SERS-Based Immunochromatographic Assay for Sensitive Detection of Escherichia coli O157:H7 Using a Novel WS2-AuDTNB Nanotag
Abstract
Highlights
- Developed a novel WS2-Au nanocomposite coupled with 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) and antibodies as sensitive surface-enhanced Raman scattering (SERS) nanotags for E. coli O157:H7 detection.
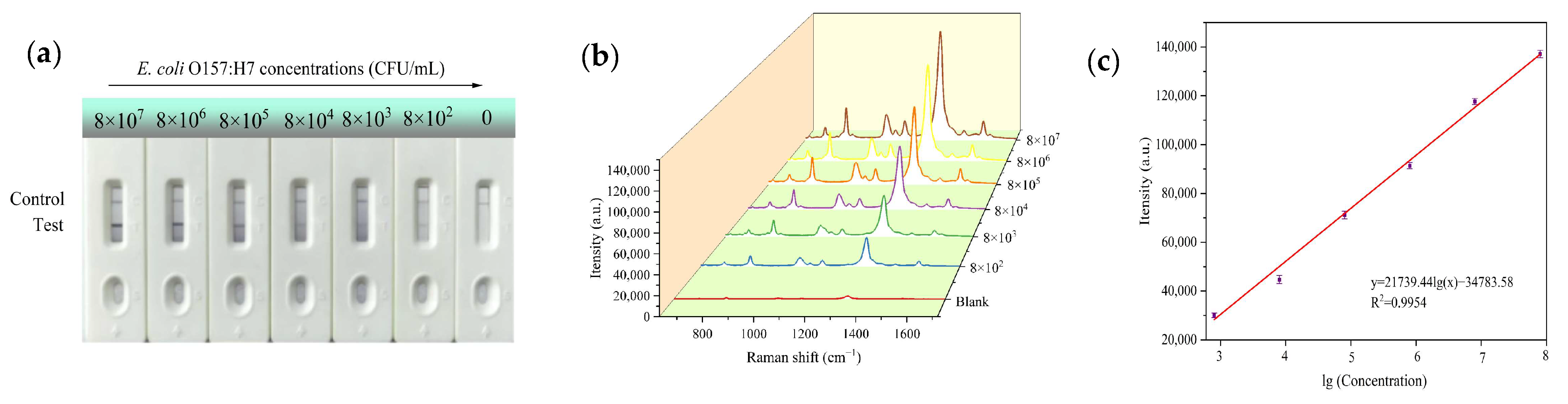
- Demonstrated a broad linear detection range (8 × 102–8 × 107 CFU/mL), a low detection limit (175 CFU/mL), high specificity and good accuracy in real food samples (milk, pork).
- A rapid assay for qualitative and quantitative detection of E. coli O157:H7, overcoming limitations of traditional ICA methods.
- A cost-effective, user-friendly surface-enhanced Raman scattering (SERS)-based immunochromatographic assay (SERS-ICA) platform with great potential for point-of-care testing and detection of other foodborne pathogens.
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Equipment
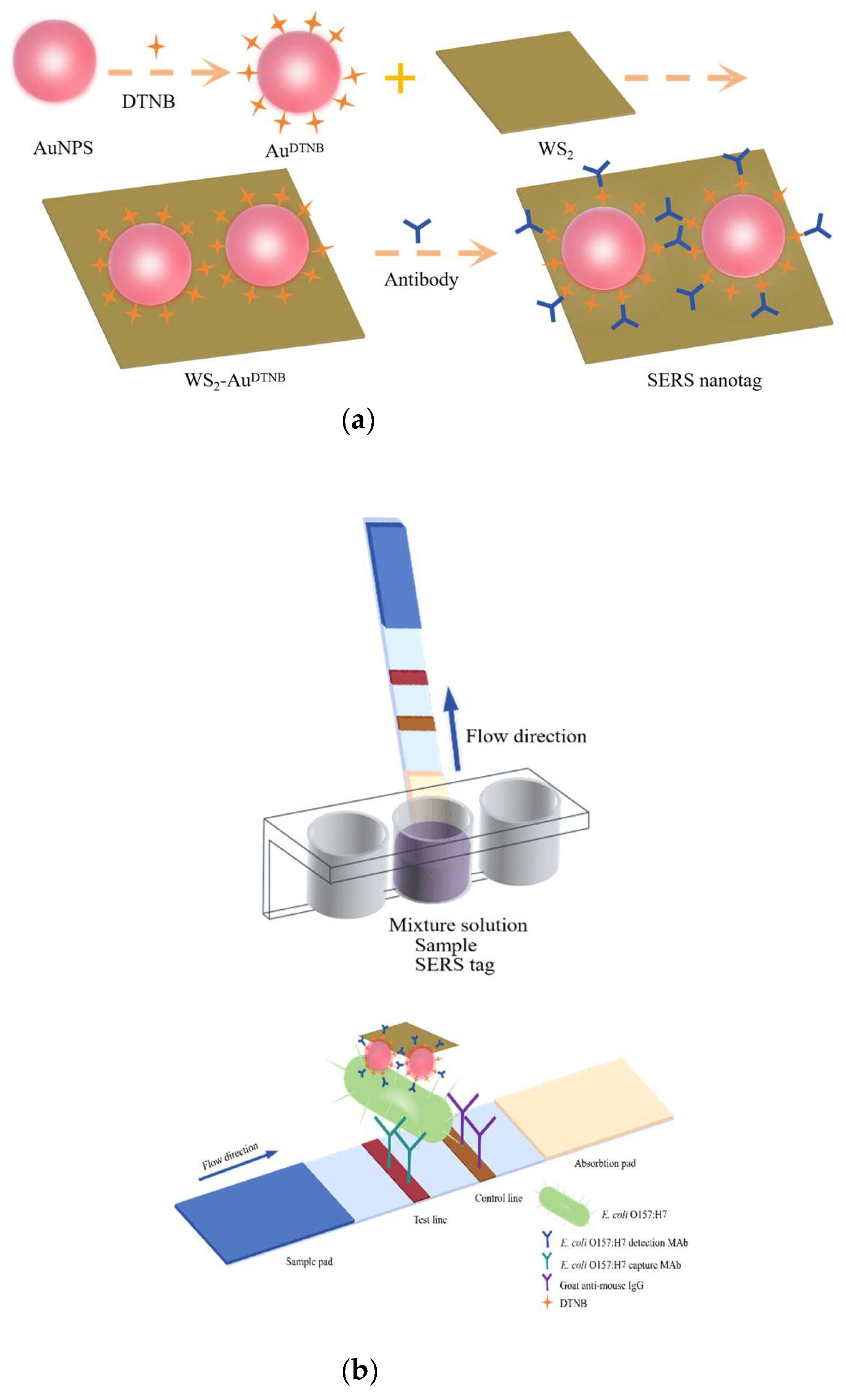
2.2. Synthesis of WS2-AuDTNB Nanotags
2.3. Preparation of Antibody-Modified WS2-AuDTNB Nanotags
2.4. SERS-ICA Test Strip Assembly
2.5. WS2-AuDTNB-Based ICA Strips for Analysis of E. coli O157:H7
2.6. Spiked Sample Preparation and Detection
3. Results
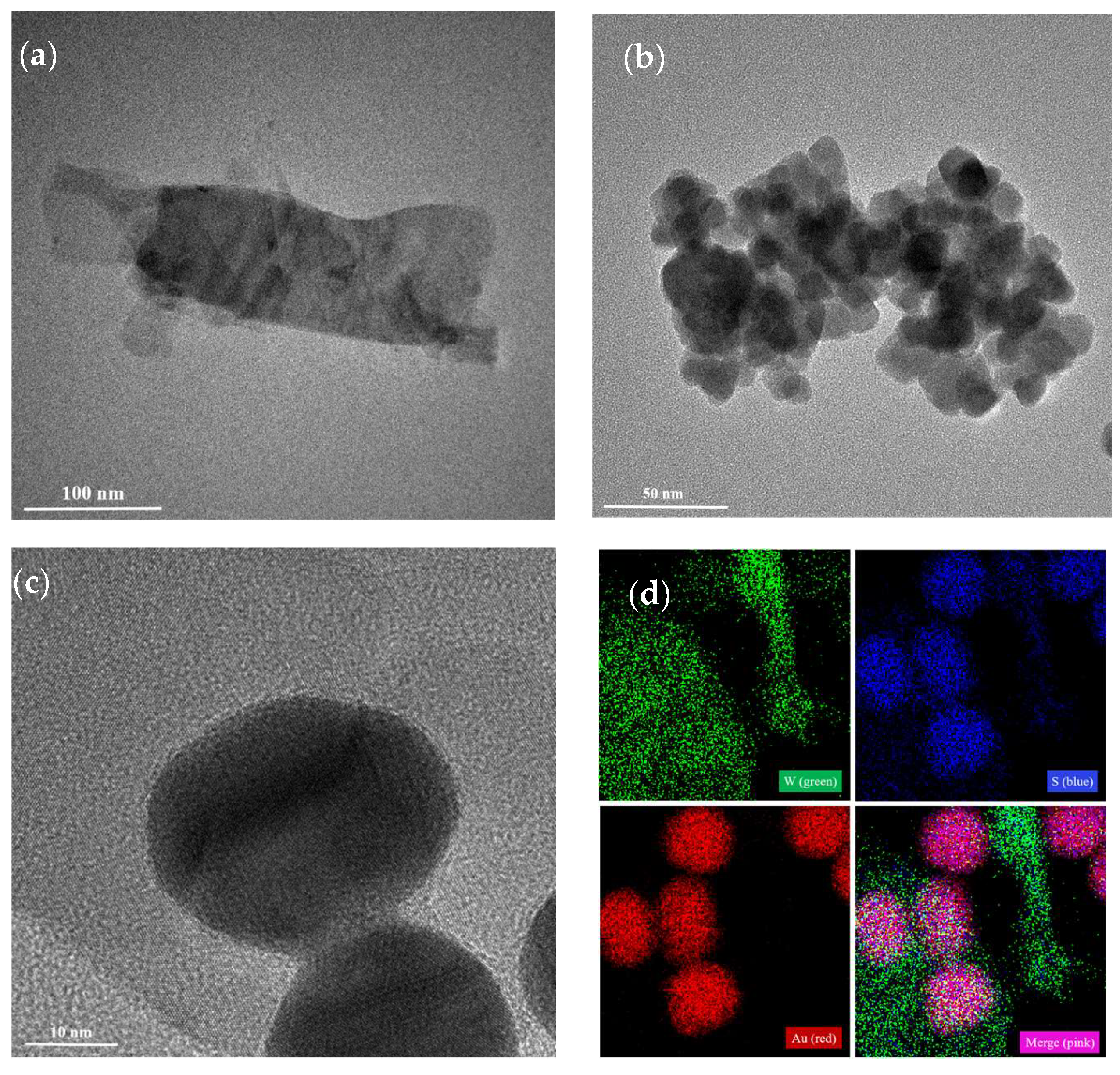
3.1. Characterization of Au, WS2-AuNPs, and SERS Nanotags
3.2. Construction of WS2-Au-Based ICA for Determination of E. coli O157:H7
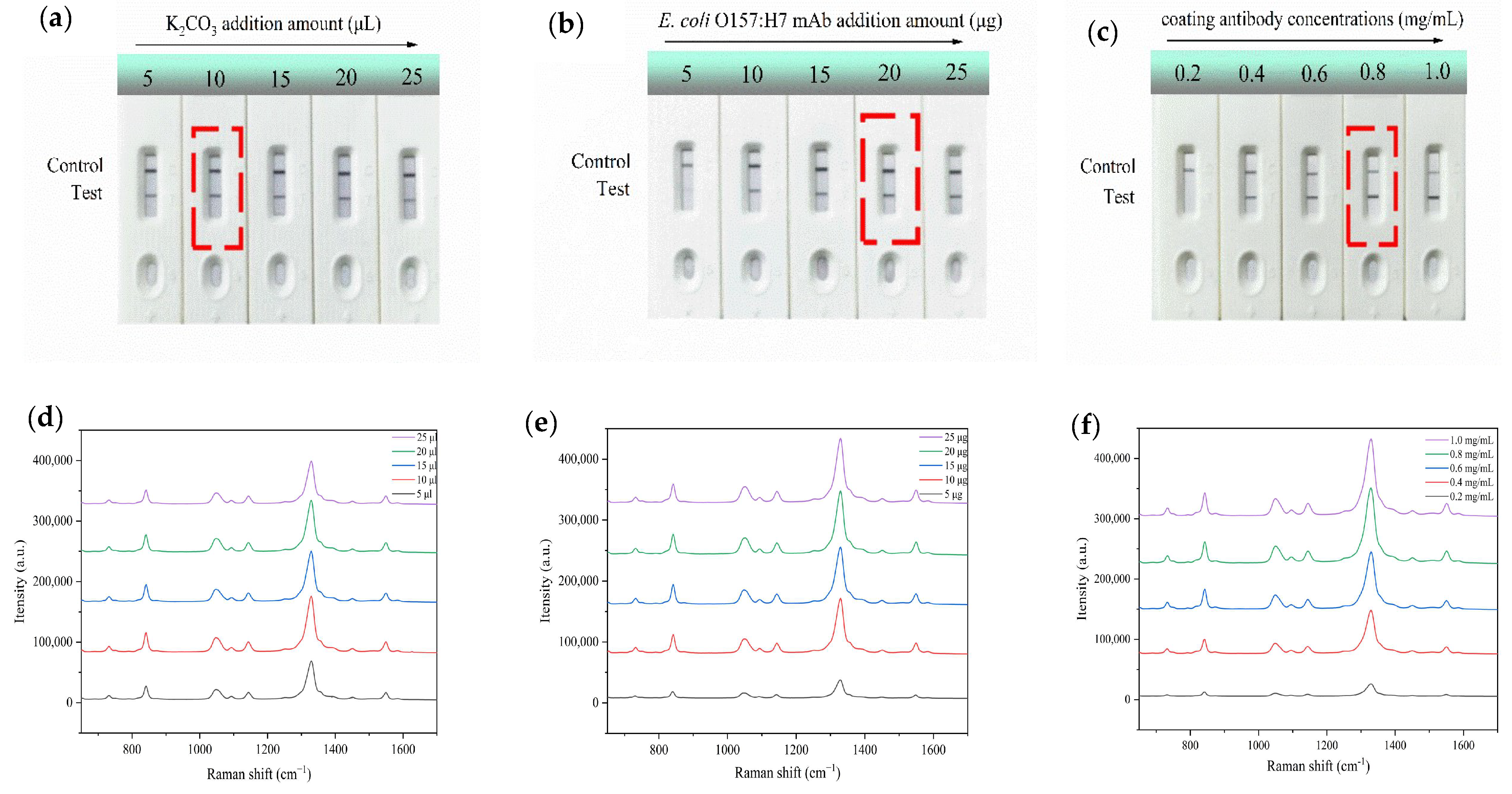
3.3. Optimization of the Assay Conditions
3.4. Sensitivity of the Assay
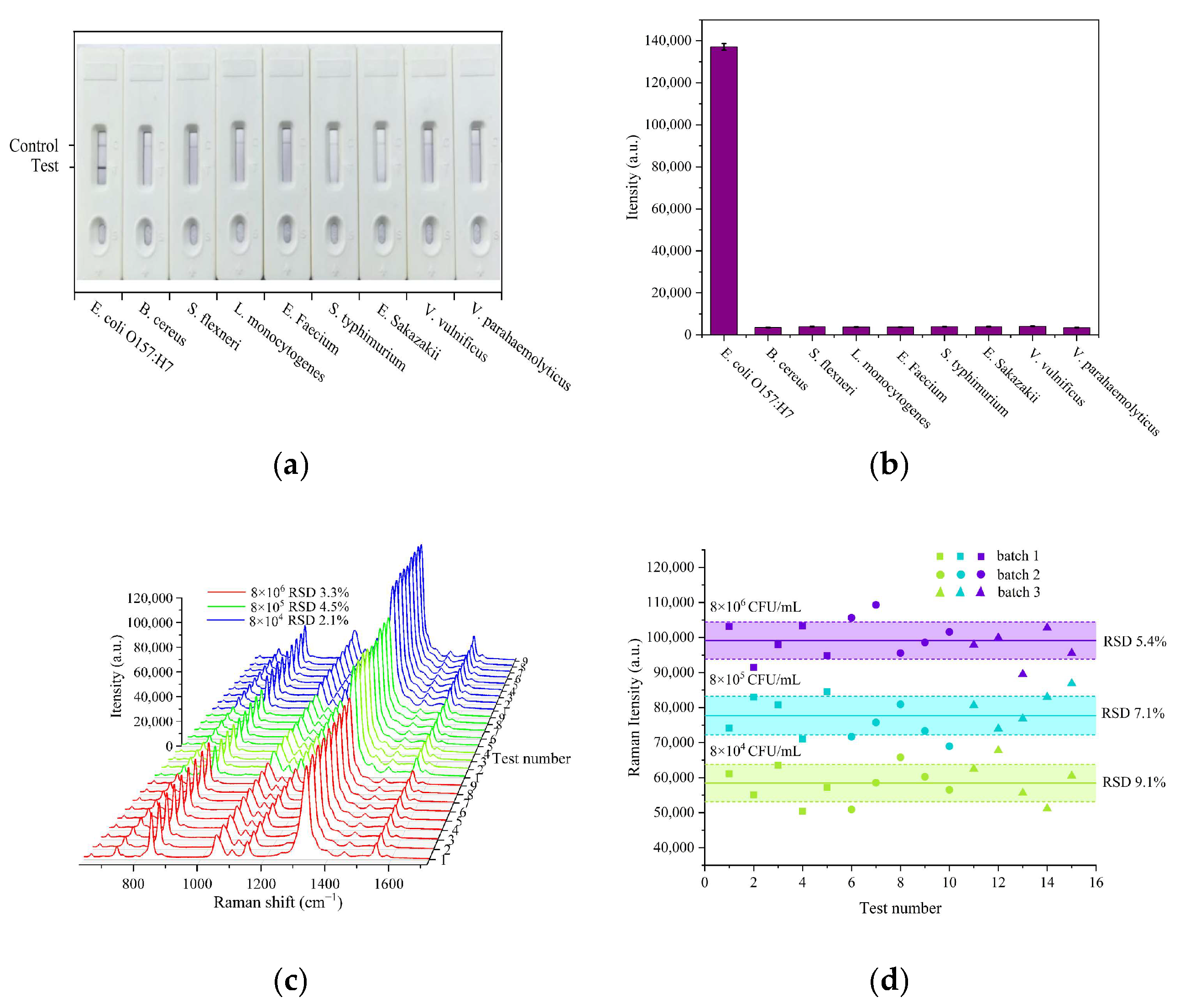
3.5. Specificity and Repeatability Evaluations
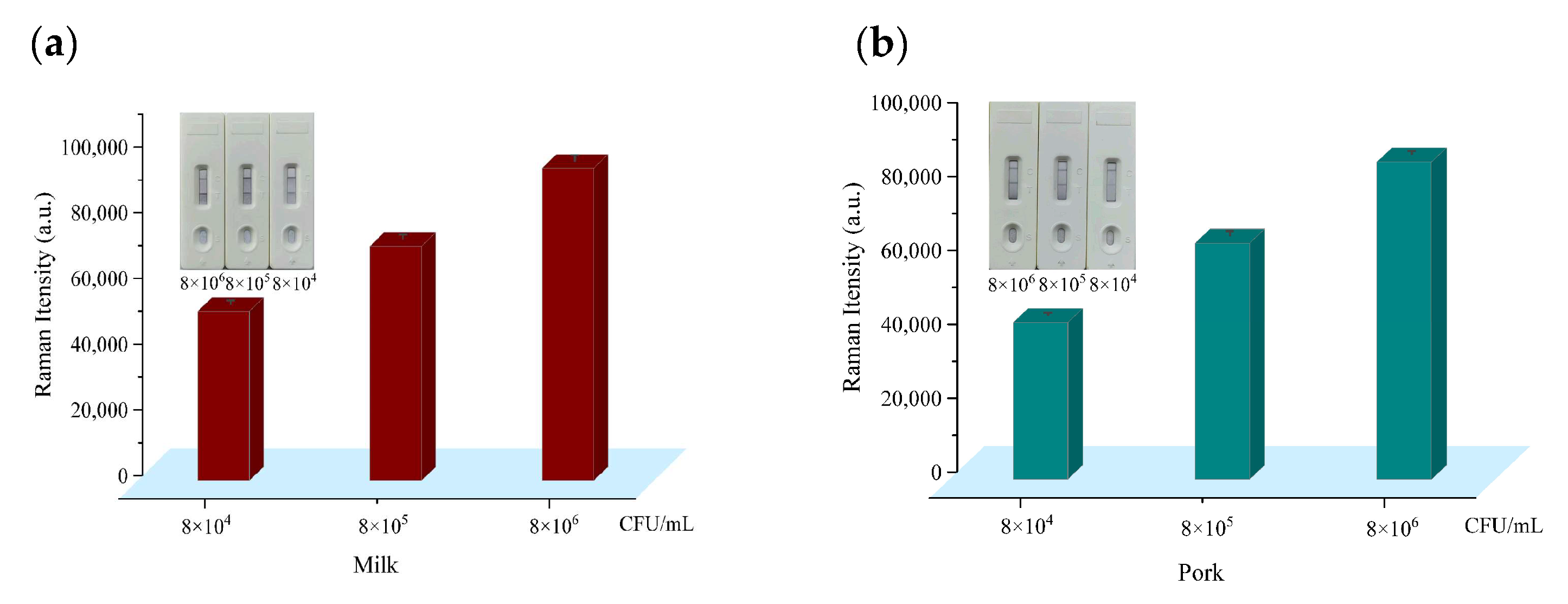
3.6. Detection of E. coli O157:H7 in Spiked Milk and Pork Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tack, D.M.; Kisselburgh, H.M.; Richardson, L.C.; Geissler, A.; Griffin, P.M.; Payne, D.C.; Gleason, B.L. Shiga Toxin-Producing Escherichia coli Outbreaks in the United States, 2010–2017. Microorganisms 2021, 9, 1529. [Google Scholar] [CrossRef] [PubMed]
- Koyun, O.Y.; Balta, I.; Corcionivoschi, N.; Callaway, T.R. Disease Occurrence in- and the Transferal of Zoonotic Agents by North American Feedlot Cattle. Foods 2023, 12, 904. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Terai, A.; Kurazono, H.; Takeda, Y.; Nishibuchi, M. Cloning and nucleotide sequencing of Vero toxin 2 variant genes from Escherichia coli O91: H21 isolated from a patient with the hemolytic uremic syndrome. Microb. Pathog. 1990, 8, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Hong, J.; Go, H.; Park, J.; Kong, M.; Ryu, S.; Kim, K.P.; Roh, E.; Park, J.K. Multiplexed Detection of Foodborne Pathogens from Contaminated Lettuces Using a Handheld Multistep Lateral Flow Assay Device. J. Agric. Food Chem. 2018, 66, 290–297. [Google Scholar] [CrossRef]
- Oluwarinde, B.O.; Ajose, D.J.; Abolarinwa, T.O.; Montso, P.K.; Du Preez, I.; Njom, H.A.; Ateba, C.N. Safety Properties of Escherichia coli O157:H7 Specific Bacteriophages: Recent Advances for Food Safety. Foods 2023, 12, 3989. [Google Scholar] [CrossRef]
- Fu, J.; Zhou, Y.; Huang, X.; Zhang, W.; Wu, Y.; Fang, H.; Zhang, C.; Xiong, Y. Dramatically Enhanced Immunochromatographic Assay Using Cascade Signal Amplification for Ultrasensitive Detection of Escherichia coli O157:H7 in Milk. J. Agric. Food Chem. 2020, 68, 1118–1125. [Google Scholar]
- Ferone, M.; Gowen, A.; Fanning, S.; Scannell, A.G.M. Microbial detection and identification methods: Bench top assays to omics approaches. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3106–3129. [Google Scholar]
- Kim, J.H.; Oh, S.W. Rapid detection for low numbers of Escherichia coli o157:h7 by real-time pcr in cabbage using a combination of filtration, short microbial enrichment, and dna concentration within 4 h. LWT-Food Sci. Technol. 2021, 139, 110520. [Google Scholar] [CrossRef]
- Kong, J.; Fan, C.; Liao, X.; Chen, A.; Yang, S.; Zhao, L.; Li, H. Accurate detection of Escherichia coli O157:H7 and Salmonella enterica serovar typhimurium based on the combination of next-generation sequencing and droplet digital PCR. LWT-Food Sci. Technol. 2022, 168, 113913. [Google Scholar] [CrossRef]
- Panchal, N.; Jain, V.; Elliott, R.; Flint, Z.; Worsley, P.; Duran, C.; Banerjee, T.; Santra, S. Plasmon-Enhanced Bimodal Nanosensors: An Enzyme-Free Signal Amplification Strategy for Ultrasensitive Detection of Pathogens. Anal. Chem. 2022, 94, 13968–13977. [Google Scholar] [CrossRef]
- Yu, Q.; Wu, T.; Tian, B.; Li, J.; Liu, Y.; Wu, Z.; Jin, X.; Wang, C.; Wang, C.; Gu, B. Recent advances in SERS-based immunochromatographic assay for pathogenic microorganism diagnosis: A review. Anal. Chim. Acta 2024, 1286, 341931. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Zhou, F.; Wang, Y.; Liao, Z.; Qian, S.; Luo, Q.; Zheng, J. Polydopamine modified colloidal gold nanotag-based lateral flow immunoassay platform for highly sensitive detection of pathogenic bacteria and fast evaluation of antibacterial agents. Talanta 2024, 278, 126525. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Xiong, S.; Huang, M.; Liu, B.; Huang, Z.; Xu, D.; Chen, X.; Xiong, Y. Recent advances in multifunctional nanomaterials enhanced immunochromatographic assays. TrAc Trend Anal. Chem. 2024, 181, 118013. [Google Scholar] [CrossRef]
- Wang, Q.; Long, M.; Lv, C.; Xin, S.; Han, X.; Jiang, W. Lanthanide-labeled fluorescent-nanoparticle immunochromatographic strips enable rapid and quantitative detection of Escherichia coli O157:H7 in food samples. Food Control 2020, 109, 106894. [Google Scholar] [CrossRef]
- Wang, R.; Kim, K.; Choi, N.; Wang, X.; Lee, J.; Jeon, J.H.; Rhie, G.; Choo, J. Highly sensitive detection of high-risk bacterial pathogens using sers-based lateral flow assay strips. Sens. Actuators B Chem. 2018, 270, 72–79. [Google Scholar] [CrossRef]
- Hong, X.; Mao, Y.; Yang, C.; Liu, Z.; Li, M.; Du, D. Contamination of Zearalenone from China in 2019 by a Visual and Digitized Immunochromatographic Assay. Toxins 2020, 12, 521. [Google Scholar] [CrossRef]
- Li, J.; Wu, T.; Wang, C.; Tu, J.; Song, X.; Shao, Y.; Wang, C.; Qi, K.; Xiao, R. Nanogapped Fe3O4-Au Surface-Enhanced Raman Scattering Tags for the Multiplex Detection of Bacteria on an Immunochromatographic Strip. ACS Appl. Nano Mater. 2022, 5, 12679–12689. [Google Scholar] [CrossRef]
- Shu, R.; Liu, S.; Huang, L.; Li, Y.; Sun, J.; Zhang, D.; Zhu, M.; Wang, J. Enzyme-Mimetic nano-immunosensors for amplified detection of food hazards: Recent advances and future trends. Biosens. Bioelectron. 2022, 217, 114577. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, C.; Li, J.; Wang, W.; Yu, Q.; Wang, C.; Wang, S. Graphene oxide-based three-dimensional au nanofilm with high-density and controllable hotspots: A powerful film-type sers tag for immunochromatographic analysis of multiple mycotoxins in complex samples. Chem. Eng. J. 2022, 448, 137760. [Google Scholar] [CrossRef]
- Mahmoudi, T.; Pourhassan-Moghaddam, M.; Shirdel, B.; Baradaran, B.; Morales-Narváez, E.; Golmohammadi, H. (Nano)tag-antibody conjugates in rapid tests. J. Mater. Chem. B 2021, 9, 5414–5438. [Google Scholar] [CrossRef]
- Wang, L.; Xu, D.; Jiang, L.; Gao, J.; Tang, Z.; Xu, Y.; Chen, X.; Zhang, H. Transition Metal Dichalcogenides for Sensing and Oncotherapy: Status, Challenges, and Perspective. Adv. Funct. Mater. 2021, 31, 2004408. [Google Scholar]
- Shorie, M.; Kumar, V.; Kaur, H.; Singh, K.; Tomer, V.K.; Sabherwal, P. Plasmonic DNA hotspots made from tungsten disulfide nanosheets and gold nanoparticles for ultrasensitive aptamer-based SERS detection of myoglobin. Mikrochim. Acta 2018, 185, 158. [Google Scholar] [CrossRef] [PubMed]
- Nisar, S.; Dastgeer, G.; Shahzadi, M.; Shahzad, Z.M.; Elah, E.; Irfan, A.; Eom, J.; Kim, H.; Kim, D. Gate-assisted MoSe2 transistor to detect the streptavidin via supporter molecule engineering. Mater. Today Nano 2023, 24, 100405. [Google Scholar]
- Cong, C.; Shang, J.; Wang, Y.; Yu, T. Optical Properties of 2D Semiconductor WS2. Adv. Opt. Mater. 2018, 6, 1700767. [Google Scholar]
- Hu, Y.; Huang, Y.; Wang, Z.; Wang, Y.; Ye, X.; Wong, W.; Li, C.; Sun, D. Gold/WS2 nanocomposites fabricated by in-situ ultrasonication and assembling for photoelectrochemical immunosensing of carcinoembryonic antigen. Mikrochim. Acta 2018, 185, 570. [Google Scholar]
- Wang, J.; Lu, Y.; Quan, W.; Hu, J.; Yang, P.; Song, G.; Fu, J.; Peng, Y.; Tong, L.; Ji, Q.; et al. Epitaxial Growth of Monolayer WS2 Single Crystals on Au(111) Toward Direct Surface-Enhanced Raman Spectroscopy Detection. ACS Nano 2024, 18, 26359–26368. [Google Scholar]
- Tang, X.; Hao, Q.; Hou, X.; Lan, L.; Li, M.; Yao, L.; Zhao, X.; Ni, Z.; Fan, X.; Qiu, T. Exploring and Engineering 2D Transition Metal Dichalcogenides toward Ultimate SERS Performance. Adv. Mater. 2024, 36, 2312348. [Google Scholar]
- Anju, K.S.; Midhun, P.S.; Kumar, K.R.; Rajeev, M.K. Tailoring surface enhanced Raman scattering platform based on pulsed laser deposited MoS2-Ag hybrid nanostructure. Mater. Chem. Phys. 2022, 276, 125286. [Google Scholar]
- Alamri, M.; Sakidja, R.; Goul, R.; Ghopry, S.; Wu, J.Z. Plasmonic Au nanoparticles on 2D MoS2/graphene van der waals heterostructures for high-sensitivity surface-enhanced raman spectroscopy. ACS Appl. Nano Mater. 2019, 2, 1412–1420. [Google Scholar]
- Yuan, H.; Yu, S.; Kim, M.; Li, J.E.; Kang, H.; Jiang, D.; Ramasamy, M.S.; Kim, D.H. Dopamine-mediated self-assembled anisotropic Au nanoworms conjugated with MoS2 nanosheets for SERS-based sensing. Sens. Actuators B Chem. 2022, 371, 132453. [Google Scholar]
- Chen, M.; Liu, D.; Du, X.; Luo, K.H.; Wang, S.; Zhou, B.; Pan, H. 2D materials: Excellent substrates for surface-enhanced Raman scattering (SERS) in chemical sensing and biosensing. TrAC Trends Anal. Chem. 2020, 130, 115983. [Google Scholar] [CrossRef]
- Song, X.; You, X.; Ren, X.; Zhang, X.; Tang, D.; Li, X. Vertically aligned Ag-decorated MoS2 nanosheets supported on polyvinyl alcohol flexible substrate enable high-sensitivity and self-cleaning SERS devices. J. Environ. Chem. Eng. 2023, 11, 109437. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, H.; Tian, Y.; Du, Y.; Ma, Y.; Zeng, S.; Gu, C.; Jiang, T.; Zhou, J. In Situ Recyclable Surface-Enhanced Raman Scattering-Based Detection of Multicomponent Pesticide Residues on Fruits and Vegetables by the Flower-like MoS2-Ag Hybrid Substrate. ACS Appl. Mater. Interfaces 2020, 12, 14386–14399. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wu, Y.C.; Yang, M.; Liu, X.; Coileáin, C.Ó.; Abid, M.; Abid, M.; Wang, J.J.; Shvets, I.; Xu, H.; et al. Surface enhanced Raman scattering of monolayer MX2 with metallic nano particles. Sci. Rep. 2016, 6, 30320. [Google Scholar] [CrossRef]
- Frens, G. Controlled Nucleation for the Regulation of the Particle Size in Monodisperse Gold Suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Zhou, K.G.; Mao, N.N.; Wang, H.X.; Peng, Y.; Zhang, H.L. A Mixed-Solvent Strategy for Efficient Exfoliation of Inorganic Graphene Analogues. Angew. Chem. Int. Ed. Engl. 2011, 50, 10839–10842. [Google Scholar] [CrossRef]
- Hwang, D.Y.; Suh, D.H. Universal surface modification of transition metal dichalcogenide decorated with metal nanoparticles for surface enhanced Raman scattering. Mater. Res. Bull. 2017, 90, 73–80. [Google Scholar] [CrossRef]
- Pramanik, A.; Davis, D.; Patibandla, S.; Begum, S.; Ray, P.; Gates, K.; Gao, Y.; Chandra Ray, P. A WS2-gold nanoparticle heterostructure-based novel SERS platform for the rapid identification of antibiotic-resistant pathogens. Nanoscale Adv. 2020, 2, 2025–2033. [Google Scholar] [CrossRef]
- Putnin, T.; Ngamaroonchote, A.; Wiriyakun, N.; Ounnunkad, K.; Laocharoensuk, R. Dually functional polyethylenimine-coated gold nanoparticles: A versatile material for electrode modification and highly sensitive simultaneous determination of four tumor markers. Microchim. Acta 2019, 186, 305. [Google Scholar] [CrossRef]
- Pang, L.; Wang, L.; Liang, Y.; Wang, Z.; Zhang, W.; Zhao, Q.; Yang, X.; Jiang, Y. G-triplex/hemin DNAzyme mediated colorimetric aptasensor for Escherichia coli O157:H7 detection based on exonuclease III-assisted amplification and aptamers-functionalized magnetic beads. Talanta 2024, 269, 125457. [Google Scholar] [CrossRef]
- Liu, H.B.; Chen, C.Y.; Zhang, C.N.; Du, X.J.; Li, P.; Wang, S. Functionalized AuMBA@Ag Nanoparticles as an Optical and SERS Dual Probe in a Lateral Flow Strip for the Quantitative Detection of Escherichia coli O157:H7. J. Food Sci. 2019, 84, 2916–2924. [Google Scholar] [PubMed]
- He, Q.; Pan, J.; Xu, Z.; Hammock, B.D.; Li, D. Development of a nanobody-based immunoassay for the detection of Escherichia coli O157:H7 in food samples. Food Chem. 2025, 473, 142987. [Google Scholar] [PubMed]
- Zhang, G.; Huang, Z.; Hu, L.; Wang, Y.; Deng, S.; Liu, D.; Peng, J.; Lai, W. Molecular Engineering Powered Dual-Readout Point-of-Care Testing for Sensitive Detection of Escherichia coli O157:H7. ACS Nano. 2023, 17, 23723–23731. [Google Scholar] [PubMed]
- Kim, J.E.; Shin, J.H.; Park, J.P. An engineered antimicrobial peptide as an alternative bioreceptor for the detection of pathogenic Escherichia coli O157:H7. J. Electroanal. Chem. 2024, 953, 118003. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, J.; Jiang, X.; Zhang, Y.; Zhang, J.; Wu, J. Bacteria-imprinted impedimetric sensor based on doping-induced nanostructured polypyrrole for determination of Escherichia coli. Mikrochim. Acta 2023, 190, 431. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Chen, Y.; Zhang, Q.; Chen, J.; Li, C.; Luo, Y.; Jin, Y.; Qi, X. SERS-Based Immunochromatographic Assay for Sensitive Detection of Escherichia coli O157:H7 Using a Novel WS2-AuDTNB Nanotag. Sensors 2025, 25, 2457. https://doi.org/10.3390/s25082457
Wang D, Chen Y, Zhang Q, Chen J, Li C, Luo Y, Jin Y, Qi X. SERS-Based Immunochromatographic Assay for Sensitive Detection of Escherichia coli O157:H7 Using a Novel WS2-AuDTNB Nanotag. Sensors. 2025; 25(8):2457. https://doi.org/10.3390/s25082457
Chicago/Turabian StyleWang, Deying, Yan Chen, Qi Zhang, Junfei Chen, Changhao Li, Yunjing Luo, Yong Jin, and Xiaohua Qi. 2025. "SERS-Based Immunochromatographic Assay for Sensitive Detection of Escherichia coli O157:H7 Using a Novel WS2-AuDTNB Nanotag" Sensors 25, no. 8: 2457. https://doi.org/10.3390/s25082457
APA StyleWang, D., Chen, Y., Zhang, Q., Chen, J., Li, C., Luo, Y., Jin, Y., & Qi, X. (2025). SERS-Based Immunochromatographic Assay for Sensitive Detection of Escherichia coli O157:H7 Using a Novel WS2-AuDTNB Nanotag. Sensors, 25(8), 2457. https://doi.org/10.3390/s25082457





