Development of a Body-Worn Textile-Based Strain Sensor: Application to Diabetic Foot Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Scope
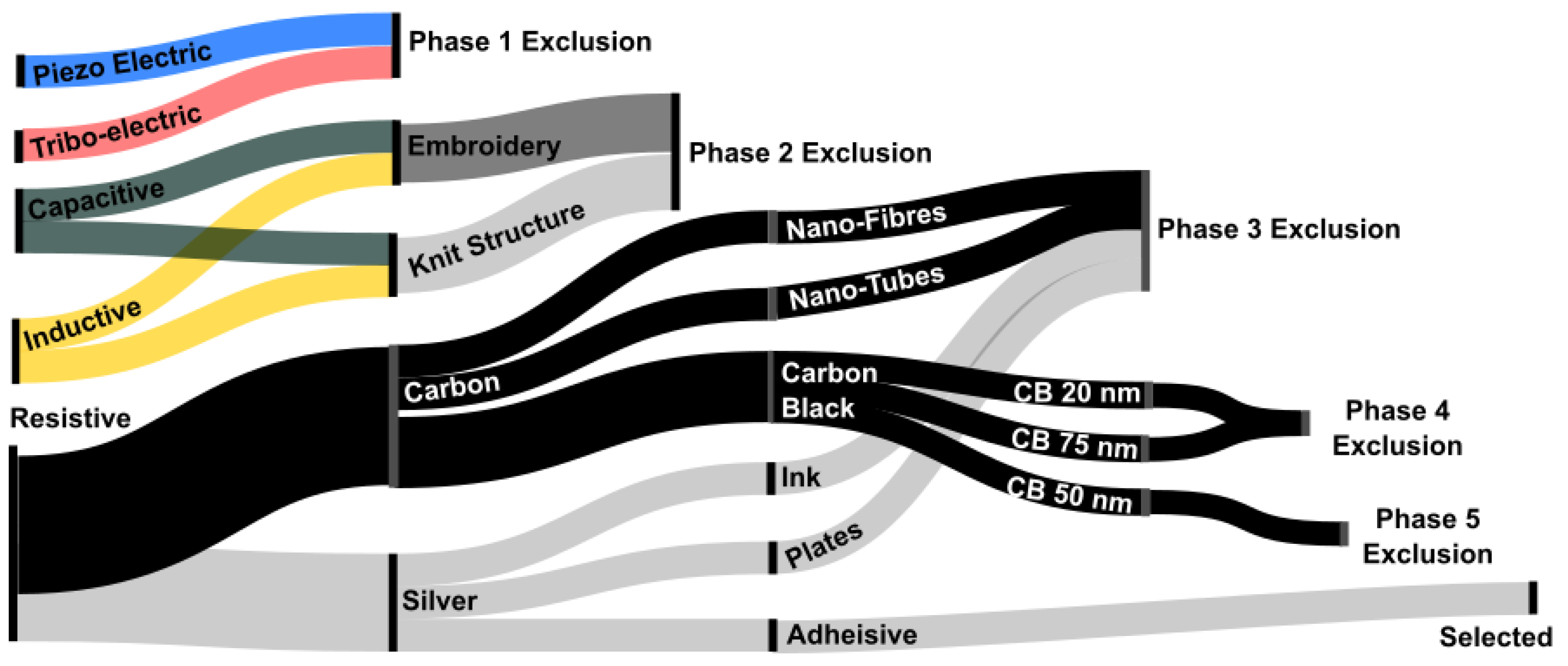
2.2. Technology Evaluation
2.2.1. Self-Generating
2.2.2. Capacitance
2.2.3. Resistive: Yarns
2.2.4. Resistive: Carbon Particles
2.2.5. Resistive: Silver Particles
2.2.6. Evaluation Summary
3. Sensor Development
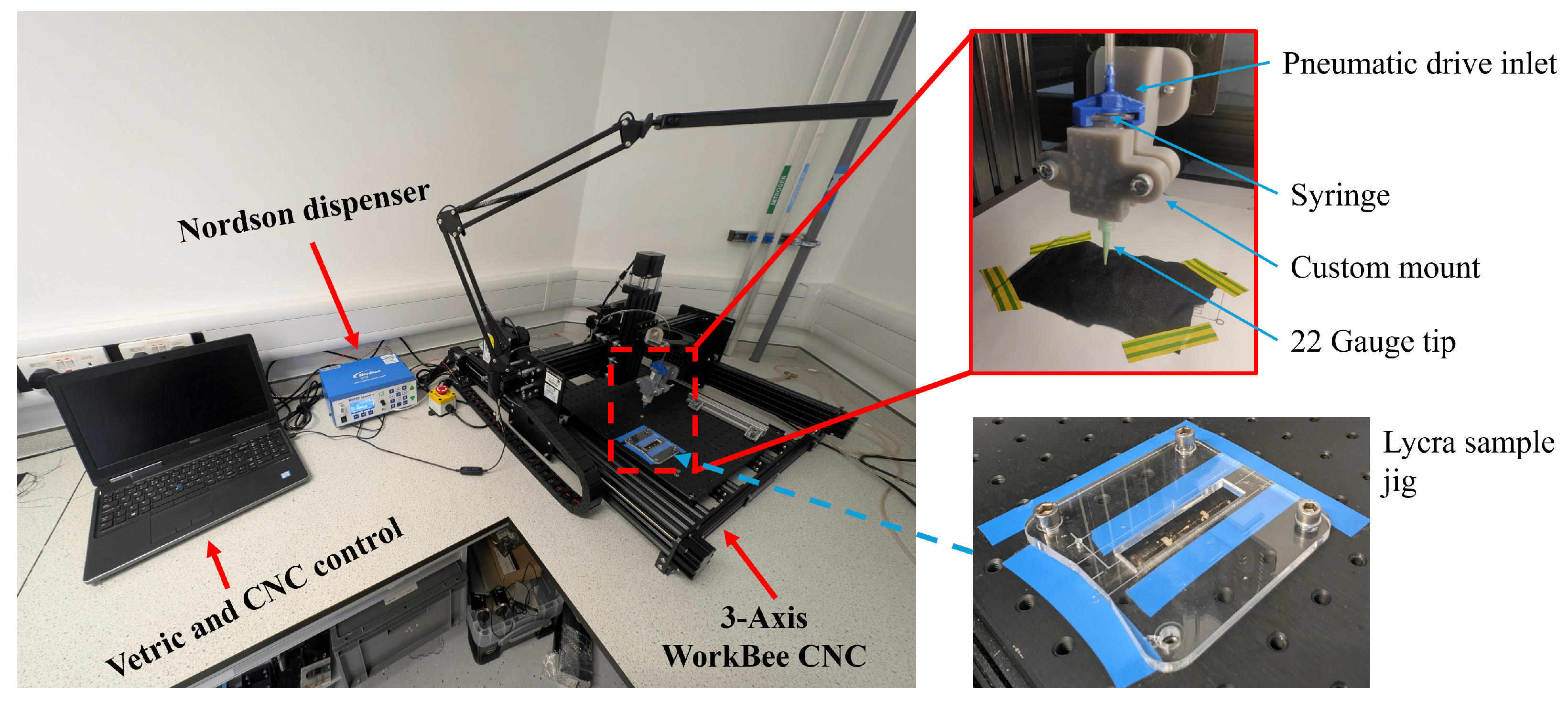
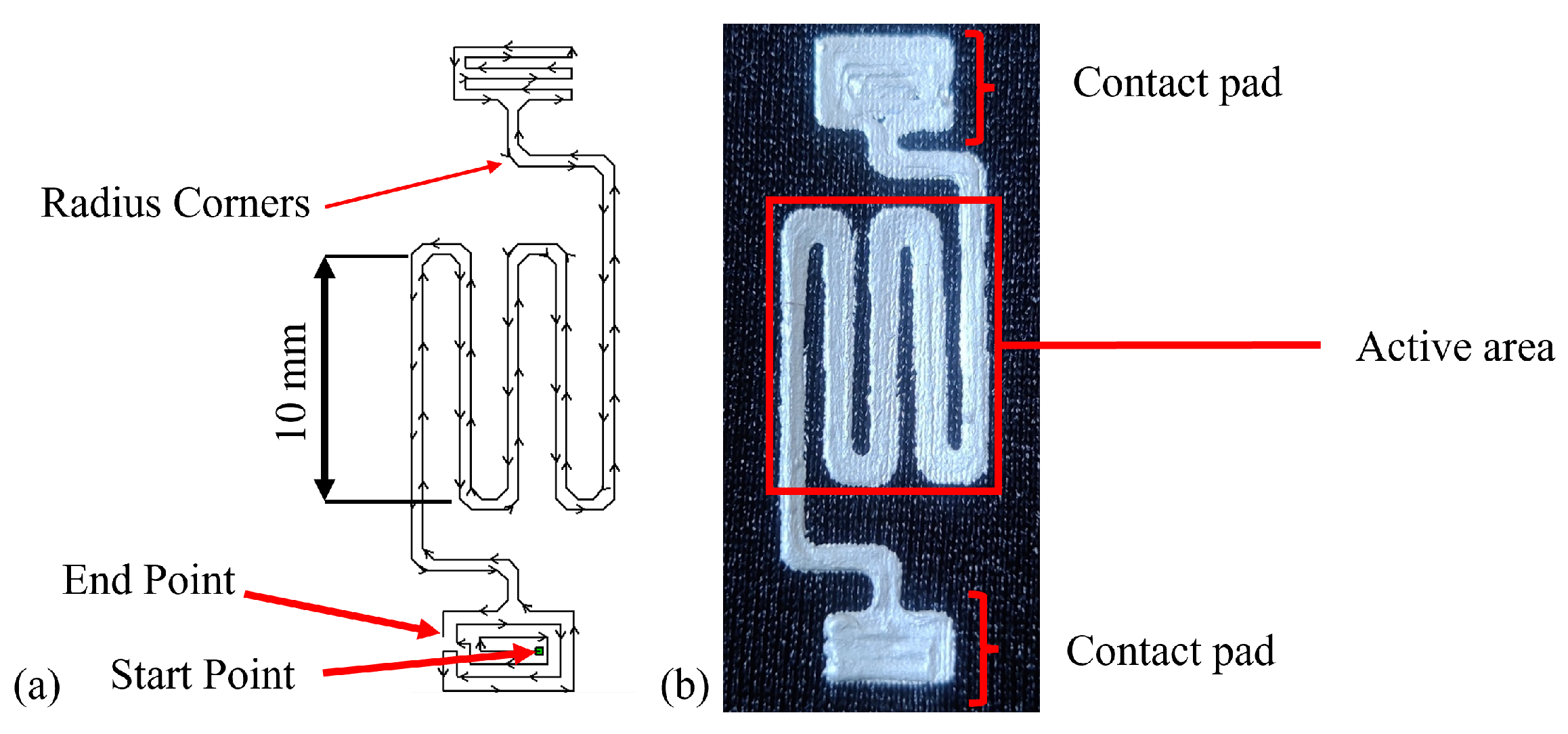
3.1. Design and Manufacture
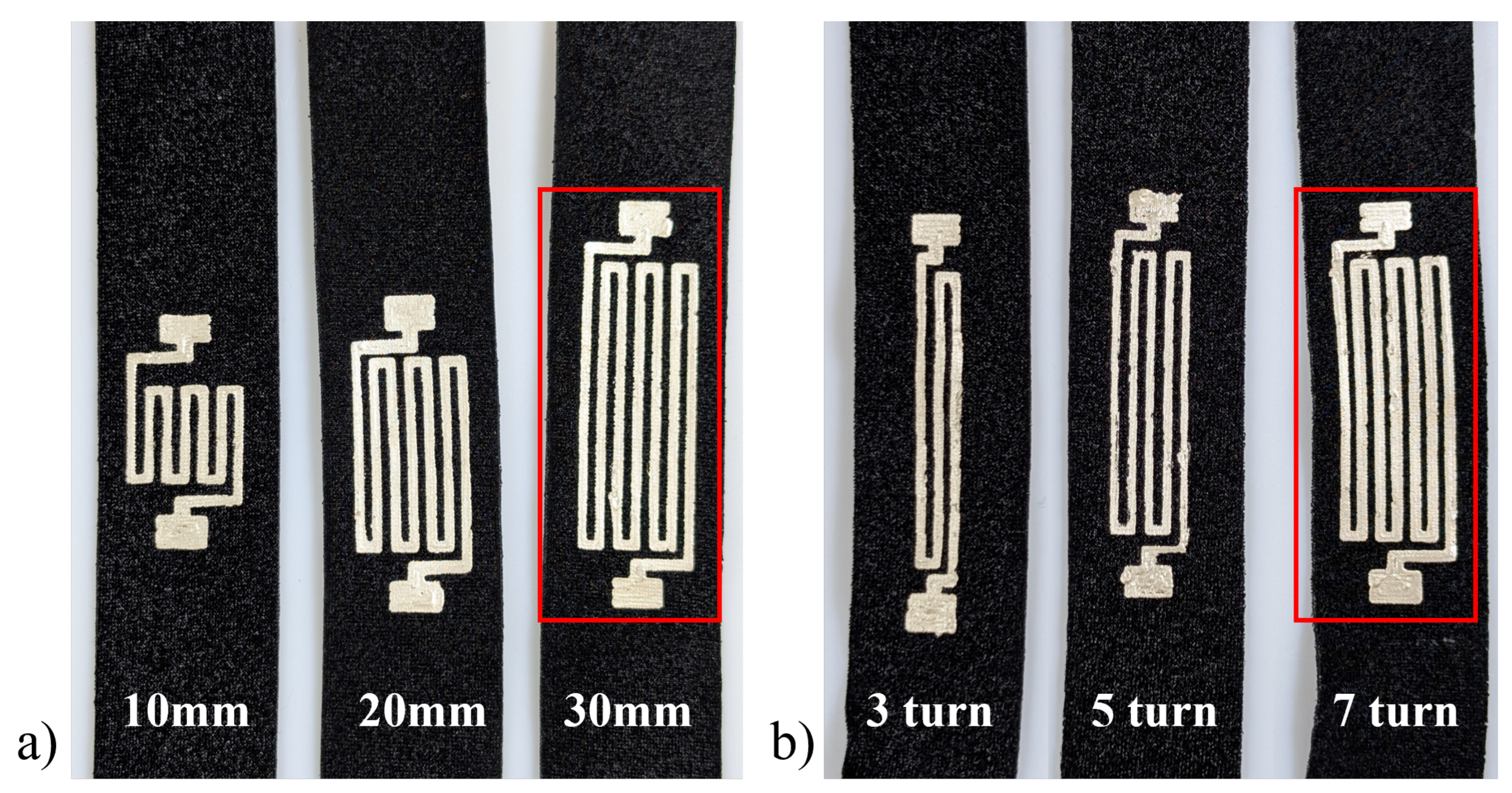
3.2. Design Parametric Testing
3.3. Robustness Improvements
3.4. Lycra Performance Evaluation
3.5. Mechanism of Action
3.6. Reinforcement of Sensing Element
3.6.1. Knitted Substrate Implementation
3.6.2. Sensor Encapsulation in Silicone
4. Final Sensor Design and Evaluation
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CB | Carbon Black |
| CNF | Carbon Nano-Fibres |
| CNT | Carbon Nano-Tubes |
| DFU | Diabetic Foot Ulcer |
| DL | Double Layer |
| DW | Double Width |
| TRL | Technology Readiness Level |
References
- Armstrong, D.G.; Tan, T.W.; Boulton, A.J.M.; Bus, S.A. Diabetic Foot Ulcers: A Review. JAMA 2023, 330, 62–75. [Google Scholar] [CrossRef]
- NDFC. National Diabetes Foot Care Audit 2018 to 2023. 2023. Available online: https://digital.nhs.uk/data-and-information/publications/statistical/national-diabetes-footcare-audit/2018-2023 (accessed on 20 May 2024).
- Tresierra-Ayala, M.A.; García Rojas, A. Association between Peripheral Arterial Disease and Diabetic Foot Ulcers in Patients with Diabetes Mellitus Type 2. Med. Univ. 2017, 19, 123–126. [Google Scholar] [CrossRef]
- Dubský, M.; Jirkovská, A.; Bem, R.; Fejfarová, V.; Skibová, J.; Schaper, N.C.; Lipsky, B.A. Risk Factors for Recurrence of Diabetic Foot Ulcers: Prospective Follow-up Analysis in the Eurodiale Subgroup. Int. Wound J. 2013, 10, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Dirac, P. The Principles of Quantum Mechanics; Comparative Pathobiology—Studies in the Postmodern Theory of Education; Clarendon Press: Oxford, UK, 1981. [Google Scholar]
- Baclawski, K. The Observer Effect. In Proceedings of the 2018 IEEE Conference on Cognitive and Computational Aspects of Situation Management (CogSIMA), Boston, MA, USA, 11–14 June 2018; pp. 83–89. [Google Scholar] [CrossRef]
- Reyzelman, A.M.; Koelewyn, K.; Murphy, M.; Shen, X.; Yu, E.; Pillai, R.; Fu, J.; Scholten, H.J.; Ma, R. Continuous Temperature-Monitoring Socks for Home Use in Patients With Diabetes: Observational Study. J. Med. Internet Res. 2018, 20, e12460. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Wang, C.; Yu, H.; Wang, H.; Zhang, Q.; Zhu, M. Human Walking-Driven Wearable All-Fiber Triboelectric Nanogenerator Containing Electrospun Polyvinylidene Fluoride Piezoelectric Nanofibers. Nano Energy 2015, 14, 226–235. [Google Scholar] [CrossRef]
- Jia, J.; Xu, C.; Pan, S.; Xia, S.; Wei, P.; Noh, H.Y.; Zhang, P.; Jiang, X. Conductive Thread-Based Textile Sensor for Continuous Perspiration Level Monitoring. Sensors 2018, 18, 3775. [Google Scholar] [CrossRef]
- Le, K.; Narayana, H.; Servati, A.; Bahi, A.; Soltanian, S.; Servati, P.; Ko, F.K. Electronic Textiles for Electrocardiogram Monitoring: A Review on the Structure–Property and Performance Evaluation from Fiber to Fabric. Text. Res. J. 2022, 93, 878–910. [Google Scholar] [CrossRef]
- White, S.; McCullough, M.B.A.; Akangah, P.M. The Structural Effects of Diabetes on Soft Tissues: A Systematic Review. Crit. Rev. Biomed. Eng. 2021, 49, 11–27. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, B.; Chen, W. Influence of Hallux Valgus Deformity on Forefoot Pressure Distribution of Chinese Diabetic Patients. Int. J. Diabetes Dev. Ctries. 2015, 35, 129–134. [Google Scholar] [CrossRef]
- Hicks, C.W.; Selvin, E. Epidemiology of Peripheral Neuropathy and Lower Extremity Disease in Diabetes. Curr. Diabetes Rep. 2019, 19, 86. [Google Scholar] [CrossRef]
- Veves, A.; Murray, H.J.; Young, M.J.; Boulton, A.J.M. The Risk of Foot Ulceration in Diabetic Patients with High Foot Pressure: A Prospective Study. Diabetologia 1992, 35, 660–663. [Google Scholar] [CrossRef] [PubMed]
- Chatwin, K.E.; Abbott, C.A.; Boulton, A.J.; Bowling, F.L.; Reeves, N.D. The Role of Foot Pressure Measurement in the Prediction and Prevention of Diabetic Foot Ulceration—A Comprehensive Review. Diabetes/Metab. Res. Rev. 2019, 36, e3258. [Google Scholar] [CrossRef] [PubMed]
- Luger, E.; Nissan, M.; Karpf, A.; Steinberg, E.; Dekel, S. Dynamic Pressures on the Diabetic Foot. Foot Ankle Int. 2001, 22, 715–719. [Google Scholar] [CrossRef]
- Perry, J.E.; Hall, J.O.; Davis, B.L. Simultaneous Measurement of Plantar Pressure and Shear Forces in Diabetic Individuals. Gait Posture 2002, 15, 101–107. [Google Scholar] [CrossRef]
- Jones, A.D.; De Siqueira, J.; Nixon, J.E.; Siddle, H.J.; Culmer, P.R.; Russell, D.A. Plantar Shear Stress in the Diabetic Foot: A Systematic Review and Meta-analysis. Diabet. Med. 2022, 39, e14661. [Google Scholar] [CrossRef]
- Jones, A.D.; Crossland, S.R.; Nixon, J.E.; Siddle, H.J.; Russell, D.A.; Culmer, P.R. STrain Analysis and Mapping of the Plantar Surface (STAMPS)—A Novel Technique of Plantar Load Analysis during Gait. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2023, 237, 841–854. [Google Scholar] [CrossRef]
- Corser, J.; Yoldi, I.; Reeves, N.D.; Culmer, P.; Venkatraman, P.D.; Orlando, G.; Turnbull, R.P.; Boakes, P.; Woodin, E.; Lightup, R.; et al. Developing a Smart Sensing Sock to Prevent Diabetic Foot Ulcers: Qualitative Focus Group and Interview Study. J. Particip. Med. 2025, 17, e59608. [Google Scholar] [CrossRef] [PubMed]
- Pitei, D.L.; Ison, K.; Edmonds, M.E.; Lord, M. Time-Dependent Behaviour of a Force-Sensitive Resistor Plantar Pressure Measurement Insole. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 1996, 210, 121–125. [Google Scholar] [CrossRef]
- Hsiao, H.; Guan, J.; Weatherly, M. Accuracy and Precision of Two In-Shoe Pressure Measurement Systems. Ergonomics 2002, 45, 537–555. [Google Scholar] [CrossRef]
- Chen, B.; Ma, H.; Qin, L.Y.; Guan, X.; Chan, K.M.; Law, S.W.; Qin, L.; Liao, W.H. Design of a Lower Extremity Exoskeleton for Motion Assistance in Paralyzed Individuals. In Proceedings of the 2015 IEEE International Conference on Robotics and Biomimetics, IEEE-ROBIO 2015, Zhuhai, China, 6–9 December 2015. [Google Scholar] [CrossRef]
- Anas, M.N. An Instrumented Insole System for Gait Monitoring and Analysis. Int. J. Online Biomed. Eng. (iJOE) 2014, 10, 30–34. [Google Scholar] [CrossRef]
- Dabiri, F.; Vahdatpour, A.; Noshadi, H.; Hagopian, H.; Sarrafzadeh, M. Electronic Orthotics Shoe: Preventing Ulceration in Diabetic Patients. In Proceedings of the 2008 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Vancouver, BC, Canada, 20–25 August 2008; pp. 771–774. [Google Scholar] [CrossRef]
- McKnight, M.; Tabor, J.; Agcayazi, T.; Fleming, A.; Ghosh, T.K.; Huang, H.; Bozkurt, A. Fully Textile Insole Seam-Line for Multimodal Sensor Mapping. IEEE Sens. J. 2020, 20, 10145–10153. [Google Scholar] [CrossRef]
- Tan, Y.; Ivanov, K.; Mei, Z.; Li, H.; Li, H.; Lubich, L.; Wang, C.; Wang, L. A Soft Wearable and Fully-Textile Piezoresistive Sensor for Plantar Pressure Capturing. Micromachines 2021, 12, 110. [Google Scholar] [CrossRef]
- Aqueveque, P.; Osorio, R.; Pastene, F.; Saavedra, F.; Pino, E. Capacitive Sensors Array for Plantar Pressure Measurement Insole Fabricated with Flexible PCB. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 4393–4396. [Google Scholar] [CrossRef]
- Guignier, C.; Camillieri, B.; Schmid, M.; Rossi, R.M.; Bueno, M.A. E-Knitted Textile with Polymer Optical Fibers for Friction and Pressure Monitoring in Socks. Sensors 2019, 19, 3011. [Google Scholar] [CrossRef] [PubMed]
- Leal-Junior, A.G.; Frizera, A.; Avellar, L.M.; Marques, C.; Pontes, M.J. Polymer Optical Fiber for In-Shoe Monitoring of Ground Reaction Forces During the Gait. IEEE Sens. J. 2018, 18, 2362–2368. [Google Scholar] [CrossRef]
- Pollard, J.P.; Le Quesne, L.P. Method of Healing Diabetic Forefoot Ulcers. Br. Med. J. 1983, 286, 436–437. [Google Scholar] [CrossRef]
- Wang, L.; Jones, D.; Chapman, G.J.; Siddle, H.J.; Russell, D.A.; Alazmani, A.; Culmer, P. A Review of Wearable Sensor Systems to Monitor Plantar Loading in the Assessment of Diabetic Foot Ulcers. IEEE Trans.-Bio-Med. Eng. 2020, 67, 1989–2004. [Google Scholar] [CrossRef]
- Castro-Martins, P.; Marques, A.; Coelho, L.; Vaz, M.; Baptista, J.S. In-Shoe Plantar Pressure Measurement Technologies for the Diabetic Foot: A Systematic Review. Heliyon 2024, 10, e29672. [Google Scholar] [CrossRef]
- Du, L.; Zhu, X.; Zhe, J. An Inductive Sensor for Real-Time Measurement of Plantar Normal and Shear Forces Distribution. IEEE Trans. Biomed. Eng. 2015, 62, 1316–1323. [Google Scholar] [CrossRef]
- Wang, L.; Jones, D.; Chapman, G.J.; Siddle, H.J.; Russell, D.A.; Alazmani, A.; Culmer, P. Design of a Digital Triaxial Force Sensor for Plantar Load Measurements. In Proceedings of the 2019 IEEE Sensors, Montreal, QC, Canada, 27–30 October 2019; pp. 1–4. [Google Scholar] [CrossRef]
- Lord, M.; Hosein, R. A Study of In-Shoe Plantar Shear in Patients with Diabetic Neuropathy. Clin. Biomech. 2000, 15, 278–283. [Google Scholar] [CrossRef]
- Mori, T.; Hamatani, M.; Noguchi, H.; Oe, M.; Sanada, H. Insole-Type Simultaneous Measurement System of Plantar Pressure and Shear Force During Gait for Diabetic Patients. J. Robot. Mechatronics 2012, 24, 766–772. [Google Scholar] [CrossRef]
- Tang, J.; Bader, D.L.; Moser, D.; Parker, D.J.; Forghany, S.; Nester, C.J.; Jiang, L. A Wearable Insole System to Measure Plantar Pressure and Shear for People with Diabetes. Sensors 2023, 23, 3126. [Google Scholar] [CrossRef]
- Amemiya, A.; Noguchi, H.; Oe, M.; Takehara, K.; Ohashi, Y.; Suzuki, R.; Yamauchi, T.; Kadowaki, T.; Sanada, H.; Mori, T. Factors Associated with Callus Formation in the Plantar Region through Gait Measurement in Patients with Diabetic Neuropathy: An Observational Case-Control Study. Sensors 2020, 20, 4863. [Google Scholar] [CrossRef] [PubMed]
- Crossland, S.R.; Siddle, H.J.; Brockett, C.L.; Culmer, P. Evaluating the Use of a Novel Low-Cost Measurement Insole to Characterise Plantar Foot Strain during Gait Loading Regimes. Front. Bioeng. Biotechnol. 2023, 11, 1187710. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; De Boer, G.; Kow, J.; Alazmani, A.; Ghajari, M.; Hewson, R.; Culmer, P. Design Methodology for Magnetic Field-Based Soft Tri-Axis Tactile Sensors. Sensors 2016, 16, 1356. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jones, D.; de Boer, G.; Kow, J.; Beccai, L.; Alazmani, A.; Culmer, P. Design and Characterization of Tri-Axis Soft Inductive Tactile Sensors. IEEE Sens. J. 2018, 18, 7793–7801. [Google Scholar] [CrossRef]
- Rajala, S.N.K.; Lekkala, J. Plantar Shear Stress Measurements—A Review. Clin. Biomech. 2014, 29, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Viry, L.; Levi, A.; Totaro, M.; Mondini, A.; Mattoli, V.; Mazzolai, B.; Beccai, L. Flexible Three-Axial Force Sensor for Soft and Highly Sensitive Artificial Touch. Adv. Mater. 2014, 26, 2659–2664. [Google Scholar] [CrossRef]
- Lee, H.K.; Chung, J.; Chang, S.I.; Yoon, E. Real-Time Measurement of the Three-Axis Contact Force Distribution Using a Flexible Capacitive Polymer Tactile Sensor. J. Micromech. Microeng. 2011, 21, 035010. [Google Scholar] [CrossRef]
- Santos, V.M.; Gomes, B.B.; Neto, M.A.; Amaro, A.M. A Systematic Review of Insole Sensor Technology: Recent Studies and Future Directions. Appl. Sci. 2024, 14, 6085. [Google Scholar] [CrossRef]
- Bahin, L.; Tourlonias, M.; Bueno, M.A.; Sharma, K.; Rossi, R.M. Smart Textiles with Polymer Optical Fibre Implementation for In-Situ Measurements of Compression and Bending. Sens. Actuators A Phys. 2023, 350, 114117. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, W.; Zhang, L.; Gong, J.; Zhao, D.; Liu, M.; Lin, L.; Meng, Q.; Thompson, R.; Sun, Y. Magnetic Properties of Smart Textile Fabrics through a Coating Method with NdFeB Flake-like Microparticles. J. Eng. Fibers Fabr. 2019, 14, 1558925019865708. [Google Scholar] [CrossRef]
- Lewis, E. Radiant Textiles: Designing Electromagnetic Textile Systems. University of Borås Studies in Artistic Research. Doctoral Thesis, University of Borås, Borås, Sweden, 2023. ISBN 978-91-89833-06-7. Available online: https://www.diva-portal.org/smash/record.jsf?pid=diva2%3A1754862&dswid=-3444 (accessed on 17 March 2025).
- Yang, P.K.; Lin, L.; Yi, F.; Li, X.; Pradel, K.C.; Zi, Y.; Wu, C.I.; He, J.H.; Zhang, Y.; Wang, Z.L. A Flexible, Stretchable and Shape-Adaptive Approach for Versatile Energy Conversion and Self-Powered Biomedical Monitoring. Adv. Mater. 2015, 27, 3817–3824. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Tao, J.; Liu, Y.; Mo, Y.; Bao, R.; Pan, C. Fully Fibrous Large-Area Tailorable Triboelectric Nanogenerator Based on Solution Blow Spinning Technology for Energy Harvesting and Self-Powered Sensing. Small 2022, 18, 2202477. [Google Scholar] [CrossRef]
- Fan, W.; He, Q.; Meng, K.; Tan, X.; Zhou, Z.; Zhang, G.; Yang, J.; Wang, Z.L. Machine-Knitted Washable Sensor Array Textile for Precise Epidermal Physiological Signal Monitoring. Sci. Adv. 2020, 6, eaay2840. [Google Scholar] [CrossRef]
- Mokhtari, F.; Cheng, Z.; Raad, R.; Xi, J.; Foroughi, J. Piezofibers to Smart Textiles: A Review on Recent Advances and Future Outlook for Wearable Technology. J. Mater. Chem. 2020, 8, 9496–9522. [Google Scholar] [CrossRef]
- Zhang, W.; Diao, D.; Sun, K.; Fan, X.; Wang, P. Study on Friction-Electrification Coupling in Sliding-Mode Triboelectric Nanogenerator. Nano Energy 2018, 48, 456–463. [Google Scholar] [CrossRef]
- Hudec, R.; Matúška, S.; Kamencay, P.; Benco, M. A Smart IoT System for Detecting the Position of a Lying Person Using a Novel Textile Pressure Sensor. Sensors 2021, 21, 206. [Google Scholar] [CrossRef]
- Gleskova, H.; Ishaku, A.; Bednar, T.; Hudec, R. Optimization of All-Textile Capacitive Sensor Array for Smart Chair. IEEE Access 2022, 10, 48615–48621. [Google Scholar] [CrossRef]
- Fobelets, K.; Thielemans, K.; Mathivanan, A.; Papavassiliou, C. Characterization of Knitted Coils for E-Textiles. IEEE Sens. J. 2019, 19, 7835–7840. [Google Scholar] [CrossRef]
- Tajitsu, Y. Development of E-Textile Sewn Together with Embroidered Fabric Having Motion-Sensing Function Using Piezoelectric Braided Cord for Embroidery. IEEE Trans. Dielectr. Electr. Insul. 2020, 27, 1644–1649. [Google Scholar] [CrossRef]
- Tajitsu, Y.; Takarada, J.; Hikichi, T.; Sugii, R.; Takatani, K.; Yanagimoto, H.; Nakanishi, R.; Shiomi, S.; Kitamoto, D.; Nakiri, T.; et al. Application of Piezoelectric PLLA Braided Cord as Wearable Sensor to Realize Monitoring System for Indoor Dogs with Less Physical or Mental Stress. Micromachines 2023, 14, 143. [Google Scholar] [CrossRef] [PubMed]
- Uno, M.O.; Morita, S.; Omori, M.; Yoshimura, K. Pressure Sensor Yarns with a Sheath-Core Structure Using Multi-Fiber Polymer. Sens. Actuators A Phys. 2022, 337, 113440. [Google Scholar] [CrossRef]
- Zhao, J.; Fu, Y.; Xiao, Y.; Dong, Y.; Wang, X.; Lin, L. A Naturally Integrated Smart Textile for Wearable Electronics Applications. Adv. Mater. Technol. 2020, 5, 1900781. [Google Scholar] [CrossRef]
- Souri, H.; Bhattacharyya, D. Wearable Strain Sensors Based on Electrically Conductive Natural Fiber Yarns. Mater. Des. 2018, 154, 217–227. [Google Scholar] [CrossRef]
- Lian, Y.; Yu, H.; Wang, M.; Yang, X.; Zhang, H. Ultrasensitive Wearable Pressure Sensors Based on Silver Nanowire-Coated Fabrics. Nanoscale Res. Lett. 2020, 15, 70. [Google Scholar] [CrossRef]
- Seyedin, S.; Razal, J.M.; Innis, P.C.; Jeiranikhameneh, A.; Beirne, S.; Wallace, G.G. Knitted Strain Sensor Textiles of Highly Conductive All-Polymeric Fibers. ACS Appl. Mater. Interfaces 2015, 7, 21150–21158. [Google Scholar] [CrossRef] [PubMed]
- Santoni SPA. Sangiacomo Star-D: Sangiacomo Electronic Knitting Machine. 2025. Available online: https://www.santoni.com/uploads/2023-10-16/STAR-D_E.pdf (accessed on 13 February 2025).
- Zhu, Y.; Assadian, M.; Ramezani, M.; Aw, K.C. Printing of Soft Stretch Sensor from Carbon Black Composites. Proceedings 2018, 2, 732. [Google Scholar] [CrossRef]
- Honnet, C.; Perner-Wilson, H.; Teyssier, M.; Fruchard, B.; Steimle, J.; Baptista, A.C.; Strohmeier, P. PolySense: Augmenting Textiles with Electrical Functionality Using In-Situ Polymerization. Conf. Hum. Factors Comput.-Syst. Proc. 2020, 2020, 1–13. [Google Scholar] [CrossRef]
- Souri, H.; Bhattacharyya, D. Highly Stretchable and Wearable Strain Sensors Using Conductive Wool Yarns with Controllable Sensitivity. Sens. Actuators A Phys. 2019, 285, 142–148. [Google Scholar] [CrossRef]
- Anike, J.C.; Le, H.H.; Brodeur, G.E.; Kadavan, M.M.; Abot, J.L. Piezoresistive Response of Integrated CNT Yarns under Compression and Tension: The Effect of Lateral Constraint. C 2017, 3, 14. [Google Scholar] [CrossRef]
- Wiranata, A.; Ohsugi, Y.; Minaminosono, A.; Mao, Z.; Kurata, H.; Hosoya, N.; Maeda, S. A DIY Fabrication Approach of Stretchable Sensors Using Carbon Nano Tube Powder for Wearable Device. Front. Robot. 2021, 8, 773056. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Gu, Y.; Liu, T.; Pecht, M. Resistive and Capacitive Strain Sensors Based on Customized Compliant Electrode: Comparison and Their Wearable Applications. Sens. Actuators A Phys. 2021, 326, 112720. [Google Scholar] [CrossRef]
- Xu, P.; Wang, S.; Lin, A.; Min, H.K.; Zhou, Z.; Dou, W.; Sun, Y.; Huang, X.; Tran, H.; Liu, X. Conductive and Elastic Bottlebrush Elastomers for Ultrasoft Electronics. Nat. Commun. 2023, 14, 623. [Google Scholar] [CrossRef]
- Muth, J.T.; Vogt, D.M.; Truby, R.L.; Mengüç, Y.; Kolesky, D.B.; Wood, R.J.; Lewis, J.A. Embedded 3D Printing of Strain Sensors within Highly Stretchable Elastomers. Adv. Mater. 2014, 26, 6307–6312. [Google Scholar] [CrossRef] [PubMed]
- Shintake, J.; Piskarev, Y.; Jeong, S.H.; Floreano, D. Ultrastretchable Strain Sensors Using Carbon Black-Filled Elastomer Composites and Comparison of Capacitive Versus Resistive Sensors. Adv. Mater. Technol. 2018, 3, 1700284. [Google Scholar] [CrossRef]
- Cholleti, E.R.; Stringer, J.; Assadian, M.; Battmann, V.; Bowen, C.; Aw, K. Highly Stretchable Capacitive Sensor with Printed Carbon Black Electrodes on Barium Titanate Elastomer Composite. Sensors 2019, 19, 42. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Wang, G.; Zhang, Y. Preparation and Performance of Graphene/Carbon Black Silicone Rubber Composites Used for Highly Sensitive and Flexible Strain Sensors. Sens. Actuators A Phys. 2021, 323, 112659. [Google Scholar] [CrossRef]
- Giffney, T.; Bejanin, E.; Kurian, A.S.; Travas-Sejdic, J.; Aw, K. Highly Stretchable Printed Strain Sensors Using Multi-Walled Carbon Nanotube/Silicone Rubber Composites. Sens. Actuators A Phys. 2017, 259, 44–49. [Google Scholar] [CrossRef]
- Matsuhisa, N.; Kaltenbrunner, M.; Yokota, T.; Jinno, H.; Kuribara, K.; Sekitani, T.; Someya, T. Printable Elastic Conductors with a High Conductivity for Electronic Textile Applications. Nat. Commun. 2015, 6, 7461. [Google Scholar] [CrossRef]
- Matsuhisa, N.; Inoue, D.; Zalar, P.; Jin, H.; Matsuba, Y.; Itoh, A.; Yokota, T.; Hashizume, D.; Someya, T. Printable Elastic Conductors by in Situ Formation of Silver Nanoparticles from Silver Flakes. Nat. Mater. 2017, 16, 834–840. [Google Scholar] [CrossRef]
- Yoon, I.S.; Kim, S.H.; Oh, Y.; Ju, B.K.; Hong, J.M. Ag Flake/Silicone Rubber Composite with High Stability and Stretching Speed Insensitive Resistance via Conductive Bridge Formation. Sci. Rep. 2020, 10, 5036. [Google Scholar] [CrossRef]
- Lu, D.; Wong, C.P. Thermal Decomposition of Silver Flake Lubricants. J. Therm. Anal. Calorim. 2000, 61, 3–12. [Google Scholar] [CrossRef]
- Zhang, R.; Lin, W.; Moon, K.s.; Wong, C.P. Fast Preparation of Printable Highly Conductive Polymer Nanocomposites by Thermal Decomposition of Silver Carboxylate and Sintering of Silver Nanoparticles. ACS Appl. Mater. Interfaces 2010, 2, 2637–2645. [Google Scholar] [CrossRef] [PubMed]
- Clement, J.L.; Jarrett, P.S. Antibacterial Silver. Met.-Based Drugs 1994, 1, 467–482. [Google Scholar] [CrossRef]
- DM-SAS-10010 Product Datasheet. Available online: https://www.dycotecmaterials.com/wp-content/uploads/2018/01/Dycotec-TDS_DM-SAS-10010-Datasheet-13.05.20.pdf (accessed on 19 September 2024).
- BS EN ISO 527-3:2018; Plastics—Determination of Tensile Properties—Part 3: Test Conditions for Films and Sheets. ISO: Geneva, Switzerland, 2018. Available online: https://www.iso.org/standard/70307.html (accessed on 17 March 2025).
- Shen, H.; Ke, H.; Feng, J.; Jiang, C.; Wei, Q.; Wang, Q. Highly Sensitive and Stretchable C-MWCNTs/PPy Embedded Multidirectional Strain Sensor Based on Double Elastic Fabric for Human Motion Detection. Nanomaterials 2021, 11, 2333. [Google Scholar] [CrossRef]
- Li, Y.; Miao, X.; Chen, J.Y.; Jiang, G.; Liu, Q. Sensing Performance of Knitted Strain Sensor on Two-Dimensional and Three-Dimensional Surfaces. Mater. Des. 2021, 197, 109273. [Google Scholar] [CrossRef]





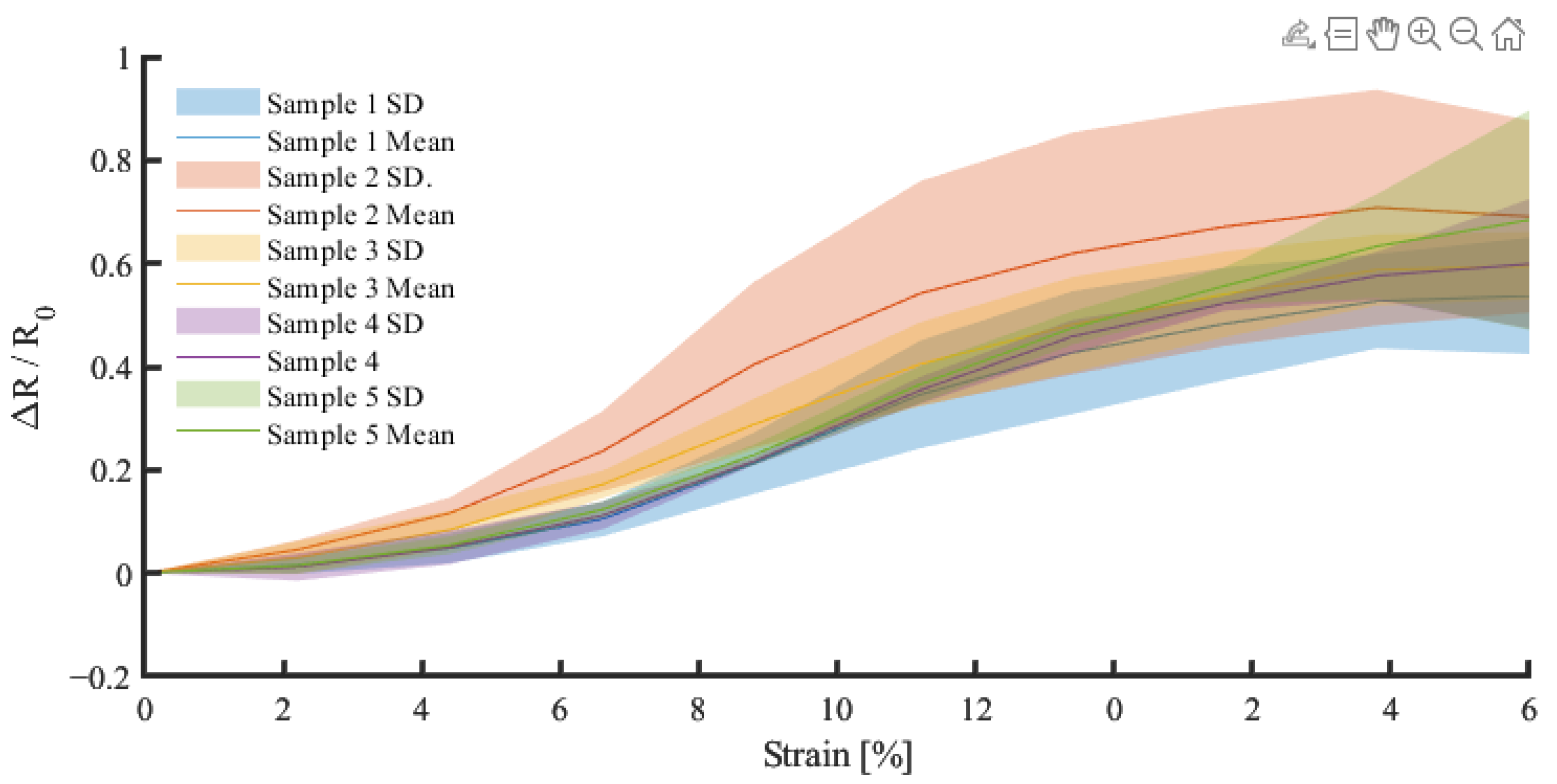
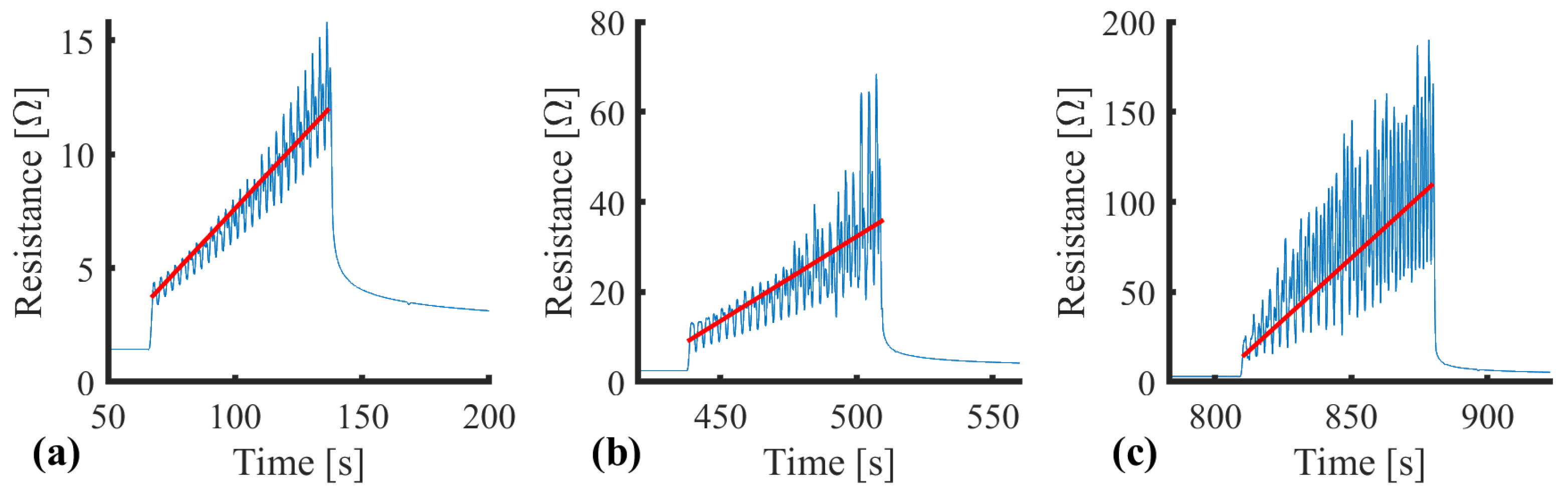

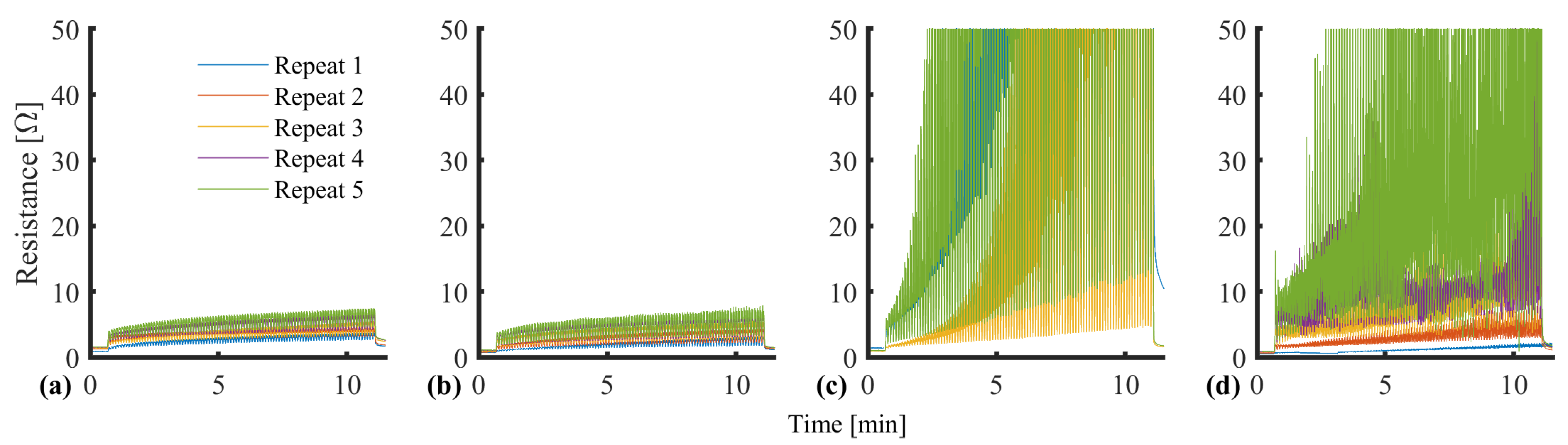
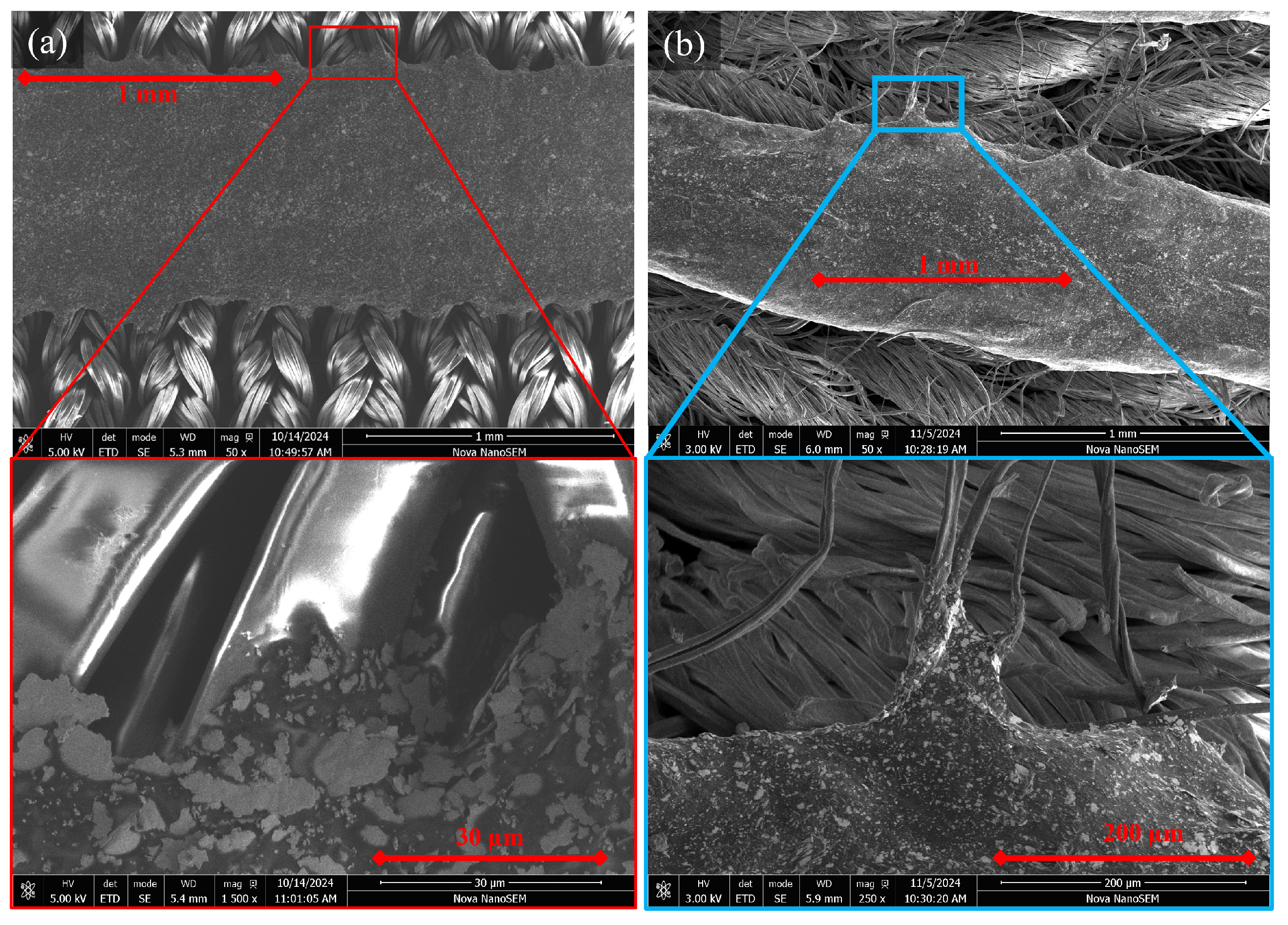



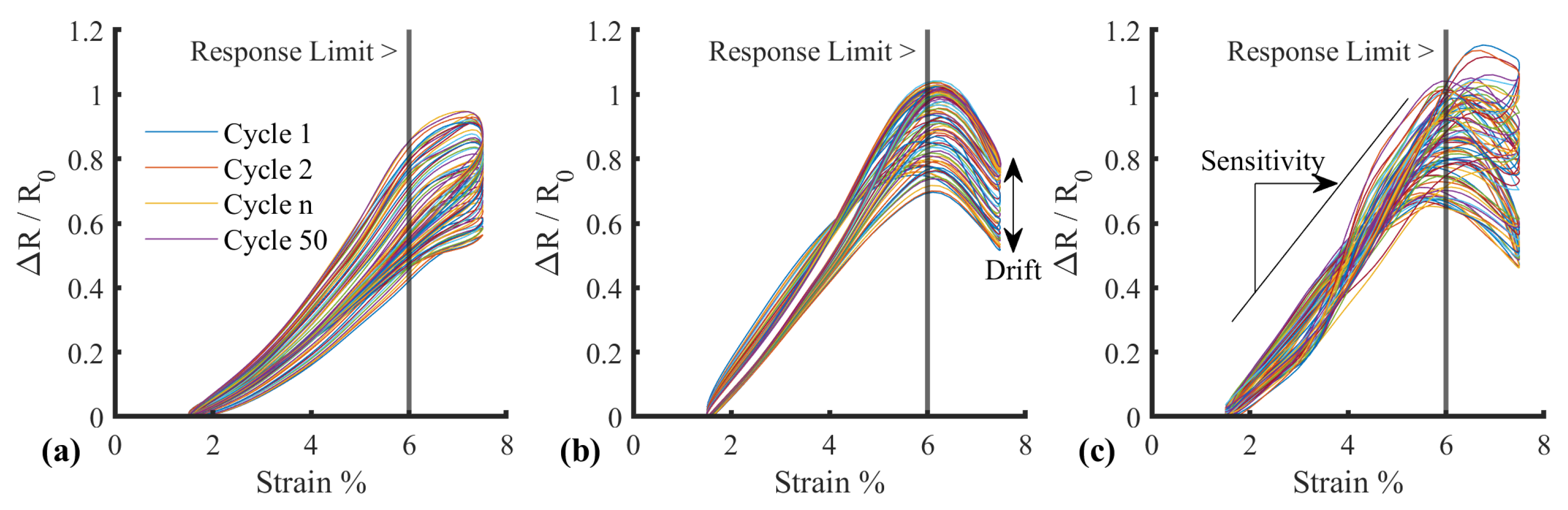
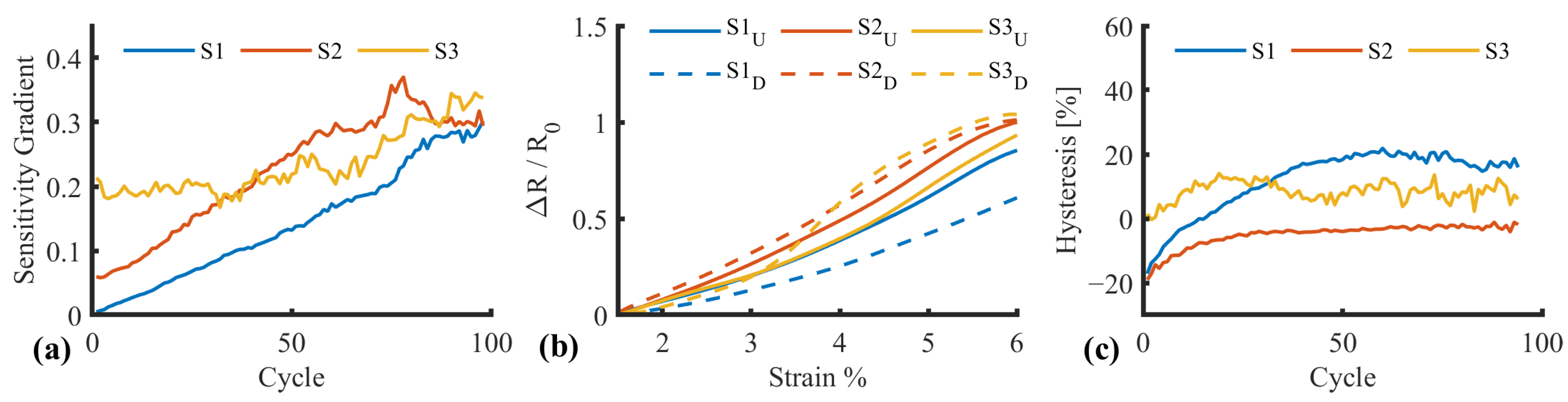
| SID | Name | Requirement |
|---|---|---|
| 1 | Robustness | Placed on the foot’s plantar surface up to 1.5×, bodyweight can be exerted during gait, requiring a robust solution. |
| 2 | Biocompatibility | Solutions must be biocompatible due to the increased risk of infection due to cracked or fissured skin increasing access through natural barriers. |
| 3 | Technology Readiness Level | With the interest of producing a real-world impact on patient quality of life. Solutions using commercially available technology will be prioritised. |
| 4 | Production Scale | The processes used in production must involve scalable technology. |
| 5 | Production Compatible | Relating to ID4 solutions must be compatible with industrial knitting machines. |
| 6 | Sensor Localisation | A discrete rather than global sensing approach for response localisation. |
| 7 | Low Profile | Due to increased sensitivity to ulcer formation of soft tissue, ridges and point forces must be avoided. |
| Parameter | Selected Value |
|---|---|
| Number of Turns | 5 |
| Length | 10 mm |
| Cure Temperature | 75 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turnbull, R.P.; Corser, J.; Orlando, G.; Venkatraman, P.D.; Yoldi, I.; Bradbury, K.; Reeves, N.D.; Culmer, P. Development of a Body-Worn Textile-Based Strain Sensor: Application to Diabetic Foot Assessment. Sensors 2025, 25, 2057. https://doi.org/10.3390/s25072057
Turnbull RP, Corser J, Orlando G, Venkatraman PD, Yoldi I, Bradbury K, Reeves ND, Culmer P. Development of a Body-Worn Textile-Based Strain Sensor: Application to Diabetic Foot Assessment. Sensors. 2025; 25(7):2057. https://doi.org/10.3390/s25072057
Chicago/Turabian StyleTurnbull, Rory P., Jenny Corser, Giorgio Orlando, Prabhuraj D. Venkatraman, Irantzu Yoldi, Kathrine Bradbury, Neil D. Reeves, and Peter Culmer. 2025. "Development of a Body-Worn Textile-Based Strain Sensor: Application to Diabetic Foot Assessment" Sensors 25, no. 7: 2057. https://doi.org/10.3390/s25072057
APA StyleTurnbull, R. P., Corser, J., Orlando, G., Venkatraman, P. D., Yoldi, I., Bradbury, K., Reeves, N. D., & Culmer, P. (2025). Development of a Body-Worn Textile-Based Strain Sensor: Application to Diabetic Foot Assessment. Sensors, 25(7), 2057. https://doi.org/10.3390/s25072057











