Recent Development in Optical Chemical Sensors Coupling with Flow Injection Analysis
Abstract
:1. Introduction
2. Absorbance
3. Reflectance
4. Fluorescence
4.1. Inorganic analysis
4.2. Organic analysis
5. Phosphorescence
5.1. Oxygen
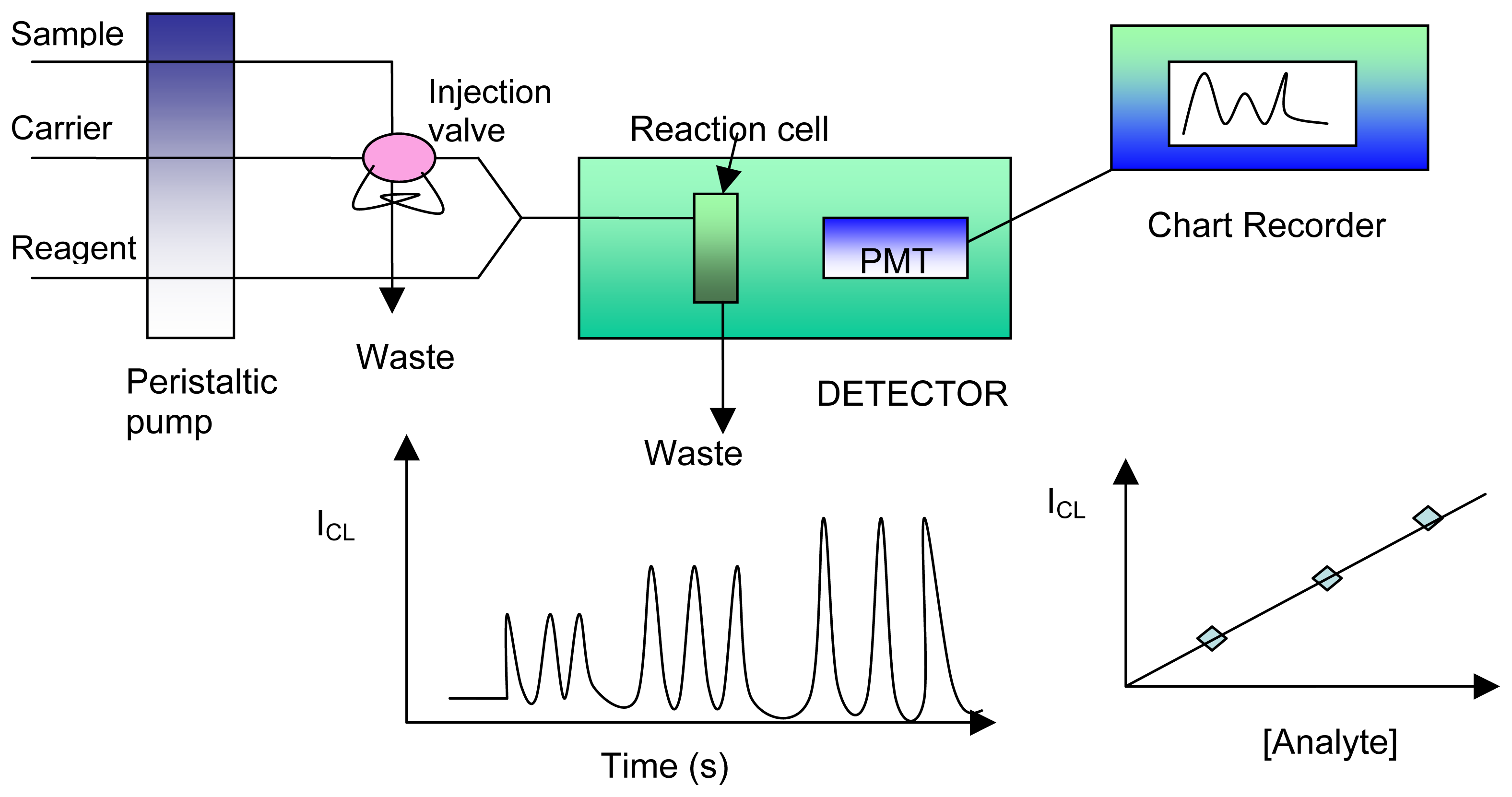
6. Chemiluminescence
6.1. Analytical applications
6.1.1. FIA-CL sensors for inorganic compounds
6.1.1.1. H2O2
6.1.1.2. Ammonium
6.1.1.3. Cyanide
6.1.1.4. Chlorine
6.1.1.5. Copper
6.1.1.6. Nitric oxide
6.1.1.7. Oxygen
6.1.1.8. Phosphate
6.1.1.9. Sulfite
6.1.2. FIA-CL sensors for organic and biological compounds
6.1.2.1. Immunoassay
6.1.2.2. Amino acids
6.1.2.3. Adrenaline and isoprenaline
6.1.2.4. Cholesterol
6.1.2.5. Ethanol
6.1.2.6. Formaldehyde
6.1.2.7. Glucose
6.1.2.8. Histamine
6.1.2.9. Hydrazine
6.1.2.10. Hydroxylamine
6.1.2.11. Oxalate
6.1.2.12. Resorcinol
6.1.2.13. Uric acid
6.1.2.14. Vitamins
6.1.3. FIA-CL sensors for drug analysis
6.1.3.1. Analgin
6.1.3.2. Berberine
6.1.3.3. Carbohydrate antigen
6.1.3.4. Ergonovine
6.1.3.5. Heroin
6.1.3.6. Hydralazine
6.1.3.7. Isoniazid
7. Conclusions
References and Notes
- Newcombe, D.T.; Cardwell, T.J.; Cattrall, R.W.; Kolev, S.D. An optical redox chemical sensor based on ferroin immobilised in a NafionR membrane. Analytica Chimica Acta 1999, 401, 137–144. [Google Scholar]
- Coo, L.dlC.; Belmonte, C.J. Nafion-PAN optical chemical sensor: optimisation by FIA. Talanta 2002, 58, 1063–1069. [Google Scholar]
- Lenarczuk, T.; Glab, S.; Koncki, R. Application of Prussian blue-based optical sensor in pharmaceutical analysis. Journal of Pharmaceutical and Biomedical Analysis 2001, 26, 163–169. [Google Scholar]
- García, S.; Albero, I.; Ortuño, J.A.; Sánchez-Pedreño, C.; Expósito, R. Flow-through bulk optrode for the spectrophotometric determination of perchlorate. Microchimica Acta 2003, 143, 59–63. [Google Scholar]
- Ruedas Rama, M.J.; Ruiz Medina, A.; Molina Díaz, A. bead injection spectroscopic flow-through renewable surface sensors with commercial flow cells as an alternative to reusable flow-through sensors. Analytica Chimica Acta 2003, 482, 209–217. [Google Scholar]
- Trinkel, M.; Trettnak, W.; Reininger, F.; benes, R.; O'Leary, P.; Wolfbeis, O.S. Study of the performance of an optochemical sensor for ammonia. Analytica Chimica Acta 1996, 320, 235–243. [Google Scholar]
- Yoshimura, K. On-line concentration and flow analysis of trace amounts of bismuth with anion-exchange method and ion-exchanger spectrophotometry. Bunseki Kagaku 1987, 36, 656–661. [Google Scholar]
- Zhu, C.; Hieftje, G.M. On-line determination of bromide ion in spent brine. Analytica Chimica Acta 1992, 256, 97–103. [Google Scholar]
- Vereda Alonso, E.; Cano Pavón, J.M.; Rios, A.; Valcárcel, M. Automatic determination of cobalt at the submicrogram per millilitre level using a flowthrough spectrophotometric sensor. Talanta 1996, 42, 1941–1947. [Google Scholar]
- Matsuoka, S.; Shiota, N.; Yoshimura, K. Determination of trace amounts of cobalt(II) by flow injection-solid phase spectrometry (FI-SPS) with 5-Br-PADAB. Analytical Sciences 2006, 22, 177–181. [Google Scholar]
- Vereda, E.; Rios, A.; Valcárcel, M. Simultaneous automatic determinationof trace amounts of copper and cobalt by use of a flow-through sensor and firt-derivative spectrometry. Analyst 1997, 122, 85–88. [Google Scholar]
- Yoshimura, K.; Matsuoka, S. 4,7-diphenyl-2,9-dimethyl-1,10-phenanthroline disulphonate and its application to the study of karst groundwater storm runoff. Analytica Chimica Acta 1992, 268, 225–233. [Google Scholar]
- Colsa Herrera, J.M.; Sánchez Rojas, F.; Bosch ojeda, C.; García de Torres, A.; Cano Pavón, J.M. Determination of trace amounts of copper using a flow-through spectrophotometric sensor. Laboratory Robotic Automation 2000, 12, 241–245. [Google Scholar]
- Jerónimo, P.C.A.; Araújo, A.N.; Montenegro, M.C.B.S.M.; Pasquini, C.; Raimundo, I.M., Jr. Direct determination of copper in urine using a sol-gel optical sensor coupled to a multicommutated flow system. Analytical and Bioanalytical Chemistry 2004, 380, 108–114. [Google Scholar]
- Ruedas Rama, M.J.; Ruiz Medina, A.; Molina Díaz, A. Resolution of biparametric mixtures using bead injection spectroscopic flow-through renewable surface sensors. Analytical Sciences 2005, 21, 1079–1084. [Google Scholar]
- Yoshimura, K. Application of ion-exchanger phase absorptiometry to flow analysis. Determination of trace amounts of chromium(VI) in water. Analyst 1988, 113, 471–474. [Google Scholar]
- Matsuoka, S.; Tennichi, Y.; Takehara, K.; Yoshimura, K. Flow analysis of micro amounts of chromium(III) and (VI) in natural water by solid phase spectrophotometry using diphenylcarbazide. Analyst 1999, 124, 787–791. [Google Scholar]
- Górski, L.; Malinowska, E. Fluoride-selective sensors based on polyurethane membranes doped with Zr(IV)-porphyrins. Analytica Chimica Acta 2005, 540, 159–165. [Google Scholar]
- Lázaro, F.; Luque de Castro, M.D.; Valcárcel, M. Integrated retention/spectrophotometric detection in flow-injection analysis. Determination of iron in water and wine. Analytica Chimica Acta 1989, 219, 231–238. [Google Scholar]
- Hase, U.; Yoshimurs, K. Determination of trace amounts of iron in highly purified water by ion-exchanger phase absorptiometry combined with flow analysis. Analyst 1992, 117, 1501–1506. [Google Scholar]
- Ayora Cañada, M. J.; Pascual reguera, M. I.; Molina Díaz, A. An integrated reaction-retention and spectrophotometric detection flow system for determination of nickel. Fresenius' Journal of Analytical Chemistry 1999, 363, 59–63. [Google Scholar]
- Amini, M. K.; Momeni-Isfahani, T.; Khorasani, J.H.; Pourhossein, M. development of an optical chemical sensor based on 2-(5-bromo-2-pyridylazo)-5-(diethylamino)phenol in Nafion for determination of nickel ion. Talanta 2004, 63, 713–720. [Google Scholar]
- Coo, L.dlC.; Martínez, I.S. nafion-based optical sensor for the determination of selenium in water samples. Talanta 2004, 64, 1317–1322. [Google Scholar]
- Ayora Cañada, M.J.; Pacual Reguera, M.I.; Molina Díaz, A. Continuous flow-through solid phase spectrophotometric determination of trace amounts of zinc. Analytica Chimica 1998, 375, 71–80. [Google Scholar]
- Schepers, D.; Schulze, G.; Frenzel, W. Spectrophotometric flow-through gas sensor for the determination of atmospheric nitrogen dioxide. Analytica Chimica Acta 1995, 308, 109–114. [Google Scholar]
- Sanz Martínez, A.; Rios, A.; Valcárcel, M. Determination of dissolved oxygen by use of a spectrophotometric flow-through sensor. Analytica Chimica Acta 1993, 284, 189–193. [Google Scholar]
- Capitán Vallvey, L.F.; Valencia, M.C.; Arana Nicolás, E. Monoparameter sensors for the determination of the antioxidants butylated hydroxyanisole and n-propyl gallate in foods and cosmetics by flow injection spectrophotometry. Analyst 2001, 126, 897–902. [Google Scholar]
- Capitán Vallvey, L.F.; Valencia, M.C.; Arana Nicolás, E. Simple resolution of butylated hydroxyanisole and n-propyl gallate in fatty foods and cosmetics samples by flow-injection solid-phase spectrophotometry. Journal of Food Science 2003, 68, 1595–1599. [Google Scholar]
- Capitán Vallvey, L.F.; Valencia, M.C.; Arana Nicolás, E. Flow-through sensor for determination of butylated hydroxytoluene in cosmetics. Analytical Letters 2002, 35, 65–81. [Google Scholar]
- Capitán Vallvey, L.F.; Valencia, M.C.; Arana Nicolás, E. Solid-phase ultraviolet absorbance spectrophotometric multisensor for the simultaneous determination of butylated hydroxytoluene and co-existing antioxidants. Analytica Chimica Acta 2004, 503, 179–186. [Google Scholar]
- Portela, D.C.; Pereira, I.M.F.; Paíga, P.; Deleure-Matos, C.; Vaz, M.C.V.F. Amperometric and spectrophotometric determination of carbaryl in natural waters and commercial formulations. Analytical and Bioanalytical Chemistry 2003, 377, 356–361. [Google Scholar]
- Agudo, M.; Rios, A.; Valcárcel, M. Automatic continuous-flow determination of paraquat at the subnanogram per millilitre level. Analytica Chimica Acta 1993, 281, 103–109. [Google Scholar]
- Cañizares, M.P.; Tena, M.T.; Luque de Castro, M.D. On line coupling of a liquid-liquid extraction flow-reversal system to a spectrophotometric flow-through sensor for the determination of polyphenols in olive oil. Analytica Chimica Acta 1996, 323, 55–62. [Google Scholar]
- Sánchez-Cabezudo, M.; Fernández-Romero, J.M.; Luque de Castro, M.D. Flow-through biosensor for sequential determination of total and prostatic acid phosphatase activity. Sensors and Actuators, B: Chemical 1995, B23, 9–15. [Google Scholar]
- Capitán-Vallvey, L.F.; Valencia, M.C.; Nicolás, E.A. Flow-through spectrophotometric sensor for the determination of sacchar in in low-calorie products. Food Additives and Contaminants 2004, 21, 32–41. [Google Scholar]
- Domínguez Vidal, A.; García Reyes, J.F.; Ortega Barrales, P.; Molina Díaz, A. UV spectrophotometric flow-through multiparameter sensor for the simultaneous determination of acetaminophen, acetylsalicylic acid, and caffeine. Analytical Letters 2002, 35, 2433–2447. [Google Scholar]
- Ruiz Medina, A.; Fernández de Córdova, M.L.; Ortega barrales, P.; Molina Díaz, A. International Journal of Pharmaceutics 2001, 216, 95–104.
- Ruiz Medina, A.; Fernández de Córdova, M.L.; Molina Díaz, A. sensitive determination of adrenaline by means of a flow-through solid phase UV spectrophotometric sensing device. Mikrochimica Acta 2000, 134, 101–105. [Google Scholar]
- Ruedas Rama, M.J.; Ruiz Medina, A.; Molina Díaz, A. A Prussian blue-based flow-through renewable surface optosensor for analysis of ascorbic acid. Microchemical Journal 2004, 78, 157–162. [Google Scholar]
- Ruiz Medina, A.; Fernández de Córdova, M.L.; Ayora Cañada, M.J.; Pascual Reguera, M.I.; Molina Díaz, A. A flow-through solid phase UV spectrophotometric biparameter sensor for the sequential determination of ascorbic acid and paracetamol. Analytica Chimica Acta 2000, 404, 131–139. [Google Scholar]
- Pascual Reguera, M.I.; Pérez Parras, G.; Molina Díaz, A. a single spectroscopic flow-through sensing device for determination of ciprofloxacin. Journal of Pharmaceutical and Biomedical Analysis 2004, 35, 689–695. [Google Scholar]
- Ortega Barrales, P.; Ruiz Medina, A.; Fernández de Córdova, M.L.; Molina Díaz, A. sensitive and simple determination of ciclofenac sodium by use of a continuous flow-injection procedure with solid-phase spectroscopic detection. Analytical Sciences 1999, 15, 985–989. [Google Scholar]
- Llorent Martínez, E.J.; García Reyes, J.F.; Ortega Barrales, P.; Molina Díaz, A. Solid-phase ultraviolet sensing system for determination of methylxanthines. Analytical and Bioanalytical Chemistry 2005, 382, 158–163. [Google Scholar]
- Ruiz Medina, A.; Fernández de Córdova, M.L.; Molina Díaz, A. A integrated flow-injection-solid phase spectrophotometric determination of minoxidil. Talanta 1999, 50, 277–282. [Google Scholar]
- Ruiz Medina, A.; Fernández de Córdova, M.L.; Molina Díaz, A. A very simple resolution of the mixture paracetamol and salicylamide by flow injection-solid phase spectrophotometry. Analytica Chimica Acta 1999, 394, 149–158. [Google Scholar]
- Ayora Cañada, M.J.; Pascual Reguera, M.I.; Molina Díaz, A. selective determination of pyridoxine in the presence of hydrosoluble vitamins using a continuous-flow solid phase sensing device with UV detection. International Journal of Pharmaceutics 2000, 202, 113–120. [Google Scholar]
- Pascual Reguera, M.I.; Ayora Cañada, M.J.; Castro Ruiz, M.S. determination of piroxicam by solid-phase spectrophotometry in a continuous flow system. European Journal of Pharmaceutical Sciences 2002, 15, 179–183. [Google Scholar]
- Hassan, S.S.M.; Mahmoud, W.H.; Othman, A.H.M. Determination of ranitidine in pharmaceutical preparations using manual and flow injection potentiometry and spectrophotometry. Analytica Chimica Acta 1996, 332, 39–48. [Google Scholar]
- Fernández de Córdova, M.L.; Ortega Barrales, P.; Rodríguez Torne, G.; Molina Díaz, A. a flow injection sensor for simultaneous determination of sulfamethoxazole and trimethoprim by using Sephadex SP C-25 for continuous on-line separation and solid phase UV transduction. Journal of Pharmaceutical and Biomedical Analysis 2003, 31, 669–677. [Google Scholar]
- Tena, M.T.; Luque de Castro, M.D.; Valcárcel, M. Flow-through photometric sensor for determination of sulfonamides. Analyst 1994, 119, 1625–1628. [Google Scholar]
- Ortega Barrales, P.; Fernández de Córdova, M.L.; Molina Díaz, A. A selective optosensor for UV spectrophotometric determination of thiamine in the presence of other vitamins B. Analytica Chimica Acta 1998, 376, 227–233. [Google Scholar]
- Pons, C.; Forteza, R.; Cerdá, V. Optical fibre reflectance sensor for the determination and speciation analysis of iron in fresh and seawater samples coupled to a multisyringe flow injection system. Analytica Chimica Acta 2005, 528, 197–203. [Google Scholar]
- Pons, C.; Forteza, R.; Cerdá, V. The use of anion-exchange disks in an optrode copules to a multi-syringe flow-injection system for the determination and speciation analysis of iron in natural water samples. Talanta 2005, 66, 210–217. [Google Scholar]
- Yusof, N.A.; Ahmad, M. A flow-through optical fibre reflectance sensor for the detection of lead ion based on immobilised gallocynine. Sensors and Actuators, B: Chemical 2003, 94, 201–209. [Google Scholar]
- Alava-Moreno, F.; Pereiro-García, R.; Díaz-García, M.E.; Sanz-Medel, A. Comparative study of two different approaches for active optical sensing of potassium with a chromoionophore. Sensors and Actuators, B: Chemical 1993, B11, 413–419. [Google Scholar]
- Miró, M.; Frenzel, W.; Estela, J.M.; Cerdá, V. A novel flow-through disk-based solid-phase extraction diffuse reflectance optrode. Application to preconcentration and determination of trace levels of nitrite. Analyst 2001, 126, 1740–1746. [Google Scholar]
- Ferrer, L.; De Armas, G.; Miró, M.; Estela, J.M.; Cerdá, V. Flow-through optical fiber sensor for automatic sulfide determination in waters by multisyringe flow injection analysis using solid-phase reflectometry. Analyst 2005, 130, 644–651. [Google Scholar]
- Ferrer, L.; De Armas, G.; Miró, M.; Estela, J.M.; Cerdá, V. Interfacing in-line gas-diffusion separation with optrode sportive preconcentration exploiting multisyringe flow injection analysis. Talanta 2005, 68, 343–350. [Google Scholar]
- Xavier, M.P.; Vallejo, B.; Marazuela, M.D.; Moreno-Bondi, M.C.; Baldini, F.; Falai, A. Fiber optic monitoring of carbamato pesticides using porous glass with covalently bound chlorophenol red. Biosensors and Bioelectronics 2000, 14, 895–905. [Google Scholar]
- Guzmán Mar, J.L.; López Martínez, L.; López de Alba, P.L.; Castrejón Durán, J.E.; Cerdá Martín, V. Optical fiber rwflectance sensor coupled to a multisyringe flow injection system for preconcentration and determination of 1-naphthylamine in water samples. Analytica Chimica Acta 2006, 573-574, 406–412. [Google Scholar]
- Reyes, J.F.G.; Barrales, P.O.; Díaz, A.M. Development of a solid surface fluorescence-based sensing system for aluminium monitoring in drinking water. Talanta 2005, 65, 1203–1208. [Google Scholar]
- Ruedas Rama, M.J.; Ruiz Medina, A.; Molina Díaz, A. Implementation of flow-through multi-sensors with bead injection spectroscopy: Fluorimetric renewable surface biparameter sensor for determination of berillium and aluminium. Talanta 2004, 62, 879–886. [Google Scholar]
- Xiao, D.; Wang, K.; Xiao, W. Synchronous fluorescence and absorbance dynamic liquid drop sensor for Cr(VI) determination at the femtomole level. Analyst 2001, 126, 1387–1392. [Google Scholar]
- Segura-Carretero, A.; Costa-fernández, J.M.; Pereiro, R.; Sanz-medel, A. Low-level mercury determination with thiamine by fluorescence optosensing. Talanta 1999, 49, 907–913. [Google Scholar]
- Yang, R.; Wang, K.; Xiao, D.; Luo, K.; Yang, X. Determination of low-level mercury based on a renewable-drops sensing technique. Fresenius' Journal of Analytical Chemistry 2000, 368, 797–802. [Google Scholar]
- Zhang, J.; Prestel, H.; Gahr, A.; Niessner, R. Development of a FIA system for the measurement of heavy metals using a fibre optic chemical sensor based on laser-induced fluorescence. Proceding of SPIE- The international Society for Optical Engineering 2000, 4077, 32–39. [Google Scholar]
- Ruedas Rama, M.J.; Ruiz Medina, A.; Molina Díaz, A. a flow-injection renewable surface sensor for the fluorimetric determination of vanadium(V) with Alizarin red S. Talanta 2005, 66, 1333–1339. [Google Scholar]
- Nomura, Y.; Nagakubo, K.; Ji, G.S.; Watanabe, A.; akimoto, T.; Mcniven, S.; Hayashi, K.; Karube, I. Continuous in situ cyanide monitoring using a highly sensitive and selective FIA system. Environmental science and Technology 2000, 34, 2618–2622. [Google Scholar]
- Chen, D.; Luque de castro, M.D.; Valcarcel, M. Fluorimetric sensor for the determination of fluoride at the nanograms per millilitre level. Analytica Chimica Acta 1990, 234, 345–352. [Google Scholar]
- Pandey, P.C. Detection of nitric oxide using an optical sensor. Indian Journal of Chemical Technology 1998, 5, 402–404. [Google Scholar]
- Masserini, R.T., Jr; Fanning, K.A. a sensor package for the simultaneous determination of nanomolar concentrations of nitrite, nitrate, and ammonia in seawater by fluorescence detection. Marine Chemistry 2000, 68, 323–333. [Google Scholar]
- Choi, M.M.F.; Tse, O.L. Flow injection analysis of water vapour based on a fluorosensor. Analytica Chimica Acta 2000, 423, 229–238. [Google Scholar]
- Gong, Z.; Zhang, Z.; Yang, X. Optosensor for cinchona alkaloids with C18 silica gel as substrate. Analyst 1997, 122, 283–285. [Google Scholar]
- Domínguez-Vidal, A.; Ortega-Barrales, P.; Molina-Díaz, A. Fast flow-injection fluorimetric determination of amiloride by using a solid sensing zone. Talanta 2002, 56, 1005–1013. [Google Scholar]
- Pandey, P.C.; Weetall, H.H. Detection of aromatic compounds based on DNA intercalation using an evanescent wave biosensor. Analytical Chemistry 1995, 67, 787–792. [Google Scholar]
- Fernández-Sánchez, J.F.; Segura Carretero, A.; Cruces-Blanco, C.; Fernández-Gutiérrez, A. Highly sensitive and selective fluorescence optosensor to detect and quantify benzo[a]pyrene in water samples. Analytica Chimica Acta 2004, 506, 1–7. [Google Scholar]
- Reyes, J.F.G.; Barrales, P.O.; Díaz, A.M. Gel-surface enhanced fluorescence sensing system coupled to a continuous-flow assembly for simultaneous monitoring of benomyl and carbendazim. Analytica Chimica Acta 2003, 493, 35–45. [Google Scholar]
- García Reyes, J.F.; Llorent Martínez, E.J.; Ortega Barrales, P.; Molina Díaz, A. Multiwavelength fluorescence based optosensor for simultaneous determination of fuberidazole, carbaryl and benomyl. Talanta 2004, 64, 742–749. [Google Scholar]
- Llorent-Martínez, E.J.; García-Reyes, J.F.; Ortega-Barrales, P.; Molina-Díaz, A. Flow-through fluorescence-based optosensor with on-line solid-phase separation for the simultaneous determination of a ternary pesticide mixture. Journal of AOAC International 2005, 88, 860–865. [Google Scholar]
- Reguera, I.P.; Rubio, M.G.; Díaz, A.M. Native fluorescence flow-through optosensor for the fast determination of diphenhydramine in pharmaceuticals. Analytical Sciences 2004, 20, 799–803. [Google Scholar]
- Ruiz-Medina, A.; Fernández de Córdova, M.L.; Molina-Díaz, A. A flow-through optosensing device with fluorimetric transduction for rapid and sensitive determination of dipyridamole in pharmaceuticals and human plasma. European Journal of Pharmaceutical Sciences 2001, 13, 385–391. [Google Scholar]
- Yang, R.H.; Wang, K.M.; Xiao, D.; Luo, K.; Yang, X.H. Flow injection renewable drops technique for assay of micro-amounts of DNA. Analytica Chimica Acta 2001, 432, 135–141. [Google Scholar]
- Dremel, B.A.A.; Schmid, R.D.; Wolfbeis, O.S. Comparison of two fibre-optic L-glutamate biosensors based on the detection of oxygen or carbon dioxide, and their application in combination with flow-injection analysis to the determination of glutamate. Analytica Chimica Acta 1991, 248, 351–359. [Google Scholar]
- Dremel, B.A.A.; Li, S.Y.; Schmid, R.D. On-line determination of glucose and lactate concentrations in animal cell culture based on fibre optic detection of oxygen in flow-injection analysis. Biosensors and Bioelectronics 1992, 7, 133–139. [Google Scholar]
- Tao, L.; Kennedy, R.T. on-line competitive immunoassay for insulin based on capillary electrophoresis with laser-induced fluorescence detection. Analytical Chemistry 1996, 68, 3899–3906. [Google Scholar]
- Ho, J.A.A.; zeng, S.C.; Huang, M.R.; Kuo, H.Y. Development of liposomal immunosensor for the measurement of isulin with femtomole detection. Analytica Chimica Acta 2006, 556, 127–132. [Google Scholar]
- Algar, S.O.; Martos, N.R.; Díaz, A.M. A continuous flow system combined with a sensing fluorimetric transductor for determination of α-naphthol. Microchemical Journal 2002, 73, 279–285. [Google Scholar]
- Ortega-Algar, S.; Ramos-Martos, N.; Molina-Díaz, A. A flow-through fluorimetric sensing device for determination of α- and β-naphthol mixtures using a partial least-squares multivariate calibration approach. Talanta 2003, 60, 313–323. [Google Scholar]
- Li, W.; Chen, J. on-line monitoring of nitrofurantoin in urine of rabbit by a flow-injection system with a fiber-optic chemical sensor. Acta Pharmaceutica Sinica 1995, 30, 599–604. [Google Scholar]
- Ortega-Algar, S.; Ramos-Martos, N.; Molina-Díaz, A. Fluorimetric flow-through sensing of quinine and quinidine. Microchimica Acta 2004, 147, 211–217. [Google Scholar]
- Tsukatani, T.; Matsumoto, K. Flow-injection fluorometric quantification of succinate in foodstuffs based on the use of an immobilized enzyme reactor. Analytica Chimica Acta 2000, 416, 197–203. [Google Scholar]
- Wortberg, M.; Midendorf, C.; Katerkamp, A.; Rump, T.; Krause, J.; Cammann, K. Flow-injection immunosensor for triazine herbicides using Eu(III) chelate label fluorescence detection. Analytica Chimica Acta 1994, 289, 177–186. [Google Scholar]
- Bart, J.C.; Judd, L.L.; Kusterbeck, A.W. Environmental immunoassay for the exploxive RDX using a fluorescent dye-labeled antigen and the continuous-flow immunosensor. Sensors and Actuators, B: Chemical 1997, 39, 411–418. [Google Scholar]
- Charles, P.T.; Bart, J.C.; Judd, L.L.; Gauger, P.R.; Ligler, F.S.; Kusterbeck, A.W. Continuous flow fluorescence based immunosensor for the detection of explosives and environmental pollutants. Proceeding of SPIE- The international Society for Optical Engineering 1997, 3105, 80–87. [Google Scholar]
- López-Flores, J.; Fernández de Córdova, M.L.; Molina-Díaz, A. Implementation of flow-through solid phase spectroscopic transduction with photochemically induced fluorescence: Determination of thiamine. Analytica Chimica Acta 2005, 535, 161–168. [Google Scholar]
- Feng, F.; Wang, K.; Chen, Z.; Chen, Q.; Lin, J.; Huang, S. Flow injection renewable drops spectrofluorimetry for sequential determinations of Vitamins B1, B2 and B6. Analytica Chimica Acta 2004, 527, 187–193. [Google Scholar]
- Badía, R.; Díaz-García, M.E. Cyclodextrin-based optosensor for the determination of wafarin in waters. Journal of Agricultural and Food Chemistry 1999, 47, 4256–4260. [Google Scholar]
- Costa-Fernández, J.M.; Díaz-García, M.E.; Sanz-Medel, A. A critical comparison of different solid supports to develop room-temperature phosphorescence sensing phases of air moisture. Sensors and Actuators, B: Chemical 1997, 38, 103–109. [Google Scholar]
- Costa-Fernández, J.M.; Díaz-García, M.E.; Sanz-Medel, A. Sol-gel immobilized room-temperature phosphorescent metal-chelate as luminescent oxygen sensing material. Analytica Chimica Acta 1998, 360, 17–26. [Google Scholar]
- Ovchinnikov, A.N.; Ogurtsov, V.I.; Trettnak, W.; Papkovsky, D.B. Enzymatic flow-injection analysis of metabolites using new type of oxygen sensor membranes and phosphorescence phase measurements. Analytical Letters 1999, 32, 701–716. [Google Scholar]
- Badía, R.; Díaz García, M.E. Tuning the performance of room temperature phosphorescence sensing materials for oxygen using factorial designs. Analytical Letters 2000, 33, 307–322. [Google Scholar]
- Costa-Fernández, J.M.; Bordel, N.; Campo, J.C.; Ferrero, F.J.; Pérez, M.A.; Sanz-Medel, A. Portable fibre optic oxygen sensor based on room-temperature phosphorescence lifetime. Mikrochimica Acta 2000, 134, 145–152. [Google Scholar]
- Díaz-García, J.; Costa-Fernández, J.M.; Bordel-García, N.; Sanz-Medel, A. Room-temperature phosphorescence fiber-optic instrumentation for simultaneous multiposition analysis of dissolved oxygen. Analytica Chimica Acta 2001, 429, 55–64. [Google Scholar]
- Duan, Y.; Chang, H.; Li, G.; Jin, W. Oxygen sensor based on palladium-porphyrin room-temperature phosphorescence quenching. Fenxi Huaxue 2003, 31, 1069–1072. [Google Scholar]
- Díaz-García, M.e.; Noval Gutiérrez, B.; Badía, R. Tailoring room-temperature phosphorescent ormosil particles for oxygen recognition in organic solvents. Sensors and Actuators B 2005, 110, 66–72. [Google Scholar]
- Gong, Z.; Zhang, Z. Room temperature phosphorescenc optosensing for gadolinium. Mikrochimica Acta 1997, 126, 117–121. [Google Scholar]
- San Vicente de la Riva, B.; Costa-Fernández, J.M.; Pereiro, R.; Sanz-Medel, A. Flow-through room temperature phosphorescence optosensing for the determination of lead in sea water. Analytica Chimica Acta 1999, 395, 1–9. [Google Scholar]
- Liu, Y.M.; garcía, R.P.; Díaz García, M.E.; Sanz-Medel, A. Room-temperature luminescence optosensing based on immobilized metal chelates: Application to iodide determination. Analytica Chimica Acta 1991, 255, 245–251. [Google Scholar]
- Traviesa-Álvarez, J.M.; Costa-Fernáncez, J.M.; Pereiro, R.; Sanz-medel, A. Flow-through solid-phase energy transfer-room temperature phosphorescence for orthophosphate determinations at trace levels. Talanta 2004, 62, 827–833. [Google Scholar]
- Papkovsky, D.; uskova, M.A.; Ponomarev, G.V.; Korpela, T.; Kulmala, S.; Guilbault, G.G. Optical sensing of sulfite with a phosphorescent probe. Analytica Chimica Acta 1998, 374, 1–9. [Google Scholar]
- Álava-Moreno, F.; Valencia-González, M.J.; Díaz-García, M.E. Room temperature phosphorescence optosensor for anthracyclines. Analyst 1998, 123, 151–154. [Google Scholar]
- Cuenca-Trujillo, R.M.; Ayora-Cañada, M.J.; Molina-Díaz, A. Determination of ciprofloxacin with a room-temperature phosphorescence flow-through sensor based on lanthanide-sensitized luminescence. Journal of AOAC International 2002, 85, 1268–1272. [Google Scholar]
- Casado-Terrones, S.; Fernández-Sánchez, J.F.; Segura-Carretero, A.; Fernández-Gutiérrez, A. The development and comparison of a fluorescence and a phosphorescence optosensors for determining the plant growth regulator 2-naphthoxyacetic acid. Sensors and Actuators, B: Chemical 2005, 107, 929–935. [Google Scholar]
- Salinas-Castillo, A.; Fernández-Sánchez, J.F.; Segura-Carretero, A.; Fernández-Gutiérrez, A. A facile flow-through phosphorimetric sensing device for simultaneous determination of naptalam and its metabolite 1-naphthylamine. Analytica Chimica Acta 2004, 522, 19–24. [Google Scholar]
- Salinas-Castillo, A.; Fernández-Sánchez, J.F.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Solid-phase phosphorescence characterization of polycyclic aromatic hydrocarbons and selective determination of benzo(a)pyrene in water samples. Analytica Chimica Acta 2005, 550, 53–60. [Google Scholar]
- Álava-Moreno, F.; Díaz-García, M.E.; Sanz-Medel, A. Room temperature phosphorescence optosensor for tetracyclines. Analytica Chimica Acta 1993, 281, 637–644. [Google Scholar]
- García-Campaña, A.M.; Baeyens, W.R.G.; Zhang, X.R.; Smet, E.; Van Der Weken, G.; Nakashima, K.; Calokerinos, A.C. Detection in the liquid phase applying chemiluminescence. Biomedical Chromatography 2000, 14, 166–172. [Google Scholar]
- García-Campaña, A.M.; Baeyens, W.R.G. Principles and recent analytical applications of chemiluminescence. Analusis 2000, 28, 686–698. [Google Scholar]
- Aboul-Enein, H.Y.; Stefan, R.I.; Van Standen, J.F.; Zhang, X.R.; García-Campaña, A.M.; Baeyens, W.R.G. Recent developments and applications of chemiluminescence sensors. Critical Reviews in Analytical Chemistry 2000, 30, 271–289. [Google Scholar]
- García-Campaña, A.M.; Baeyens, W.R.G. Chemiluminescence in Analytical Chemistry; Marcel Dekker: NY, 2001; pp. 567–591. [Google Scholar]
- Zhang, X.R.; Baeyens, W.R.G.; García-Campaña, A.M.; Ouyang, J. Recent developments in chemiluminescence sensors. Trac-Trends Analytical Chemistry 1999, 18, 384–391. [Google Scholar]
- Zhang, Z.; Zhang, S.; Zhang, X. Recent developments and applications of chemiluminescence sensors. Analytica Chimica Acta 2005, 541, 37–47. [Google Scholar]
- Qin, W. Flow injection chemiluminescence-bases chemical sensors. Analytical Letters 2002, 35, 2207–2220. [Google Scholar]
- Hool, K.; Nieman, T.A. Chemiluminescence analysis in flowing streams with luminol immnobilized on silica and controlled-pore glass. Analytical Chemistry 1987, 59, 869–872. [Google Scholar]
- Nieman, T.A. Immobilized and solid-state reagent systems for luminol chemiluminescence in flow systems. Mikrochimica Acta 1988, 3, 239–247. [Google Scholar]
- Downey, T.M.; Nieman, T.A. Chemiluminescence detection using regenerable tris(2,2′-bypyridyl)ruthenium(II) immobilized in nafion. Analytical Chemistry 1992, 64, 261–268. [Google Scholar]
- Kuniyoshi, A.; Hatta, K.; Suzuki, T.; Masuda, A.; Yamada, M. Chemiluminiscence sensor with Mn(II)-tetrakis(4-sulfonatophenyl)-porphyrin immobilized on dioctadecyldimethylammonium chloride bilayer membranas incorporated into PVC film. Analytical Letter 1996, 29, 673–685. [Google Scholar]
- Ponten, E.; glad, B.; stigbrand, M.; Sjogren, A.; Irgum, K. Non-porous spherical polymer particles for luminescent solid phase detection. Analytica Chimica Acta 1996, 320, 87–97. [Google Scholar]
- Zhang, Z.J.; Qin, W. Fiber optic chemical sensors based on chemiluminescence and bioluminescence. Fenxi Kexue Xuebao 1997, 13, 72–77. [Google Scholar]
- Aboul-Enein, H.Y.; Stefan, R.I.; Van Standen, J.F. Chemiluminescence based (bio)sensors- An overview. Critical Review Analytical Chemistry 1999, 29, 323–331. [Google Scholar]
- Coulet, P.R.; Blum, L.J.; Gautier, S.M. Luminescence-based fibre-optic probles. Sensors and Actuators, B: Chemical 1993, B11, 57–61. [Google Scholar]
- Karube, I.; Yokoyama, K. trends in biosensor research and development. Sensors and Actuators, B: Chemical 1993, B13, 12–15. [Google Scholar]
- Sanz-Medel, A. Solid surface photoluminescence and flow analysis: A happy marriage. Analytica Chimica Acta 1993, 283, 367–378. [Google Scholar]
- Busch, M.; Hobel, W.; Polster, J. Sofware FIACRE: Bioprocess monitoring on the basis of flow injection analysis using simultaneously a ures optrode and a glucose luminescence sensor. Journal of Biotechnology 1993, 31, 327–343. [Google Scholar]
- Ruzicka, J.; Pollema, C.H.; Scudder, K.M. Jet ring cell: A tool for flow injection spectroscopy and microscopy on a renewable solid support. Analytical Chemistry 1993, 65, 3566–3570. [Google Scholar]
- Jorgensen, A.M.; Mogensen, K.B.; Kutter, J.P.; Geschke, O. A biochemical microdevice with an integrated chemiluminescence detector. Sensors and Actuators B 2003, 90, 15–21. [Google Scholar]
- Miró, M.; Estela, J.M.; Cerdá, V. Potentials of multisyringe flor injection analysis for chemiluminescence detection. Analytica Chimica Acta 2005, 541, 57–68. [Google Scholar]
- Janasek, D.; Spohn, U.; Beckmann, D. Novel chemiluminometric H2O2 sensors for the selective flow injection analysis. Sensors and actuators B 1998, 51, 107–113. [Google Scholar]
- Qin, W.; Zhang, Z.; Li, B.; Liu, S. Chemiluminescence flow-sensing system for hydrogen peroxide with immobilized reagents. Analytica Chimica Acta 1998, 372, 357–363. [Google Scholar]
- Li, B.X.; Qin, W.; Zhang, Z.J. Flow-injection chemiluminescence sensor for hydrogen peroxide with immobilized reagents. Chinese Chemical Letters 1998, 9, 471–472. [Google Scholar]
- Janasek, D.; Spohn, U.; Kiesow, A.; Heilmann, A. Enzyme modified membranes protected by plasma polymer layers. Sensors and actuators B 2001, 78, 228–231. [Google Scholar]
- Kiba, N.; Tokizawa, T.; Kato, S.; Tachibana, M.; Tani, K.; Koizumi, H.; Edo, M.; Yonezawa, E. Flow-through micro sensor using immobilized peroxidise with chemiluminometric FIA system for determining hydrogen peroxide. Analytical Sciences 2003, 19, 823–827. [Google Scholar]
- Lin, J.M.; yamada, M. Oxidation reaction between periodate and polyhydroxyl compounds and its application to chemiluminescence. Analytical Chemistry 1999, 71, 1760–1766. [Google Scholar]
- Li, B.X.; Qin, W.; Zhang, Z.J. Chemiluminescence flow sensor for determination of the ammonium ion. Chinese Chemical Letters 1998, 9, 743–745. [Google Scholar]
- Lu, J.; Qin, W.; Zhang, Z.; Feng, M.; Wang, Y. A flow-injection type chemiluminescence-based sensor for cyanide. Analytica Chimica Acta 1995, 304, 369–373. [Google Scholar]
- Liu, S.N.; Qin, W.; Zhang, Z.J. Flow-injection chemiluminescence sensor for the determination of free chlorine in tap water. Chinese Chemical Letters 1996, 7, 1023–1026. [Google Scholar]
- Qin, W.; Zhang, Z.; Liu, S. Flow-injection chemiluminescence sensor for the determination of free chlorine in tap water. Analytical Letters 1997, 30, 11–19. [Google Scholar]
- Qin, W.; Zhang, Z.; Liu, H. Chemiluminescence flow-through sensor for copper based on an anodic stripping voltammetric flow cell and ion-exchange column with immobilized reagents. Analytical Chemistry 1998, 70, 3579–3584. [Google Scholar]
- Gibson, C.; Byrne, P.; Gray, D.; MacCraith, B.; Paul, B.; Tyrrell, E. Design of a micro-fluidic sensor for high sensitivity copper(II) sensing applications. Proceeding of SPIE-The international Society for Optical Engineering 2002, 4876, 615–622. [Google Scholar]
- Yao, D.; Vlessidis, A.G.; Evmiridis, N.P. On-line monitoring of nitric oxide complexed with porphyrine-bearing biochemical materials by using flow injection with chemiluminescence detection. Analytica Chimica Acta 2001, 435, 273–280. [Google Scholar]
- Dai, K.; Evmiridis, N.P.; Vlessidis, A.; Zhou, Y. Chemiluminescence detection of peroxynitrite with flow injection. Proceeding of SPIE-The international Society for Optical Engineering 2001, 4414, 104–107. [Google Scholar]
- Okamoto, T.; Tanaka, K.; Goto, H.; Lin, J.M.; Yamada, M.; Asano, Y. Luminol immobilized anion-exchange resin as an indicador phase for a chemiluminescence oxigen gas sensor. Analytical Communication 1999, 36, 181–183. [Google Scholar]
- Nakamura, H.; Ikebukuro, K.; McNiven, S.; Krube, I.; Yamamoto, H.; Hayashi, K.; Suzuki, M.; Kubo, I. a chemiluminescent FIA biosensor for phosphate ion monitoring using pyruvate oxidase. Biosensors & Bioelectronics 1997, 12, 959–966. [Google Scholar]
- Nakamura, H.; Tanaka, H.; Hasegawa, M.; Masuda, Y.; Arikawa, Y.; Nomura, Y.; Ikebukuro, K.; Karube, I. An automatic flor-injection análisis system for determining phosphate ion in river water using pyruvate oxidase G (from Aerococcus viridans). Talanta 1999, 50, 799–807. [Google Scholar]
- Nakamura, H.; Hasegawa, M.; Nomura, Y.; Arikawa, Y.; Matsukawa, R.; Ikebukuro, K.; Karube, I. Development of a highly sensitive chemiluminescence flow-injection analysis sensor for phosphate-ion detection using maltose phosphorylase. Journal of Biotechnology 1999, 75, 127–133. [Google Scholar]
- Nakamura, H.; Hasegawa, M.; Nomura, Y.; Ikebukuro, K.; Arikawa, Y.; Karube, I. Improvement of a CL-FIA system using maltose phosphorylase for the determination of phosphate-ion in freshwater. Analytical Letters 2003, 36, 1805–1817. [Google Scholar]
- Morais, I.P.A.; Miró, M.; Manera, M.; Estela, J.M.; Cerdá, V.; Souto, M.R.S.; Rangel, A.O.S.S. Flor-through solid-phase based optical sensor for the multisyringe flor injection trace determination of orthophosphate in waters with chemiluminescence detection. Analytica Chimica Acta 2004, 506, 17–24. [Google Scholar]
- Lin, J.M.; Qu, F.; Yamada, M. Chemiluminescent investigation of tris(2,2′-bipyridyl)ruthenium(II) immobilized on a cationic ion-exchange resin and its application to analysis. Analytical and Bioanalytical Chemistry 2002, 374, 1159–1164. [Google Scholar]
- Zheng, X.; Zhang, Z. Flow-injection chemiluminescence detecting sulfite with in situ electrogenerated Mn3+ as the oxidant. Sensors and Actuators B 2002, 84, 142–147. [Google Scholar]
- García-Campaña, A.M.; Baeyens, W.R.G.; Cuadros-Rodríguez, L.; Alés Barrero, F.; Bosque-Sendra, J.M.; Gámiz-Gracia, L. Potencial of chemiluminescence and bioluminescence in organic analysis. Current Organic Chemistry 2002, 6, 1–20. [Google Scholar]
- Osipov, A.P.; Zaitseva, N.V.; Egorov, A.M. Chemiluminescent immunoenzyme biosensor with a thin-layer flow-through cell. Application for study of a real-time biomolecular antigen-antibody interaction. Biosensor & Bioelectronics 1996, 11, 881–887. [Google Scholar]
- Martin, A.F.; Nieman, T.A. Chemiluminescence biosensors using tris(2,2′-bipyridyl)ruthenium(II) and dehydrogenases immobilized in cation exchange polymers. Biosensor & Bioelectronics 1997, 12, 479–489. [Google Scholar]
- Chen, X.; Zhang, X.E.; Chai, Y.Q.; Hu, W.P.; Zhang, Z.P.; Zhang, X.M.; Cass, A.E.G. DNA optical sensor: a rapid method for the detection of DNA hybridization. Biosensor & Bioelectronics 1998, 13, 451–458. [Google Scholar]
- Marquette, C.A.; Coulet, P.R.; Blum, L.J. Semi-automated membrane based chemiluminescent immunosensor for flow injection analysis of okadaic acid in mussels. Analytica Chimica Acta 1999, 398, 173–182. [Google Scholar]
- Janasek, D.; Spohn, U. A chemiluminometric FIA procedure for the enzymatic determination of L-aspartate. Sensors and Actuators B 2001, 74, 163–167. [Google Scholar]
- Blankestein, G.; preuschoff, F.; Spohn, U.; Mohr, K.H.; Kula, M.R. Determination of L-glutamate and L-glutamine by flow-injection analysis and chemiluminescence detection: Comparison of an enzyme column and enzyme membrane sensor. Analytica Chimica Acta 1993, 271, 231–237. [Google Scholar]
- Spohn, U.; Preuschoff, F.; Blankestein, G.; Janasek, D.; Kula, M.R.; Hacker, A. Chemiluminometric enzyme sensors for flow-injection analysis. Analytica Chimica Acta 1995, 303, 109–120. [Google Scholar]
- Janasek, D.; Spohn, U. Chemiluminometric flow injection analysis procedures for the enzymatic determination of L-alanine, α-ketoglutarate and L-glutamate. Biosensors & Bioelectronics 1999, 14, 123–129. [Google Scholar]
- Kiba, N.; Ito, S.; Tachibana, M.; Tani, K.; Koizumi, H. Flow-through chemiluminescence sensor using immobilized oxidases for the selective determination of L- glutamate in flow-injection system. Analytical Sciences 2001, 17, 929–933. [Google Scholar]
- Kiba, N.; Miwa, T.; Tachibana, M.; Tani, K.; Koizumi, H. Chemiluminometric sensor for simultaneous determination of L-glutamate and L-Lysine with immobilized oxidases in a flow injection system. Analytical Chemistry 2002, 74, 1269–1274. [Google Scholar]
- Kiba, N.; Koga, A.; Tachibana, M.; Tani, K.; Koizumi, H.; kayama, T.; Yamamura, A.; Matsumoto, K.; Okuda, T.; Yokotsuka, K. Flow-injection determination of L-histidine with an immobilized histidine oxidase from Brevibacillus borstelensis KAIT-B-=22 and chemiluminescence detection. Analytical Sciences 2006, 22, 95–98. [Google Scholar]
- Berger, A.; Blum, L.J. Enhancement of the response of a lactate oxidase/peroxidise-based fiberoptic sensor by compartmentalization of the enzyme layer. Enzyme and Microbial technology 1994, 16, 979–984. [Google Scholar]
- Zhou, G.J.; Zhang, G.F.; Chen, H.Y. Development of integrated chemiluminescence flow sensor for the determination of adrenaline and isoprenaline. Analytica Chimica Acta 2002, 463, 257–263. [Google Scholar]
- Huang, Y.; Zhang, C.; Zhang, Z. Chemiluminescence flow biosensor system for cholesterol with immobilized reagents. Analytical Sciences 1999, 15, 867–870. [Google Scholar]
- Xie, X.; Suleiman, A.A.; Guilbault, G.G. Flow-injection determination of ethanol by fiber-optic chemiluminescence measurement. Analytica Chimica Acta 1992, 266, 325–329. [Google Scholar]
- Song, Z.; Hou, S. On-line monitoring of formaldehyde in water and air using chemiluminescence detection. International Journal Environmental Analytical Chemistry 2003, 83, 807–817. [Google Scholar]
- Martin, A.F.; Nieman, T.A. Glucose quantitation using an immobilized glucose dehydrogenase enzyme reactor and a tris(2,2(000B4)-bipyridyl)ruthenium(II) chemiluminescent sensor. Analytica Chimica Acta 1993, 281, 475–481. [Google Scholar]
- Cattaneo, M.V.; Luong, J.H.T. On-line chemiluminescence assay using FIA and fiber optics for urinary and blood glucose. Enzyme and Microbial Technology 1993, 15, 424–428. [Google Scholar]
- Blum, L.J. Chemiluminescence sensor flow injection analysis of the glucose in drinks with a bienzyme fiberoptic biosensor. Enzyme and Microbial Technology 1993, 15, 407–411. [Google Scholar]
- Wilson, R.; Kremeskotter, J.; Schiffrin, D.J. Electrochemiluminescence detection of glucose oxidase as a model for flow injection immunoassays. Biosensors & Bioelectronics 1996, 11, 805–810. [Google Scholar]
- Li, Q.; Wang, Y.; Zhang, X.; Luo, G. preparation of immobilized glucose oxidase column by sol-gel technique and its application to the chemiluminescent glucose sensor. Fenxi Huaxue 1999, 27, 1274–1277. [Google Scholar]
- Kiba, N.; Inoue, Y.; Tachibana, M.; Tani, K.; Koizumi, H. Simultaneous determination of d-glucose and 3-hydroxybutyrate by chemiluminescence detection with immobilized encimes in a flow injection system. Analytical Sciences 2003, 19, 1203–1206. [Google Scholar]
- Marquette, C.A.; Degiuli, A.; Blum, L.J. Fiberoptic biosensors based on chemiluminescent reactions. Applied Biochemistry and Biotechnology-Part A Enzyme Engieriering and Biotechnology 2000, 89, 107–115. [Google Scholar]
- Sekiguchi, Y.; Nishikawa, A.; Makita, H.; Yamamura, A.; Matsumoto, K.; Kiba, N. Flow-through chemiluminescence sensor using immobilized histamine oxidase from Arthrobacter crystallopoietes KAIT-B-007 and preoxidase for selective determination of histamine. Analytical Sciences 2001, 17, 1161–1164. [Google Scholar]
- Song, Z.; Wang, L.; Zhao, T. Chemiluminescence flow sensor for hydrazine with immobilized reagents. Analytical Letters 2001, 34, 399–413. [Google Scholar]
- Zhang, S.C.; Zhou, G.J.; Ju, H.X. Chemiluminescence sensor for the determination of hydroxilamine by electrostatically immobilizing luminol and periodate. Chemical Research in Chinese Universities 2003, 19, 155–159. [Google Scholar]
- Downey, T.M.; Nieman, T.A. Chemiluminescence detection using regenerable tris(2,2′-bipyridyl)ruthenium(II) immobilized in Nafion. Analytical Chemistry 1992, 64, 261–268. [Google Scholar]
- Song, Z.; Wang, L. Resorcinol chemosensor based on detection of chemiluminescence with immobilized reagents. Microchemical Journal 2001, 68, 47–52. [Google Scholar]
- Song, Z.H.; Hou, S. Chemiluminescence assay for uric acid in human serum and urine using flow-injection with immobilized reagents technology. Analytical Bioanalytical Chemistry 2002, 372, 327–332. [Google Scholar]
- Zhang, Z.; Qin, W. Chemiluminescence flow sensor for the determination of ascorbic acid with immobilized reagents. Talanta 1996, 43, 119–124. [Google Scholar]
- Wang, F.; Qin, W.; Zhang, Z. Flow-injection chemiluminescence sensor for the determination of ascorbic acid. Fénix Huaxue 1997, 25, 1257–1258. [Google Scholar]
- Song, Z.; Hou, S. Determination of picomole amounts of thiamine through flow-injection analysis based on the supression of luminol-KIO4 chemiluminescence system. Journal of Pharmaceutical and Biomedical Analysis 2002, 28, 683–691. [Google Scholar]
- Song, Z.; Hou, S. Flow through sensor for the chemiluminescence determination of thiamine. Chemia Analityczna 2002, 47, 747–757. [Google Scholar]
- Wang, L.; Song, Z.H. Chemiluminescence flow-through sensor for the determination of vitamin B2 using controlled-reagent-release technology. Yaoxue Xuebao 2002, 37, 793–797. [Google Scholar]
- Qin, W.; Zhang, Z.; Liu, H. Chemiluminescence flow sensor for the determination of vitamin B12. Analítica Chimica Acta 1997, 357, 127–132. [Google Scholar]
- Song, Z.; Zhang, Z. A chemiluminescence biosensor for amygdalin. Fenxi Huaxue 2000, 28, 966–967. [Google Scholar]
- Huang, Y.; Chen, Z.; Zhang, Z. Flow-injection determination of vitamin K3 by a chemiluminescence sensor. Analytical Sciences 1999, 15, 1227–1230. [Google Scholar]
- Song, Z.; Zhou, X. Chemiluminescence flow sensor for folic acid with immobilized reagents. Spectrochimica Acta Part A 2001, 57, 2567–2574. [Google Scholar]
- Song, Z.; Wang, L. Chemiluminescence investigation of detection of rutin in medicine and human urine using controlled-reagent-release technology. Journal of Agricultural and Food Chemistry 2001, 49, 5697–5701. [Google Scholar]
- Huang, Y.M.; Zhang, C.; Zhang, X.R.; Zhang, Z.J. A novel chemiluminescence flow-through sensor for the determination of analgin. Fresenius Journal Analytical Chemistry 1999, 365, 381–383. [Google Scholar]
- Song, Z.; Zhang, N. In vitro monitoring sub-nanogram amounts analgin in human urine by its inhibitory of the luminol-periodate chemiluminescence reaction using reagent immobilization release technique. Bioorganic & Medicinal Chemistry 2002, 10, 2091–2097. [Google Scholar]
- Zhao, L.; baoxin, L.; Zhang, Z.; Lin, J.M. Chemiluminescent flow-through sensor for automated dissolution testing of analgin tablets using manganese dioxide as oxidate. Sensors and actuators B 2004, 97, 266–271. [Google Scholar]
- Song, Z.; Zhao, T.; Wang, L.; Xiao, Z. Chemiluminescence flow sensor for berberine with immobilized reagents. Bioorganic & Medicinal Chemistry 2001, 9, 1701–1705. [Google Scholar]
- Lin, J.; Yan, F.; Hu, X.; Ju, H. Chemiluminescent immunosensor for CA19-9 based on antigen immobilization on a cross-linked chitosan membrane. Journal of Immunological Methods 2004, 291, 165–174. [Google Scholar]
- Gong, Z.; Huang, Y.; Zhang, Z. Flow injection chemiluminescence sensor for the determination of ergonovine maleate. Fenxi Huaxue 2004, 32, 641–643. [Google Scholar]
- Zhuang, Y.; Zhang, D.; Ju, H. Sensitive determination of heroin based on electrogenerated chemiluminescence of tris(2,2′-bipyridyl)ruthenium(II) immobilized in zeolite Y modified carbon paste electrode. Analyst 2005, 130, 534–540. [Google Scholar]
- Xiong, Y.; Zhou, H.; Zhang, Z.; He, D.; He, C. Determination of hydralazine with flow injection chemiluminescence sensor using molecularly imprinted polymer as recognition element. Journal of Pharmaceutical and Biomedical Analysis 2006, 41, 694–700. [Google Scholar]
- Zhang, S.; Li, H. Flow-injection chemiluminescence sensor for the determination of isoniazid. Analítica Chimica Acta 2001, 444, 287–294. [Google Scholar]
- Song, Z.; Lü, J.; Zhao, T. Chemiluminescence sensor for isoniazid with controlled-reagent-release technology. Talanta 2001, 53, 1171–1177. [Google Scholar]
- Xiong, Y.; Zhou, H.; Zhang, Z.; He, D.; He, C. Flow-injection chemiluminescence sensor for determination of isoniazid in urine sample based on molecularly imprinted polymer. Spectrochimica Acta Part A Molecular and Biomolecular Spectroscopy 2006, in press Available on-line 7 March. [Google Scholar]
| Analyte | Remarks | Ref. |
|---|---|---|
| Inorganic species | ||
| Ammonia | A pH-sensitive dye (bromophenol blue) was immobilized as an ion pair with cetyltrimethylammonium in a silicone matrix | [6] |
| Bismuth | By forming iodide complexes and retaining them in a flow-through cell containing Sephadex QAE A-25 anion exchanger as support; in rocks and metals | [7] |
| Bromide | Fiber-optic sensor; selective oxidation to bromine in a flow reactor containing a packed bed of chloramine-T; in spent brine | [8] |
| Cobalt | Using pyridoxal-4-phenylthiosemicarbazone; in pharmaceutical preparations | [9] |
| Cobalt | The complex formed with 5-Br-PADAB was on-line protonated and concentrated on an AG 50W-X2 cation-exchanger in a flow through cell; in water samples | [10] |
| Cobalt and copper | Use of a diode-array detector accommodated in a flow-through sensor and pyridoxal-4-phenylthiosemicarbazone as reagent immobilized in C18; in steels | [11] |
| Copper | The increase in the absorbance of the coloured complex with 4,7-diphenyl-2,9-dimethyl-1,10-phenanthroline disulphonate, which was concentrated on-line on to an ion exchanger packed in a flow-through cell, could be measured continuously with a spectrophotometer at 484 nm; in water | [12] |
| Copper | Based on retention of Cu(I)-1,2 ciclohexanedione thiosemicarbazone complex on C18 bonded phase beads packed in a flow-cell; in catalysts and alloys | [13] |
| Copper | Multicommutated flow system incorporating a sol–gel optical sensor; by physical entrapment of 4-(2-pyridylazo)resorcinol (PAR) in sol–gel thin films by means of a base-catalysed process; at 500 nm; optical transduction was based on a dual-colour light-emitting diode (LED) (green/red) light source and a photodiode detector; in urine | [14] |
| Copper and zinc | The flow-cell (Hellma 138-OS) is filled by injecting in the flow system 300 μL of a homogeneous bead suspension of an anion exchanger gel (Sephadex QAE A-25) previously loaded with the chromogenic reagent 2-carboxyl-2-hydroxy-5-sulfoformazylbenzene (Zincon); a sequential reaction of Cu(II) and Zn(II) with Zincon to form two complexes is performed on the bead sensing support and the absorbance is monitored at 627 nm, after two successive injections from the mixture solution; the sample containing these metal ions is injected into the first carrier (deionized water, pH 5.9), and Cu(II) selectively reacts with Zincon on the beads, developing the analytical signal, then, 600 μL of 2 M HCl is injected to decompose the complex, and the carrier solution is changed; at pH 11 (second carrier) both Cu(II) and Zn(II) react with the chromogenic reagent, the absorbance now corresponding to both analytes. The eluent is again injected to descompose both complexes; in waters, pharmaceuticals, soils and human hair samples | [15] |
| Chromium(VI) | The product of the reaction with 1,5-diphenylcarbazide was introduced into a carrier solution stream in the flow system; the increase in absorption by the coloured complex sorbed on a cation exchanger, with which the light-path of a flow-through cell had been partly filled, could be measured directly with high precision; in natural waters | [16] |
| Chromium(III) and (VI) | The chromium in sample solution was concentrated on cation-exchange resin packed in a flow-through cell as a reaction product of Cr(VI) with diphenylcarbazide; the absorbance increase caused by the accumulation of the complex on cation-exchange resin was continuously measured; by using peroxodisulfate as an oxidizing agent, the chromium(III) in sample solution was completely oxidized to Cr(VI). The Cr(III) concentration was calculated by the difference; in natural water | [17] |
| Fluoride | Using Tecoflex polyurethane as a polymeric matrix for fluoride-selective membranes doped with Zr(IV)-octaethyl-(OEP) or Zr(IV)-tetraphenylporphyrins (TPP) | [18] |
| Iron | By forming Fe(III)-thiocyanate complex and retention on Dowex 1-X2-200 anion exchanger located in the flow cell; in water and wine | [19] |
| Iron | After the sample had been introduced onto a small cation-exchange column, the iron concentrated on the column was eluted with an acetate carrier solution, and then mixed with the other carrier stream which contained a pulse of 4,7-diphenyl-1,10-phenanthroline disulfonate (DPPS) reagent solution; the coloured iron–DPPS complex, formed in the stream, was introduced into a flow-through cell, the light path of which had been partly filled with anion exchanger. The increase in attenuance due to the sorption of the coloured complex was measured continuously; in highly purified water | [20] |
| Nickel | Based on the reaction with 1-(2-pyridylazo)-2-naphtol immobilized on a cationic resin; at 566 nm | [21] |
| Nickel | Based on immobilization of 2-(5-bromo-2- pyridylazo)-5-(diethylamino)phenol (Br-PADAP) in Nafion membrane; in vegetable oil and chocolate samples | [22] |
| Selenium | Optical chemical sensor responsive to selenium (SeO32−); its matrix was nafion membrane suffused with an organic ligand p-amino-p'-methoxydiphenylamine or variamine blue; the method of analysis was flow injection where in the membrane was fixed in a flow-through demountable measuring cell and connected to a computer-controlled simple spectrophotometer; in water samples | [23] |
| Zinc | Using 1-(2-pyridylazo)-2-naphtol immobilized on a Dowex cation exchanger placed in a flow-through cell; in human hair, pharmaceutical and cosmetic preparations and waters | [24] |
| Atmospheric nitrogen dioxide | Relies on membrane based analyte collection and trapping in a temporally halted liquid absorber and in situ spectrophotometric detection of the reaction product formed in solution | [25] |
| Dissolved oxygen | Methylene Blue immobilized on a cationic exchange resin packed into a flow-cell located in the spectrophotometer, where the leuco form of the dye is formed by flowing a dithionate solution; in waters | [26] |
| Organic species | ||
| Antioxidants: butylated hydroxyanisole and n-propyl gallate | Based on the transient retention behaviour of these compounds in a flow-through cell packed with C-18 silica using ethanol–water mixtures as a carrier, and on the intrinsic absorbance monitored at 290 and 283 nm, respectively; in several food and cosmetic samples | [27][28] |
| Butylated hydroxytoluene | Based on transient retention of this compound in a flow-through cell packed with C18 silica using ethanol:water mixture as a carrier; at 274 nm; in cosmetics | [29] |
| Butylated hydroxytoluene and co-existing antioxidants | Butylated hydroxytoluene (BHT)/n-propyl gallate (n-PG) and butylated hydroxytoluene (BHT)/butylated hydroxyanisole (BHA), in food and cosmetics samples; based on the different residence times of each antioxidant when the flow cell is packed to a height of 25mm with silica C18 using methanol-water 50:50% (v/v) as a carrier with a flow rate of 1.25 and 1.10 mL min-1, respectively. The determination of each antioxidant is based on the measurement of its absorbance at its maximum wavelengths using a DAD detector at 30 and 180s for the mixture n-PG-BHT and 90 and 220s for BHA-BHT. | [30] |
| Carbaryl | Samples are injected directly into the system where they are subjected to alkaline hydrolysis thus forming 1-naphthol; based on the coupling of 1-naphthol with phenylhydrazine hydrochloride to produce a red complex which has maximum absorbance at 495 nm; in spiked natural waters and commercial formulations | [31] |
| Paraquat | Based on integration of preconcentration, reaction and detection in the flow cell; in water samples and soils | [32] |
| Polyphenols | Coupling of a liquid-liquid extraction approach based on flow-reversal to a low-through sensor; the basis of the sensor is the use of Folin-Ciocalteau reagent immobilized on an anionic exchange resin packed in a flow cell; in olive oil | [33] |
| Total and prostatic acid phosphatase activity | Hydrolysis of p-nitrophenyl-phosphate catalysed by the analyte and monitoring at 405 nm; in serum | [34] |
| Saccharin | Based on the transient adsorption of the sweetener on Sephadex G-25 solid phase packed to a height of 20 mm in the flow cell. The optimal transient retention of the synthetic sweetener, in terms of sensitivity and sampling frequency, was obtained when pH 2.75 citric acid-sodium citrate buffer 5 × 10-3 M was used as a carrier at a flow-rate of 1.5 mL min-1; measuring its intrinsic absorbance at 217 nm; in low-calorie products | [35] |
| Pharmaceutical products | ||
| Acetylsalicylic acid, caffeine and paracetamol | The use of a solid phase (C18 silica gel) placed in an on-line microcolumn provides the sequential arrival of analytes to the detection solid zone (also C18 silica gel beads placed in a flow cell); in pharmaceutical preparations | [36] |
| Acetylsalicylic acid and salicylic acid | Salicylic acid: By monitoring of its intrinsic absorbance at 297 nm sorbed on Sephadex QAE A-25 resin; indirect determination of acetylsalicylic acid previous hydrolysis on-line to salicylic acid; in pharmaceutical preparations | [37] |
| Adrenaline | Bases of direct fixation on a solid support, Sephadex QAE A-25, and continuous monitoring of its intrinsic absorbance at 287 nm; different medical formulations | [38] |
| Ascorbic acid | By a simple Bead Injection Spectroscopy-Flow Injection Analysis (BIS-FIA) system with spectrophotometric detection; based on the decrease of absorbance obtained (720 nm) when Prussian blue (PB) is reduced by ascorbic acid; commercial available flow-cell (Hellma 138-OS) is used and an appropriate volume of homogeneous bead suspension of Sephadex QAE A-25 was injected to fill this flow-cell for each measurement; PB is injected into the carrier and immobilized on beads, when sample is injected, reaching the bead surface where PB is sorbed, ascorbic acid converts it to Prussian white form, which is transparent, producing the discoloration of the detection zone; at the end of the analysis, beads are discarded by reversing the flow and instantaneously transported out of the system; in fruit juices, pharmaceuticals, sweets and conservative liquids | [39] |
| Ascorbic acid and paracetamol | Based on alternate use of two carrier/self-eluting agents; the selective and sequential sorption of both on an active support (Sephadex QAE A-25) is performed and their respective UV intrinsic absorbances monitored; in pharmaceuticals | [40] |
| Ciprofloxacin | Based on its transient retention/concentration on Sephadex SP C-25 cation-exchange gel beads packed in the flow cell and the continuous monitoring of its native absorbance on the solid phase at 277 nm. The procedure is carried out without any derivatisation. Formic acid/NaOH 1.75 M at pH 2.2 is used as carrier solution in a simple monochannel FIA manifold. When the analytical signal reached the maximum value, ciprofloxacin was eluted from the solid support by the carrier solution itself; in pharmaceuticals | [41] |
| Diclofenac sodium | Based on retention on a Sephadex QAE A-25 anion-exchange resin packed in a flow-through cell of 1 mm of optical path length; at 281 nm; in pharmaceutical preparations | [42] |
| Methylxanthines | Two methods were developed to determine caffeine (CF) and theophylline (TP) in pharmaceuticals and CF and theobromine (TB) in food and beverages. The sensor is based on transient and sequential retention of the analytes on a hydrophobic sensing solid zone (octadecyl silane C18 gel) and detection of their intrinsic UV absorbance. Temporary sequencing of the arrival of the analytes at the sensing zone is achieved by on-line separation of one of the analytes using a pre-column of the same particulate material, placed just before the flow cell. After TB or TP had been carried toward the sensing zone (by the appropriate carrier solution), produced its transitory signal, and been eluted by the carrier, an appropriate eluting solution (25% MeOH) was used to elute CF, which was strongly retained on the minicolumn, so that its transient signal could be recorded | [43] |
| Minoxidil | Concentrated on Sephadex SP-C25 ion-exchanger packed in a flow cell; at 282 nm; in pharmaceutical preparations | [44] |
| Paracetamol and salicylamide | Using anionic exchanger (Sephadex QAE A-25) packed in a flow-through cell; at 300 nm; in pharmaceutical preparations | [45] |
| Pyridoxine | Continuously monitored at 290 nm when it is transiently retained on Sephadex SP-C25 cation exchanger gel beads placed in the detection area of a flow cell; in pharmaceuticals | [46] |
| Piroxicam | Based on its transient retention and concentration on Sephadex DEAE-A-25 anion-exchange gel beads packed in the flow cell and the continuous monitoring of its native absorbance on the solid phase at 354 nm; in formulations | [47] |
| Ranitidine | Formation of a yellow di(N-nitroso)ranitidine chromophore by reaction with excess nitrite in acetate of pH 4.8 and in the presence of Cu2+/Br- or micelles as catalyst; in pharmaceutical dosage forms | [48] |
| Sulfamethoxazole and trimethoprim | Using the different kinetics of retention/elution of analytes on Sephadex SP C-25; in pharmaceuticals | [49] |
| Sulfonamides | Based on integration of spectrophotometric detection and retention of the product of the Bratton-Marshall reaction for the determination of sulphanilamide, sulfamethazine, sulfaquinoxaline and sulfathiazole; in spiked milks and waters | [50] |
| Thiamine | In the presence of riboflavin, pyridoxine and hydroxocobalamine; the active solid phase of the optosensor was the cationic resin Sephadex CMC-25 placed in a quartz cell of 1 mm of optical path length; monitored at 247 nm; in pharmaceuticals | [51] |
| Analite | Remarks | Ref. |
|---|---|---|
| Gadolinium | RTP behaviour in aqueous solution induced by the transient adsorption of the complex formed by 1,4-bis (1′-phenyl-3′-methyl-5′-pyrazolone-4′-)butanedione (1,4) with Gd (III) on the chelating resin Chelex 100 (packed in a flow cell); in synthetic sample. | [106] |
| Lead | The chelates formed between Pb(II) and 8-hydroxy-5-quinolinesulphonic acid, 8-hydroxy-7-quinolinesulphonic acid and 8-hydroxy-7-iodo-5-quinolinesulphonic acid exhibit strong RTP if retained on the surface of anion exchange resin beads; based on the on-line formation, in a FI system, of such RTP lead chelates and their transient immobilization on an anion exchange resin, three flow-through optosensing systems are investigated for lead in sea water. | [107] |
| Iodide | The chelate of Al(III) with quinolin-8-ol-5-sulphonic acid immobilized on an anion-exchange resin is the sensing phase; both RTP and RTF measurements provided excellent calibration linearity. The detection limits for iodide were 10 and 5 μg mL-1 for RTP and RTF measurement modes, respectively. RTP optosensing allows the determination of iodide in the presence of relatively large amounts of other halides (Br-, Cl-), and hence it overcomes the classical problem of fluorescence quenching-based iodide optical sensors. | [108] |
| Orthophosphate | Flow-through solid-phase RTP method based on the energy transfer from a phosphor molecule (acting as a donor) to an orthophosphate dye-indicator (acting as an acceptor); injection in a flow system of 1 mL sample treated to form phosphomolybdenum blue from the orthophosphate; after injection, the phosphomolybdenum blue is on-line co-immobilised onto a polymeric resin containing adsorbed erythrosine B. This selected donor molecule exhibits strong RTP in a de-oxygenated aqueous media when retained on the surface of polymeric resin beads. | [109] |
| Sulfite | Spectral-luminescent properties and quenching behavior of the covalent conjugate of the Pt(II) complex of coproporphyrin-I and bovine serum albumin. Quenching of phosphorescence by sulfite was studied in detail in an acidic range as a function of pH; FI system based on the PtCP-BSA conjugate immobilized on a preactivated Biodyne ABC membrane and placed in a flow-cell, with an acidic carrier buffer and fiber-optic phosphorescent detector. | [110] |
| Anthracyclines | Based on the anthracycline-europium chelate RTP energy transfer. The sensor is based on the transient immobilization on a non-ionic resin (packed in a flow-through cell) of the anthracycline-europium chelate; in clinical samples (urine and pharmaceutical preparations). | [111] |
| Ciprofloxacin | Direct measurement of the sensitized luminescence of the europium-ciprofloxacin chelate immobilized on a cationic exchanger; flow-through RTP optosensor; in pharmaceutical formulations. | [112] |
| 2-naphthoxyacetic acid (β-NOA) | Two luminescence methods based on a flow-through optical sensor:The fluorescence optosensor is based on the on-line immobilization of β-NOA on a non-ionic resin (Amberlite XAD-7) solid support in a continuous-FI and the phosphorescence one on the on-line immobilization of β-NOA on silica gel solid support in a continuous-FI. Fluorescence and phosphorescence intensities were measured at λexc/em = 328/348 and 276/516 nm, respectively. | [113] |
| Naptalam | Simultaneous determination of pesticide N-1-naphthylphthlamic acid and its metabolite 1-naphthylamine; the system works as a rapid phosphorimetry-biparameter sensor. It is based in the on-line immobilization of the analytes onto a non-ionic resin solid support (Amberlite XAD 7) in a continuous flow system, followed by the measurement of their native phosphorescence; in drinking and mineral waters | [114] |
| Polycyclic aromatic hydrocarbons (PAHs) | PAHs immobilized onto diferent non-ionic resins solids supports (Amberlite XAD2, Amberlite XAD4, AmberliteXAD7, Silica Gel) coupled to a continuous flow system and the applications for the selective determination of benzo(a)pyrene (BaP). The phosphorescent characterization of 15 PAHs, described as major pollutants by the EPA (naphthalene, acenaphthylene, acenaphthene, fluorene, phenanthrene, anthracene, fluoranthene, pyrene, chrysene, benzo(a)anthracene, benzo(k)fluoranthene, benzo(b)fluoranthene, benzo(a)pyrene, indeno(1,2,3-cd) pyrene, benzo(g,h,i)perylene and dibenzo(a,h)anthracene) has been carried out. | [115] |
| Tetracyclines | Based on the tetracycline-europium chelate RTP energy transfer. The sensor is developed in conjunction with a FIA system and is based on the transient immobilization on a non-ionic resin (packed in a flow-through cell) of the tetracycline-europium chelate. The analytical performance characteristics of the proposed sensor for semiautomated analysis and control of very low levels of tetracycline were as follows: the detection limits for tetracycline, oxytetracycline and chlortetracycline were 0.25, 0.30 and 0.40 ng ml-1, respectively, with a relative standard deviation of 1% for determination of 0.24 μg ml-1 of each antibiotic; in clinical samples (urine and pharmaceutical preparations). | [116] |
© 2006 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Ojeda, C.B.; Rojas, F.S. Recent Development in Optical Chemical Sensors Coupling with Flow Injection Analysis. Sensors 2006, 6, 1245-1307. https://doi.org/10.3390/s6101245
Ojeda CB, Rojas FS. Recent Development in Optical Chemical Sensors Coupling with Flow Injection Analysis. Sensors. 2006; 6(10):1245-1307. https://doi.org/10.3390/s6101245
Chicago/Turabian StyleOjeda, Catalina Bosch, and Fuensanta Sánchez Rojas. 2006. "Recent Development in Optical Chemical Sensors Coupling with Flow Injection Analysis" Sensors 6, no. 10: 1245-1307. https://doi.org/10.3390/s6101245
APA StyleOjeda, C. B., & Rojas, F. S. (2006). Recent Development in Optical Chemical Sensors Coupling with Flow Injection Analysis. Sensors, 6(10), 1245-1307. https://doi.org/10.3390/s6101245




