Study of Voltammetric Determination of Carcinogenic 1-Nitropyrene and 1-Aminopyrene Using a Glassy Carbon Paste Electrode
Abstract
:Introduction
Experimental
Reagents
Apparatus
Procedures
Results and Discussion
Linear scan voltammetry of 1-aminopyrene
Differential pulse voltammetry of 1-aminopyrene
Voltammetry of 1-aminopyrene with adsorptive accumulation
Linear sweep voltammetry of 1-nitropyrene
Differential pulse voltammetry of 1-nitropyrene
Conclusions
Acknowledgments
References
- Moreira, J.C.; Barek, J. Analysis of carcinogenic nitrated polycyclic aromatic hydrocarbons. Quimica Nova. 1995, 18, 362. [Google Scholar]
- Jacob, J.; Karcher, W.; Belliardo, J.J.; Dumler, R.; Boenke, A. Review. Polycyclic aromatic compounds of environmental and occupational importance - their occurrence, toxicity and the development of high-purity certified reference materials. Part III. Fresenius J. Anal. Chem. 1991, 340, 755. [Google Scholar]
- Cvacka, J.; Barek, J.; Fogg, A.G.; Moreira, J.C.; Zima, J. Review. HPLC of nitrated polycyclic aromatic hydrocarbons. Analyst. 1998, 123, 9. [Google Scholar]
- http://www-cie.iarc.fr/htdocs/monographs/vol46/46-14.htm (January 28, 2004).
- Stanton, C.A.; Chow, F.L.; Phillips, D.H.; Grover, P.L.; Garner, R.C.; Martin, C.N. Evidence for n-(deoxyguanosin-8-yl)-1-aminopyrene as a major DNA adduct in female rats treated with 1-nitropyrene. Carcinogenesis 1985, 6, 535. [Google Scholar]
- Barek, J.; Fogg, A.G.; Muck, A.; Zima, J. Polarography and voltammetry at mercury electrodes. Crit. Rev. Anal. Chem. 2001, 31, 291. [Google Scholar]
- Svancara, I.; Vytras, K.; Barek, J.; Zima, J. Carbon paste electrodes in modern electroanalysis. Crit. Rev. Anal. Chem. 2001, 31, 311. [Google Scholar]
- Kalcher, K.; Kauffmann, J.-M.; Wang, J.; Svancara, I.; Vytras, K.; Neuhold, C.; Yang, Z. Sensors based on carbon paste in electrochemical analysis: a review with particular emphasis on the period 1990-1993. Electroanalysis. 1995, 7, 5. [Google Scholar]
- Mayer, M.; Ruzicka, J. Flow injection based renewable electrochemical sensor system. Anal.Chem. 1996, 68, 3808. [Google Scholar]
- Svancara, I.; Hvizdalova, M.; Vytras, K.; Kalcher, K.; Novotny, R. A microscopic study on carbon paste electrodes. Electroanalysis 1996, 8, 61. [Google Scholar]
- Wang, J.; Kirgöz, U.A.; Mo, J.W.; Lu, J.; Muck, A. Glassy carbon paste electrodes. Electrochem.Commun. 2001, 3, 203. [Google Scholar]
- Seddon, B.J.; Osborne, M.D.; Lagger, G.; Dryfe, R.A.W.; Loyal, U.; Schäfer, H.; Girault, H.H. Micro-glassy carbon inks for thick-film electrodes. Electrochim. Acta. 1997, 42, 1883. [Google Scholar]
- Rodriguez, M.C.; Rivas, G.A. Glassy carbon paste electrodes modified with polyphenol oxidase. Anal. Chim. Acta. 2002, 459, 43. [Google Scholar]
- Ricci, F.; Goncalves, C.; Amine, A.; Gorton, L.; Palleschi, G.; Moscone, D. Electroanalytical study of Prussian Blue modified glassy carbon paste electrodes. Electroanalysis. 2003, 15, 1204. [Google Scholar]
- Muck, A.; Barek, J.; Zima, J.; Wang, J. Determination of 1-nitropyrene and 1-aminopyrene using a glassy carbon paste electrode. In US-CZ Workshop on Electrochemical Sensors, Book of abstracts; p. p.23. Czech Chemical Society: Prague, 2001. [Google Scholar]
- Oppenhelmer, L.; Cappizi, T.P.; Weppelman, R.M.; Metha, H. Determining the lowest limit of reliable assay measurement. Anal. Chem. 1983, 55, 638. [Google Scholar]
- Schwartz, L.M. Lowest limit of reliable assay measurement with nonlinear calibration. Anal. Chem. 1983, 55, 1424. [Google Scholar]
- Ebel, S.; Kamm, U. Statistical definition of the limit of determination. Fresenius Z. Anal. Chem. 1984, 318, 293. [Google Scholar]


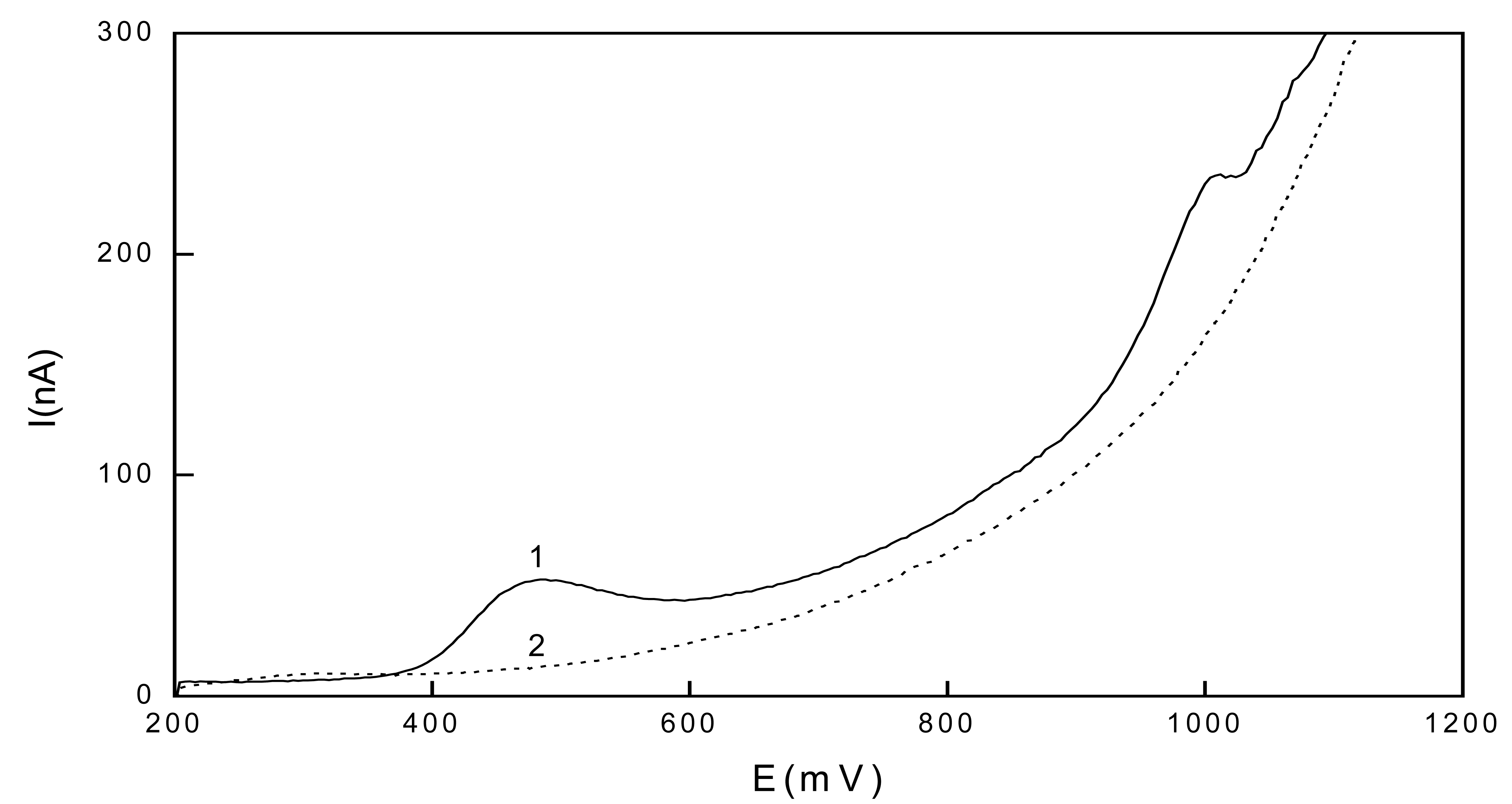

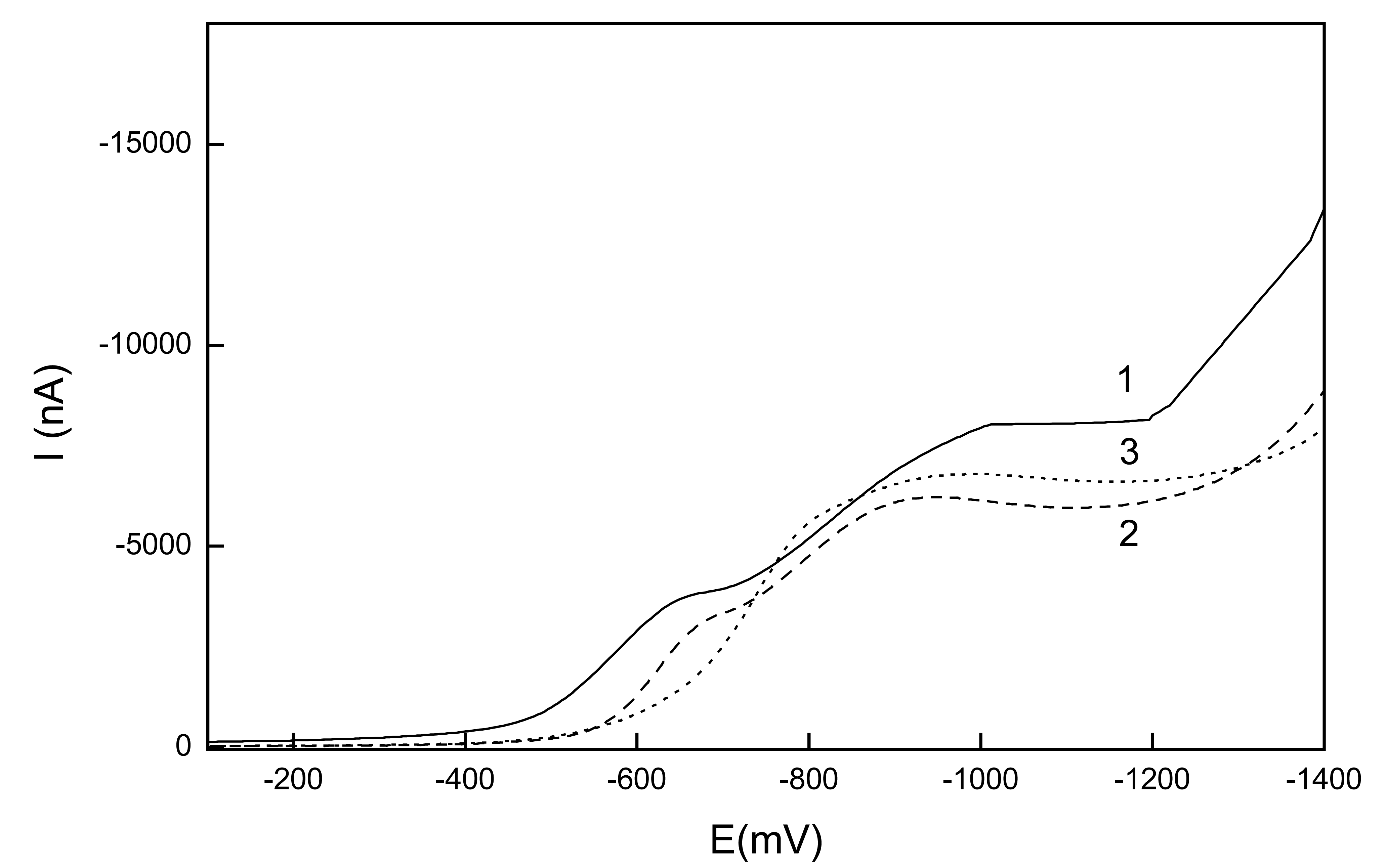
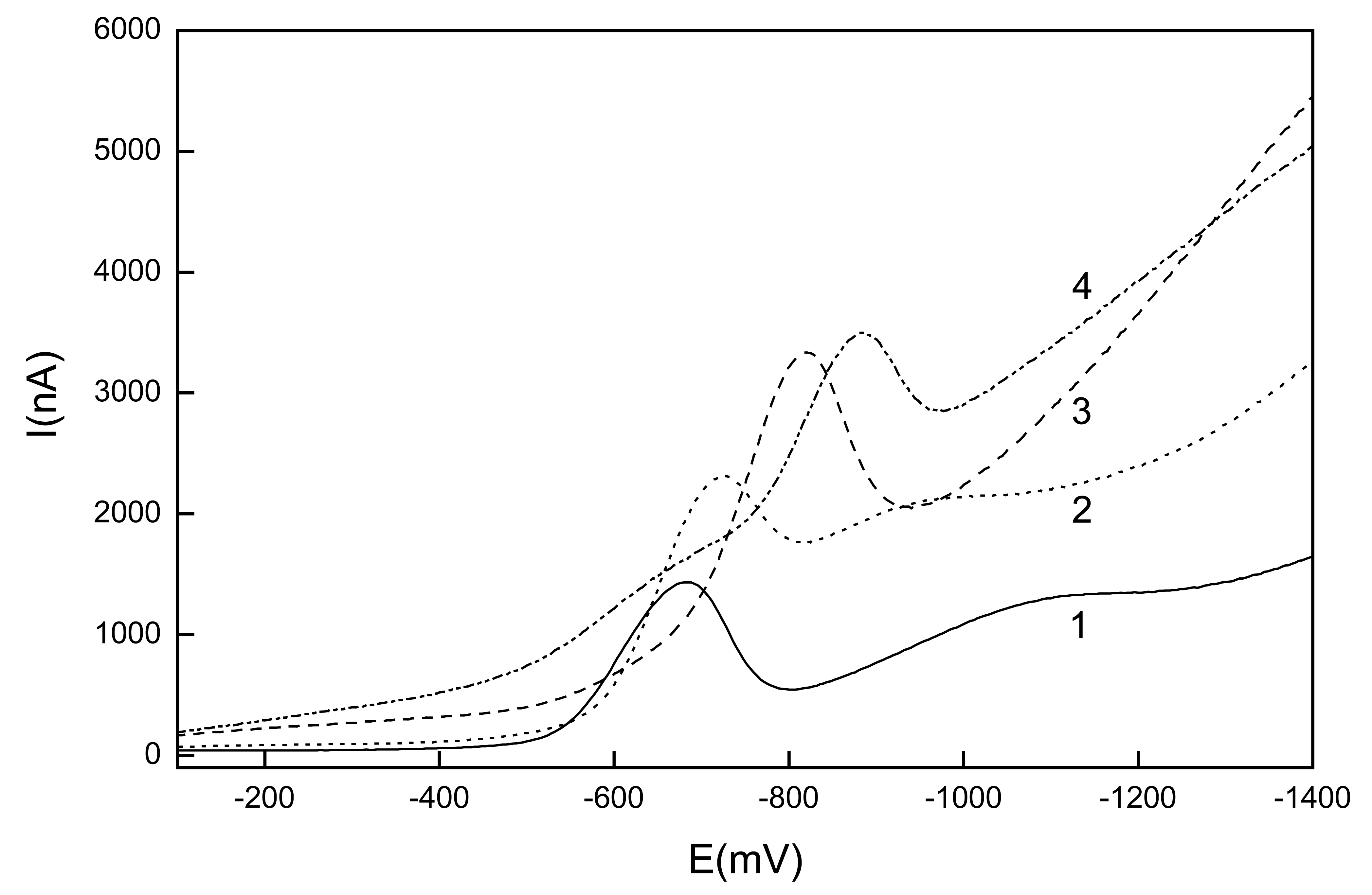
| c (mol.l-1) | Slope (mA.mol-1.L) | Intercept (nA) | R | LQ (mol.L-1) |
|---|---|---|---|---|
| LSV, BR buffer pH 2: MeOH (9:1 v/v) | ||||
| (2-10).10-5 | 6.86 | 80.0 | 0.9942 | -- |
| (2-10).10-6 | 7.91 | 6.2 | 0.9994 | 3.10-6 |
| DPV, BR buffer pH 2: MeOH (9:1 v/v) | ||||
| (2-10).10-5 | 6.33 | 43.01 | 0.9963 | -- |
| (2-10).10-6 | 9.98 | -0.85 | 0.9958 | 1.10-6 |
| AAV, BR buffer pH 2: MeOH (9:1 v/v), tacc= 180 s | ||||
| (2-10).10-7 | 20.5 | 0.3 | 0.9967 | 1.10-7 |
| c (mol.L-1) | Slope (mA.mol-1.L) | Intercept (nA) | R | LQ (mol.L-1) |
|---|---|---|---|---|
| LSV, BR buffer pH 3: MeOH (1:9 v/v) | ||||
| (2-10).10-5 | 26.2 | 267.8 | 0.9924 | 2. 10-5 |
| DPV, BR buffer pH 3: MeOH (1:9 v/v) | ||||
| (2-10).10-5 | 9.13 | 17.9 | 0.9935 | -- |
| (2-10).10-6 | 11.40 | 5.3 | 0.9901 | 2.10-6 |
© 2004 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Barek, J.; Muck, A.; Wang, J.; Zima, J. Study of Voltammetric Determination of Carcinogenic 1-Nitropyrene and 1-Aminopyrene Using a Glassy Carbon Paste Electrode. Sensors 2004, 4, 47-57. https://doi.org/10.3390/s40500047
Barek J, Muck A, Wang J, Zima J. Study of Voltammetric Determination of Carcinogenic 1-Nitropyrene and 1-Aminopyrene Using a Glassy Carbon Paste Electrode. Sensors. 2004; 4(5):47-57. https://doi.org/10.3390/s40500047
Chicago/Turabian StyleBarek, Jirí, Alexandr Muck, Joseph Wang, and Jirí Zima. 2004. "Study of Voltammetric Determination of Carcinogenic 1-Nitropyrene and 1-Aminopyrene Using a Glassy Carbon Paste Electrode" Sensors 4, no. 5: 47-57. https://doi.org/10.3390/s40500047
APA StyleBarek, J., Muck, A., Wang, J., & Zima, J. (2004). Study of Voltammetric Determination of Carcinogenic 1-Nitropyrene and 1-Aminopyrene Using a Glassy Carbon Paste Electrode. Sensors, 4(5), 47-57. https://doi.org/10.3390/s40500047




