Copper Nanoclusters Anchored on Crumpled N-Doped MXene for Ultra-Sensitive Electrochemical Sensing
Abstract
Highlights
- 3D crumpled MXene with strain wrinkles enhances conductivity and prevents restacking.
- Cu nanoclusters (3.0 wt%) synergize N-doping for noble metal-like catalytic activity.
- Ultrasensitive dual-analyte non-enzymatic detection of DA/UA with anti-interference capability and rapid response.
- Novel material design strategies provide new approaches for the development of future high-performance sensors.
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Monolayer Ti3C2Tx Nanosheets
2.3. Synthesis of Cu-N/Ti3C2Tx, N/Ti3C2Tx, and Cu-Ti3C2Tx Nanosheets
2.4. Preparation of Three Modified Electrodes
2.5. Material Characterizations and Electrochemical Measurements
3. Results and Discussion
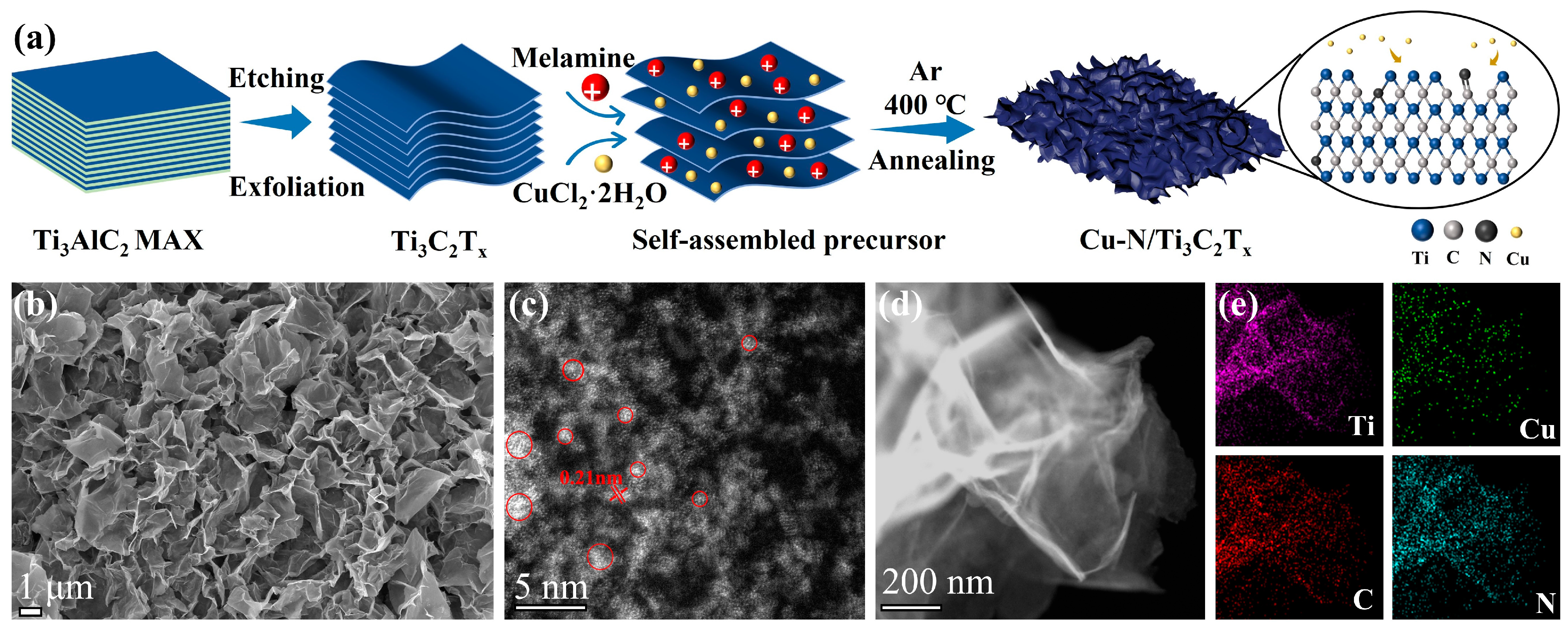
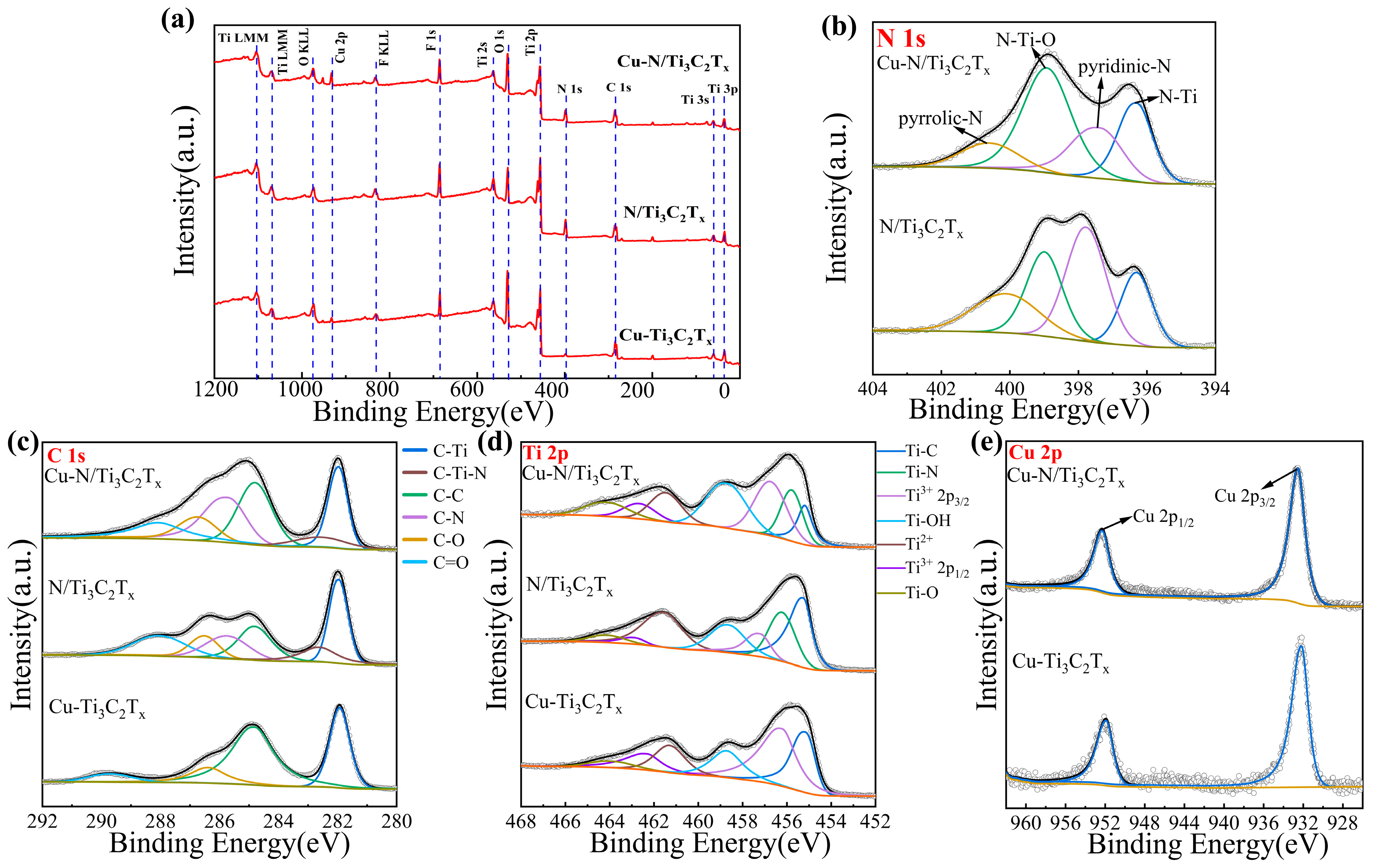
3.1. Characterization of Cu-N/Ti3C2Tx
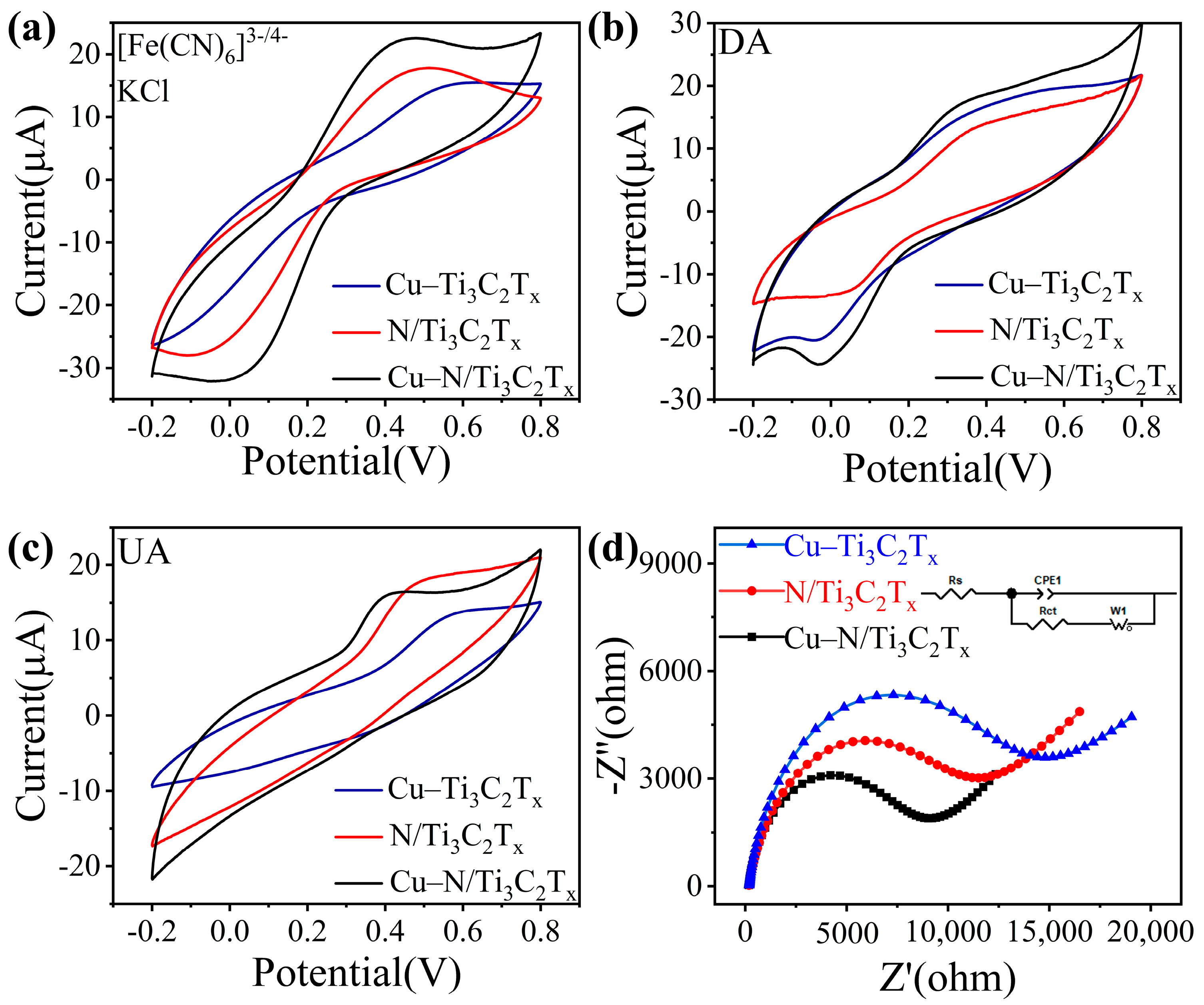
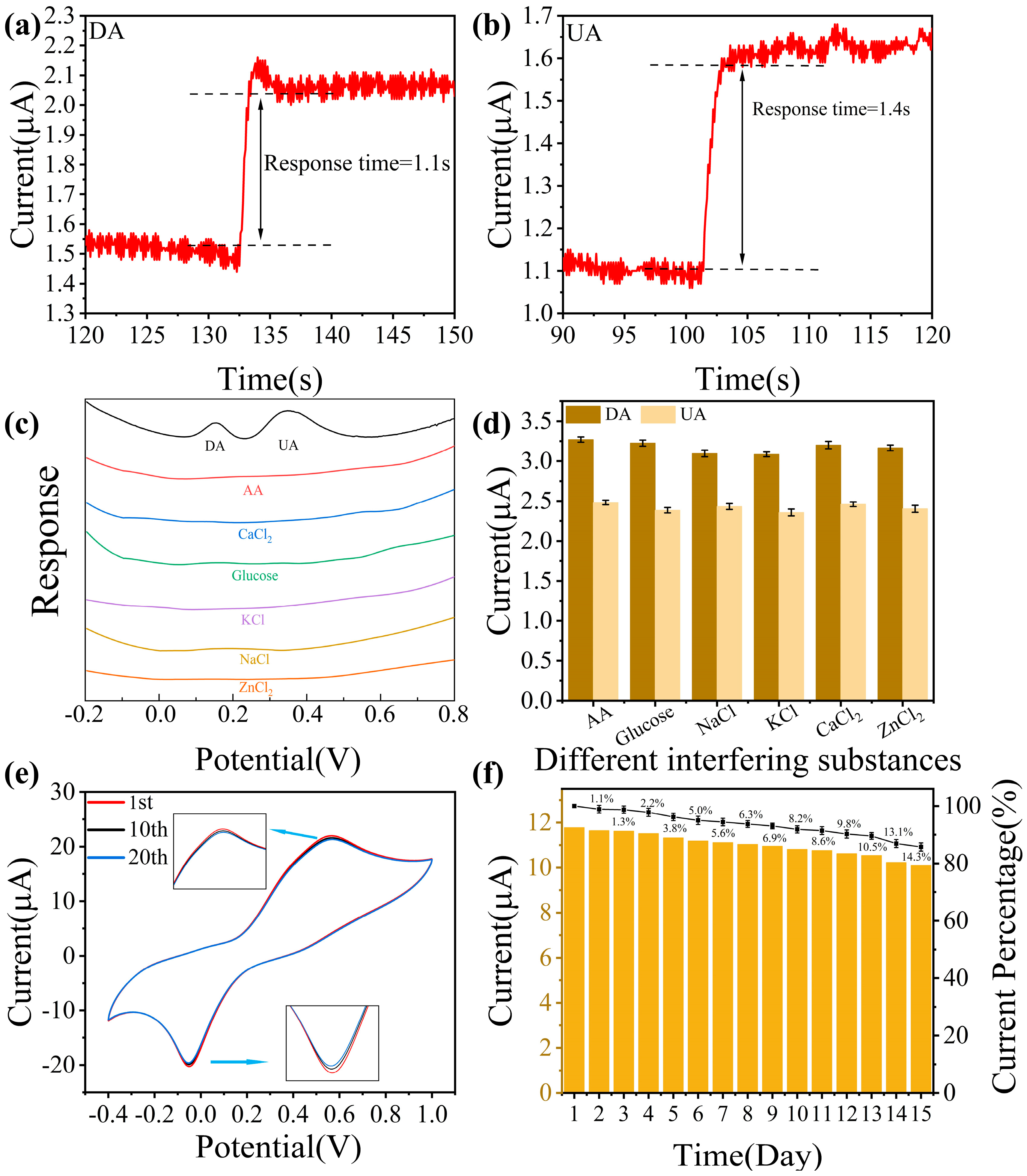
3.2. Analysis of Electrochemical Sensing Responses to DA and UA
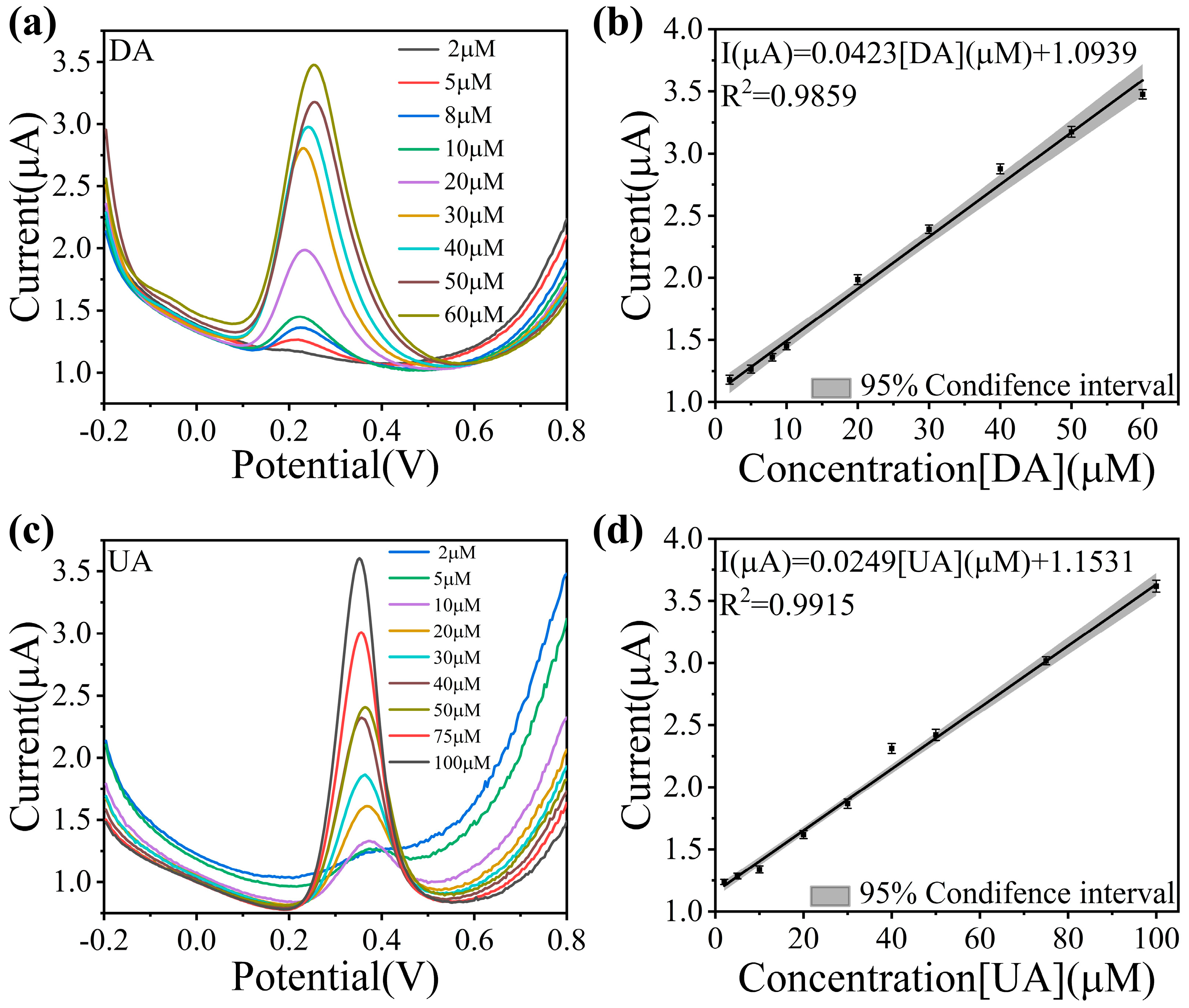
3.3. Analysis of Electrochemical Sensing Performance to DA and UA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sriram, B.; Kogularasu, S.; Wang, S.-F.; Sheu, J.-K. Deep Eutectic Solvent-Mediated Synthesis of Spinel Zinc Chromite Nanoparticles: A Simple Label-Free Electrochemical Sensor for Dopamine and Ascorbic Acid. ACS Appl. Nano Mater. 2023, 6, 17593–17602. [Google Scholar] [CrossRef]
- Shukla, R.P.; Aroosh, M.; Matzafi, A.; Ben-Yoav, H. Partially Functional Electrode Modifications for Rapid Detection of Dopamine in Urine. Adv. Funct. Mater. 2021, 31, 2004146. [Google Scholar] [CrossRef]
- Shi, Z.; Wu, X.; Zou, Z.; Yu, L.; Hu, F.; Li, Y.; Guo, C.; Li, C.M. Screen-Printed Analytical Strip Constructed with Bacteria-Templated Porous N-Doped Carbon Nanorods/Au Nanoparticles for Sensitive Electrochemical Detection of Dopamine Molecules. Biosens. Bioelectron. 2021, 186, 113303. [Google Scholar] [CrossRef]
- Hu, F.X.; Hu, T.; Chen, S.; Wang, D.; Rao, Q.; Liu, Y.; Dai, F.; Guo, C.; Yang, H.B.; Li, C.M. Single-Atom Cobalt-Based Electrochemical Biomimetic Uric Acid Sensor with Wide Linear Range and Ultralow Detection Limit. Nano-Micro Lett. 2021, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Li, Q.; Cai, J.; Yang, T.; Chen, J.; Hou, X. Electrochemical Detection Mechanism of Dopamine and Uric Acid on Titanium Nitride-Reduced Graphene Oxide Composite with and without Ascorbic Acid. Sens. Actuators B Chem. 2019, 298, 126872. [Google Scholar] [CrossRef]
- Sriram, B.; Shajahan, S.; Hsu, Y.; Wang, S.-F.; Haija, M.A. Electrochemical Behavior of a Copper Tungstate-Fabricated Disposable Strip for the Rapid and Real-Time Detection of Uric Acid. ACS Appl. Nano Mater. 2023, 6, 1–9. [Google Scholar] [CrossRef]
- Xie, X.; Wang, D.P.; Guo, C.; Liu, Y.; Rao, Q.; Lou, F.; Li, Q.; Dong, Y.; Li, Q.; Yang, H.B.; et al. Single-Atom Ruthenium Biomimetic Enzyme for Simultaneous Electrochemical Detection of Dopamine and Uric Acid. Anal. Chem. 2021, 93, 4916–4923. [Google Scholar] [CrossRef]
- Wu, Y.; Deng, P.; Tian, Y.; Feng, J.; Xiao, J.; Li, J.; Liu, J.; Li, G.; He, Q. Simultaneous and Sensitive Determination of Ascorbic Acid, Dopamine and Uric Acid via an Electrochemical Sensor Based on PVP-Graphene Composite. J. Nanobiotechnol. 2020, 18, 112. [Google Scholar] [CrossRef]
- Mei, H.; Peng, J.; Wang, T.; Zhou, T.; Zhao, H.; Zhang, T.; Yang, Z. Overcoming the Limits of Cross-Sensitivity: Pattern Recognition Methods for Chemiresistive Gas Sensor Array. Nano-Micro Lett. 2024, 16, 269. [Google Scholar] [CrossRef]
- Li, Q.; Qiu, Y.; Han, W.; Zheng, Y.; Wang, X.; Xiao, D.; Mao, M.; Li, Q. Determination of Uric Acid in Biological Samples by High Performance Liquid Chromatography-Electrospray Ionization-Tandem Mass Spectrometry and Study on Pathogenesis of Pulmonary Arterial Hypertension in Pulmonary Artery Endothelium Cells. RSC Adv. 2018, 8, 25808–25814. [Google Scholar] [CrossRef]
- Nie, Z.; Long, X.; Bouroubi, N.E.H.; Liu, H.; Cao, S.; Chen, Y.; Zheng, X.; Xia, J. Biosynthesis and Detection of Domoic Acid from Diatom Pseudo-Nitzschia: A Review. Curr. Pharm. Biotechnol. 2023, 24, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, H.; Wu, Y.; Jiang, W.; Chen, X.; Zeng, M.; Yang, J.; Su, Y.; Hu, N.; Yang, Z. Target Discrimination, Concentration Prediction, and Status Judgment of Electronic Nose System Based on Large-Scale Measurement and Multi-Task Deep Learning. Sens. Actuators B Chem. 2022, 351, 130915. [Google Scholar] [CrossRef]
- Ma, Z.; Xu, Y.; Li, P.; Cheng, D.; Zhu, X.; Liu, M.; Zhang, Y.; Liu, Y.; Yao, S. Self-Catalyzed Surface Reaction-Induced Fluorescence Resonance Energy Transfer on Cysteine-Stabilized MnO2 Quantum Dots for Selective Detection of Dopamine. Anal. Chem. 2021, 93, 3586–3593. [Google Scholar] [CrossRef]
- Kim, J.; Park, H.; Ryu, J.; Jeon, O.; Paeng, I.R. COMPETITIVE ENZYME-LINKED IMMUNOSORBENT ASSAY FOR A SELECTIVE AND SENSITIVE DETERMINATION OF DOPAMINE IN THE PRESENCE OF ASCORBIC ACID AND URIC ACID. J. Immunoass. Immunochem. 2009, 31, 33–44. [Google Scholar] [CrossRef]
- Balkourani, G.; Brouzgou, A.; Tsiakaras, P. A Review on Recent Advancements in Electrochemical Detection of Dopamine Using Carbonaceous Nanomaterials. Carbon 2023, 213, 118281. [Google Scholar] [CrossRef]
- Ni, W.; Wang, T.; Wu, Y.; Liu, X.; Li, Z.; Yang, R.; Zhang, K.; Yang, J.; Zeng, M.; Hu, N.; et al. Multi-Task Deep Learning Model for Quantitative Volatile Organic Compounds Analysis by Feature Fusion of Electronic Nose Sensing. Sens. Actuators B Chem. 2024, 417, 136206. [Google Scholar] [CrossRef]
- Qin, Z.; Tang, B.; Zhang, G.; Zhu, C.; Jiang, K.; Zhang, B.; Xuan, F.-Z. Single-Atom Ni-N4 for Enhanced Electrochemical Sensing. Nano Res. 2024, 17, 7658–7664. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, T.; Li, J.; Shi, Y.; Hou, M.; Yang, Y.; Zhang, Z.; Lei, S. Two-Dimensional Covalent Organic Frameworks for Electrocatalysis: Achievements, Challenges, and Opportunities. Nano Res. 2023, 16, 8570–8595. [Google Scholar] [CrossRef]
- Matsoso, B.J.; Lerotholi, T.; Coville, N.J.; Jones, G. Designing Parameters of Surfactant-Free Electrochemical Sensors for Dopamine and Uric Acid on Nitrogen Doped Graphene Films: Evaluating the Correlation between the Content of N-Configurations and the Sensing for Electrochemically Active Molecules. Johns. Matthey Technol. Rev. 2019, 63, 76–88. [Google Scholar] [CrossRef]
- Azizpour Moallem, Q.; Beitollahi, H. Electrochemical Sensor for Simultaneous Detection of Dopamine and Uric Acid Based on a Carbon Paste Electrode Modified with Nanostructured Cu-Based Metal-Organic Frameworks. Microchem. J. 2022, 177, 107261. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, L.; Ni, W.; Cheng, W.; Yang, Z.; Xu, S.; Wang, T.; Zhang, B.; Xuan, F. NiO/ZnO Nanocomposites for Multimodal Intelligent MEMS Gas Sensors. ACS Sens. 2025, acssensors.4c02789. (in press). [Google Scholar] [CrossRef]
- Yue, J.-Y.; Song, L.-P.; Wang, Y.-T.; Yang, P.; Ma, Y.; Tang, B. Fluorescence/Colorimetry/Smartphone Triple-Mode Sensing of Dopamine by a COF-Based Peroxidase-Mimic Platform. Anal. Chem. 2022, 94, 14419–14425. [Google Scholar] [CrossRef]
- Xin, M.; Li, J.; Ma, Z.; Pan, L.; Shi, Y. MXenes and Their Applications in Wearable Sensors. Front. Chem. 2020, 8, 297. [Google Scholar] [CrossRef] [PubMed]
- Shekhirev, M.; Shuck, C.E.; Sarycheva, A.; Gogotsi, Y. Characterization of MXenes at Every Step, from Their Precursors to Single Flakes and Assembled Films. Prog. Mater. Sci. 2021, 120, 100757. [Google Scholar] [CrossRef]
- Pei, Y.; Zhang, X.; Hui, Z.; Zhou, J.; Huang, X.; Sun, G.; Huang, W. Ti3C2TX MXene for Sensing Applications: Recent Progress, Design Principles, and Future Perspectives. ACS Nano 2021, 15, 3996–4017. [Google Scholar] [CrossRef] [PubMed]
- Chu, T.; Wang, G.; Zhang, X.; Jia, Y.; Dai, S.; Liu, X.; Zhang, L.; Yang, X.; Zhang, B.; Xuan, F.-Z. High-Density Dual-Structure Single-Atom Pt Electrocatalyst for Efficient Hydrogen Evolution and Multimodal Sensing. Nano Lett. 2024, 24, 9666–9674. [Google Scholar] [CrossRef]
- Rong, C.; Su, T.; Li, Z.; Chu, T.; Zhu, M.; Yan, Y.; Zhang, B.; Xuan, F.-Z. Elastic Properties and Tensile Strength of 2D Ti3C2Tx MXene Monolayers. Nat Commun 2024, 15, 1566. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, X.; Xin, X.; Tang, Z.-R.; Xu, Y.-J. Ti3C2Tx-Based Three-Dimensional Hydrogel by a Graphene Oxide-Assisted Self-Convergence Process for Enhanced Photoredox Catalysis. ACS Nano 2019, 13, 295–304. [Google Scholar] [CrossRef]
- Mao, X.; Qin, Z.; Ge, S.; Rong, C.; Zhang, B.; Xuan, F. Strain Engineering of Electrocatalysts for Hydrogen Evolution Reaction. Mater. Horiz. 2023, 10, 340–360. [Google Scholar] [CrossRef]
- Zhang, X.; Miao, J.; Zhang, P.; Zhu, Q.; Jiang, M.; Xu, B. 3D Crumbled MXene for High-Performance Supercapacitors. Chin. Chem. Lett. 2020, 31, 2305–2308. [Google Scholar] [CrossRef]
- Jiang, Y.; Liang, P.; Tang, M.; Sun, S.; Min, H.; Han, J.; Shen, X.; Yang, H.; Chao, D.; Wang, J. A High-Throughput Screening Permeability Separator with High Catalytic Conversion Kinetics for Li–S Batteries. J. Mater. Chem. A 2022, 10, 22080–22092. [Google Scholar] [CrossRef]
- Bhamore, J.R.; Jha, S.; Mungara, A.K.; Singhal, R.K.; Sonkeshariya, D.; Kailasa, S.K. One-Step Green Synthetic Approach for the Preparation of Multicolor Emitting Copper Nanoclusters and Their Applications in Chemical Species Sensing and Bioimaging. Biosens. Bioelectron. 2016, 80, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, E.; Hajian, A.; Rezaei, M.; Afkhami, A.; Amine, A.; Bagheri, H. A Novel Platform Based on Graphene Nanoribbons/Protein Capped Au-Cu Bimetallic Nanoclusters: Application to the Sensitive Electrochemical Determination of Bisphenol A. Microchem. J. 2019, 145, 242–251. [Google Scholar] [CrossRef]
- Das, N.K.; Ghosh, S.; Priya, A.; Datta, S.; Mukherjee, S. Luminescent Copper Nanoclusters as a Specific Cell-Imaging Probe and a Selective Metal Ion Sensor. J. Phys. Chem. C 2015, 119, 24657–24664. [Google Scholar] [CrossRef]
- Wang, D.; Nie, Y.; Wang, P.; Ma, Q. In Situ Synthesis of Cu Nanoclusters/CeO2 Nanorod as Aggregated Induced ECL Probe for Triple-Negative Breast Cancer Detection. Talanta 2023, 258, 124400. [Google Scholar] [CrossRef]
- Li, Q.; Wang, X.; Chen, L.; Liu, X.; Ma, J.; Wang, L.; Wang, W. Cu/Cu2O Nanoparticles Modified Ti3C2 MXene with in-Situ Formed TiO2-X for Detection of Hydrogen Peroxide. Ceram. Int. 2023, 49, 9632–9641. [Google Scholar] [CrossRef]
- Li, Q.; Fu, S.; Wang, X.; Wang, L.; Liu, X.; Gao, Y.; Li, Q.; Wang, W. Electrochemical and Photoelectrochemical Detection of Hydrogen Peroxide Using Cu2O/Cu Nanowires Decorated with TiO2− x Deriving from MXenes. ACS Appl. Mater. Interfaces 2022, 14, 57471–57480. [Google Scholar] [CrossRef]
- Nasrollahpour, H.; Sánchez, B.J.; Sillanpää, M.; Moradi, R. Metal Nanoclusters in Point-of-Care Sensing and Biosensing Applications. ACS Appl. Nano Mater. 2023, 6, 12609–12672. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, P.; Duan, X.; Gao, X.; Gao, L. Advances in Precise Synthesis of Metal Nanoclusters and Their Applications in Electrochemical Biosensing of Disease Biomarkers. Nanoscale 2025, 17, 3616–3634. [Google Scholar] [CrossRef]
- Chu, T.; Rong, C.; Zhou, L.; Mao, X.; Zhang, B.; Xuan, F. Progress and Perspectives of Single-Atom Catalysts for Gas Sensing. Adv. Mater. 2023, 35, 2206783. [Google Scholar] [CrossRef]
- Shahzad, F.; Alhabeb, M.; Hatter, C.B.; Anasori, B.; Man Hong, S.; Koo, C.M.; Gogotsi, Y. Electromagnetic Interference Shielding with 2D Transition Metal Carbides (MXenes). Science 2016, 353, 1137–1140. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, J.; Yu, Q.; Li, Q.; Fan, R.; Li, C.; Fan, Y.; Zhao, C.; Cheng, W.; Ji, P.; et al. Metal/MXene Composites via in Situ Reduction. Nat. Synth 2025, 4, 252–261. [Google Scholar] [CrossRef]
- Xie, S.; Shu, J.; Huang, H.; Li, J.; Yue, R.; Xu, J. Engineering the Highly Efficient Heterogeneous Catalyst Based on PdCu Nanoalloy and Nitrogen-Doped Ti3C2Tx MXene for Ethanol Electrooxidation. J. Colloid Interface Sci. 2023, 639, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; You, S.; Yao, Y.; Chen, H.; Wang, Y.; Liu, Y. An Electroactive Single-Atom Copper Anchored MXene Nanohybrid Filter for Ultrafast Water Decontamination. J. Mater. Chem. A 2021, 9, 25964–25973. [Google Scholar] [CrossRef]
- Bao, W.; Liu, L.; Wang, C.; Choi, S.; Wang, D.; Wang, G. Facile Synthesis of Crumpled Nitrogen-Doped MXene Nanosheets as a New Sulfur Host for Lithium–Sulfur Batteries. Adv. Energy Mater. 2018, 8, 1702485. [Google Scholar] [CrossRef]
- Li, Z.; Yu, L.; Milligan, C.; Ma, T.; Zhou, L.; Cui, Y.; Qi, Z.; Libretto, N.; Xu, B.; Luo, J.; et al. Two-Dimensional Transition Metal Carbides as Supports for Tuning the Chemistry of Catalytic Nanoparticles. Nat. Commun. 2018, 9, 5258. [Google Scholar] [CrossRef]
- Fang, Y.; Yang, X.; Chen, T.; Xu, G.; Liu, M.; Liu, J.; Xu, Y. Two-Dimensional Titanium Carbide (MXene)-Based Solid-State Electrochemiluminescent Sensor for Label-Free Single-Nucleotide Mismatch Discrimination in Human Urine. Sens. Actuators B Chem. 2018, 263, 400–407. [Google Scholar] [CrossRef]
- Li, Z.; Wang, P.; Liang, Z.; Wang, D.; Ma, Q. Cu NCs@MXene-Luminescent Faraday Cage and Cu Nanocone/Bi NPs Array-Based Saliva Exosome Assay for Asthma Evaluation. Chem. Eng. J. 2024, 493, 152650. [Google Scholar] [CrossRef]
- Lu, C.; Yang, L.; Yan, B.; Sun, L.; Zhang, P.; Zhang, W.; Sun, Z. Nitrogen-Doped Ti3C2 MXene: Mechanism Investigation and Electrochemical Analysis. Adv. Funct Mater. 2020, 30, 2000852. [Google Scholar] [CrossRef]
- Gu, H.; Yue, W.; Hu, J.; Niu, X.; Tang, H.; Qin, F.; Li, Y.; Yan, Q.; Liu, X.; Xu, W.; et al. Asymmetrically Coordinated Cu–N1C2 Single-Atom Catalyst Immobilized on Ti3C2Tx MXene as Separator Coating for Lithium–Sulfur Batteries. Adv. Energy Mater. 2023, 13, 2204014. [Google Scholar] [CrossRef]
- Liu, H.; Hu, Z.; Liu, Q.; Sun, P.; Wang, Y.; Chou, S.; Hu, Z.; Zhang, Z. Single-Atom Ru Anchored in Nitrogen-Doped MXene (Ti3C2Tx) as an Efficient Catalyst for the Hydrogen Evolution Reaction at All pH Values. J. Mater. Chem. A 2020, 8, 24710–24717. [Google Scholar] [CrossRef]
- Ramalingam, V.; Varadhan, P.; Fu, H.; Kim, H.; Zhang, D.; Chen, S.; Song, L.; Ma, D.; Wang, Y.; Alshareef, H.N.; et al. Heteroatom-Mediated Interactions between Ruthenium Single Atoms and an MXene Support for Efficient Hydrogen Evolution. Adv. Mater. 2019, 31, 1903841. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Han, F.; Xie, L.; Zhang, C.; Li, T.; Wang, H.; Lai, W.-F.; Luo, S.; Wei, W.; Wang, Z.; et al. A MXene of Type Ti3C2Tx Functionalized with Copper Nanoclusters for the Fluorometric Determination of Glutathione. Microchim. Acta 2020, 187, 38. [Google Scholar] [CrossRef]
- Rajendrachari, S.; Basavegowda, N.; Vinaykumar, R.; Narsimhachary, D.; Somu, P.; Lee, M.-J. Electrocatalytic Determination of Methyl Orange Dye Using Mechanically Alloyed Novel Metallic Glass Modified Carbon Paste Electrode by Cyclic Voltammetry. Inorg. Chem. Commun. 2023, 155, 111010. [Google Scholar] [CrossRef]
- Yoon, Y.; Lee, M.; Kim, S.K.; Bae, G.; Song, W.; Myung, S.; Lim, J.; Lee, S.S.; Zyung, T.; An, K. A Strategy for Synthesis of Carbon Nitride Induced Chemically Doped 2D MXene for High-Performance Supercapacitor Electrodes. Adv. Energy Mater. 2018, 8, 1703173. [Google Scholar] [CrossRef]
- Jiang, D.; Wei, M.; Du, X.; Qin, M.; Shan, X.; Chen, Z. One-Pot Synthesis of ZnO Quantum Dots/N-Doped Ti3C2 MXene: Tunable Nitrogen-Doping Properties and Efficient Electrochemiluminescence Sensing. Chem. Eng. J. 2022, 430, 132771. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, W.; Zhang, J.; Li, Y.; Tang, W.; Xu, Q. NiCo-Embedded in Hierarchically Structured N-Doped Carbon Nanoplates for the Efficient Electrochemical Determination of Ascorbic Acid, Dopamine, and Uric Acid. RSC Adv. 2015, 5, 65532–65539. [Google Scholar] [CrossRef]
- Ma, Y.; Wei, Z.; Wang, Y.; Ding, Y.; Jiang, L.; Fu, X.; Zhang, Y.; Sun, J.; Zhu, W.; Wang, J. Surface Oxygen Functionalization of Carbon Cloth toward Enhanced Electrochemical Dopamine Sensing. ACS Sustain. Chem. Eng. 2021, 9, 16063–16072. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Liu, X.; Song, K.; Wang, H.; Wang, J.; Li, R.; Liu, S.; Peng, Z. A Novel Electrochemical Sensor Based on Pd Confined Mesoporous Carbon Hollow Nanospheres for the Sensitive Detection of Ascorbic Acid, Dopamine, and Uric Acid. Molecules 2024, 29, 2427. [Google Scholar] [CrossRef]
- You, Q.; Guo, Z.; Zhang, R.; Chang, Z.; Ge, M.; Mei, Q.; Dong, W.-F. Simultaneous Recognition of Dopamine and Uric Acid in the Presence of Ascorbic Acid via an Intercalated MXene/PPy Nanocomposite. Sensors 2021, 21, 3069. [Google Scholar] [CrossRef]
- Yao, W.; Guo, H.; Liu, H.; Li, Q.; Wu, N.; Li, L.; Wang, M.; Fan, T.; Yang, W. Highly Electrochemical Performance of Ni-ZIF-8/ N S-CNTs/CS Composite for Simultaneous Determination of Dopamine, Uric Acid and L-Tryptophan. Microchem. J. 2020, 152, 104357. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, P.; Gao, B.; Yuan, M.; Yu, J.; Wang, Z.; Chen, X. Self-Reduction of Bimetallic Nanoparticles on Flexible MXene-Graphene Electrodes for Simultaneous Detection of Ascorbic Acid, Dopamine, and Uric Acid. Microchem. J. 2023, 185, 108177. [Google Scholar] [CrossRef]
- Shang, L.; Li, R.; Li, H.; Yu, S.; Sun, X.; Yu, Y.; Ren, Q. The Simultaneous Detection of Dopamine and Uric Acid In Vivo Based on a 3D Reduced Graphene Oxide–MXene Composite Electrode. Molecules 2024, 29, 1936. [Google Scholar] [CrossRef] [PubMed]
- Murugan, N.; Jerome, R.; Preethika, M.; Sundaramurthy, A.; Sundramoorthy, A.K. 2D-Titanium Carbide (MXene) Based Selective Electrochemical Sensor for Simultaneous Detection of Ascorbic Acid, Dopamine and Uric Acid. J. Mater. Sci. Technol. 2021, 72, 122–131. [Google Scholar] [CrossRef]
- Liu, K.; Chen, Y.; Dong, X.; Huang, H. Simultaneous Voltammetric Determination of Dopamine and Uric Acid Based on MOF-235 Nanocomposite. Inorg. Chem. Commun. 2022, 142, 109584. [Google Scholar] [CrossRef]
- Jia, D.; Yang, T.; Wang, K.; Wang, H.; Wang, E.; Chou, K.-C.; Hou, X. Ti3C2Tx Coated with TiO2 Nanosheets for the Simultaneous Detection of Ascorbic Acid, Dopamine and Uric Acid. Molecules 2024, 29, 2915. [Google Scholar] [CrossRef]
- Gong, J.; Tang, H.; Wang, M.; Lin, X.; Wang, K.; Liu, J. Novel Three-Dimensional Graphene Nanomesh Prepared by Facile Electro-Etching for Improved Electroanalytical Performance for Small Biomolecules. Mater. Des. 2022, 215, 110506. [Google Scholar] [CrossRef]
| Analyte | Concentration Added (µM) | Found (µM) a | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| DA | 10 | 10.39 | 103.9 | 3.27 |
| 30 | 29.45 | 98.2 | 2.41 | |
| 50 | 51.19 | 102.4 | 3.68 | |
| UA | 10 | 10.12 | 101.2 | 1.92 |
| 30 | 29.71 | 99.1 | 4.01 | |
| 50 | 49.46 | 98.9 | 1.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Rong, C.; Ge, S.; Wang, T.; Zhang, B.; Xuan, F.-Z. Copper Nanoclusters Anchored on Crumpled N-Doped MXene for Ultra-Sensitive Electrochemical Sensing. Sensors 2025, 25, 2508. https://doi.org/10.3390/s25082508
Yang H, Rong C, Ge S, Wang T, Zhang B, Xuan F-Z. Copper Nanoclusters Anchored on Crumpled N-Doped MXene for Ultra-Sensitive Electrochemical Sensing. Sensors. 2025; 25(8):2508. https://doi.org/10.3390/s25082508
Chicago/Turabian StyleYang, Hanxue, Chao Rong, Shundong Ge, Tao Wang, Bowei Zhang, and Fu-Zhen Xuan. 2025. "Copper Nanoclusters Anchored on Crumpled N-Doped MXene for Ultra-Sensitive Electrochemical Sensing" Sensors 25, no. 8: 2508. https://doi.org/10.3390/s25082508
APA StyleYang, H., Rong, C., Ge, S., Wang, T., Zhang, B., & Xuan, F.-Z. (2025). Copper Nanoclusters Anchored on Crumpled N-Doped MXene for Ultra-Sensitive Electrochemical Sensing. Sensors, 25(8), 2508. https://doi.org/10.3390/s25082508







