Systematic Review of Commercially Available Clinical CMUT-Based Systems for Use in Medical Ultrasound Imaging: Products, Applications, and Performance
Abstract
1. Introduction
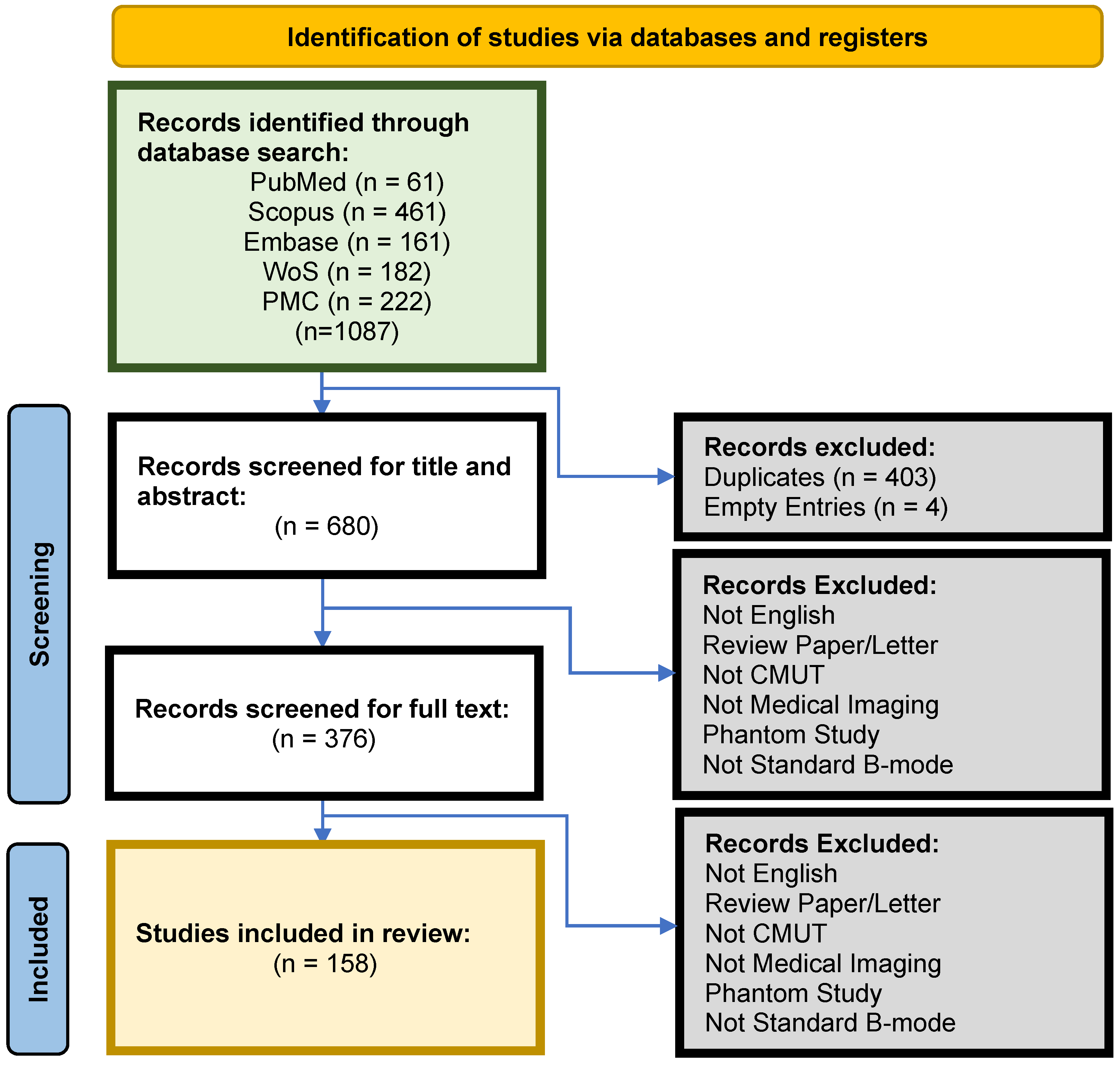
2. Methods
2.1. Pre-Search
2.2. Search Strategy
2.3. Eligibility Criteria
2.4. Data Extraction
3. Results
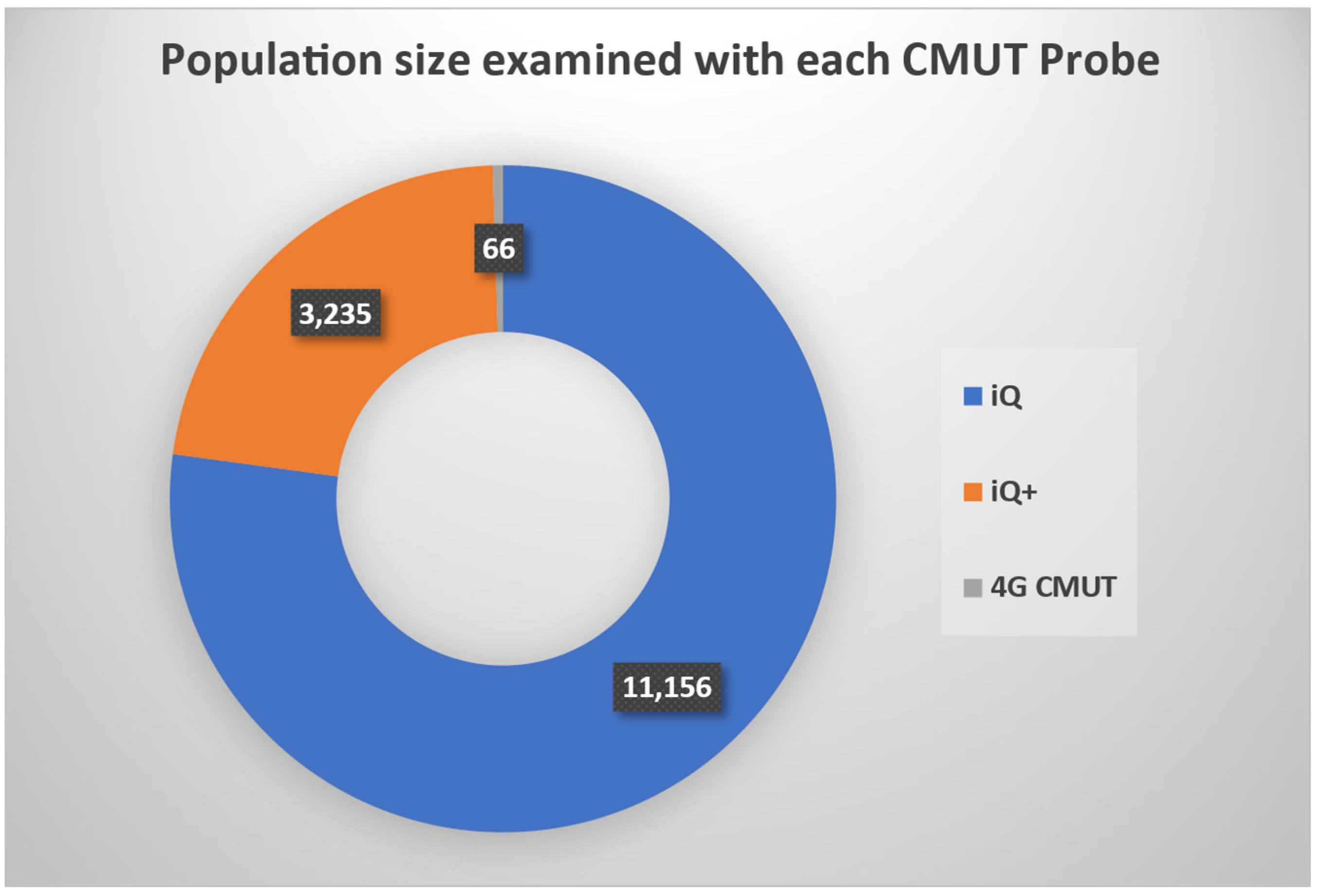
3.1. Commercially Available CMUT Products
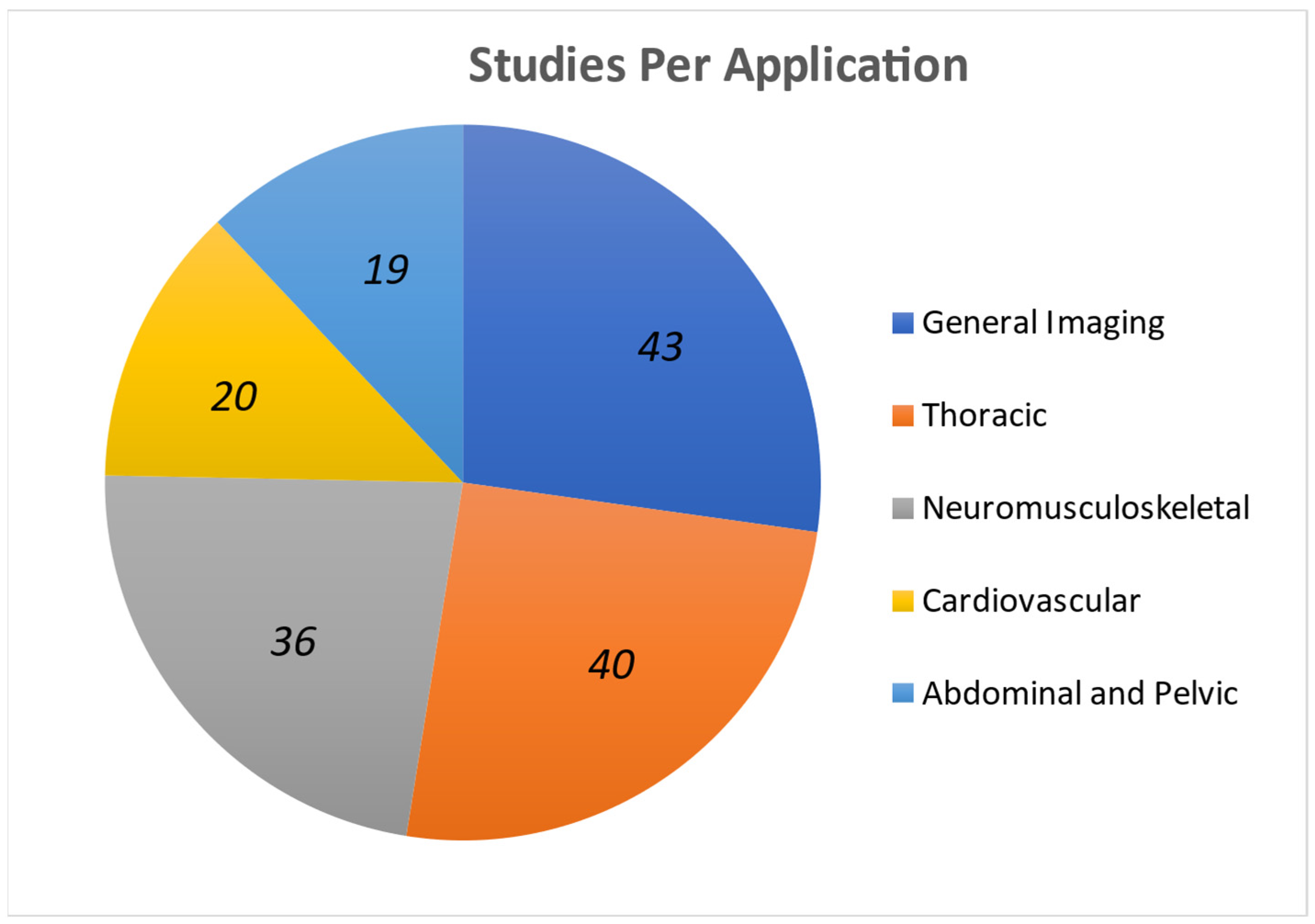
3.2. Applications and Performance
- Diagnostic Performance: a measure of the performance or accuracy of a diagnostic tool or method in detecting a condition. The performance metrics of this measure included the following:
- ∘
- Sensitivity (Se): the proportion of true positives among all patients with a condition/disease.
- ▪
- where TP = True Positives and FN = False Negatives;
- ∘
- Specificity (Sp): proportion of true negatives among all patients without a condition.
- ▪
- where TN = True Negatives and FP = False Positives;
- ∘
- Diagnostic Accuracy (DA): overall proportion of correctly identified cases, both true positives and true negatives. The general formula for DA is as follows:
- ▪
- ∘
- Positive Predictive Value (PPV): probability that a condition tested positive exists.
- ▪
- ∘
- Negative Predictive Value (NPV): probability that a condition tested negative does not exist.
- ▪
- ∘
- Area Under the Curve (AUC): area underneath the Receiver Operating Curve (ROC), which is a graphical representation of the true positive rate versus the false negative rate where their sum is equal to one, and they range from [0,1] and [0,1], respectively. AUC is ideally equal to 1 and at least larger than 0.5;
- ∘
- Diagnostic Duration (Time): time taken to complete a diagnostic procedure or obtain results;
- ∘
- Feasibility (Yes/No): determines whether a diagnostic method is practical and can be implemented effectively in clinical settings;
- ∘
- Cannulation or Injection Accuracy: rate or accuracy of successful cannulation or injection relying on a diagnostic tool/method for guidance;
- Correlation: a measure of the degree of similarity or agreement between test results and a reference standard, or the consistency between different observers and test conditions. Performance metrics included the following:
- ∘
- Inter-observer and Intra-observer Agreement (Kappa Agreement (k), ICC): this entailed two categories of agreement/reliability—agreement among different observers/operators/raters (interrater) over an identical exam and consistency of a single rater across repeated exams (intrarater);
- ▪
- Kappa Agreement/Cohen’s Kappa (k): agreement between two different raters on categorical assessments. It ranges from −1 (complete disagreement) to +1 (complete agreement), with 0 indicating random chance agreement. Standard k is usually employed for nominal categorical assessments, while variants are adapted for ordinal data or assessments by more than two raters;
- ▪
- Intraclass Correlation Coefficient (ICC): reliability or consistency across two or more different raters on continuous measurements. This metric was also employed by studies for intrarater reliability. It typically ranges from 0 (no reliability) to 1 (perfect reliability);
- ∘
- Correlation with a reference standard (Pearson’s Correlation/Spearman’s Rank Correlation):
- ▪
- Pearson’s Correlation (r): measures linear relationship between two continuous assessment variables;
- ▪
- Spearman’s Rank Correlation: measures correlation of the rank of two variables. It indicates the degree to which the variables are monotonically related, even if their relationship is not linear;
- ∘
- Measurement Variability/Reproducibility: fluctuation of results of tests repeated under similar conditions.
- Values ≤ 0: no or poor agreement;
- Values 0.01–0.20: poor or slight agreement;
- Values 0.21–0.40: fair agreement;
- Values 0.41–0.60: moderate agreement;
- Values 0.61–0.80: substantial agreement;
- Values 0.81–1.00: almost perfect agreement;
- Image Quality: a measure of the clarity, resolution, and usefulness of images produced by diagnostic tools.
- ∘
- Image Resolution: spatial (e.g., axial and lateral) technical image details;
- ∘
- Image Clarity: delineation of structure, sharpness and presence of artefacts;
- ∘
- Interpretability: images deemed useful for diagnosis;
- Learning Experience: a measure of improvements in US training, knowledge, interpretation and technical skills related to diagnostic procedures, often before and after training or experience. Metrics included the following:
- ∘
- Skill Acquisition and Retention (Objective Structured Clinical Exams (OSCE), Exam Scores, Theoretical/Practical Tests): assesses how well trainees acquire and retain US skills over time, often through examination;
- ∘
- US and Anatomical Knowledge Improvement (Exam Scores): assesses, often through exams, the improvement in anatomical or US-specific knowledge after training;
- ∘
- Training Effectiveness (Experts vs. Novices): assesses the performance of a trainee post-training against experts;
- ∘
- Feasibility of Teleguided Learning: assesses the feasibility or performance of a trainee or a novice in conducting US examination and/or interpreting US findings with teleguidance;
- Satisfaction: a measure of user confidence, ease of use, and operational efficiency. Metrics included the following:
- ∘
- User Satisfaction Scores (Surveys, User Feedback): evaluates subjective satisfaction in using the US tool, often through surveys or user feedback;
- ∘
- Confidence in Self-Assessment (Confidence Percentage, Surveys): measures how well users feel they performed.
3.2.1. Thoracic
3.2.2. Cardiovascular
3.2.3. Abdominal and Pelvic
3.2.4. Neuromusculoskeletal
3.2.5. General Imaging
3.3. Future Work of Modern Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Carovac, A.; Smajlovic, F.; Junuzovic, D. Application of Ultrasound in Medicine. Acta Inform. Medica 2011, 19, 168–171. [Google Scholar] [CrossRef]
- Sceusa, D.K. 1065: Ultrasound of Abdominal Organ Transplantation. Ultrasound Med. Biol. 2009, 35, S115. [Google Scholar] [CrossRef]
- Bethune, M.; Alibrahim, E.; Davies, B.; Yong, E. A pictorial guide for the second trimester ultrasound. Australas. J. Ultrasound Med. 2013, 16, 98–113. [Google Scholar] [CrossRef]
- Lucas, V.S.P.; Burk, R.S.; Creehan, S.B.; Grap, M.J.P. Utility of High-Frequency Ultrasound: Moving Beyond the Surface to Detect Changes in Skin Integrity. Plast. Surg. Nurs. 2014, 34, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Van Schaijk, R. CMUT and PMUT: New Technology Platform for Medical Ultrasound. November 2018. Available online: https://www.engineeringsolutions.philips.com/app/uploads/2019/03/CMUT-and-PMUT-Rob-van-Schaijk-November-2018.pdf (accessed on 15 July 2022).
- Sabbadini, L.; Germano, R.; Hopkins, E.; Haukoos, J.S.; Kendall, J.L. Ultrasound hypotension protocol time-motion study using the multifrequency single transducer versus a multiple transducer ultrasound device. West. J. Emerg. Med. 2021, 22, 775–781. [Google Scholar] [CrossRef]
- Yole Developpement. Ultrasound Sensing Technologies for Medical, Industrial and Consumer Applications 2018 Report by Yole Developpement. 2018. Available online: https://www.slideshare.net/slideshow/ultrasound-sensing-technologies-for-medical-industrial-and-consumer-applications-2018-report-by-yole-developpement-107577886/107577886 (accessed on 15 July 2022).
- Saeidi, N.; Selvam, K.; Vogel, K.; Baum, M.; Wiemer, M. Capacitive Micromachined Ultrasonic Transducers for Medical and Non-medical Applications; Fraunhofer ENAS: Chemnitz, Germany, 2019; pp. 1–18. [Google Scholar]
- Yole Developpement. From Technologies to Markets—Ultrasound Sensing Technologies 2020. 2020. Available online: https://medias.yolegroup.com/uploads/2020/11/YDR20134-Ultrasound-Sensing-Technologies-2020-Sample.pdf (accessed on 18 July 2022).
- Roh, Y. Ultrasonic transducers for medical volumetric imaging. Jpn. J. Appl. Phys. 2014, 53, 07KA01. [Google Scholar] [CrossRef]
- Bhuyan, A.; Choe, J.W.; Lee, B.C.; Cristman, P.; Oralkan, O.; Khuri-Yakub, B.T. Miniaturized, wearable, ultrasound probe for on-demand ultrasound screening. In Proceedings of the IEEE International Ultrasonics Symposium, New York, NY, USA, 18–21 October 2011; pp. 1060–1063. [Google Scholar] [CrossRef]
- Gross, D.; Boulmé, A.; Sénégond, N.; Férin, G.; Legros, M.; Roman, B.; Teston, F.; Certon, D. Dual Mode Transducers Based on cMUTs Technology. In Proceedings of the Acoustics 2012 Nantes Conference, Nantes, France, 23–27 April 2012. [Google Scholar]
- Stephens, D.N.; Truong, U.T.; Nikoozadeh, A.; Oralkan, Ö.; Seo, C.H.; Cannata, J.; Dentinger, A.; Thomenius, K.; de la Rama, A.; Nguyen, T.; et al. First In Vivo Use of a Capacitive Micromachined Ultrasound Transducer Array–Based Imaging and Ablation Catheter. J. Ultrasound Med. 2012, 31, 247–256. [Google Scholar] [CrossRef]
- Brenner, K.; Ergun, A.S.; Firouzi, K.; Rasmussen, M.F.; Stedman, Q.; Khuri-Yakub, B.P. Advances in Capacitive Micromachined Ultrasonic Transducers. Micromachines 2019, 10, 152. [Google Scholar] [CrossRef]
- Manwar, R.; Kratkiewicz, K.; Avanaki, K. Overview of ultrasound detection technologies for photoacoustic imaging. Micromachines 2020, 11, 692. [Google Scholar] [CrossRef]
- Hitachi. Technology Innovation Finance, Public, Healthcare: Research & Development: Hitachi Review. 2018. Available online: https://www.hitachihyoron.com/rev/archive/2018/r2018_03/25/index.html (accessed on 17 July 2022).
- Kolo Medical Inc. Products Series. Available online: http://www.kolomedical.com/xdwxl (accessed on 17 July 2022).
- Verasonics, Inc. Verasonics®. CMUT Transducers—Verasonics. Available online: https://verasonics.com/cmut-hf-transducers/ (accessed on 17 July 2022).
- Savoia, A.S.; Caliano, G. MEMS-Based Transducers (CMUT) and Integrated Electronics for Medical Ultrasound Imaging. In Lecture Notes in Electrical Engineering; Springer: Cham, Switzerland, 2018; Volume 431, pp. 421–429. [Google Scholar] [CrossRef]
- Burleson, S.L.; Swanson, J.F.; Shufflebarger, E.F.; Wallace, D.W.; Heimann, M.A.; Crosby, J.C.; Pigott, D.C.; Gullett, J.P.; Thompson, M.A.; Greene, C.J. Evaluation of a novel handheld point-of-care ultrasound device in an African emergency department. Ultrasound J. 2020, 12, 53. [Google Scholar] [CrossRef]
- Zhuang, S.; Zhao, D.; Chen, L.; Zhai, L. A 50-MHz CMUT Probe for Medical Ultrasound Imaging. In Proceedings of the 2018 IEEE International Ultrasonics Symposium (IUS), Kobe, Japan, 22–25 October 2018; pp. 1–4. [Google Scholar] [CrossRef]
- Zhao, D.; Zhuang, S.; Daigle, R. A commercialized high frequency CMUT probe for medical ultrasound imaging. In Proceedings of the 2015 IEEE International Ultrasonics Symposium, Taipei, Taiwan, 21–24 October 2015. [Google Scholar] [CrossRef]
- Vermon. Innovations—Vermon MEMS. Available online: https://vermon-mems.com/innovations/#ressource (accessed on 20 July 2022).
- Basset, O.; Bouakaz, A.; Sénégond, N.; Toulemonde, M.; Guillermin, R.; Fouan, D.; Lin, F.; Tourniaire, F.; Cristea, A.; Novell, A.; et al. Ultrasound imaging using CMUT—Techniques developed in the frame of the ANR BBMUT project. IRBM 2015, 36, 126–132. [Google Scholar] [CrossRef]
- Certon, D.; Legros, M.; Gross, D.; Vince, P.; Gens, F.; Gregoire, J.; Coutier, C.; Novell, A.; Bouakaz, A. Ultrasound pre-clinical platform for diagnosis and targeted therapy. In Proceedings of the 2014 IEEE International Ultrasonics Symposium (IUS), Chicago, IL, USA, 3–6 September 2014; pp. 329–332. [Google Scholar] [CrossRef]
- Wagner, P.; Daft, C.; Panda, S.; Ladabaum, I. 5G-1 Two Approaches to Electronically Scanned 3D Imaging Using cMUTs. In Proceedings of the 2006 IEEE Ultrasonics Symposium, Vancouver, Canada, 3–6 October 2006; pp. 685–688. [Google Scholar]
- Daft, C.; Wagner, P.; Bymaster, B.; Panda, S.; Patel, K.; Ladabaum, I. cMUTs and electronics for 2D and 3D imaging: Monolithic integration, in-handle chip sets and system implications. In Proceedings of the IEEE Ultrasonics Symposium, Rotterdam, The Netherlands, 18–21 September 2005. [Google Scholar] [CrossRef]
- Savoia, A.; Caliano, G.; Pappalardo, M. A CMUT probe for medical ultrasonography: From microfabrication to system integration. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2012, 59, 1127–1138. [Google Scholar] [CrossRef]
- Savoia, A.; Caliano, G.; Mauti, B.; Pappalardo, M. Performance optimization of a high frequency CMUT probe for medical imaging. In Proceedings of the 2011 IEEE International Ultrasonics Symposium (IUS), Orlando, FL, USA, 18–21 October 2011; pp. 600–603. [Google Scholar]
- Cid, X.; Wang, A.; Heiberg, J.; Canty, D.; Royse, C.; Li, X.; El-Ansary, D.; Yang, Y.; Haji, K.; Haji, D.; et al. Point-of-care lung ultrasound in the assessment of patients with COVID-19: A tutorial. Australas. J. Ultrasound Med. 2020, 23, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Bennett, D.; De Vita, E.; Mezzasalma, F.; Lanzarone, N.; Cameli, P.; Bianchi, F.; Perillo, F.; Bargagli, E.; Mazzei, M.A.; Volterrani, L.; et al. Portable pocket-sized ultrasound scanner for the evaluation of lung involvement in coronavirus disease 2019 patients. Ultrasound Med. Biol. 2021, 47, 19–24. [Google Scholar] [CrossRef]
- Pugliese, C.M.; Adegbite, B.R.; Edoa, J.R.; Mombo-Ngoma, G.; Obone-Atome, F.A.; Heuvelings, C.C.; Bélard, S.; Kalkman, L.C.; Leopold, S.J.; Hänscheid, T.; et al. Point-of-care ultrasound to assess volume status and pulmonary oedema in malaria patients. Infection 2021, 50, 65–82. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Weng, I.; Graglia, S.; Lew, T.; Gandhi, K.; Lalani, F.; Chia, D.; Duanmu, Y.; Jensen, T.; Lobo, V.; et al. Point-of-CareUltrasound Predicts Clinical Outcomes in Patients WithCOVID-19. J. Ultrasound Med. 2021, 41, 1367–1375. [Google Scholar] [CrossRef]
- Knight, T.; Edwards, L.; Rajasekaran, A.; Clare, S.; Lasserson, D. Point-of-care lung ultrasound in the assessment of suspected COVID-19: A retrospective service evaluation with a severity score. Acute Med. J. 2020, 19, 192–200. [Google Scholar] [CrossRef]
- Haji-Hassan, M.; Lenghel, L.M.; Bolboacă, S.D. Hand-Held Ultrasound of the Lung: A Systematic Review. Diagnostics 2021, 11, 1381. [Google Scholar] [CrossRef]
- Tung-Chen, Y.; de Gracia, M.M.; Díez-Tascón, A.; Alonso-González, R.; Agudo-Fernández, S.; Parra-Gordo, M.L.; Ossaba-Vélez, S.; Rodríguez-Fuertes, P.; Llamas-Fuentes, R. Correlation between Chest Computed Tomography and Lung Ultrasonography in Patients with Coronavirus Disease 2019 (COVID-19). Ultrasound Med. Biol. 2020, 46, 2918–2926. [Google Scholar] [CrossRef]
- Calvo-Cebrián, A.; Alonso-Roca, R.; Rodriguez-Contreras, F.J.; Rodríguez-Pascual, M.d.L.N.; Calderín-Morales, M.d.P. Usefulness of Lung Ultrasound Examinations Performed by Primary Care Physicians in Patients with Suspected COVID-19. J. Ultrasound Med. 2020, 40, 741–750. [Google Scholar] [CrossRef]
- Chiem, A.T.; Lim, G.W.; Tabibnia, A.P.; Takemoto, A.S.; Weingrow, D.M.; Shibata, J.E. Feasibility of patient-performed lung ultrasound self-exams (Patient-PLUS) as a potential approach to telemedicine in heart failure. ESC Hear. Fail. 2021, 8, 3997–4006. [Google Scholar] [CrossRef]
- Pivetta, E.; Girard, E.; Locascio, F.; Lupia, E.; Martin, J.D.; Stone, M. Self-Performed Lung Ultrasound for Home Monitoring of a Patient Positive for Coronavirus Disease 2019. Chest 2020, 158, E93–E97. [Google Scholar] [CrossRef] [PubMed]
- Tung-Chen, Y. Lung ultrasound in the monitoring of COVID-19 infection. Clin. Med. 2020, 20, e62–e65. [Google Scholar] [CrossRef]
- Shokoohi, H.; Duggan, N.M.; Sánchez, G.G.-D.; Torres-Arrese, M.; Tung-Chen, Y. Lung ultrasound monitoring in patients with COVID-19 on home isolation. Am. J. Emerg. Med. 2020, 38, 2759.e5–2759.e8. [Google Scholar] [CrossRef]
- Kumar, A.; Weng, Y.; Graglia, S.; Chung, S.; Duanmu, Y.; Lalani, F.; Gandhi, K.; Lobo, V.; Jensen, T.; Nahn, J.; et al. Interobserver Agreement of Lung Ultrasound Findings of COVID-19. J. Ultrasound Med. 2021, 40, 2369–2376. [Google Scholar] [CrossRef] [PubMed]
- Leiphrakpam, P.D.; Weber, H.R.; McCain, A.; Matas, R.R.; Duarte, E.M.; Buesing, K.L. A novel large animal model of smoke inhalation-induced acute respiratory distress syndrome. Respir. Res. 2021, 22, 198. [Google Scholar] [CrossRef]
- Leviter, J.; Auerbach, M.; Amick, M.; O’Marr, J.; Battipaglia, T.; Amendola, C.; Riera, A. Point-of-Care Ultrasound Curriculum for Endotracheal Tube Confirmation for Pediatric Critical Care Transport Team Through Remote Learning and Teleguidance. Air Med J. 2022, 41, 222–227. [Google Scholar] [CrossRef]
- Pivetta, E.; Cara, I.; Paglietta, G.; Scategni, V.; Labarile, G.; Tizzani, M.; Porrino, G.; Locatelli, S.; Calzolari, G.; Morello, F.; et al. Diaphragmatic point-of-care ultrasound in COVID-19 patients in the emergency department—A proof-of-concept study. J. Clin. Med. 2021, 10, 5291. [Google Scholar] [CrossRef]
- Kok, B.; Schuit, F.; Lieveld, A.; Azijli, K.; Nanayakkara, P.W.; Bosch, F. Comparing lung ultrasound: Extensive versus short in COVID-19 (CLUES): A multicentre, observational study at the emergency department. BMJ Open 2021, 11, e048795. [Google Scholar] [CrossRef]
- Liu, W.; A Zagzebski, J.; Hall, T.J.; Madsen, E.L.; Varghese, T.; A Kliewer, M.; Panda, S.; Lowery, C.; Barnes, S. Acoustic backscatter and effective scatterer size estimates using a 2D CMUT transducer. Phys. Med. Biol. 2008, 53, 4169–4183. [Google Scholar] [CrossRef]
- Bima, P.; Pivetta, E.; Baricocchi, D.; Giamello, J.D.; Risi, F.; Vesan, M.; Chiarlo, M.; De Stefano, G.; Ferreri, E.; Lauria, G.; et al. Lung Ultrasound Improves Outcome Prediction over Clinical Judgment in COVID-19 Patients Evaluated in the Emergency Department. J. Clin. Med. 2022, 11, 3032. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.B.; Hasara, S.; Coker, P. Identification of a branchial cleft anomaly via handheld point-of-care ultrasound. J. Ultrason. 2022, 22, 67–69. [Google Scholar] [CrossRef]
- Pivetta, E.; Goffi, A.; Tizzani, M.; Locatelli, S.M.; Porrino, G.; Losano, I.; Leone, D.; Calzolari, G.; Vesan, M.; Steri, F.; et al. Lung Ultrasonography for the Diagnosis of SARS-CoV-2 Pneumonia in the Emergency Department. Ann. Emerg. Med. 2020, 77, 385–394. [Google Scholar] [CrossRef]
- Pafitanis, G.; Pawa, A.; Mohanna, P.-N.; Din, A.H. The Butterly iQ: An ultra-simplified color Doppler ultrasound for bedside pre-operative perforator mapping in DIEP flap breast reconstruction. J. Plast. Reconstr. Aesthetic Surg. 2020, 73, 983–1007. [Google Scholar] [CrossRef]
- Marini, T.J.; Castaneda, B.; Iyer, R.; Baran, T.M.; Nemer, O.; Dozier, A.M.; Parker, K.J.; Zhao, Y.; Serratelli, W.; Matos, G.; et al. Breast Ultrasound Volume Sweep Imaging: A New Horizon in Expanding Imaging Access for Breast Cancer Detection. J. Ultrasound Med. 2022, 42, 817–832. [Google Scholar] [CrossRef]
- Sagreiya, H.; Jacobs, M.; Akhbardeh, A. Novel Quantitative Tool for Assessing Pulmonary Disease Burden in COVID-19 Using Ultrasound. Med Phys. 2022, 49. [Google Scholar] [CrossRef]
- Figueroa, J.F.; Pokrzywa, C.J.; Brandolino, A.; Murphy, P.; A De Moya, M.; Carver, T.M.W. The Time Course of Recurrent Pneumothorax Development after Thoracostomy Tube Removal in Trauma Patients: An Ultra-Portable Ultrasound Study (UPUS Trial). J. Am. Coll. Surg. 2022, 235, S98. [Google Scholar] [CrossRef]
- Dewar, Z.E.; Ko, S.; Rogers, C.; Oropallo, A.; Augustine, A.; Pamula, A.; Berry, C.L. Prehospital portable ultrasound for safe and accurate prehospital needle thoracostomy: A pilot educational study. Ultrasound J. 2022, 14, 1–6. [Google Scholar] [CrossRef]
- Marini, T.J.; Castaneda, B.; Parker, K.; Baran, T.M.; Romero, S.; Iyer, R.; Zhao, Y.T.; Hah, Z.; Park, M.H.; Brennan, G.; et al. No sonographer, no radiologist: Assessing accuracy of artificial intelligence on breast ultrasound volume sweep imaging scans. PLOS Digit. Health 2022, 1, e0000148. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, R.; Chiem, A. 377 Peer-Instructed Teleguidance Ultrasound in Undergraduate Medical Education: A Randomized Control Equivalence Study. Ann. Emerg. Med. 2022, 80, S163. [Google Scholar] [CrossRef]
- Gibbons, R.C.; Magee, M.; Goett, H.; Murrett, J.; Genninger, J.; Mendez, K.; Tripod, M.; Tyner, N.; Costantino, T.G. Lung Ultrasound vs. Chest X-Ray Study for the Radiographic Diagnosis of COVID-19 Pneumonia in a High-Prevalence Population. J. Emerg. Med. 2021, 60, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Schilp, C.M.; Meijer, L.; Stocker, M.; Langermans, J.A.M.; Bakker, J.; Stammes, M.A. A Comparative Study of Chest CT With Lung Ultrasound After SARS-CoV-2 Infection in the Assessment of Pulmonary Lesions in Rhesus Monkeys (Macaca Mulatta). Front. Veter- Sci. 2021, 8, 748635. [Google Scholar] [CrossRef]
- Neves, S.E.; Fatima, H.; Walsh, D.P.; Mahmood, F.; Chaudhary, O.; Matyal, R. Role of Ultrasound-Guided Evaluation of Dyspnea in the Coronavirus Disease 2019 Pandemic. J. Cardiothorac. Vasc. Anesthesia 2020, 34, 3197–3202. [Google Scholar] [CrossRef]
- Pivetta, E.; Ravetti, A.; Paglietta, G.; Cara, I.; Buggè, F.; Scozzari, G.; Maule, M.M.; Morello, F.; Locatelli, S.; Lupia, E. Feasibility of Self-Performed Lung Ultrasound with Remote Teleguidance for Monitoring at Home COVID-19 Patients. Biomedicines 2022, 10, 2569. [Google Scholar] [CrossRef]
- Tung-Chen, Y.; Hernández, A.G.; Vargas, A.M.; Doblado, L.D.; Merino, P.E.G.; Alijo, Á.V.; Jiménez, J.H.; Rojas, Á.G.; Prieto, S.G.; Abreu, E.V.G.; et al. Impact of lung ultrasound during the SARS-CoV-2 pandemic: Distinction between viral and bacterial pneumonia. Reumatol. Clínica 2022, 18, 546–550. [Google Scholar] [CrossRef]
- Duggan, N.M.; Jowkar, N.; Ma, I.W.Y.; Schulwolf, S.; Selame, L.A.; Fischetti, C.E.; Kapur, T.; Goldsmith, A.J. Novice-performed point-of-care ultrasound for home-based imaging. Sci. Rep. 2022, 12, 1–8. [Google Scholar] [CrossRef]
- Schaad, S.; Brahier, T.; Hartley, M.-A.; Cordonnier, J.-B.; Bosso, L.; Espejo, T.; Pantet, O.; Hugli, O.; Carron, P.-N.; Meuwly, J.-Y.; et al. Point-of-care lung ultrasonography for early identification of mild COVID-19: A prospective cohort of outpatients in a Swiss screening center. BMJ Open 2022, 12, e060181. [Google Scholar] [CrossRef]
- Tung-Chen, Y.; Algora-Martín, A.; Llamas-Fuentes, R.; Rodríguez-Fuertes, P.; Virto, A.M.M.; Sanz-Rodríguez, E.; Alonso-Martínez, B.; Núñez, M.A.R. Point-of-care ultrasonography in the initial characterization of patients with COVID-19. Med. Clin. 2021, 156, 477–484. [Google Scholar] [CrossRef]
- Tung-Chen, Y.; Rivera-Núñez, M.A.; Martínez-Virto, A.M. Lung ultrasound in the frontline diagnosis of COVID-19 infection. Med. Clin. 2020, 155, 232. [Google Scholar] [CrossRef]
- Golombeck, D.; Khandokar, R.; Fu, D.; Provenzale, A.; McGee, M.; Lin, M.; Rossi, D.; Maybaum, S. Acquisition of High-Quality Pulmonary Ultrasound Images in the Heart Failure Clinic Following a Short Period of Training. J. Hear. Lung Transplant. 2024, 43, S211–S212. [Google Scholar] [CrossRef]
- Edelman, J.; Taylor, H.; Goss, A.-M.; Tisovszky, N.; Sun, K.M.; O’toole, S.; Herriotts, K.; Inglis, E.; Johnson, C.; Penfold, S.; et al. Point-of-care ultrasound as a diagnostic tool in respiratory assessment in awake paediatric patients: A comparative study. Arch. Dis. Child. 2023, 109, 287–291. [Google Scholar] [CrossRef]
- Sieber, S.; Garbe, J.; Böhm, S.; Eisenmann, S. Pneumothorax detection with thoracic ultrasound as the method of choice in interventional pulmonology—A retrospective single-center analysis and experience. BMC Pulm. Med. 2023, 23, 227. [Google Scholar] [CrossRef]
- Elmi, N.; Sadri, Y.; Myslik, F.; Chenkin, J.; Cherniak, W. Self-administered at-home lung ultrasound with remote guidance in patients without clinical training. Respir. Res. 2024, 25, 111. [Google Scholar] [CrossRef]
- Mulye, A.; Bhasin, A.; Borger, B.; Fant, C. Virtual immediate feedback with POCUS in Belize. Front. Digit. Health 2023, 5, 1268905. [Google Scholar] [CrossRef]
- Haji-Hassan, M.; Călinici, T.; Drugan, T.; Bolboacă, S.D. Effectiveness of Ultrasound Cardiovascular Images in Teaching Anatomy: A Pilot Study of an Eight-Hour Training Exposure. Int. J. Environ. Res. Public Health 2022, 19, 3033. [Google Scholar] [CrossRef]
- Sanchez, S.; Miller, M.; Asha, S. Assessing the validity of two-dimensional carotid ultrasound to detect the presence and absence of a pulse. Resuscitation 2020, 157, 67–73. [Google Scholar] [CrossRef]
- Wang, L.; Harrison, J.; Dranow, E.; Khor, L. Point of Care Ultrasound of the Jugular Venous Pulse in the Upright Position (u2 Jvp) Predicts Elevated Right Atrial Pressure and Pulmonary Capillary Wedge Pressure on Right Heart Catheterization in Obese and Non-obese Patients. Circulation 2020, 142, A16244. [Google Scholar] [CrossRef]
- Scott, T.; van Waart, H.; Vrijdag, X.C.E.; Mullins, D.; Mesley, P.; Mitchell, S.J. Arterial blood gas measurements during deep open-water breath-hold dives. J. Appl. Physiol. 2021, 130, 1490–1495. [Google Scholar] [CrossRef]
- Wang, L.; Harrison, J.; Dranow, E.; Khor, L. Abstract 16212: Ultrasound Jugular Venous Pulsation Outperforms Visual Assessment and Ivc Collapsibility for Central Venous Pressures in Obese Patients. Circulation 2020, 142. [Google Scholar] [CrossRef]
- Khor, L.L.C.; Wang, L.; Harrison, J.; Alijev, N.; Dranow, E. Ultrasound of the internal jugular vein (uJVP) with point of care ultrasound (pocus) correlates with elevated right atrial pressure (RAP) on right heart catheterization (RHC) in predicting 6-month mortality. J. Am. Coll Cardiol. 2021, 77, 708. [Google Scholar] [CrossRef]
- Dua Niyyar, V.; Buch, K.; Rawls, F.; Broxton, R. Effectiveness of Ultrasound-Guided Cannulation of AVF on Infiltration Rates: A Single-Center Quality Improvement Study. Am. J. Kidney Dis. 2021, 77, 581. [Google Scholar] [CrossRef]
- Gulati, U.; Ray, K.; Dasgupta, S.; Jerusik, B. 124 Comparison of First-Pass Peripheral Intravenous Cannulation Using a Handheld Ultrasound Device to Using a Traditional High-End Ultrasound System: A Randomized Controlled Trial. Ann. Emerg. Med. 2021, 78, S51. [Google Scholar] [CrossRef]
- Karimpour, K.; Brenner, R.J.; Dong, G.Z.; Cleve, J.; Martina, S.; Harris, C.; Graf, G.J.; Kistler, B.J.; Hoang, A.H.; Jackson, O.; et al. Comparison of Newer Hand-Held Ultrasound Devices for Post-Dive Venous gas Emboli Quantification to Standard Echocardiography. Front. Physiol. 2022, 13, 907651. [Google Scholar] [CrossRef]
- Jujo, S.; Lee-Jayaram, J.J.; Sakka, B.I.; Nakahira, A.; Kataoka, A.; Izumo, M.; Kusunose, K.; Athinartrattanapong, N.; Oikawa, S.; Berg, B.W. Pre-clinical medical student cardiac point-of-care ultrasound curriculum based on the American Society of Echocardiography recommendations: A pilot and feasibility study. Pilot Feasibility Stud. 2021, 7, 1–15. [Google Scholar] [CrossRef]
- Jujo, S.; Sakka, B.I.; Lee-Jayaram, J.J.; Kataoka, A.; Izumo, M.; Kusunose, K.; Nakahira, A.; Oikawa, S.; Kataoka, Y.; Berg, B.W. Medical student medium-term skill retention following cardiac point-of-care ultrasound training based on the American Society of Echocardiography curriculum framework. Cardiovasc. Ultrasound 2022, 20, 1–12. [Google Scholar] [CrossRef]
- Mchechesi, I.; Simemeza, T.M.; Chikuwadzo, B.; Keller, M. Zimbabwe telehealth pilot program identifies high risk patients in an underserved population using point of care echocardiograms and digital electrocardiograms. J. Am. Coll Cardiol. 2021, 77, 3024. [Google Scholar] [CrossRef]
- Ladha, P.; Truong, E.; Kanuika, P.; Allan, A.; Kishawi, S.; Ho, V.P.; Claridge, J.A.; Brown, L.R. Diagnostic Adjunct Techniques in the Assessment of Hypovolemia: A Prospective Pilot Project. J. Surg. Res. 2023, 293, 1–7. [Google Scholar] [CrossRef]
- Pettit, N.A.; Pedroja, B.S.; Li, H.F.; Sutcliffe, M. Brief training in ultrasound equips novice clinicians to accurately and reliably measure jugular venous pressure in obese patients. Australas. J. Ultrasound Med. 2023, 26, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Stec, S.; Wileczek, A.; Reichert, A.; Śledź, J.; Kosior, J.; Jagielski, D.; Polewczyk, A.; Zając, M.; Kutarski, A.; Karbarz, D.; et al. Shared Decision Making and Cardioneuroablation Allow Discontinuation of Permanent Pacing in Patients with Vagally Mediated Bradycardia. J. Cardiovasc. Dev. Dis. 2023, 10, 392. [Google Scholar] [CrossRef]
- Willems, L.; Vermeulen, J.; Wiegerinck, A.; Fekkes, S.; Reijnen, M.; Warlé, M.; De Korte, C.; Thijssen, D. Construct Validity and Reproducibility of Handheld Ultrasound Devices in Carotid Artery Diameter Measurement. Ultrasound Med. Biol. 2022, 49, 866–874. [Google Scholar] [CrossRef]
- Lowell, M.J.; Kropf, C.W.; Thomas, J.; Valentini, N.; Schmid, S.A.; Hunt, N. Pericardiocentesis During Transport for Cardiac Tamponade Complicating Acute Type A Aortic Dissection. Air Med J. 2024, 43, 445–449. [Google Scholar] [CrossRef]
- Huang, O.; Palmeri, M.L. TPU Based Deep Learning Image Enhancement for Real-Time Point-of-Care Ultrasound. IEEE Trans. Comput. Imaging 2024, 10, 461–468. [Google Scholar] [CrossRef]
- Aguero, P.; Barnes, R.F.; Flores, A.; von Drygalski, A. Teleguidance for Patient Self-Imaging of Hemophilic Joints Using Mobile Ultrasound Devices. J. Ultrasound Med. 2022, 42, 701–712. [Google Scholar] [CrossRef]
- Hoffman, S.; Desai, S.; Sikorski, M.; Fatupaito, G.; Tupua, S.; Thomsen, R.; Rambocus, S.; Nimarota-Brown, S.; Punimata, L.; Sialeipata, M.; et al. Point-of-Care Ultrasound by Nonexpert Operators Demonstrates High Sensitivity and Specificity in Detecting Gallstones: Data from the Samoa Typhoid Fever Control Program. Am. J. Trop. Med. Hyg. 2022, 106, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Alfuraih, A.M.; Alrashed, A.I.; Almazyad, S.O.; Alsaadi, M.J. Abdominal aorta measurements by a handheld ultrasound device compared with a conventional cart-based ultrasound machine. Ann. Saudi Med. 2021, 41, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Erlick, M.B.; Marini, T.; Drennan, K.; Dozier, A.; Castaneda, B.; Baran, T.; Toscano, M. Assessment of a Brief Standardized Obstetric Ultrasound Training Program for Individuals Without Prior Ultrasound Experience. Ultrasound Q. 2022, 39, 124–128. [Google Scholar] [CrossRef]
- Ash, S.; Carr, D.; Keeling, G.; Giza, G. Abstracts from the 40th Annual Dialysis Conference Held in Kansas City, Missouri, 8–11 February 2020. Perit. Dial. Int. J. Int. Soc. Perit. Dial. 2020, 40, 2S–18S. [Google Scholar] [CrossRef]
- Houze, A.; Baek, I.; Amerling, R. Point-of-service ultrasound (POSUS) in nephrology. Perit. Dial. Int. 2020, 40, 4S–5S. [Google Scholar]
- Wright, H.; Brar, H.; Henry, F.; Corrigan, D.; De, S. Can handheld point of care ultrasound probes reliably measure transabdominal prostate volume? A prospective randomized study. J. Urol. 2022, 207, 1362734. [Google Scholar] [CrossRef]
- Manzar, S.; Bhat, R. Feasibility of handheld ultrasound to assess heart rate in newborn nursery. Resuscitation 2022, 179, 78–82. [Google Scholar] [CrossRef]
- Skjøt-Arkil, H.; Heltborg, A.; Lorentzen, M.H.; Cartuliares, M.B.; Hertz, M.A.; Graumann, O.; Rosenvinge, F.S.; Petersen, E.R.B.; Østergaard, C.; Laursen, C.B.; et al. Improved diagnostics of infectious diseases in emergency departments: A protocol of a multifaceted multicentre diagnostic study. BMJ Open 2021, 11, e049606. [Google Scholar] [CrossRef]
- Bui, M.; Fernandez, A.; Ramsukh, B.; Noel, O.; Prashad, C.; Bayne, D. Training and Implementation of Handheld Ultrasound Technology at Georgetown Public Hospital Corporation: A Low-cost Intervention to Improve Diagnostic Evaluation in a Resource-limited Urology Clinic. J. Endourol. 2022, 36, A1–A315. [Google Scholar] [CrossRef]
- Rothberg, J.M.; Ralston, T.S.; Rothberg, A.G.; Martin, J.; Zahorian, J.S.; Alie, S.A.; Sanchez, N.J.; Chen, K.; Chen, C.; Thiele, K.; et al. Ultrasound-on-chip platform for medical imaging, analysis, and collective intelligence. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef]
- Asikhia, O.; Bhatnagar, N.; Au, A.; Lewiss, R.; Fields, M.; Chang, A.; Maloney, K.; Chu, T.; Bollinger, E.; Tam, A. 351 The Accuracy of Handheld Ultrasound in the Evaluation of Symptomatic Pregnant Patients in the Emergency Department. Ann. Emerg. Med. 2022, 80, S151. [Google Scholar] [CrossRef]
- Pokaprakarn, T.; Prieto, J.C.; Price, J.T.; Kasaro, M.P.; Sindano, N.; Shah, H.R.; Peterson, M.; Akapelwa, M.M.; Kapilya, F.M.; Sebastião, Y.V.; et al. AI Estimation of Gestational Age from Blind Ultrasound Sweeps in Low-Resource Settings. NEJM Évid. 2022, 1. [Google Scholar] [CrossRef]
- Bhagavatula, S.; Thompson, D.; Dominas, C.; Haider, I.; Jonas, O. Self-Expanding Anchors for Stabilizing Percutaneously Implanted Microdevices in Biological Tissues. Micromachines 2021, 12, 404. [Google Scholar] [CrossRef]
- Rittenhouse, K.J.; Vwalika, B.; Sebastião, Y.; Pokaprakarn, T.; Sindano, N.; Shah, H.; Stringer, E.M.; Kasaro, M.P.; Cole, S.R.; Stringer, J.S.A.; et al. Accuracy of portable ultrasound machines for obstetric biometry. Ultrasound Obstet. Gynecol. 2024, 63, 772–780. [Google Scholar] [CrossRef]
- Jalfon, M.; Gardezi, M.; Heckscher, D.; Shaheen, D.; Maciejewski, K.R.; Li, F.; Rickey, L.; Foster, H.; Cavallo, J.A. Agreement and Reliability of Patient-measured Postvoid Residual Bladder Volumes. Urology 2023, 184, 62–68. [Google Scholar] [CrossRef]
- Araujo, K.G.; Yoshida, A.; Juliato, C.R.T.; Sarian, L.O.; Derchain, S. Performance of a handheld point of care ultrasonography to assess IUD position compared to conventional transvaginal ultrasonography. Eur. J. Contracept. Reprod. Health Care 2024, 29, 69–75. [Google Scholar] [CrossRef]
- Wachira, J.; Matheka, D.M.; Masheti, S.A.; Githemo, G.K.; Shah, S.; Haldeman, M.S.; Ramos, M.; Bergman, K. A training program for obstetrics point-of-care ultrasound to 514 rural healthcare providers in Kenya. BMC Med Educ. 2023, 23, 1–8. [Google Scholar] [CrossRef]
- Duminuco, A.; Cupri, A.; Massimino, R.; Leotta, S.; Milone, G.A.; Garibaldi, B.; Giuffrida, G.; Garretto, O.; Milone, G. Handheld Ultrasound or Conventional Ultrasound Devices in Patients Undergoing HCT: A Validation Study. J. Clin. Med. 2023, 12, 520. [Google Scholar] [CrossRef] [PubMed]
- Bui, M.; Fernandez, A.; Ramsukh, B.; Noel, O.; Prashad, C.; Bayne, D. Training and implementation of handheld ultrasound technology at Georgetown Public Hospital Corporation in Guyana: A virtual learning cohort study. J. Educ. Evaluation Health Prof. 2023, 20, 11. [Google Scholar] [CrossRef]
- Merkel, D.; Züllich, T.F.; Schneider, C.; Yousefzada, M.; Beer, D.; Ludwig, M.; Weimer, A.; Künzel, J.; Kloeckner, R.; Weimer, J.M. Prospective Comparison of Handheld Ultrasound Devices from Different Manufacturers with Respect to B-Scan Quality and Clinical Significance for Various Abdominal Sonography Questions. Diagnostics 2023, 13, 3622. [Google Scholar] [CrossRef]
- Motamedi, D.; Bauer, A.H.; Patel, R.; Morgan, T.A. Problem Solved. J. Ultrasound Med. 2020, 40, 1693–1704. [Google Scholar] [CrossRef] [PubMed]
- Draghi, F.; Lomoro, P.; Bortolotto, C.; Mastrogirolamo, L.; Calliada, F. Comparison between a new ultrasound probe with a capacitive micromachined transducer (CMUT) and a traditional one in musculoskeletal pathology. Acta Radiol. 2020, 61, 1653–1660. [Google Scholar] [CrossRef]
- Corte, G.; Bayat, S.; Tascilar, K.; Valor, L.; Schuster, L.; Knitza, J.; Schett, G.; Kleyer, A.; Simon, D. POS1394 accuracy and performance of a handheld ultrasound device to assess articular and periarticular pathologies in patients with inflammatory arthritis. Ann. Rheum. Dis. 2021, 80, 979–980. [Google Scholar] [CrossRef]
- Dzihic, E.; To, J.K.; Horton, D.; Vu, A.N.; Browne, A.W. Clinical comparison of ophthalmic ultrasound with a portable multipurpose ultrasound. Invest. Ophthalmol. Vis. Sci. 2021, 62, 8. [Google Scholar]
- Tajfirouz, D.; Judge, C.; Chen, J.J.; McClelland, C. Proceedings of the 43nd Annual Upper Midwest Neuro-Ophthalmology Group Meeting, July 23, 2021 and Second Virtual Upper Midwest Neuro-Ophthalmology Group Meeting. Neuro-Ophthalmology 2021, 45, 411–416. [Google Scholar] [CrossRef]
- Hagemeijer, N.; Sato, G.; Bhimani, R.; Lubberts, B.; Elghazy, M.A.; Kerkhoffs, G.; Guss, D.; DiGiovanni, C.W. Diagnosing Syndesmosis Instability: Dynamic Ultrasound versus Arthroscopy: A Cadaveric Study. Foot Ankle Orthop. 2020, 5, 2473011420S0024. [Google Scholar] [CrossRef]
- Hagemeijer, N.; Bhimani, R.; Saengsin, J.; Lubberts, B.; Waryasz, G.R.; Kerkhoffs, G.; DiGiovanni, C.W. Portable Dynamic Ultrasonography is a Useful Tool for the Evaluation of Suspect Syndesmotic Instability: A Cadaveric Study. Foot Ankle Orthop. 2020, 5, 2473011420S0024. [Google Scholar] [CrossRef]
- Hagemeijer, N.; Lubberts, B.; Saengsin, J.; Bhimani, R.; Sato, G.; Waryasz, G.R.; Kerkhoffs, G.; DiGiovanni, C.W. Portable Dynamic Ultrasonography Versus Fluoroscopy for the Evaluation of Syndesmotic Instability: A Cadaveric Study. Foot Ankle Orthop. 2020, 5, 2473011420S0004. [Google Scholar] [CrossRef]
- Flores, A.R.; Srinivasan, V.M.; Seeley, J.; Huggins, C.; Kan, P.; Burkhardt, J.-K. Safety, Feasibility, and Patient-Rated Outcome of Sonolucent Cranioplasty in Extracranial-Intracranial Bypass Surgery to Allow for Transcranioplasty Ultrasound Assessment. World Neurosurg. 2020, 144, e277–e284. [Google Scholar] [CrossRef]
- Wheat, S.W.; Stryjewska, B.; Cartwright, M.S. A Hand-Held Ultrasound Device for the Assessment of Peripheral Nerves in Leprosy. J. Neuroimaging 2021, 31, 76–78. [Google Scholar] [CrossRef]
- Grobelski, J.; Recker, F.; Wilsmann-Theis, D.; Hartung, W.; Karakostas, P.; Brossart, P.; Schäfer, V. Establishment and validation of a didactic musculoskeletal ultrasound course for dermatologists using an innovative handheld ultrasound system—The MUDE study (Musculoskeletal Ultrasound in Dermatology). JDDG J. Der Dtsch. Dermatol. Gesellschaft 2021, 19, 1753–1759. [Google Scholar] [CrossRef]
- Li, J.J.; Kim, J.J.; Young, C.; Nausheen, F. Comparing the Effectiveness and Image Quality of Musculoskeletal Ultrasound of First-Year Medical Students After Training by Student Tutors Versus Ultrasound Instructors: A Pilot Study. Cureus 2022, 14, e26890. [Google Scholar] [CrossRef]
- Cook, A.E.; Inkpen, P. Education in the Time of COVID: At-a-Distance Training in Neuromusculoskeletal Ultrasonography. Arch. Rehabilitation Res. Clin. Transl. 2020, 3, 100098. [Google Scholar] [CrossRef]
- Yu, K.; Niu, X.; He, B. Neuromodulation Management of Chronic Neuropathic Pain in the Central Nervous System. Adv. Funct. Mater. 2020, 30, 1908999. [Google Scholar] [CrossRef]
- Zhao, D.; Zhuang, S.; Weng, L. One-probe solution in medical ultrasound imaging with CMUT technology. In Proceedings of the 2016 IEEE International Ultrasonics Symposium (IUS), Tours, France, 18–21 September 2016; pp. 1–3. [Google Scholar]
- Manzar, S. Obtaining Neonatal Head Ultrasound Using Butterfly iQ. J. Clin. Neonatol. 2021, 10, 146. [Google Scholar] [CrossRef]
- Blenkinsop, G.; A Heller, R.; Carter, N.J.; Burkett, A.; Ballard, M.; Tai, N. Remote ultrasound diagnostics disrupting traditional military frontline healthcare delivery. BMJ Mil. Health 2021, 169, 456–458. [Google Scholar] [CrossRef]
- Eslick, I.G.; Reid, K.; Eslick, G.D.; Reid, C. Diagnosing a fracture using a smartphone during the COVID-19 pandemic. J. Paediatr. Child Health 2022, 58, 1272–1273. [Google Scholar] [CrossRef]
- Surek, C.C. A New Target for Temple Volumization? An Anatomical and Ultrasound-Guided Study of the Intermediate Temporal Fat Pad. Aesthetic Surg. J. 2021, 41, 1339–1343. [Google Scholar] [CrossRef]
- Mahapatra, S.; Balamurugan, M.; Chung, K.; Kuppoor, V.; Curry, E.; Aghabaglou, F.; Kaovasia, T.P.; Acord, M.; Ainechi, A.; Kim, J.; et al. Automatic detection of cotton balls during brain surgery: Where deep learning meets ultrasound imaging to tackle foreign objects. In Medical Imaging 2021: Ultrasonic Imaging and Tomography; Byram, B.C., Ruiter, N.V., Eds.; Progress in Biomedical Optics and Imaging; SPIE—International Society Optical Engineering: Bellingham, WA, USA, 2021; Volume 11602, pp. 295–302. [Google Scholar]
- Dhaliwal, G.S.B.; Swanson, D.L. Bilateral Subcutaneous Lipomas: The Potential of Point-Of-Care Ultrasonography as a Diagnostic Tool in Dermatology. Dermatol. Surg. 2021, 47, 1328–1329. [Google Scholar] [CrossRef]
- Aguero, P.; Flores, A.; Von Drygalski, A. Implementation of tele-guidance for patient self-imaging of arthropathic joints using mobile ultrasound devices-results from a pilot study. Res. Pract. Thromb. Haemost. 2021, 5, e12591. [Google Scholar] [CrossRef]
- Bu, Y.; Borna, A.; Schwindt, P.; Zeng, X.; Baum, E.; Rao, R.; Kimball, D.; Shah, V.; Coleman, T.; Huang, M.; et al. Capturing the Magnetic Signature of Small Fiber Neuronal Activity. Neuromodulation 2021, 24, e32–e33. [Google Scholar] [CrossRef]
- Rodriguez, P.A.; Salimi, N.; Abdelfattah, A.; Yanez, D.; Gonzalez, A.; Alian, A. The use of handheld ultrasound device for neuraxial placement in obstetric patients. Anesth. Analg. 2022, 134, 81. [Google Scholar]
- Bhimani, R.; Ashkani-Esfahani, S.; Mirochnik, K.; Lubberts, B.; DiGiovanni, C.W.; Tanaka, M.J. Utility of Diagnostic Ultrasound in the Assessment of Patellar Instability. Orthop. J. Sports Med. 2022, 10, e1777–e1787. [Google Scholar] [CrossRef]
- Bhimani, R.; Lubberts, B.; DiGiovanni, C.W.; Tanaka, M.J. Dynamic Ultrasound Can Accurately Quantify Severity of Medial Knee Injury: A Cadaveric Study. Arthrosc. Sports Med. Rehabil. 2022, 4, e1777–e1787. [Google Scholar] [CrossRef]
- Ferreira, G.F.; Sevilla, D.; Oliveira, C.N.; Junior, L.C.N.; Arliani, G.G.; Oliveira, V.O.; Filho, M.V.P. Comparison of the effect of hyaluronic acid injection versus extracorporeal shockwave therapy on chronic plantar fasciitis: Protocol for a randomized controlled trial. PLoS ONE 2021, 16, e0250768. [Google Scholar] [CrossRef]
- Krishnan, S.R.; Arafa, H.M.; Kwon, K.; Deng, Y.; Su, C.-J.; Reeder, J.T.; Freudman, J.; Stankiewicz, I.; Chen, H.-M.; Loza, R.; et al. Continuous, noninvasive wireless monitoring of flow of cerebrospinal fluid through shunts in patients with hydrocephalus. npj Digit. Med. 2020, 3, 13. [Google Scholar] [CrossRef]
- Alfuraih, A.M.; Alqarni, M.A.; Alhuthaili, H.S.; Mubaraki, M.Y.; Alotaibi, N.N.; Almusalim, F.M. Reproducibility and feasibility of a handheld ultrasound device compared to a standard ultrasound machine in muscle thickness measurements. Australas. J. Ultrasound Med. 2023, 26, 13–20. [Google Scholar] [CrossRef]
- Thomas, J.O.; To, J.K.; Vu, A.N.; Horton, D.; Dzihic, E.; Browne, A.W. Imaging performance of portable and conventional ultrasound imaging technologies for ophthalmic applications. PLoS ONE 2024, 19, e0300451. [Google Scholar] [CrossRef]
- Elliott-Burke, T.; Dillon, T.; Bailey, J.; Miller, S.; Joos, R.; Stein, A.B. Lumbar multifidus muscle ultrasound imaging: Is handheld technology reliable? Musculoskelet. Sci. Pract. 2023, 65, 102771. [Google Scholar] [CrossRef]
- Ramanujam, V.; Tian, L.; Chow, C.; Kendall, M.C. Three-Dimensional Imaging of Commonly Performed Peripheral Blocks: Using a Handheld Point-of-Care Ultrasound System. Anesthesiol. Pain Med. 2023, 13, e134797. [Google Scholar] [CrossRef]
- Loffredo, L.; Pignatelli, P. Radial artery pseudo-aneurysm detected with a portable handheld ultrasound device in a COVID-19 patient. Kardiol. Polska 2023, 81, 66–67. [Google Scholar] [CrossRef]
- Kolnik, S.E.; Sahota, A.; Wood, T.R.; German, K.; Puia-Dumitrescu, M.; Mietzsch, U.; Dighe, M.; Law, J.B. Cranial Point-of-Care Ultrasound for Neonatal Providers. J. Ultrasound Med. 2024, 43, 1089–1097. [Google Scholar] [CrossRef]
- E Park, K.; Mehta, P.; Tran, C.; O Parikh, A.; Zhou, Q.; Zhang-Nunes, S. A comparison of five point-of-care ultrasound devices for use in ophthalmology and facial aesthetics. Ultrasound 2023, 32, 28–35. [Google Scholar] [CrossRef]
- Wang, P.-H.; Wang, P.-H.; Lin, H.-Y.; Lin, H.-Y.; Chang, P.-Y.; Chang, P.-Y.; Lien, W.-C.; Lien, W.-C. Focused Assessment with Sonography for Trauma. J. Med. Ultrasound 2021, 29, 151–153. [Google Scholar] [CrossRef]
- Dewar, Z.E.; Wu, J.; Hughes, H.; Adnani, A.; Christiansen, G.; Ovedovitz, L.; Rittenberger, J.C. A comparison of handheld ultrasound versus traditional ultrasound for acquisition of RUSH views in healthy volunteers. J. Am. Coll. Emerg. Physicians Open 2020, 1, 1320–1325. [Google Scholar] [CrossRef]
- Salimi, N.; Gonzalez-Fiol, A.; Yanez, D.; Fardelmann, K.; Harmon, E.; Kohari, K.; Abdel-Razeq, S.; Magriples, U.; Alian, A. Ultrasound Image Quality Comparison Between a Handheld Ultrasound Transducer and Mid-Range Ultrasound Machine. POCUS J. 2022, 7, 154–159. [Google Scholar] [CrossRef]
- Le, M.-P.T.; Voigt, L.; Nathanson, R.; Maw, A.M.; Johnson, G.; Dancel, R.; Mathews, B.; Moreira, A.; Sauthoff, H.; Gelabert, C.; et al. Comparison of four handheld point-of-care ultrasound devices by expert users. Ultrasound J. 2022, 14, 1–9. [Google Scholar] [CrossRef]
- Russell, F.M.; Herbert, A.; Ferre, R.M.; Zakeri, B.; Echeverria, V.; Peterson, D.; Wallach, P. Development and implementation of a point of care ultrasound curriculum at a multi-site institution. Ultrasound J. 2021, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.M.; Pogson, K.B.; Friedman, V.E.; Corley, J.L.; Canario, D.A.H.; Johnson, C.S. Ultrasound as a Learning Tool in Bachelor-Level Anatomy Education. Med. Sci. Educ. 2020, 31, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Der Sarkissian, S.; Frew, J.W. Ultrasound-guided de-roofing of epithelialised tunnels of hidradenitis suppurativa. Australas. J. Dermatol. 2021, 62, 360–363. [Google Scholar] [CrossRef]
- Williams, Z.J.; Sage, A.; Valberg, S.J. Hand-Held Point-of-Care Ultrasound: A New Tool for Veterinary Student Self-Driven Learning in the Time of COVID-19. J. Veter-Med Educ. 2022, 49, 306–311. [Google Scholar] [CrossRef]
- Weigelt, M.A.; Hilerowicz, Y.; Leichter, J.A.; Lev-Tov, H. Sonographic Evaluation of Hidradenitis Suppurativa with Smartphone-Linked Portable Ultrasound. Dermatology 2021, 237, 378–382. [Google Scholar] [CrossRef]
- Venigalla, T.; Yarramsetty, D.; Wallace, P. Advancing medicine with pocus. J. Gen. Intern. Med. 2022, 37, 129–664. [Google Scholar] [CrossRef]
- Saxena, M.; Tan, T.X.; Duanmu, Y.; Ashenburg, N.; Ghaziaskar, Z. Seeing (virtually) is believing: The utility of teleultrasound education for the focused assessment with sonography for trauma exam. Acad. Emerg. Med. 2021, 28, S403. [Google Scholar] [CrossRef]
- Carpenter, S.L.; Wong, B.; Carthy, M.M.; Lipner, K.V.; Herbst, M.K. SAEM Annual Meeting Abstracts: Incorporating butterfly iq devices into undergraduate medical education and graduate medical education curricula. Acad. Emerg. Med. 2020, 27, S318. [Google Scholar]
- Wu, S.L.-L.; Parkman, T.; Deciccio, D.; Rose-Petruck, C.; Dunsiger, S.; Anderson, A.; Fletcher, J.; Galvin, W.; Becker, B.M. SAEM Annual Meeting Abstracts: Comparing the accuracy of microfocus X-ray to standard ultrasound for locating glass foreign bodies. Acad. Emerg. Med. 2020, 27, S193. [Google Scholar]
- Nydam, R.L.; Finch, C.; Simons, E.L.; Townsend, K.B.; Grow, W.A. Ultrasound in the First Two Years of Medical School: Enhanced Anatomical Learning via Clinically Based, Hands-On Workshops. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Ienghong, K.; Cheung, L.W.; Tiamkao, S.; Bhudhisawasdi, V.; Apiratwarakul, K. Development and Remodeling of Point-of-Care Ultrasound Education for Emergency Medicine Residents in Resource Limited Countries during the COVID-19 Pandemic. Tomography 2021, 7, 721–733. [Google Scholar] [CrossRef]
- Boivin, Z.; Carpenter, S.; Lee, G.; Chimileski, B.; Harrison, J.; Choudhary, D.; Herbst, M. Evaluation of a Required Vertical Point-of-Care Ultrasound Curriculum for Undergraduate Medical Students. Cureus 2022, 14, e30002. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, M.R.; Lawrence, M.B.; Chen, F.; Ross, E.M. Piloting a New Curriculum: Guided At-Home Pediatric Regional Anesthesia Education Using a Portable Ultrasound. Cureus 2021, 13, e17933. [Google Scholar] [CrossRef] [PubMed]
- Slader, M.; Young, H.; Barker, M.; Prentice, K.; Bogaard, K.; Yuan, C.; Saadat, S.; Lahham, S. A comparison of handheld and standard ultrasound in Swiss medical students. World J. Emerg. Med. 2022, 13, 85–90. [Google Scholar] [CrossRef]
- O’brien, N.F.; Fonseca, Y.; Johnson, H.C.; Postels, D.; Birbeck, G.L.; Chimalizeni, Y.; Seydel, K.B.; Gushu, M.B.; Phiri, T.; June, S.; et al. Mechanisms of Transcranial Doppler Ultrasound phenotypes in paediatric cerebral malaria remain elusive. Malar. J. 2022, 21, 196. [Google Scholar] [CrossRef]
- Nix, K.; Liu, E.L.; Oh, L.; Duanmu, Y.; Fong, T.; Ashenburg, N.; Liu, R.B. A Distance-Learning Approach to Point-of-Care Ultrasound Training (ADAPT): A Multi-Institutional Educational Response During the COVID-19 Pandemic. Acad. Med. 2021, 96, 1711–1716. [Google Scholar] [CrossRef] [PubMed]
- Haghighat, L.; Israel, H.; Jordan, E.; Bernstein, E.L.; Varghese, M.; Cherry, B.M.; Van Tonder, R.; Honiden, S.; Liu, R.; Sankey, C. Development and Evaluation of Resident-Championed Point-of-Care Ultrasound Curriculum for Internal Medicine Residents. POCUS J. 2021, 6, 103–108. [Google Scholar] [CrossRef]
- Ienghong, K.; Cheung, L.W.; Tiamkao, S.; Bhudhisawasdi, V.; Apiratwarakul, K. The Utilization of Handheld Ultrasound Devices in a Prehospital Setting. Prehosp. Disaster Med. 2022, 37, 355–359. [Google Scholar] [CrossRef]
- Onen, B.; Ghaffari, E.; Gaied, J.; De Butts, R.; Bowen, J.; Lasserson, D. The use of live and continuous training ‘ultrarounds’ to enhance use of point-of-care ultrasound on a busy ambulatory assessment unit. Futur. Heal. J. 2022, 9, S36–S37. [Google Scholar] [CrossRef]
- Haldeman, M.S.; Kunka, E.; Makasa, M.; Birkland, B. Resident perception on the impact of point-of-care ultrasound in clinical care at a family medicine training program in Zambia. Ultrasound J. 2022, 14, 18. [Google Scholar] [CrossRef]
- Grewal, S.; Houston, A.; Bacon, J.; Balderama, E.; Elhassan, M.G. Point-of-Care Ultrasound Curriculum for Internal Medicine Residents During the COVID-19 Era: A Pilot Study. Cureus 2022, 14, e25944. [Google Scholar] [CrossRef] [PubMed]
- Pazmiño, P.; Del Vecchio, D. Static Injection, Migration, and Equalization (SIME): A New Paradigm for Safe Ultrasound-Guided Brazilian Butt Lift: Safer, Faster, Better. Aesthetic Surg. J. 2023, 43, 1295–1306. [Google Scholar] [CrossRef]
- Signor, E.; Gerstenberger, J.; Cotton, J.; Colbert-Getz, J.; Lappé, K. Integrating a self-directed ultrasound curriculum for the internal medicine clerkship. Ultrasound J. 2024, 16, 1–6. [Google Scholar] [CrossRef]
- Katende, A.; Oehri, J.; Urio, V.Z.; Mahundi, E.; Wilson, L.; Myovela, V.; Mlula, C.; Chitimbwa, C.; Mbawala, C.; Faustine, F.; et al. Use of a Handheld Ultrasonographic Device to Identify Heart Failure and Pulmonary Disease in Rural Africa. JAMA Netw. Open 2024, 7, e240577. [Google Scholar] [CrossRef] [PubMed]
- Wubben, B.M.; Yun, H.I. The Performance Characteristics of Handheld, Non-Piezoelectric Point-of-Care Ultrasound (POCUS) in the Emergency Department. Diagnostics 2023, 14, 17. [Google Scholar] [CrossRef]
- Weimer, J.M.; Beer, D.; Schneider, C.; Yousefzada, M.; Gottwald, M.; Züllich, T.F.; Weimer, A.; Jonck, C.; Buggenhagen, H.; Kloeckner, R.; et al. Inter-System Variability of Eight Different Handheld Ultrasound (HHUS) Devices—A Prospective Comparison of B-Scan Quality and Clinical Significance in Intensive Care. Diagnostics 2023, 14, 54. [Google Scholar] [CrossRef]
- Höhne, E.; Recker, F.; Brossart, P.; Schäfer, V.S. Teledidactic Versus Hands-on Teaching of Abdominal, Thoracic, and Thyroid Ultrasound—The TELUS II Study. J. Gen. Intern. Med. 2024, 39, 1803–1810. [Google Scholar] [CrossRef]
- Baion, D.E.; La Ferrara, A.; Maserin, D.; Caprioli, S.; Albano, R.; Malara, F.; Locascio, F.; Galluzzo, E.; Luison, D.; Lombardo, M.; et al. Mono- and bi-plane sonographic approach for difficult accesses in the emergency department—A randomized trial. Am. J. Emerg. Med. 2023, 74, 49–56. [Google Scholar] [CrossRef]
- Gillon, J.T.; Liu, E.L.; Dutreuil, V.; Cohen, S.G.; Shah, L.A. Comparison of in-person versus virtual ultrasound instruction for pediatric residents. BMC Med Educ. 2024, 24, 203. [Google Scholar] [CrossRef]
- Jørgensen, L.T.; Præsius, S.K.; Stuart, M.B.; Jensen, J.A. Row–Column Beamformer for Fast Volumetric Imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2023, 70, 668–680. [Google Scholar] [CrossRef]
- Cal, E.M.; Gunnell, E.; Olinger, K.; Benefield, T.; Nelson, J.; Maggioncalda, E.; McGinty, K. Utility of tele-guidance for point-of-care ultrasound: A single center prospective diagnostic study. J. Ultrasound 2024, 27, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Parkman, T.; Dunsinger, S.; Deciccio, D.; Anderson, A.; Lash, E.; Fletcher, J.; Galvin, W.; Rose-Petruck, F.; Becker, B.; et al. Comparing the Accuracy of Micro-Focus X-ray Technology to Standard Clinical Ultrasound for Locating Small Glass Foreign Bodies in Soft Tissue. Appl. Sci. 2023, 13, 6551. [Google Scholar] [CrossRef]
- Kolo Medical. Kolo Medical to Release a SiliconWaveTM Ultra-High Performance Ultrasound Transducer at IEEE. Available online: http://www.kolomedical.com/xwzx (accessed on 20 March 2022).
- Clevert, D.-A.D.A.; Schwarze, V.; Nyhsen, C.; D’Onofrio, M.; Sidhu, P.; Brady, A.P.A.P. ESR statement on portable ultrasound devices. Insights Imaging 2019, 10, 1. [Google Scholar]
- Gibson, L.E.; Bittner, E.A.; Chang, M.G. Handheld ultrasound devices: An emerging technology to reduce viral spread during the COVID-19 pandemic. Am. J. Infect. Control. 2020, 48, 968–969. [Google Scholar] [CrossRef]
- Hilbert-Carius, P.; Struck, M.F.; Rudolph, M.; Knapp, J.; Rognås, L.; Adler, J.; Slagt, C.; Jacobsen, L.; Pich, H.; Christian, M.D.; et al. Point-of-care ultrasound (POCUS) practices in the helicopter emergency medical services in Europe: Results of an online survey. Scand. J. Trauma Resusc. Emerg. Med. 2021, 29, 124. [Google Scholar] [CrossRef]
| Company | Probe | Price | Center Frequency | Transducer Type | Portability | Application | Features | References |
|---|---|---|---|---|---|---|---|---|
| Clinical | ||||||||
| Hitachi | Mappie | No longer available | 10 MHz | US Platform-Compatible Probe | Detecting Breast Cancer | [16] | ||
| 4G CMUT probe (SML44) | 11 MHz | Adjustable | US Platform-Compatible Single-Probe | General Imaging | -High Doppler and Color Doppler sensitivity -High-resolution imaging of deep structures | |||
| Butterfly Network | iQ, iQ+ | USD 2999 | 5 MHz | Adjustable | Handheld Single-Probe | General Imaging | -Cloud Storage -AI guidance for user training | [20] |
| iQ3 | USD 3899 | 6 MHz | -Cloud storage -3D imaging capabilities -AI guidance for user training | |||||
| Research | ||||||||
| Kolo Medical | L62-38 | 50 MHz | Linear | US Platform-Compatible | Ultrahigh resolution for dermatology, ophthalmology, and rheumatology | Ultrahigh/high frequency US | [21] | |
| L38-22 | 30 MHz | Superficial imaging in Dermatology and MSK | [8,17] | |||||
| L30-14 | 22 MHz | Neonatal, Paediatric, and MSK applications | ||||||
| M17-4 | 8–10 MHz | General imaging | ||||||
| Kolo Medical + Verasonics | L22-8v | 15 MHz | MSK and small parts imaging | [18,22] | ||||
| L38-33v | 30 MHz | Pre-clinical and superficial imaging | ||||||
| Vermon | CMUT Catheter for Therapy (Interstitial CMUT) | 6 MHz | Catheter | US Platform-Compatible Probe US | IVUS + tumor ablation and interstitial surgery | Compatible with MRI guidance | [23] | |
| 128-element array probe | 5 MHz | Linear | Imaging and characterization of liver tissue | [24] | ||||
| CMUT Intracardiac imaging catheter (CMUT-based 1D ICE) | 7.5 MHz | Catheter | Intracardiac echocardiography (ICE) | Integrated with an ASIC chip | [23] | |||
| Dual-Mode CMUT probe | 1 M or 15–20 MHz | Linear | Diagnosis and targeted therapy | [25] | ||||
| Siemens | 9 MHz linear array | 9 MHz | Linear | Neuromusculoskeletal | Electronically Scanned 3D imaging | [26] | ||
| 3.5 MHz curved array | 3.5 MHz | Curved | Abdominal Imaging | 40 mm radius of curvature | [27] | |||
| ACULAB | HF12 CMUT Probe | 13.6 MHz | Linear | Imaging of the carotid artery | High frequency and small probe head | [28] | ||
| Reverse fabricated CMUT Probe | 12 MHz | Linear | Vascular, small parts, rheumatology, and anesthesiology imaging | Multichannel analogue front-end electronic circuits housing | [29] | |||
| Study Design | POS | C-S | RCT | FS | ROS | CS | VET | CAD |
|---|---|---|---|---|---|---|---|---|
| n | 10,326 | 1554 | 1679 | 453 | 134 | 82 | 170 | 64 |
| Cite | n | Comparator | Outcome Measure | Performance |
|---|---|---|---|---|
| [41] | 3 | Symptomatic Improvement | Diagnostic Performance | US findings lagged behind symptomatic improvements |
| [30] | - | - | - | No conclusive result |
| [42] | 13 | Interobserver | Correlation | Substantial interobserver correlation in identifying abnormal US scans; moderate correlation for COVID-19 manifestations (except consolidations and pleural thickening/effusions) |
| [37] | 61 | Chest X-ray | Correlation | Significant association of LUS severity scale with appropriate referral (p = 0.001); with chest X-ray (p = 0.034) |
| [40] | 1 | Clinical Diagnosis | Diagnostic Performance | Confirmed clinical findings and guided therapy by predicting patient outcomes |
| [43] | 21 | - | - | Guided placement of pulmonary, carotid, and femoral artery catheters |
| [38] | 44 | Expert vs. Non-expert | Correlation, Learning Experience | Expert agreement: 87%; Kappa agreement of 0.49 for LUS results; 98% of participants were confident in self-exam |
| [39] | 1 | Chest Radiography | Correlation | Detected findings unseen in radiography, correlated with clinical diagnosis |
| [22] | - | High-Frequency (10 MHz) PZT Probe | Image Quality | Improved spatial (axial and lateral) resolution compared to conventional probes |
| [44] | - | - | Learning Experience | No conclusive result |
| [36] | 51 | - | Diagnostic Performance | Se = 100.0%, Sp = 78.6% |
| [45] | 118 | DUS Offline vs. Bedside | Correlation | Mean absolute variability = 4.2% (95% CI: 2.8–5.6%); moderate correlation |
| [46] | 202 | 12, 8, and 6 Zone LUS for COVID-19 | Diagnostic Performance | 6-zone LUS was the best screening tool (Se = 94.1%, Sp = 83.5%); 12-zone LUS had the highest Sp (Sp = 92.3%) |
| [47] | 1 | VFX13-5 Probe vs. Conventional Probe | Image Quality | Resolution improved at 5 MHz (p < 0.05); elevated side lobe artifacts were observed at 8 MHz. |
| [31] | 18 | Standard High-end US (Venue GO) | Correlation | No statistically significant difference between LUS scores |
| [34] | 100 | POCUS vs. Clinical Outcomes | Diagnostic Performance | 92% accuracy in diagnosing COVID-19; good discriminatory performance for mortality and critical care admission (AUC = 0.80, 0.80, 0.82); mortality risk: 2.5% (lowest quartile) vs. 42.9% (highest quartile) |
| [48] | 393 | HOME-CoV and 4C mortality score | - | No conclusive result |
| [49] | 1 | N/A | - | No conclusive result |
| [50] | 228 | RT-PCR | Diagnostic Performance | Se = 94.4%, Sp = 95%; higher sensitivity than RT-PCR (80%) |
| [51] | - | Computed Tomographic Angiography (CTA) | Correlation | Correlated with CTA output. Feasible for pre-operative perforators mapping in DIEP flap breast reconstruction |
| [52] | 170 | Standard of Care | Diagnostic Performance, Correlation | Se = 97%, Sp = 100%, and 97.6% agreement with Standard of Care (κ = 0.95, p < 0.0001); 100% detection rate for sonographically visible cancers |
| [53] | 52 | ML vs. Radiologist Assessment | Correlation | Perfect correlation |
| [54] | 20 | N/A | Diagnostic Performance | rPTX successfully identified and imaging is useful within 4 h from TT-removal |
| [55] | - | US NT vs. Landmark Technique | Diagnostic Performance | Accuracy: 67% vs. 85% (p = 0.019); time: 19.9 s vs. 10.7 s (p = 0.001) |
| [56] | 115 | Novice with BiQ vs. Expert with High-end US | Diagnostic Performance, Correlation | Se = 100%; Sp = 86%; Cohen’s κ = 0.73 (95% CI: 0.57–0.9, p < 0.0001) |
| [57] | - | Teleguidance vs. Traditional Teaching | Learning Experience, Satisfaction | No difference in performance and similar satisfaction |
| [58] | 110 | Chest X-ray with and without Atelectasis | Diagnostic Performance | Se = 100%, and Sp = 13.5% (95% CI); with Atelectasis: Se = 67.2%, and Sp = 29.7%; without Atelectasis: Se = 56.3%, and Sp = 43.2% |
| [59] | 28 | Chest CT | Diagnostic Performance | High Se and lower diagnostic efficacy for mild–moderate disease: not an alternative for CT in COVID-19 assessment |
| [60] | 3 | Chest X-ray | - | No conclusive result |
| [61] | 21 | Expert Review | Image Quality, Learning Experience | Only 5.9% of 728 zones were low quality; longer scanning time (>7 min) improved accuracy |
| [62] | 40 | Chest X-ray | Diagnostic Performance | AUC = 89.2% (95% CI: 75.0–100%; p < 0.001); Se = 93.3%, and Sp = 80% (p < 0.001) |
| [63] | 30 | Medically untrained vs. POCUS Experts | Image Quality | Novices acquired interpretable, expert-quality LUS clips at home with minimal training (median 7 out of 8 zones, IQR 6–8; p = 0.42) |
| [64] | 134 | Clinical Findings | Diagnostic Performance | Se = 45.5%, Sp = 77.3%, AUC = 63.9% vs. Se = 72.7%, Sp = 79.8%, and AUC = 80.3% |
| [65] | 96 | Chest X-ray | Diagnostic Performance, Correlation | Se = 71.7% vs. 62.2%, Sp = 65.1% vs. 71.4%, and the presence of bilateral B-lines showed the greatest likelihood ratio for accurately identifying COVID-19 |
| [66] | 1 | - | - | No conclusive result |
| [67] | 31 | - | Image Quality, Diagnostic Performance | 90% of images were of good quality after 2.5 h of training; 5 min per exam; B-lines were significantly associated with weight gain, a clinical marker of worsening heart failure |
| [68] | 100 | Chest X-ray | Diagnostic Performance, Correlation | Se = 98.51% (95% CI 91.96–99.96%), Sp = 87.9% (95% CI 71.8–96.6%), DA = 95% (PPV = 94.3%, NPV = 96.7%), 30% scans were normal (97% correlation with normal Chest X-ray), and 70% scans were abnormal (90% correlation with abnormal Chest X-ray) |
| [69] | 754 | Chest Radiography | Diagnostic Performance | Diagnosed 96.6% of pneumothorax cases on initial imaging, comparable to chest radiography. Only one case was missed by US. Chest radiography use decreased from 98.2% to 25.8% after US introduction. Increased confirmatory investigations post-US compared to chest radiography |
| [70] | 18 | Expert vs. Non-clinician | Correlation, Satisfaction | 96% of the 1339 scans were deemed interpretable (k = 0.67) | 100% of surveyed participants found the experience positive and reported ease of operation |
| [71] | 11 | Untrained (Teleguidance vs. No Assistance) | Image Quality, Learning Experience | All images were deemed adequate for clinical decision-making | Teleguidance through social media app improved diagnostic accuracy and practitioner confidence |
| Cite | n | Comparator | Outcome Measure | Performance |
|---|---|---|---|---|
| [81] | 6 | Pre- and Post-training Comparison | Learning Experience | Skill test score improvement: pre-training to 8-week post-training: +2.11 points (95% CI: 1.22–3.00, ES: 1.13); knowledge test improvement: +19.6 points (95% CI: 15.4–23.8, ES: 2.24) |
| [29] | 1 | LA435 PZT Probe | Image Quality | No conclusive result |
| [82] | 54 | Pre-training vs. Post-training | Learning Experience | Improvement between pre- and 8-week post-training: mean difference = 2.11 (95% CI, 1.22–3.00); ICC for interrater reliability = 0.93 (95% CI, 0.76–0.97) |
| [80] | 75 | Vivid q™ and O’Dive™ | Diagnostic Performance, Correlation | Moderate agreement produced a smaller number of quality images and less Se and Sp than Vivid q but higher than O’Drive. Overall, it is not a replacement for Venous gas Emboli assessment |
| [73] | 23 | Physical Examination | Diagnostic Performance | Se = 91% (95% CI: 89–93%), Sp = 90% (95% CI: 86–93%), PPV = 97% (95% CI: 95–98%), NPV = 77% (95% CI: 72–81%), and DA = 0.94 (95% CI: 0.91–0.96, p < 0.001) |
| [74] | 72 | Right heart catheterization | Diagnostic Performance | Se = 70.6%; Sp = 85.5%; NPV = 90.4% |
| [76] | 41 | Right heart catheterization | Diagnostic Performance | DA: (non-obese = 0.923 and obese = 0.852) |
| [83] | 159 | - | - | No conclusive result |
| [77] | 76 | Right Heart Catheterization (RHC) vs. Point-of-Care Ultrasound (POCUS) uJVP | Correlation | HR for uJVP ≥10 cm = 3.21 (95% CI: 1.05–9.82, p = 0.041); HR for Right Atrial Pressure (RAP) ≥ 10 mmHg = 3.22 (95% CI: 1.05–9.86, p = 0.04). Neither uJVP nor RAP was predictive of 90 or 180-day HF hospitalizations |
| [78] | 42 | - | Diagnostic Performance | Ultrasound-guided AVF cannulation was feasible and showed a reduction in infiltration rates from 14% to 10.2%, with further protocol improvements lowering rates to 1.7% |
| [79] | 59 | High-End US System: Mindray TE7 | Diagnostic Performance | First-pass cannulation success rate: Mindray TE7 = 92.59% vs. standard US = 68.75% (p = 0.023), indicating significantly better first-attempt success using Mindray TE7 |
| [72] | 1 | - | - | No conclusive result |
| [75] | 1 | - | - | Guided arterial injection |
| [84] | 44 | Clinical hypovolemia techniques | Correlation | No significant correlation between clinical hypovolemia and Adjunct Diagnostic Techniques (κ = −0.045), US-IVC (κ = −0.009), or US-C (κ = 0.029) |
| [85] | 26 | Experienced Physical Examination | Correlation, Learning Experience | Good correlation (r = 0.73) with an average error of 0.06; ICC = 0.83 (95% CI: 0.44–0.96); moderate-to-high confidence novice satisfaction |
| [86] | 20 | - | Diagnostic Performance | 12/17 patients had successful pacemaker transplants without major complications. No conclusive result on overall accuracy |
| [87] | 20 | Telemed vs. Lumify vs. high-end US (Terason 3300) | Correlation | ICC for inter-observer variability: Telemed = 0.901, Lumify = 0.827, and Butterfly iQ = 0.684 |
| [88] | 2 | Non-intervention | Diagnostic Performance | BiQ+ was feasible for guiding pericardiocentesis in transit. No conclusive performance statistics provided |
| [89] | - | ML + BiQ+ Algorithm vs. Conventional Processing | Image Quality | Real-time processing speeds: 60–80 FPS on 288 × 240 input size and 25 FPS on 464 × 208 × 2. Minimal image degradation: mean weight error 0.0054% |
| [90] | 10 | Teleguidance vs. No Assistance (Untrained Operators) | Image Quality | Teleguidance group: 100% correctly acquired images with significantly improved quality over time: 76–80% at 2–6 weeks and 53% at 3–4 months follow-up. Time per view: 1–1.5 min, comparable between groups |
| Cite | n | Comparator | Outcome Measure | Performance |
|---|---|---|---|---|
| [24] | - | PZT Probe | Correlation | Adequate correlation with cellular features, but no conclusive quantitative result |
| [95] | 19 | Clinical Diagnosis | Diagnostic Performance | US findings confirmed diagnosis of 50% of the patients and determined new diagnoses in the other half |
| [93] | 114 | EPIQ 7 (Philips, US) | Correlation | Nearly perfect correlation (ICC > 0.8) for intra- and inter-operator reproducibility of aortic measurements, except for inter-operator reproducibility at the proximal location (ICC = 0.467) |
| [97] | 30 | Pulse Oximetry (PO) | Correlation | Pearson correlation coefficient r = 0.75, p < 0.000197 |
| [91] | 120 | Nonexperts vs. Experts | Diagnostic Performance | Se = 85.7% (95% CI: 42.1–99.6); Sp = 95.5% (95% CI: 88.9–98.8) |
| [94] | 194 | - | Image Quality | 88.2% excellent/adequate image quality; 96.4% diagnostically interpretable images; 3 h training |
| [96] | 78 | Clarius C3 | Correlation | ICC = 0.78 (95% CI: 0.62–0.88, p = 0.044) vs. ICC = 0.71 (95% CI: 0.51–0.83, p = 0.011) |
| [99] | - | Before vs. After Training | Diagnostic Performance | Bladder evaluation and characterization: 25% vs. 53% | Hydronephrosis evaluation: 40% vs. 90% | Diagnosis of pelvic structures: 100% |
| [92] | 17 | Clinical Diagnosis | Diagnostic Performance | Confirmed 70% of findings and determined 30% new findings | Challenging to learn |
| [101] | 50 | Mindray M9 with a curvilinear 2–5 MHz probe | Diagnostic Performance | Se = 92% (95% CI: 73–99%), Sp = 100% (95% CI: 87–100%), and Diagnostic Accuracy = 96% (95% CI: 85–100%) |
| [102] | 4695 | Expert Standard fetal biometry | Learning experience | Novices successfully measured symphysial-fundal height. AI estimator gestational age estimator: Mean Squared Error (MSE) difference = −0.8 days (95% CI: −1.1 to −0.5) |
| [103] | 1 | - | - | No conclusive result |
| [104] | 818 | High-specification US machine (HSUM) | Correlation | ICC ≥ 0.989. Mean gestational age estimation error: −0.20 days (95% CI, −0.60 to 0.20) in the first trimester (1T); −0.68 days (95% CI, −0.93 to −0.44) in the second/third trimesters (2/3T). Slightly higher mean differences observed with an alternative handheld device but within acceptable limits |
| [105] | 50 | Standard bladder scanner (Verathon BVI 9400 and Verathon BladderScan Prime systems) | Correlation | High ICC (0.95 to 0.98) for triplicate measurements using both the standard bladder scanner and the study device. Patient preference: 84% preferred self-measurement |
| [106] | 70 | Conventional transvaginal US (GE Logiq P9, Toshiba Xario SSA-660A) | Diagnostic Performance | Se: 92.9% (95% CI: 66.1–99.8) for detecting mispositioned IUDs. Sp: 96.4% (95% CI: 87.7–99.6). PPV: 86.7%. NPV: 98.2%. DA: 95.7%. k = 0.87 |
| [107] | - | - | Learning Experience | Mean pre-test scores improved from 52.8% to 90.6% post-test |
| [108] | 16 | CUD | Diagnostic Performance, Correlation | Right hepatic lobe enlargement: Se = 95%, Sp = 87% with CUD| Gallbladder wall thickening: Se = 100% and Sp = 98% | Strong correlation with CUD for measuring the right hepatic lobe (r = 0.912), left hepatic lobe (r = 0.843), portal vein diameter (r = 0.724), and spleen size (r = 0.983) | Substantial agreement in detecting ascites (p < 0.0001), gallbladder wall thickening (p < 0.0009), and portal vein flow direction (p < 0.0001) |
| [109] | - | - | Learning Experience | All 14 participants scored 100% on clinical exam |
| [110] | 48 | High-end US (Canon Aplio i900) vs. Apinion Minisono | Claruis C3 HD3 | iSiniQ 30A | Kosmos | mSonics MU 1 | Philips Lumify | SonoSite iViz | Sonostar Uprobe-C4PL | Vscan Air | Youkey Q7 | BiQ+ | Image Quality, Correlation | Among top performers, avg. score 3.83/5 (Acceptable or better in 96%) | High reproducibility and clinical significance score = 3.97/5 |
| Cite | n | Comparator | Outcome Measure | Performance |
|---|---|---|---|---|
| [124] | 1 | - | - | No conclusive result |
| [116] | 8 | Arthroscopy | Correlation | US detected increased translation but failed to distinguish injury stages |
| [117] | 10 | Fluoroscopy | Correlation | US showed moderate correlation with fluoroscopy, but only US could measure the change in distal tibiofibular clear space (TFCS) |
| [118] | 8 | Fluoroscopy | Correlation | TFCS distance correlated. US was more sensitive in evaluation |
| [115] | 1 | Ophthalmologic examination | Diagnostic Performance | Scans confirmed examination findings |
| [125] | - | L12-3v and L25e | Image Quality | L12-3v: better in lateral and axial resolution; deeper penetration. L25e: better in detail and contrast resolution |
| [126] | 1 | - | - | Best images acquired using pediatric abdomen mode |
| [121] | - | Musculoskeletal | Learning Experience | No conclusive result |
| [113] | 32 | Samsung HS40 | Correlation | 97% agreement between BiQ and CUD in B-mode imaging. κ = 0.90 (95% CI: 0.89–0.94). No Power Doppler (PD) signal detected by BiQ |
| [127] | 1 | - | Learning Experience | Teleguidance was successful for patient care by a US-inexperienced physician; good clinical images and outcomes were obtained |
| [120] | 8 | GE Logiq E (CUD) | Correlation | Pearson product-moment correlation = 0.76 (p < 0.001) |
| [112] | 66 | L64 | Diagnostic Performance, Image Quality | Similar diagnostic performance; higher image panoramicity and deep structure definition; worse superficial structure evaluation and Doppler signal |
| [128] | 1 | - | Diagnostic Performance | Detected a cortical bulge on the dorsal aspect of the distal right radius using a Butterfly iQ device connected to an iPhone. Confirmed as a torus fracture on radiographs |
| [129] | 20 | - | Diagnostic Performance | Saline successfully injected into the Intermediate Temporal Fat Pad (ITFP) 90% of the time using ultrasound guidance. Study focused on anatomical and clinical localization of ITFP for filler injections |
| [130] | - | - | Diagnostic Performance | No conclusive result |
| [119] | 7 | - | Diagnostic Performance | Surgery was successful in all patients |
| [122] | - | Expert US Instructors (UI) vs. Student Tutors (ST) | Diagnostic Performance | Identification accuracy = 89.2%; no significant difference between both groups |
| [131] | 1 | - | - | - |
| [132] | 132 | Teleguidance vs. Unguided | Learning Experience, Diagnostic Performance | Training 2-6w: 80% adequately acquired images | Landmark identification: 55% | Perfect image quality | Teleguidance did not affect speed |
| [133] | - | - | - | - |
| [114] | 1 | Conventional PZT Ophthalmic Probe | Diagnostic Performance | Similar diagnostic performance |
| [123] | 1 | - | Learning Experience | No conclusive results |
| [134] | 139 | US guidance vs. landmark technique | Diagnostic Performance | 3.1 min (3.2) vs. 6.3 (7.5); p = 0.009 | 1.0 (0.6) vs. 2 (1.3); p = 0.29 | 1.0 (0.8) vs. 3.0 (1.3); p < 0.001 | −0.15 (0.37) 95% confidence interval [−0.07, −0.23] |
| [25] | 1 | - | Image Quality | Vein clearly distinguished |
| [26] | 1 | - | Image Quality | Clear 3D view of the leg vein, containing high anatomical information |
| [135] | 10 | - | Diagnostic Performance | Dynamic scanning with BiQ identified complete medial patellofemoral complex injury: Se = 77.8%, Sp = 100%, and Acc = 88.9% |
| [136] | 8 | - | Diagnostic Performance | Diagnostic Accuracy = 0.97; Se = 100%; Sp = 94.1%; dynamic BiQ quantified medial knee injury severity |
| [137] | 80 | - | - | No conclusive result |
| [138] | 7 | - | Image Quality | Identified shunt location and measured skin thickness accurately |
| [139] | 33 | Hitachi HI VISION Avius | Correlation | Intra-operator reproducibility was almost perfect for both operators on both machines (ICC > 0.80) | Inter-system reproducibility ICC ranged from 0.815 to 0.927 | Measurement difference: 1.8% to 6.6%. Mean muscle thickness difference: clinically acceptable |
| [140] | 7 | EyeCubed v3 | Accutome B-Scan Pro | Diagnostic Performance, Image Quality | Both imaged features as small as 0.1 mm with comparable resolution | Similar imaging quality | 1/3 retina specialists showed a statistically significant preference for COU regarding resolution, detail, and diagnostic confidence |
| [141] | 42 | SonoSite M-Turbo | Correlation | Excellent inter-device reliability: ICC = 0.92 (95% CI: 0.87–0.94) | ICC (BiQ): ICC = 0.85 (95% CI: 0.73–0.92) | ICC (SonoSite): ICC = 0.89 (95% CI: 0.82–0.93) |
| [142] | 10 | CUD | Correlation, Diagnostic Performance | Successfully constructed 3D images for all 10 peripheral nerve blocks | 3D imaging provided enhanced visualization of local anesthetic spread, improving needle direction and placement of anesthetics | Effective postoperative analgesia was achieved in all patients without complications | Time per scan: <5 s. |
| [143] | 19 | CT | Diagnostic Performance | Successfully detected the presence of a radial artery pseudo-aneurysm | Effective monitoring of HHUS, allowing for timely intervention | Findings consistent with CT |
| [144] | 82 | US guidance vs. Landmark Technique | Diagnostic Performance | 3.1 min (3.2) vs. 6.3 min (7.5); p = 0.009 (shorter procedure time) Insertion attempts: 1.0 (0.6) vs. 2 (1.3); p = 0.29 Needle redirections: 1.0 (0.8) vs. 3.0 (1.3); p < 0.001 Depth: −0.15 (0.37) 95% CI [−0.07, −0.23] |
| [145] | 3 | Clarius L15 | Clarius L20 | Lumify | Vscan Air | Image Quality | L20 > L15 > Vscan Air > BiQ+ > Lumify. L20 had the best overall image quality for retina, orbicularis oculi muscle, and lacrimal gland imaging. L20 was also the best in vascular imaging. Longer battery life and better stability noted for Lumify and BiQ+ |
| Cite | n | Comparator | Outcome Measure | Performance |
|---|---|---|---|---|
| [147] | 50 | Phillips Sparq | Image Quality, Diagnostic Performance | No significant difference in RUSH exam time (249.4 s vs. 251.4 s, p = 0.81); similar image quality (82% vs. 86%, p = 0.786); κ = 0.69. |
| [150] | - | Lumify and HHUS | Image Quality, Learning Experience | Selected for cost and single-probe ability to visualize deep and superficial structures but preferred Lumify for imaging heart, lung, and abdomen; novice US medical students could accurately identify anatomical structures with good image quality after 6 h workshops. |
| [151] | - | - | Learning Experience | Post-test scores significantly improved (p = 0.0002 for content; p = 0.0001 for ultrasound proficiency); no conclusive result for long-term knowledge retention. |
| [21] | 1 | - | - | No conclusive results. |
| [100] | - | Traditional Ultrasound Machines | Image Quality, Satisfaction | Comparable image quality to traditional ultrasound devices; real-time AI guidance improved ease of use; no conclusive result for full clinical diagnostic accuracy. |
| [152] | - | Physical Examination | Diagnostic Performance | Ultrasound-guided de-roofing identified larger disease areas than clinical examination; reduced recurrence rates (14% vs. 30%); no conclusive result for direct diagnostic performance. |
| [6] | 29 | GE LOGIQ S7 Expert with: SP-D, C1-5-D, and ML5-15 transducers | Image Quality, Diagnostic Performance | UHP was performed faster by 2 min with similar diagnostic image quality. |
| [20] | 33 | - | Image Quality | Good image quality for most applications but poorer cardiac imaging due to phased-array limitations. No conclusive result for cardiac function accuracy. |
| [98] | 1000 | Standard Diagnostic Methods | Diagnostic Performance | OCUS was useful in early detection of pneumonia and pyelonephritis but required additional confirmation through CT/MRI; Se and Sp not consistently quantified; no conclusive result for direct diagnostic performance. |
| [32] | 110 | Clinical Diagnosis | Image Quality, Diagnostic Performance | IVC collapsibility index (CI) ≥ 50% correlated with hypovolemia signs; lung ultrasound B-patterns associated with pulmonary oedema; no conclusive result for general diagnostic accuracy. |
| [149] | 3 | Vscan Air| Lumify|Kosmos | Satisfaction, Image Quality | Lumify had the best image quality; Vscan Air rated highest for ease of use; no single handheld device was superior in all categories. |
| [153] | 1 | Expert vs. non-expert | Learning Experience, Satisfaction | Veterinary students successfully obtained diagnostic-quality images; Color Doppler and M-mode were used; no conclusive result for comparative performance or long-term retention. |
| [154] | 16 | - | Diagnostic Performance | Sonographic lesions were identified in 10 patients (62.5%). Subclinical lesions were identified in 2 patients (12.5%). |
| [155] | - | Traditional PZT transducers | Image Quality | CMUT-based imaging enhanced 3D volume scanning with higher resolution and contrast; improved penetration depth compared to PZT; no conclusive result for diagnostic accuracy in clinical applications. |
| [156] | - | In-person vs. Virtually guided | Image Quality, Diagnostic Performance | 100% of participants successfully completed the FAST exam; Tele-ultrasound group took significantly longer (341 s vs. 62 s, p < 0.001); no conclusive result for long-term retention or diagnostic accuracy improvement. |
| [157] | - | - | Satisfaction | Convenient cloud storage, personalized cloud folders and easy image access, and prompt feedback from instructors | L: Apple-only compatibility. |
| [158] | 60 | Micro Focus X-ray Imaging | Diagnostic Performance | Identification Accuracy: 62.3% vs. 97.6%. |
| [159] | - | - | Learning Experience | Improved anatomy understanding in 90% of the participants. |
| [27] | - | Siemens PZT Probes | Image Quality | CMUT integration improves contrast resolution and 3D imaging, but no quantified diagnostic accuracy was reported (no conclusive result). |
| [148] | 74 | Sonosite M-Turbo | Image Quality | No differences in resolution or quality but better detail. Mean differences: (Resolution) 1.7, (Detail) 1.6, and (Quality) 1.1). |
| [160] | - | - | Learning Experience, Satisfaction | 100% completion rate; 98% found POCUS training important for emergency medicine; most useful activities: bedside ultrasound and image review; no conclusive result for diagnostic accuracy improvement. |
| [161] | 18 | Sonosite M-Turbo | - | - |
| [162] | 2 | - | Diagnostic Performance, Satisfaction | Trainees successfully obtained ultrasound images of 12 regional block sites; virtual guidance rated highly for hands-on skill improvement; no conclusive result for superiority over traditional workshops. |
| [163] | 604 | Hitachi Arietta V70 | Learning Experience, Correlation | Equivalent education tool as a standard US machine. No statistically significant differences in any measurements, including number of correctly acquired and clinically useful images, except for faster EPSS vascular setup time and lower image quality median score. |
| [164] | 174 | - | Diagnostic Performance | TCD patterns correlated with patient outcomes; Hyperemia and Posterior High Flow had best prognosis (PCPC 1–2 in 77–89%); Low Flow had worst outcomes (PCPC 1–2 in 42%, p < 0.001); no conclusive result for clinical treatment impact. |
| [165] | 29 | - | Satisfaction | 61.9% of educators were satisfied with image acquisition and interpretation. |
| [166] | 40 | - | Learning Experience, Satisfaction | Significant improvement in confidence and knowledge after workshops; increased likelihood of POCUS use in cardiac section; no conclusive result for long-term retention or clinical impact. |
| [167] | 169 | Prehospital vs. Hospital | Diagnostic Performance | Prehospital diagnosis confirmed in 66 cases (39.1%); overall diagnostic accuracy = 75.8%; no conclusive result on impact of prehospital POCUS on patient outcomes. |
| [28] | - | PZT Probe | Image Quality | Better axial resolution than PZT probes; inferior Doppler imaging performance; no quantitative diagnostic accuracy provided (no conclusive result). |
| [168] | 5 | Expert Review | Learning Experience | POCUS confidence improved over time; trainees found POCUS useful for early treatment decisions; no conclusive result on direct impact of training on patient care. |
| [169] | 366 | - | Satisfaction, Learning Experience | 95.6% of scans assisted in decision-making; 65.8% changed patient management (most common change: medication adjustment); no conclusive result on direct patient outcome improvement. |
| [170] | 23 | Senior Residents vs. Interns | Learning Experience | 91% of residents were highly interested in POCUS; confidence improved in all POCUS skills (p < 0.01) except pulmonary embolism (p = 0.084); image interpretation skills improved only for pneumothorax detection (p < 0.05); no conclusive result on image acquisition improvement. |
| [171] | 4150 | Expansion Vibration Lipofilling | Diagnostic Performance | Confirmed subcutaneous-only fat graft placement | Operative times were 6 min shorter | US guidance safter | Lower complications. |
| [172] | 110 | - | Learning Experience | Students scored an average of 1.79/2 on image acquisition; 78% average on quiz; 72% reported improved clinical reasoning, 69% improved pathophysiology understanding, and 55% improved patient care; no conclusive result on impact on real patient care. |
| [173] | 438 | Untrained vs. Senior Physicians | Learning Experience | 4 h of training is sufficient for substantial agreement in sonographic findings in diagnosing heart failure (85%) and moderate for diagnosing tuberculosis (78%) | Se and Sp for pleural effusion: 88% and 95% | Se and Sp for heart failure: 82% and 84%. |
| [174] | 381 | Radiology imaging (CT, MRI, US) | Diagnostic Performance | Overall sensitivity: 86.4% (95% CI: 77.0–92.5), specificity: 82.3% (95% CI: 74.1–88.3); cardiac image quality lower than lung (p = 0.002); no conclusive result on impact in emergency care. |
| [175] | 40 | 8 HHUS devices vs. Canon Aplio i900 | Image Quality, Correlation | GE Vscan Air achieved highest scores in B-scan quality (3.90 ± 0.65) and clinical significance (4.03 ± 0.73) | Significant variability in B-scan quality among the eight HHUS devices tested | All HHUS devices performed acceptably for certain clinical applications | Some devices showed limitations that could impact their clinical utility. |
| [176] | - | Teledidactic vs. Hands-on Teaching | Learning Experience | No significant difference in final exam scores (p > 0.05); Teledidactic students performed better in FAST (p = 0.015) and aorta (p = 0.017) modules; no conclusive result on impact in real patient care. |
| [177] | 422 | Mono-plane vs. Bi-plane | Diagnostic Performance | 1st attempt success rate: 68.3% vs. 73.3% (p = 0.395) | Success rate: 100% for both | Median time: 45 s vs. 35 s (p = 0.03). |
| [178] | 50 | In-person Sonosite vs. Remote Guidance BiQ | Correlation, Learning Experience | No significant difference in assessment scores (p = 0.349); no significant difference in assessment duration (482.6 s vs. 432.6 s, p = 0.346); no conclusive result on whether virtual training improves real-world ultrasound proficiency. |
| [179] | - | Dual Stage vs. Traditional Beamforming Techniques | Image Quality | Beamforming was up to 9.23 times faster while maintaining equivalent image quality; no significant degradation in FWHM or FWTM; no conclusive result on clinical diagnostic performance. |
| [180] | 30 | Untrained (Teleguidance vs. No assistance) | Diagnostic Performance, Image Quality | Heart (3.46 s vs. 134.1 s), right kidney (55.1 s vs. 149.3 s), and gallbladder (48.5 s vs. 169.3 s) | Higher predicted mean image quality scores for the heart (3.46 vs. 1.86), right kidney (4.49 vs. 1.58), and gallbladder (3.93 vs. 1.5) compared to the control group (p < 0.0001 for all). |
| [181] | 60 | Micro-focus X-ray imaging | Diagnostic Performance, Correlation | Micro-Focus X-ray identified 97.6% of foreign bodies with 96.5% inter-rater reliability; POCUS identified only 62.3% of foreign bodies with 70.8% inter-rater reliability; no conclusive result on impact beyond detection rates. |
| Cite | Y | Future Work |
|---|---|---|
| [139] | 23 | Explore the use of HHUS in pathological conditions associated with muscle wasting, such as sarcopenia and cachexia, and validate these findings in a broader range of clinical scenarios and populations, including elderly and critically ill patients. Additionally, further studies could investigate the ergonomics and user-friendliness of HHUS in diverse healthcare settings. |
| [104] | 24 | Focus on understanding the underlying causes of the modest underestimation trend and exploring the use of HHUS in a broader range of clinical scenarios and populations. Additionally, studies could investigate the impact of operator training and experience on measurement accuracy, as well as the long-term outcomes of using portable US machines in routine prenatal care. |
| [171] | 23 | Focus on refining the technique further and expanding its application to other types of body contouring surgeries. Additionally, long-term studies are needed to evaluate the durability of the aesthetic results and the overall patient satisfaction with this method. Integrating Static Injection, Migration, and Equalization into standard clinical practice could set a new benchmark for safety and outcomes in cosmetic surgery. |
| [140] | 24 | Focus on larger clinical studies to further validate the findings and explore the use of portable US devices in various ophthalmic conditions. Additionally, integrating these devices into routine clinical workflows could enhance their utility and impact on patient care. Expanding the evaluation to include a broader range of pathologies and more diverse patient populations will help establish the robustness and generalizability of these findings |
| [67] | 24 | Focus on larger, multicenter studies to validate these findings and explore the long-term impact of LUS on patient outcomes, such as reducing hospitalizations and improving quality of life for heart failure patients. Additionally, expanding training programs to include other healthcare providers and settings could further enhance the adoption and utility of LUS in managing heart failure. |
| [172] | 24 | Focus on expanding this curriculum to other clinical disciplines and evaluating its long-term impact on students’ clinical practice. Additionally, the use of advanced technologies such as AI and augmented reality for US training could further enhance the learning experience and reduce the need for faculty involvement. Integrating such curricula into medical education programs can help produce more competent and confident physicians, ultimately improving patient care. |
| [68] | 24 | Focus on larger-scale studies to further validate these results and explore the use of POCUS in other clinical scenarios. Additionally, developing standardized training programs for pediatricians and other healthcare providers on the use of POCUS will be crucial for its widespread adoption. The potential for POCUS to provide earlier detection of respiratory pathologies and reduce the reliance on chest x-ray makes it a valuable tool in pediatric care. |
| [105] | 24 | Focus on longitudinal assessments of postvoid residual bladder volume self-measurement reliability and agreement with provider measurements over time. Additionally, further studies are needed to evaluate the accuracy of patient self-measured postvoid residual bladder volume compared to bladder catheterization volumes, the gold standard for bladder volume measurement. Integrating portable US devices into remote health monitoring systems could enhance patient autonomy and reduce the need for frequent clinical visits, ultimately improving patient outcomes and healthcare efficiency. |
| [173] | 24 | Focus on optimizing training protocols to further improve diagnostic accuracy and exploring the use of telemedicine to support remote diagnoses. Additionally, expanding the study to include a broader range of conditions and settings will help validate these findings and enhance the generalizability of the results. Integrating handheld ultrasonographic devices into routine clinical practice could significantly improve healthcare access and outcomes in underserved areas. |
| [84] | 24 | Explore the integration of multiple diagnostic modalities to improve the assessment of hypovolemia and fluid responsiveness. Additionally, further studies should investigate the role of acute cardiac dysfunction in the management of hypovolemia and the development of more reliable and comprehensive diagnostic tools. Enhanced training and standardized protocols for the use of US in critical care could also improve the consistency and accuracy of these assessments. |
| [174] | 24 | Focus on improving the diagnostic accuracy of HHUS for conditions where its sensitivity is currently lower. Additionally, integrating HHUS into routine ED workflows and training programs can enhance its effectiveness and reliability, ultimately improving patient care and outcomes. Further studies comparing different HHUS platforms and their performance across various clinical settings will help refine best practices and guide the adoption of these technologies in emergency medicine. |
| [175] | 24 | Focus on optimizing the use of these devices and exploring their integration with artificial intelligence to enhance diagnostic accuracy. Additionally, further studies comparing HHUS devices in different clinical scenarios and settings will help establish standardized guidelines for their use. As HHUS technology continues to evolve, ensuring consistent quality and reliability across devices will be crucial for their widespread adoption in critical care environments. |
| [106] | 24 | Focus on larger-scale studies to validate these results further and explore the use of POCUS in other gynecological and obstetric applications. Additionally, training programs for gynecologists on the use of POCUS could enhance its adoption and improve patient care, especially in low-resource settings where access to conventional US may be limited. |
| [176] | 24 | Focus on larger-scale studies to validate these results and explore the long-term retention of skills acquired through teledidactic methods. Additionally, the development of standardized curricula and assessment tools for teledidactic US education will be crucial for ensuring consistent and high-quality training across different institutions. The adoption of portable US devices like the BiQ in teledidactic programs could further enhance the accessibility and effectiveness of US education worldwide. |
| [69] | 23 | Focus on further optimizing TUS protocols, training, and technology to address its limitations and enhance its diagnostic accuracy. Expanding the use of TUS in other clinical settings and interventions could also be explored to maximize its benefits across various medical fields. |
| [141] | 23 | Explore the reliability of handheld US technology in measuring other musculoskeletal and pathological structures. Additionally, training programs for clinicians on the use of handheld US devices could enhance their adoption and integration into routine practice, ultimately improving patient care and clinical outcomes. |
| [142] | 23 | Explore the broader application of this technology in other regional anesthesia procedures and in settings where access to full-sized US equipment is limited. Additionally, further research could focus on refining the 3D imaging capabilities of handheld devices to enhance their utility in complex or anatomically challenging cases. |
| [177] | 23 | Explore the impact of operator experience on the effectiveness of these techniques, as well as the potential benefits of bi-plane imaging in other clinical scenarios beyond PIV catheterization. |
| [178] | 24 | Explore the long-term retention of skills acquired through virtual instruction and investigate the applicability of this training method in other medical specialties. Additionally, developing standardized assessment tools for evaluating US skills could improve the consistency and reliability of training outcomes. |
| [179] | 23 | Explore further optimization of the beamforming algorithm, as well as its application to other types of US transducers and imaging scenarios. Additionally, expanding the method’s use in clinical settings could validate its utility and performance in a broader range of medical applications. |
| [143] | 23 | Explore the broader use of handheld US devices in diagnosing other vascular conditions and their role in outpatient follow-up care. Additionally, research into the integration of these devices into routine clinical workflows could enhance their adoption in various medical settings, particularly in resource-limited environments. |
| [107] | 23 | Explore the long-term impact of such training on patient outcomes, as well as the integration of POCUS into routine obstetric care. Additionally, similar training models could be adapted for other clinical applications of POCUS, further expanding the reach of portable US technology in resource-limited settings. |
| [85] | 23 | Explore the long-term retention of these skills and assess the impact of US-based JVP measurement on clinical outcomes. Expanding this training to include a wider range of diagnostic applications for point-of-care US (POCUS) could further enhance the diagnostic capabilities of novice clinicians. |
| [86] | 23 | Explore long-term outcomes of Cardioneuroablation and the role of advanced imaging techniques, such as those provided by portable US devices, in guiding these procedures. |
| [87] | 23 | Focus on improving the inter-observer reliability of devices like the BiQ through enhanced training programs or technological improvements. Additionally, expanding the use of these devices to other vascular measurements and exploring their use in patients with known cardiovascular conditions could further validate their utility in clinical practice. |
| [70] | 24 | Focus on expanding this model to include more diverse patient populations, different pathologies, and other types of US exams. Additionally, further exploration into improving the inter-rater reliability and the effectiveness of remote training methods could enhance the overall utility of self-administered US in home-based care. |
| [108] | 23 | Focus on larger-scale studies to further validate these findings and explore the use of HHUS in other transplant-related complications or in different clinical settings. Additionally, integrating HHUS with telemedicine could enhance its utility in remote or underserved areas, providing critical diagnostic capabilities where conventional US is not available. |
| [180] | 24 | Explore the application of this technology in different clinical scenarios, including emergency medicine and home healthcare. Additionally, further studies could assess the long-term effectiveness of teleguidance in various populations and explore ways to optimize the technology for broader clinical adoption. |
| [144] | 24 | Explore the long-term outcomes of infants diagnosed with intracranial hemorrhage using cranial POCUS and evaluate the impact of this early detection on neurodevelopmental outcomes. Additionally, expanding the training program to include more neonatal intensive care units and different types of POCUS applications could further validate the utility of handheld US devices in neonatal care. |
| [109] | 23 | Explore the long-term impact of such training on patient care and outcomes, as well as the potential for expanding the program to include other medical specialties. Additionally, further studies could investigate the integration of telemedicine with POCUS training to provide continuous support and guidance for healthcare providers in remote areas. |
| [52] | 23 | Focus on scaling this approach in different geographic regions, evaluating its long-term impact on breast cancer outcomes, and exploring its applicability to other types of US imaging. Additionally, further studies could investigate the cost-effectiveness of widespread Volume Sweep Imaging deployment compared to traditional breast cancer screening methods in middle-income countries. |
| [145] | 24 | Explore the clinical implications of using these devices in actual patient settings, particularly in ophthalmology and facial aesthetics, and assess their long-term reliability and user satisfaction. Additionally, further studies could evaluate the impact of device selection on clinical outcomes, particularly in procedures where imaging precision is critical. |
| [181] | 23 | Focus on optimizing micro-focus X-ray imaging technology for broader clinical use, evaluating its effectiveness in other types of foreign body detection, and exploring its integration with artificial intelligence to further enhance diagnostic accuracy. Additionally, studies involving human subjects could validate these findings and assess the practical feasibility of implementing micro-focus X-ray imaging in various clinical environments. |
| [88] | 24 | Focus on developing guidelines for the use of US-guided pericardiocentesis in transport, particularly in patients with aortic dissection, and exploring the use of advanced imaging techniques to better assess the risks of intervention in these cases. Additionally, further studies could investigate the outcomes of patients who undergo pericardiocentesis during transport to identify factors that may improve survival rates in such critical conditions. |
| [89] | 24 | Explore the application of this technology in more complex imaging scenarios, such as harmonic imaging and 3D US. Additionally, further optimization of deep learning models for different clinical applications could enhance the versatility and effectiveness of handheld US devices in various medical fields. |
| [90] | 23 | Focus on expanding the study to a larger cohort, exploring the long-term outcomes of such an approach, and developing standardized protocols for patient training and teleguidance in various clinical settings. |
| [71] | 23 | Focus on scaling this approach, evaluating its long-term impact on patient outcomes, and exploring ways to integrate it into routine healthcare practices in low- and middle-income countries. Additionally, studies could investigate the effectiveness of different training models and feedback mechanisms to optimize the learning curve for local providers. |
| [110] | 23 | Focus on expanding the range of clinical applications, exploring the use of these devices in more specialized sonographic questions, and further comparing their performance with other emerging handheld technologies. Additionally, studies could investigate the long-term impact of using handheld devices on diagnostic accuracy and patient outcomes in various clinical settings. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sewify, A.; Antico, M.; Alzubaidi, L.; Alwzwazy, H.A.; Roots, J.; Pivonka, P.; Fontanarosa, D. Systematic Review of Commercially Available Clinical CMUT-Based Systems for Use in Medical Ultrasound Imaging: Products, Applications, and Performance. Sensors 2025, 25, 2245. https://doi.org/10.3390/s25072245
Sewify A, Antico M, Alzubaidi L, Alwzwazy HA, Roots J, Pivonka P, Fontanarosa D. Systematic Review of Commercially Available Clinical CMUT-Based Systems for Use in Medical Ultrasound Imaging: Products, Applications, and Performance. Sensors. 2025; 25(7):2245. https://doi.org/10.3390/s25072245
Chicago/Turabian StyleSewify, Ahmed, Maria Antico, Laith Alzubaidi, Haider A. Alwzwazy, Jacqueline Roots, Peter Pivonka, and Davide Fontanarosa. 2025. "Systematic Review of Commercially Available Clinical CMUT-Based Systems for Use in Medical Ultrasound Imaging: Products, Applications, and Performance" Sensors 25, no. 7: 2245. https://doi.org/10.3390/s25072245
APA StyleSewify, A., Antico, M., Alzubaidi, L., Alwzwazy, H. A., Roots, J., Pivonka, P., & Fontanarosa, D. (2025). Systematic Review of Commercially Available Clinical CMUT-Based Systems for Use in Medical Ultrasound Imaging: Products, Applications, and Performance. Sensors, 25(7), 2245. https://doi.org/10.3390/s25072245








