar]
- Mayhew, J.; Johnston, D.; Berwick, J.; Jones, M.; Coffey, P.; Zheng, Y. Spectroscopic analysis of neural activity in brain: increased oxygen consumption following activation of barrel cortex. Neuroimage 2000, 12, 664–675. [Google Scholar]
- Jones, M.; Berwick, J.; Johnston, D.; Mayhew, J. Concurrent optical imaging spectroscopy and laser-Doppler flowmetry: the relationship between blood flow, oxygenation, and volume in rodent barrel cortex. Neuroimage 2001, 13, 1002–1015. [Google Scholar]
- Malonek, D.; Dirnagl, U.; Lindauer, U.; Yamada, K.; Kanno, I.; Grinvald, A. Vascular imprints of neuronal activity: relationships between the dynamics of cortical blood flow, oxygenation, and volume changes following sensory stimulation. Proc. Natl. Acad. Sci. USA 1997, 94, 14826–14831. [Google Scholar]
- Duong, T.Q.; Kim, D.S.; Ugurbil, K.; Kim, S.G. Spatiotemporal dynamics of the BOLD fMRI signals: toward mapping submillimeter cortical columns using the early negative response. Magn. Reson. Med. 2000, 44, 231–242. [Google Scholar]
- Kim, D.S.; Duong, T.Q.; Kim, S.G. High-resolution mapping of iso-orientation columns by fMRI. Nat. Neurosci. 2000, 3, 164–169. [Google Scholar]
- Logothetis, N.K.; Guggenberger, H.; Peled, S.; Pauls, J. Functional imaging of the monkey brain. Nat. Neurosci. 1999, 2, 555–562. [Google Scholar]
- Yacoub, E.; Shmuel, A.; Pfeuffer, J.; Van De Moortele, P.F.; Adriany, G.; Ugurbil, K.; Hu, X. Investigation of the initial dip in fMRI at 7 Tesla. NMR Biomed. 2001, 14, 408–412. [Google Scholar]
- Buxton, R.B. The elusive initial dip. Neuroimage 2001, 13, 953–958. [Google Scholar]
- Lindauer, U.; Royl, G.; Leithner, C.; Kuhl, M.; Gold, L.; Gethmann, J.; Kohl-Bareis, M.; Villringer, A.; Dirnagl, U. No evidence for early decrease in blood oxygenation in rat whisker cortex in response to functional activation. Neuroimage 2001, 13, 988–1001. [Google Scholar]
- Ernst, T.; Hennig, J. Observation of a fast response in functional MR. Magn. Reson. Med. 1994, 32, 146–149. [Google Scholar]
- Hu, X.; Le, T.H.; Ugurbil, K. Evaluation of the early response in fMRI in individual subjects using short stimulus duration. Magn. Reson. Med. 1997, 37, 877–884. [Google Scholar]
- Menon, R.S.; Ogawa, S.; Hu, X.; Strupp, J.P.; Anderson, P.; Ugurbil, K. BOLD based functional MRI at 4 Tesla includes a capillary bed contribution: echo-planar imaging correlates with previous optical imaging using intrinsic signals. Magn. Reson. Med. 1995, 33, 453–459. [Google Scholar]
- Silva, A.C.; Lee, S.P.; Iadecola, C.; Kim, S.G. Early temporal characteristics of cerebral blood flow and deoxyhemoglobin changes during somatosensory stimulation. J. Cereb. Blood Flow Metab. 2000, 20, 201–206. [Google Scholar]
- Hathout, G.M.; Varjavand, B.; Gopi, R.K. The early response in fMRI: a modeling approach. Magn. Reson. Med. 1999, 41, 550–554. [Google Scholar]
- Devor, A.; Dunn, A.K.; Andermann, M.L.; Ulbert, I.; Boas, D.A.; Dale, A.M. Coupling of total hemoglobin concentration, oxygenation, and neural activity in rat somatosensory cortex. Neuron 2003, 39, 353–359. [Google Scholar]
- Logothetis, N.K. The ins and outs of fMRI signals. Nat. Neurosci. 2007, 10, 1230–1232. [Google Scholar]
- Metzger, H.; Erdmann, W.; Thews, G. Effect of short periods of hypoxia, hyperoxia, and hypercapnia on brain O 2 supply. J. Appl. Physiol. 1971, 31, 751–759. [Google Scholar]
- Buerk, D. G.; Atochin, D. N.; Riva, C. E. Simultaneous tissue PO2, nitric oxide, and laser Doppler blood flow measurements during neuronal activation of optic nerve. Adv Exp Med Biol 1998, 454, 159–164. [Google Scholar]
- Metzger, H. Effects of direct stimulation on cerebral cortex oxygen tension level. Microvasc. Res. 1979, 17, 80–89. [Google Scholar]
- Silver, I.A. Cellular microenvironment in relation to local blood flow. Ciba. Found Symp. 1978, 56, 49–67. [Google Scholar]
- Lowry, J.P.; Boutelle, M.G.; O'Neill, R.D.; Fillenz, M. Characterization of carbon paste electrodes in vitro for simultaneous amperometric measurement of changes in oxygen and ascorbic acid concentrations in vivo. Analyst 1996, 121, 761–766. [Google Scholar]
- Lowry, J.P.; Fillenz, M. Evidence for uncoupling of oxygen and glucose utilization during neuronal activation in rat striatum. J. Physiol. 1997, 498, 497–501. [Google Scholar]
- Ances, B.M.; Buerk, D.G.; Greenberg, J.H.; Detre, J.A. Temporal dynamics of the partial pressure of brain tissue oxygen during functional forepaw stimulation in rats. Neurosci. Lett. 2001, 306, 106–110. [Google Scholar]
- Masamoto, K.; Omura, T.; Takizawa, N.; Kobayashi, H.; Katura, T.; Maki, A.; Kawaguchi, H.; Tanishita, K. Biphasic changes in tissue partial pressure of oxygen closely related to localized neural activity in guinea pig auditory cortex. J. Cereb. Blood Flow Metab. 2003, 23, 1075–1084. [Google Scholar]
- Offenhauser, N.; Thomsen, K.; Caesar, K.; Lauritzen, M. Activity-induced tissue oxygenation changes in rat cerebellar cortex: interplay of postsynaptic activation and blood flow. J. Physiol. 2005, 565, 279–294. [Google Scholar]
- Masamoto, K.; Vazquez, A.; Wang, P.; Kim, S.G. Trial-by-trial relationship between neural activity, oxygen consumption, and blood flow responses. Neuroimage 2008, 1, 442–450. [Google Scholar]
- Vazquez, A.L.; Masamoto, K.; Kim, S.G. Dynamics of oxygen delivery and consumption during evoked neural stimulation using a compartment model and CBF and tissue P(O2) measurements. Neuroimage 2008, 42, 49–59. [Google Scholar]
- Shmuel, A.; Yacoub, E.; Chaimow, D.; Logothetis, N.K.; Ugurbil, K. Spatio-temporal point-spread function of fMRI signal in human gray matter at 7 Tesla. Neuroimage 2007, 35, 539–552. [Google Scholar]
- Martindale, J.; Mayhew, J.; Berwick, J.; Jones, M.; Martin, C.; Johnston, D.; Redgrave, P.; Zheng, Y. The hemodynamic impulse response to a single neural event. J. Cereb. Blood Flow Metab. 2003, 23, 546–555. [Google Scholar]
- Jones, M.; Hewson-Stoate, N.; Martindale, J.; Redgrave, P.; Mayhew, J. Nonlinear coupling of neural activity and CBF in rodent barrel cortex. Neuroimage 2004, 22, 956–965. [Google Scholar]
- Hewson-Stoate, N.; Jones, M.; Martindale, J.; Berwick, J.; Mayhew, J. Further nonlinearities in neurovascular coupling in rodent barrel cortex. Neuroimage 2005, 24, 565–574. [Google Scholar]
- Sheth, S.A.; Nemoto, M.; Guiou, M.; Walker, M.; Pouratian, N.; Toga, A.W. Linear and nonlinear relationships between neuronal activity, oxygen metabolism, and hemodynamic responses. Neuron 2004, 42, 347–355. [Google Scholar]
- Heckman, G.M.; Bouvier, S.E.; Carr, V.A.; Harley, E.M.; Cardinal, K.S.; Engel, S.A. Nonlinearities in rapid event-related fMRI explained by stimulus scaling. Neuroimage 2007, 34, 651–660. [Google Scholar]
- Wan, X.; Riera, J.; Iwata, K.; Takahashi, M.; Wakabayashi, T.; Kawashima, R. The neural basis of the hemodynamic response nonlinearity in human primary visual cortex: Implications for neurovascular coupling mechanism. Neuroimage 2006, 32, 616–625. [Google Scholar]
- Martindale, J.; Berwick, J.; Martin, C.; Kong, Y.; Zheng, Y.; Mayhew, J. Long duration stimuli and nonlinearities in the neural-haemodynamic coupling. J. Cereb. Blood Flow Metab. 2005, 25, 651–661. [Google Scholar]
- Ances, B.M.; Zarahn, E.; Greenberg, J.H.; Detre, J.A. Coupling of neural activation to blood flow in the somatosensory cortex of rats is time-intensity separable, but not linear. J. Cereb. Blood Flow Metab. 2000, 20, 921–930. [Google Scholar]
- Logothetis, N.K.; Wandell, B.A. Interpreting the BOLD signal. Annu. Rev. Physiol. 2004, 66, 735–769. [Google Scholar]
- Monosov, I.E.; Trageser, J.C.; Thompson, K.G. Measurements of simultaneously recorded spiking activity and local field potentials suggest that spatial selection emerges in the frontal eye field. Neuron 2008, 57, 614–625. [Google Scholar]
- Belitski, A.; Gretton, A.; Magri, C.; Murayama, Y.; Montemurro, M.A.; Logothetis, N.K.; Panzeri, S. Low-frequency local field potentials and spikes in primary visual cortex convey independent visual information. J. Neurosci. 2008, 28, 5696–5709. [Google Scholar]
- Viswanathan, A.; Freeman, R.D. Neurometabolic coupling in cerebral cortex reflects synaptic more than spiking activity. Nat. Neurosci. 2007, 10, 1308–1312. [Google Scholar]
- Nir, Y.; Dinstein, I.; Malach, R.; Heeger, D.J. BOLD and spiking activity. Nat. Neurosci. 2008, 11, 523–524. [Google Scholar]
- Logothetis, N.K.; Pauls, J.; Augath, M.; Trinath, T.; Oeltermann, A. Neurophysiological investigation of the basis of the fMRI signal. Nature 2001, 412, 150–157. [Google Scholar]
- Goense, J.B.; Logothetis, N.K. Neurophysiology of the BOLD fMRI signal in awake monkeys. Curr. Biol. 2008, 18, 631–640. [Google Scholar]
- Rauch, A.; Rainer, G.; Logothetis, N.K. The effect of a serotonin-induced dissociation between spiking and perisynaptic activity on BOLD functional MRI. Proc. Natl. Acad. Sci. USA 2008, 105, 6759–6764. [Google Scholar]
- Caesar, K.; Gold, L.; Lauritzen, M. Context sensitivity of activity-dependent increases in cerebral blood flow. Proc. Natl. Acad. Sci. USA 2003, 100, 4239–4244. [Google Scholar]
- Lund, J.S. Anatomical organization of macaque monkey striate visual cortex. Annu. Rev. Neurosci. 1988, 11, 253–288. [Google Scholar]
- Alonso Bde, C.; Lowe, A.S.; Dear, J.P.; Lee, K.C.; Williams, S.C.; Finnerty, G.T. Sensory inputs from whisking movements modify cortical whisker maps visualized with functional magnetic resonance imaging. Cereb. Cortex. 2008, 18, 1314–1325. [Google Scholar]
- Feng, Z.C.; Roberts, E.L., Jr.; Sick, T.J.; Rosenthal, M. Depth profile of local oxygen tension and blood flow in rat cerebral cortex, white matter and hippocampus. Brain Res. 1988, 445, 280–288. [Google Scholar]
- Metzger, H.; Heuber, S. Local oxygen tension and spike activity of the cerebral grey matter of the rat and its response to short intervals of O2 deficiency or CO2 excess. Pflugers Arch. 1977, 370, 201–209. [Google Scholar]
- LaManna, J.C.; McCracken, K.A.; Patil, M.; Prohaska, O.J. Stimulus-activated changes in brain tissue temperature in the anesthetized rat. Metab. Brain Dis. 1989, 4, 225–237. [Google Scholar]
- Lecoq, J.; Tiret, P.; Charpak, S. Analysis of the spatio-temporal relationship between neuronal activity, local blood flow and oxygen partial pressure in the rat olfactory bulb. SFN Conf., San Diego, Nov. 3-7, 2007; 301, p. 10.
- Woolsey, T.A.; Van der Loos, H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res. 1970, 17, 205–242. [Google Scholar]
- Li, J.; Gilmour, G.; Bannerman, D.; De Flippi, G.; Mchugh, S.; Lowry, J. P.; Tricklebank, M. Establishing a controlled whisker-barrel evoked potential model of neural activation to be used in combination with real-time oxygen voltammetry in the anaesthetised rat. SFN Conf., San Diego, Nov. 3-7, 2007; 401, p. 27.
- Friston, K. Neurophysiology: the brain at work. Curr. Biol. 2008, 18, R418–R420. [Google Scholar]
© 2008 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bartlett, K.; Saka, M.; Jones, M. Polarographic Electrode Measures of Cerebral Tissue Oxygenation: Implications for Functional Brain Imaging. Sensors 2008, 8, 7649-7670. https://doi.org/10.3390/s8127649
Bartlett K, Saka M, Jones M. Polarographic Electrode Measures of Cerebral Tissue Oxygenation: Implications for Functional Brain Imaging. Sensors. 2008; 8(12):7649-7670. https://doi.org/10.3390/s8127649
Chicago/Turabian StyleBartlett, Kate, Mohamad Saka, and Myles Jones. 2008. "Polarographic Electrode Measures of Cerebral Tissue Oxygenation: Implications for Functional Brain Imaging" Sensors 8, no. 12: 7649-7670. https://doi.org/10.3390/s8127649
APA StyleBartlett, K., Saka, M., & Jones, M. (2008). Polarographic Electrode Measures of Cerebral Tissue Oxygenation: Implications for Functional Brain Imaging. Sensors, 8(12), 7649-7670. https://doi.org/10.3390/s8127649



