Membrane Permeability Monitoring to Antipsychotic Olanzapine Using Platinum Black-Modified Electrodes
Abstract
1. Introduction
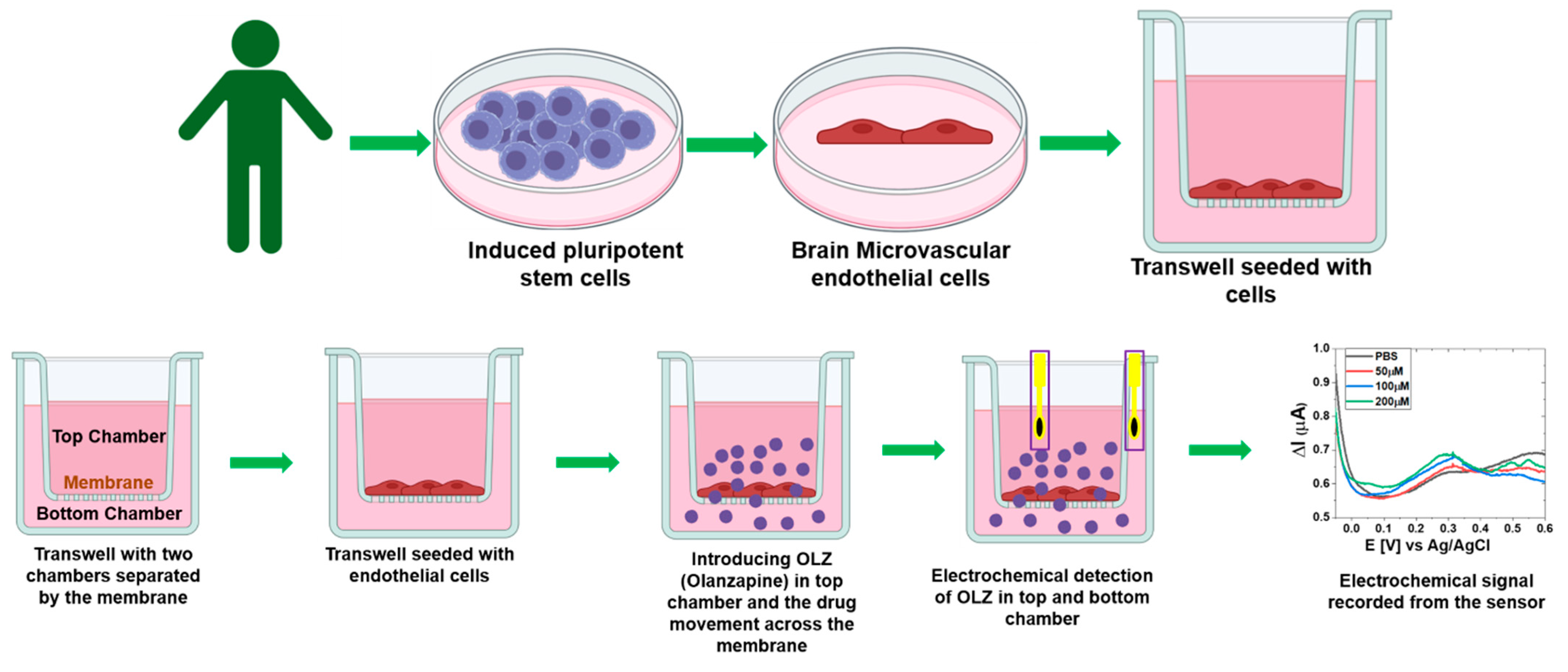
2. Materials and Methods
3. Results and Discussion
3.1. Olanzapine Sensing
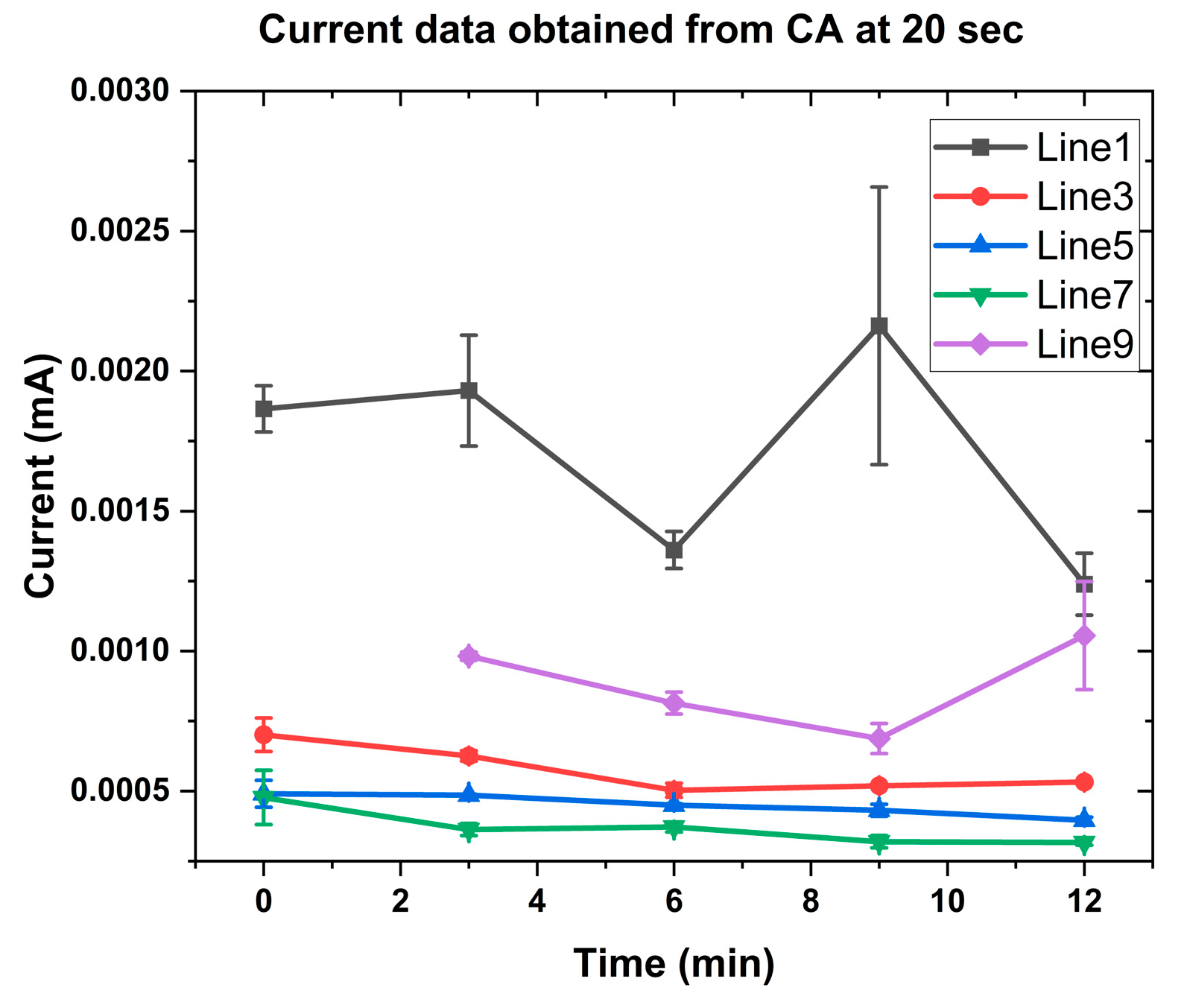
3.2. Rate Constant for Precision Dosing of OLZ
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meftah, A.M.; Deckler, E.; Citrome, L.; Kantrowitz, J.T. New Discoveries for an Old Drug: A Review of Recent Olanzapine Research. Postgrad. Med. 2020, 132, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Chen, S.; Li, D.; Chen, Q.; Zhu, M.; Wang, M.; Fu, X.; Lin, P. Metabolic Effects and Clinical Outcomes of Olanzapine in Schizophrenia: A Systematic Review and Meta-Analysis. Heliyon 2024, 10, e40424. [Google Scholar] [CrossRef] [PubMed]
- Carli, M.; Kolachalam, S.; Longoni, B.; Pintaudi, A.; Baldini, M.; Aringhieri, S.; Fasciani, I.; Annibale, P.; Maggio, R.; Scarselli, M. Atypical Antipsychotics and Metabolic Syndrome: From Molecular Mechanisms to Clinical Differences. Pharmaceuticals 2021, 14, 238. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, H.Y. Update on Typical and Atypical Antipsychotic Drugs. Annu. Rev. Med. 2013, 64, 393–406. [Google Scholar] [CrossRef]
- Lieberman, J.A.; Stroup, T.S.; McEvoy, J.P.; Swartz, M.S.; Rosenheck, R.A.; Perkins, D.O.; Keefe, R.S.E.; Davis, S.M.; Davis, C.E.; Lebowitz, B.D.; et al. Effectiveness of Antipsychotic Drugs in Patients with Chronic Schizophrenia. N. Engl. J. Med. 2005, 353, 1209–1223. [Google Scholar] [CrossRef]
- Tollefson, G.D.; Beasley, C.M.; Tran, P.V.; Street, J.S.; Krueger, J.A.; Tamura, R.N.; Graffeo, K.A.; Thieme, M.E. Olanzapine versus Haloperidol in the Treatment of Schizophrenia and Schizoaffective and Schizophreniform Disorders: Results of an International Collaborative Trial. Am. J. Psychiatry 1997, 154, 457–465. [Google Scholar] [CrossRef]
- Andreasen, N.C. Symptoms, Signs, and Diagnosis of Schizophrenia. Lancet 1995, 346, 477–481. [Google Scholar] [CrossRef]
- Bymaster, F.P.; Hemrick-Luecke, S.K.; Perry, K.W.; Fuller, R.W. Neurochemical Evidence for Antagonism by Olanzapine of Dopamine, Serotonin, Alpha 1-Adrenergic and Muscarinic Receptors in Vivo in Rats. Psychopharmacology 1996, 124, 87–94. [Google Scholar] [CrossRef]
- Catlow, J.T.; Barton, R.D.; Clemens, M.; Gillespie, T.A.; Goodwin, M.; Swanson, S.P. Analysis of Olanzapine in Human Plasma Utilizing Reversed-Phase High-Performance Liquid Chromatography with Electrochemical Detection. J. Chromatogr. B Biomed. Appl. 1995, 668, 85–90. [Google Scholar] [CrossRef]
- Xu, H.; Yang, F. The Interplay of Dopamine Metabolism Abnormalities and Mitochondrial Defects in the Pathogenesis of Schizophrenia. Transl. Psychiatry 2022, 12, 464. [Google Scholar] [CrossRef]
- Yun, S.; Yang, B.; Anair, J.D.; Martin, M.M.; Fleps, S.W.; Pamukcu, A.; Yeh, N.-H.; Contractor, A.; Kennedy, A.; Parker, J.G. Antipsychotic Drug Efficacy Correlates with the Modulation of D1 Rather than D2 Receptor-Expressing Striatal Projection Neurons. Nat. Neurosci. 2023, 26, 1417–1428. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Blood–Brain Barrier Delivery. Drug Discov. Today 2007, 12, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Tu, K.-H.; Yu, L.-S.; Sie, Z.-H.; Hsu, H.-Y.; Al-Jamal, K.T.; Wang, J.T.-W.; Chiang, Y.-Y. Development of Real-Time Transendothelial Electrical Resistance Monitoring for an In Vitro Blood-Brain Barrier System. Micromachines 2021, 12, 37. [Google Scholar] [CrossRef]
- Kincses, A.; Vigh, J.P.; Petrovszki, D.; Valkai, S.; Kocsis, A.E.; Walter, F.R.; Lin, H.-Y.; Jan, J.-S.; Deli, M.A.; Dér, A. The Use of Sensors in Blood-Brain Barrier-on-a-Chip Devices: Current Practice and Future Directions. Biosensors 2023, 13, 357. [Google Scholar] [CrossRef]
- Paradis, A.; Leblanc, D.; Dumais, N. Optimization of an in Vitro Human Blood–Brain Barrier Model: Application to Blood Monocyte Transmigration Assays. MethodsX 2016, 3, 25–34. [Google Scholar] [CrossRef]
- Wang, J.-S.; Taylor, R.; Ruan, Y.; Donovan, J.L.; Markowitz, J.S.; Lindsay De Vane, C. Olanzapine Penetration into Brain Is Greater in Transgenic Abcb1a P-Glycoprotein-Deficient Mice than FVB1 (Wild-Type) Animals. Neuropsychopharmacology 2004, 29, 551–557. [Google Scholar] [CrossRef]
- Luptáková, D.; Vallianatou, T.; Nilsson, A.; Shariatgorji, R.; Hammarlund-Udenaes, M.; Loryan, I.; Andrén, P.E. Neuropharmacokinetic Visualization of Regional and Subregional Unbound Antipsychotic Drug Transport across the Blood–Brain Barrier. Mol. Psychiatry 2021, 26, 7732–7745. [Google Scholar] [CrossRef]
- Ben-Shachar, D.; Livne, E.; Spanier, I.; Leenders, K.L.; Youdim, M.B. Typical and Atypical Neuroleptics Induce Alteration in Blood-Brain Barrier and Brain 59FeCl3 Uptake. J. Neurochem. 1994, 62, 1112–1118. [Google Scholar] [CrossRef]
- Pardridge, W.M.; Crawford, I.L.; Connor, J.D. Permeability Changes in the Blood-Brain Barrier Induced by Nortriptyline and Chlorpromazine. Toxicol. Appl. Pharmacol. 1973, 26, 49–57. [Google Scholar] [CrossRef]
- Dipta, P.; Sarsenbayeva, A.; Shmuel, M.; Forno, F.; Eriksson, J.W.; Pereira, M.J.; Abalo, X.M.; Wabitsch, M.; Thaysen-Andersen, M.; Tirosh, B. Macrophage-Derived Secretome Is Sufficient to Confer Olanzapine-Mediated Insulin Resistance in Human Adipocytes. Compr. Psychoneuroendocrinol. 2021, 7, 100073. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and Function of the Blood-Brain Barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Cecchelli, R.; Berezowski, V.; Lundquist, S.; Culot, M.; Renftel, M.; Dehouck, M.-P.; Fenart, L. Modelling of the Blood–Brain Barrier in Drug Discovery and Development. Nat. Rev. Drug Discov. 2007, 6, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER Measurement Techniques for In Vitro Barrier Model Systems. SLAS Technol. 2015, 20, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Holzreuter, M.A.; Segerink, L.I. Innovative Electrode and Chip Designs for Transendothelial Electrical Resistance Measurements in Organs-on-Chips. Lab. Chip 2024, 24, 1121–1134. [Google Scholar] [CrossRef]
- Dusci, L.J.; Peter Hackett, L.; Fellows, L.M.; Ilett, K.F. Determination of Olanzapine in Plasma by High-Performance Liquid Chromatography Using Ultraviolet Absorbance Detection. J. Chromatogr. B 2002, 773, 191–197. [Google Scholar] [CrossRef]
- Bonde, S.L.; Bhadane, R.P.; Gaikwad, A.; Gavali, S.R.; Katale, D.U.; Narendiran, A.S. Simultaneous Determination of Olanzapine and Fluoxetine in Human Plasma by LC-MS/MS: Its Pharmacokinetic Application. J. Pharm. Biomed. Anal. 2014, 90, 64–71. [Google Scholar] [CrossRef]
- Basavaiah, K.; Rajendraprasad, N.; Vinay, K.B. Isocratic High-Performance Liquid Chromatographic Assay of Olanzapine: Method Development and Validation. Int. Sch. Res. Not. 2014, 2014, 616941. [Google Scholar] [CrossRef]
- Jenkins, A.J.; Sarconi, K.M.; Raaf, H.N. Determination of Olanzapine in a Postmortem Case. J. Anal. Toxicol. 1998, 22, 605–609. [Google Scholar] [CrossRef]
- Patel, D.S.; Sharma, N.; Patel, M.C.; Patel, B.N.; Shrivastav, P.S.; Sanyal, M. LC–MS/MS Assay for Olanzapine in Human Plasma and Its Application to a Bioequivalence Study. Acta Pharm. Sin. B 2012, 2, 481–494. [Google Scholar] [CrossRef]
- Hussien, L.A.E.A.; Ghani, M.F.A.; El-Alamein, A.M.A.; Mohamed, E.H. Stability-Indicating Methods for the Determination of Olanzapine in Presence of Its Degradation Products. Eur. J. Chem. 2014, 5, 311–320. [Google Scholar] [CrossRef]
- Arvand, M.; Pourhabib, A. Surfactant-Assisted Voltammetric Determination of Olanzapine at Amine Functionalized TiO2/Multi-Walled Carbon Nanotubes Nanocomposite. J. Anal. Chem. 2019, 74, 1096–1103. [Google Scholar] [CrossRef]
- Heres, S.; Kraemer, S.; Bergstrom, R.F.; Detke, H.C. Pharmacokinetics of Olanzapine Long-Acting Injection: The Clinical Perspective. Int. Clin. Psychopharmacol. 2014, 29, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Azab, S.M.; Fekry, A.M. Role of Green Chemistry in Antipsychotics’ Electrochemical Investigations Using a Nontoxic Modified Sensor in McIlvaine Buffer Solution. ACS Omega 2019, 4, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.M.; Mohamed, M.A.; Salem, W.M. New Voltammetric Analysis of Olanzapine in Tablets and Human Urine Samples Using a Modified Carbon Paste Sensor Electrode Incorporating Gold Nanoparticles and Glutamine in a Micellar Medium. Anal. Methods 2015, 7, 581–589. [Google Scholar] [CrossRef]
- Shukla, R.P.; Belmaker, R.H.; Bersudsky, Y.; Ben-Yoav, H. A Platinum Black-Modified Microelectrode for in Situ Olanzapine Detection in Microliter Volumes of Undiluted Serum. J. Neural. Transm. 2020, 127, 291–299. [Google Scholar] [CrossRef]
- Qiang, L.; Vaddiraju, S.; Rusling, J.F.; Papadimitrakopoulos, F. Highly Sensitive and Reusable Pt-Black Microfluidic Electrodes for Long-Term Electrochemical Sensing. Biosens. Bioelectron. 2010, 26, 682–688. [Google Scholar] [CrossRef]
- Seo, H.-K.; Park, D.-J.; Park, J.-Y. Fabrication and Characterization of Platinum Black and Mesoporous Platinum Electrodes for in-Vivo and Continuously Monitoring Electrochemical Sensor Applications. Thin Solid. Films 2008, 516, 5227–5230. [Google Scholar] [CrossRef]
- Goh, A.; Roberts, D.; Wainright, J.; Bhadra, N.; Kilgore, K.; Bhadra, N.; Vrabec, T. Evaluation of Activated Carbon and Platinum Black as High-Capacitance Materials for Platinum Electrodes. Sensors 2022, 22, 4278. [Google Scholar] [CrossRef]
- Li, Y.; Sella, C.; Lemaître, F.; Guille Collignon, M.; Thouin, L.; Amatore, C. Highly Sensitive Platinum-Black Coated Platinum Electrodes for Electrochemical Detection of Hydrogen Peroxide and Nitrite in Microchannel. Electroanalysis 2013, 25, 895–902. [Google Scholar] [CrossRef]
- Stanca, S.E.; Hänschke, F.; Ihring, A.; Zieger, G.; Dellith, J.; Kessler, E.; Meyer, H.-G. Chemical and Electrochemical Synthesis of Platinum Black. Sci. Rep. 2017, 7, 1074. [Google Scholar] [CrossRef]
- Konopka, S.J.; McDuffie, B. Diffusion Coefficients of Ferri- and Ferrocyanide Ions in Aqueous Media, Using Twin-Electrode Thin-Layer Electrochemistry. Anal. Chem. 1970, 42, 1741–1746. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley: New York, NY, USA; Weinheim, Germany, 2001; ISBN 978-0-471-04372-0. [Google Scholar]
- Wayu, M.B.; Pannell, M.J.; Leopold, M.C. Layered Xerogel Films Incorporating Monolayer-Protected Cluster Networks on Platinum-Black-Modified Electrodes for Enhanced Sensitivity in First-Generation Uric Acid Biosensing. ChemElectroChem 2016, 3, 1245–1252. [Google Scholar] [CrossRef]
- Matthiesen, I.; Voulgaris, D.; Nikolakopoulou, P.; Winkler, T.E.; Herland, A. Continuous Monitoring Reveals Protective Effects of N-Acetylcysteine Amide on an Isogenic Microphysiological Model of the Neurovascular Unit. Small 2021, 17, e2101785. [Google Scholar] [CrossRef] [PubMed]
- Bednarek, R. In Vitro Methods for Measuring the Permeability of Cell Monolayers. Methods Protoc. 2022, 5, 17. [Google Scholar] [CrossRef]
- Salama, H.A.; Mahmoud, A.A.; Kamel, A.O.; Abdel Hady, M.; Awad, G.A.S. Phospholipid Based Colloidal Poloxamer–Nanocubic Vesicles for Brain Targeting via the Nasal Route. Colloids Surf. B Biointerfaces 2012, 100, 146–154. [Google Scholar] [CrossRef]
- McMartin, K.E.; Sebastian, C.S.; Dies, D.; Jacobsen, D. Kinetics and Metabolism of Fomepizole in Healthy Humans. Clin. Toxicol. 2012, 50, 375–383. [Google Scholar] [CrossRef]
- Currie, G.M. Pharmacology, Part 2: Introduction to Pharmacokinetics. J. Nucl. Med. Technol. 2018, 46, 221–230. [Google Scholar] [CrossRef]
- Menichetti, R.; Kanekal, K.H.; Bereau, T. Drug–Membrane Permeability across Chemical Space. ACS Cent. Sci. 2019, 5, 290–298. [Google Scholar] [CrossRef]
- Dutta, A.; Vreeken, J.; Ghiringhelli, L.M.; Bereau, T. Data-Driven Equation for Drug–Membrane Permeability across Drugs and Membranes. J. Chem. Phys. 2021, 154, 244114. [Google Scholar] [CrossRef]
- Blume, L.-F.; Denker, M.; Gieseler, F.; Kunze, T. Temperature Corrected Transepithelial Electrical Resistance (TEER) Measurement to Quantify Rapid Changes in Paracellular Permeability. Pharmazie 2010, 65, 19–24. [Google Scholar] [PubMed]
- Vigh, J.P.; Kincses, A.; Ozgür, B.; Walter, F.R.; Santa-Maria, A.R.; Valkai, S.; Vastag, M.; Neuhaus, W.; Brodin, B.; Dér, A.; et al. Transendothelial Electrical Resistance Measurement across the Blood–Brain Barrier: A Critical Review of Methods. Micromachines 2021, 12, 685. [Google Scholar] [CrossRef] [PubMed]
- Bard, A.J.; Faulkner, L.R. Basic Potential Step Methods. In Electrochemical Methods: Fundamentals and Applications; Wiley: New York, NY, USA; Weinheim, Germany, 2001; pp. 156–225. [Google Scholar]


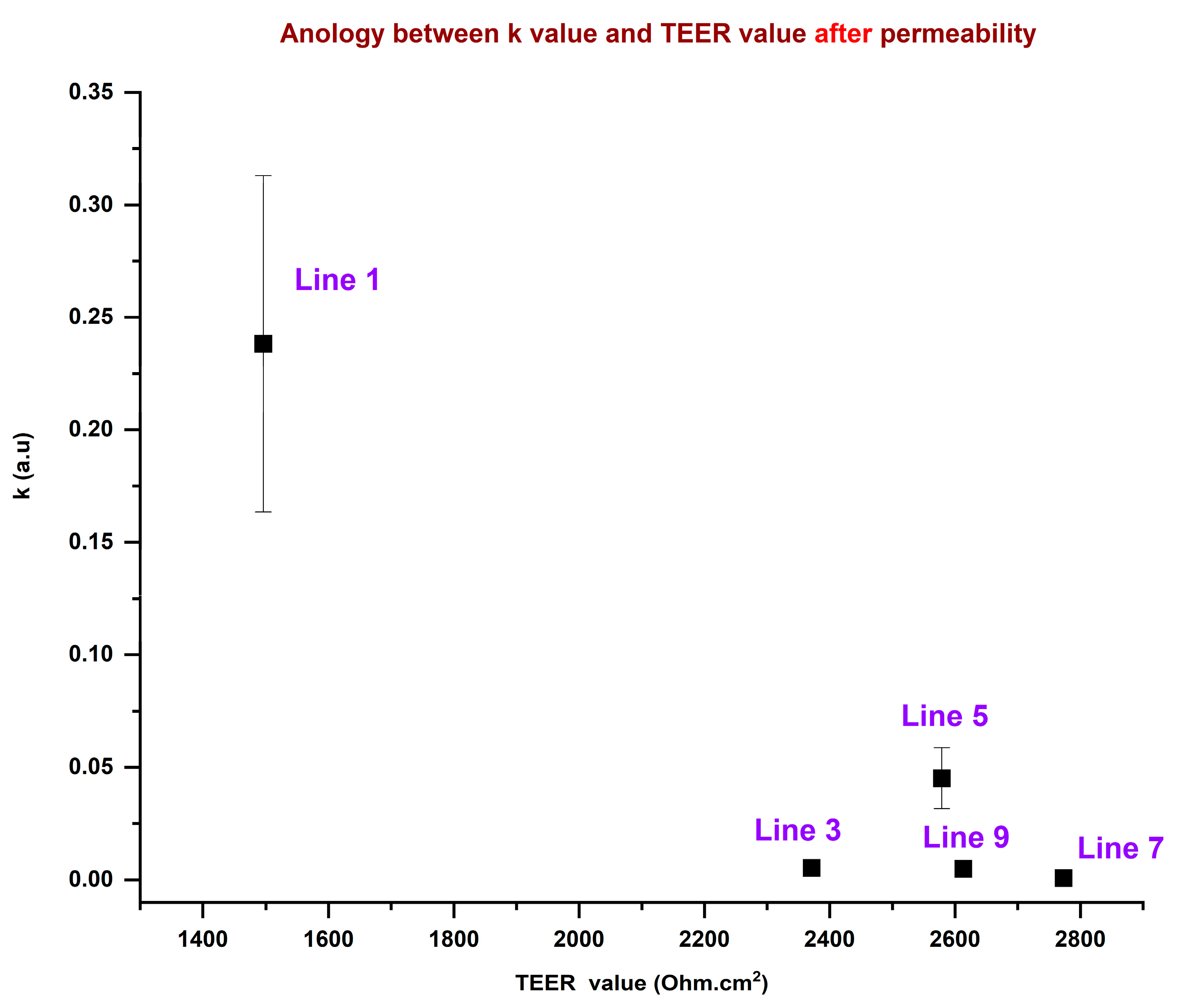
| Lines | Line 1 (Ω cm2) | Line 3 (Ω cm2) | Line 5 (Ω cm2) | Line 7 (Ω cm2) | Line 9 (Ω cm2) |
|---|---|---|---|---|---|
| Before Permeability | 1879 | 2689 | 2877 | 3145 | 2851 |
| After Permeability | 1496 | 2371 | 2613 | 2773 | 2579 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilango, M.S.; Desagani, D.; Jagadeesan, S.; Snezhko, A.; Vatine, G.; Ben-Yoav, H. Membrane Permeability Monitoring to Antipsychotic Olanzapine Using Platinum Black-Modified Electrodes. Sensors 2025, 25, 2266. https://doi.org/10.3390/s25072266
Ilango MS, Desagani D, Jagadeesan S, Snezhko A, Vatine G, Ben-Yoav H. Membrane Permeability Monitoring to Antipsychotic Olanzapine Using Platinum Black-Modified Electrodes. Sensors. 2025; 25(7):2266. https://doi.org/10.3390/s25072266
Chicago/Turabian StyleIlango, Murugaiya Sridar, Dayananda Desagani, Srikanth Jagadeesan, Alexander Snezhko, Gad Vatine, and Hadar Ben-Yoav. 2025. "Membrane Permeability Monitoring to Antipsychotic Olanzapine Using Platinum Black-Modified Electrodes" Sensors 25, no. 7: 2266. https://doi.org/10.3390/s25072266
APA StyleIlango, M. S., Desagani, D., Jagadeesan, S., Snezhko, A., Vatine, G., & Ben-Yoav, H. (2025). Membrane Permeability Monitoring to Antipsychotic Olanzapine Using Platinum Black-Modified Electrodes. Sensors, 25(7), 2266. https://doi.org/10.3390/s25072266







