Microfluidic Biosensors: Enabling Advanced Disease Detection
Abstract
1. Introduction
2. Microfluidics and Microfluidic Biosensors
2.1. Materials for Microfluidic Chip Preparation
2.2. Surface Ttreatment of Microfluidic Chip Channels
2.2.1. Anti-Pollution Protection Treatments
2.2.2. Functionalization for Bio-Selectivity
2.3. Principles of Microfluidic Biosensor Technology
2.3.1. Flow Channel Microfluidic Biosensors
2.3.2. Digital Microfluidic Biosensors
2.3.3. Paper-Based Microfluidic Biosensors
3. Applications of Microfluidic Biosensors
3.1. Cancer Liquid Biopsy
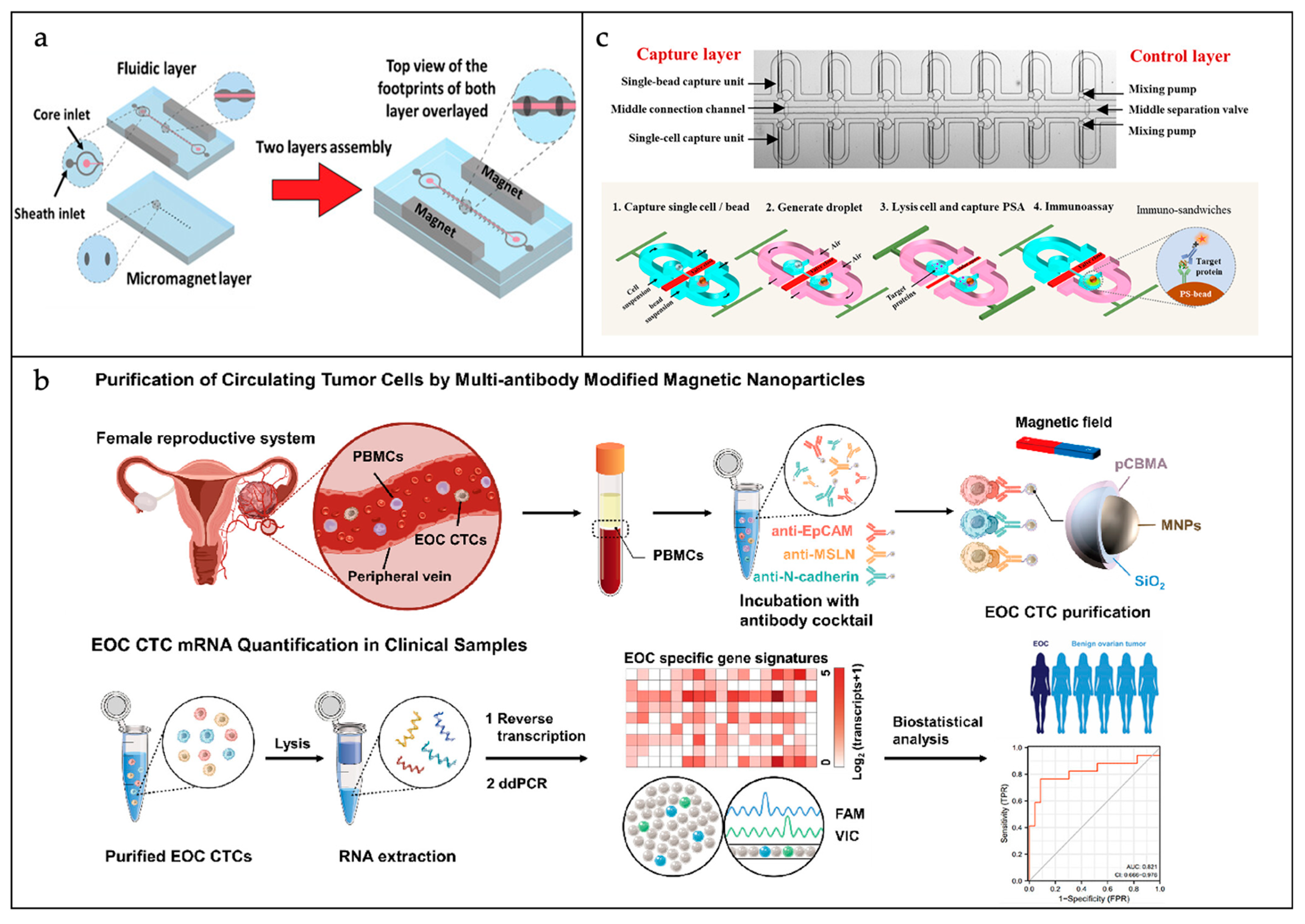
3.1.1. CTCs
3.1.2. ctDNA
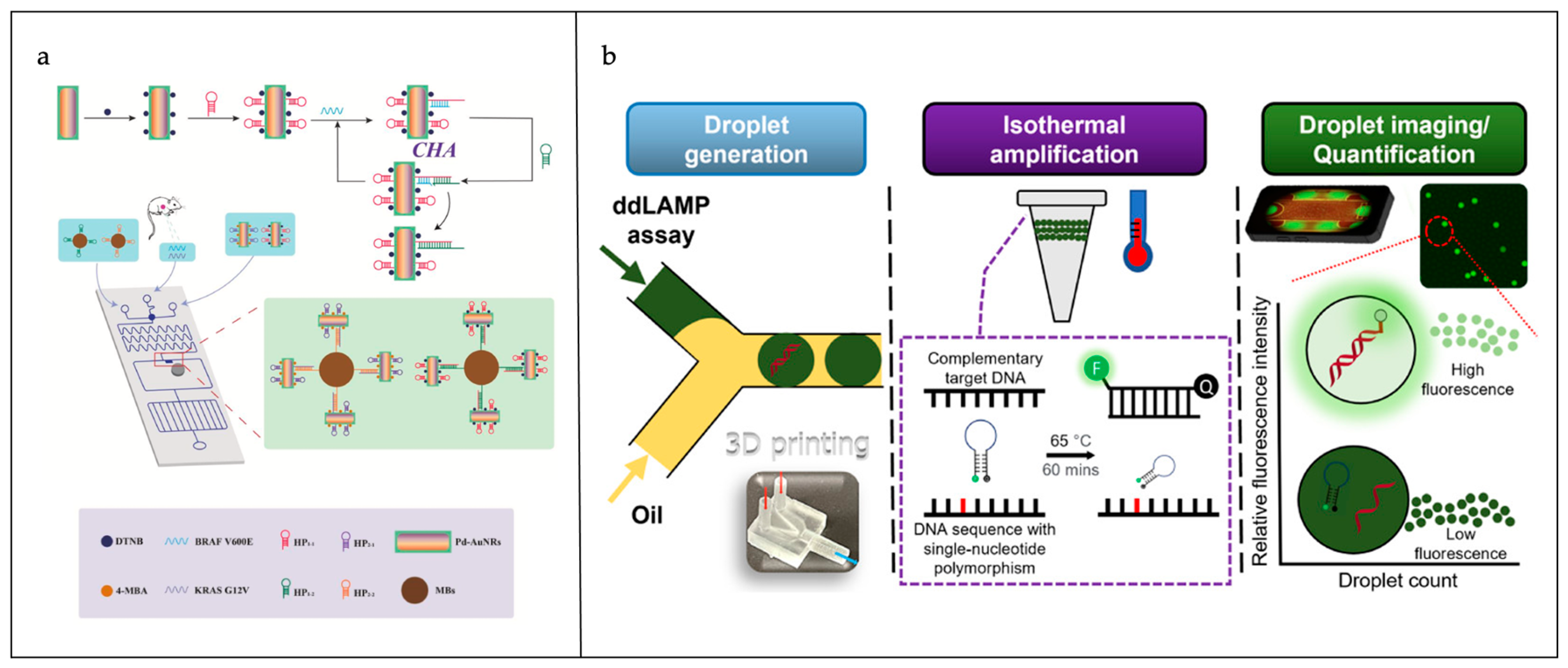
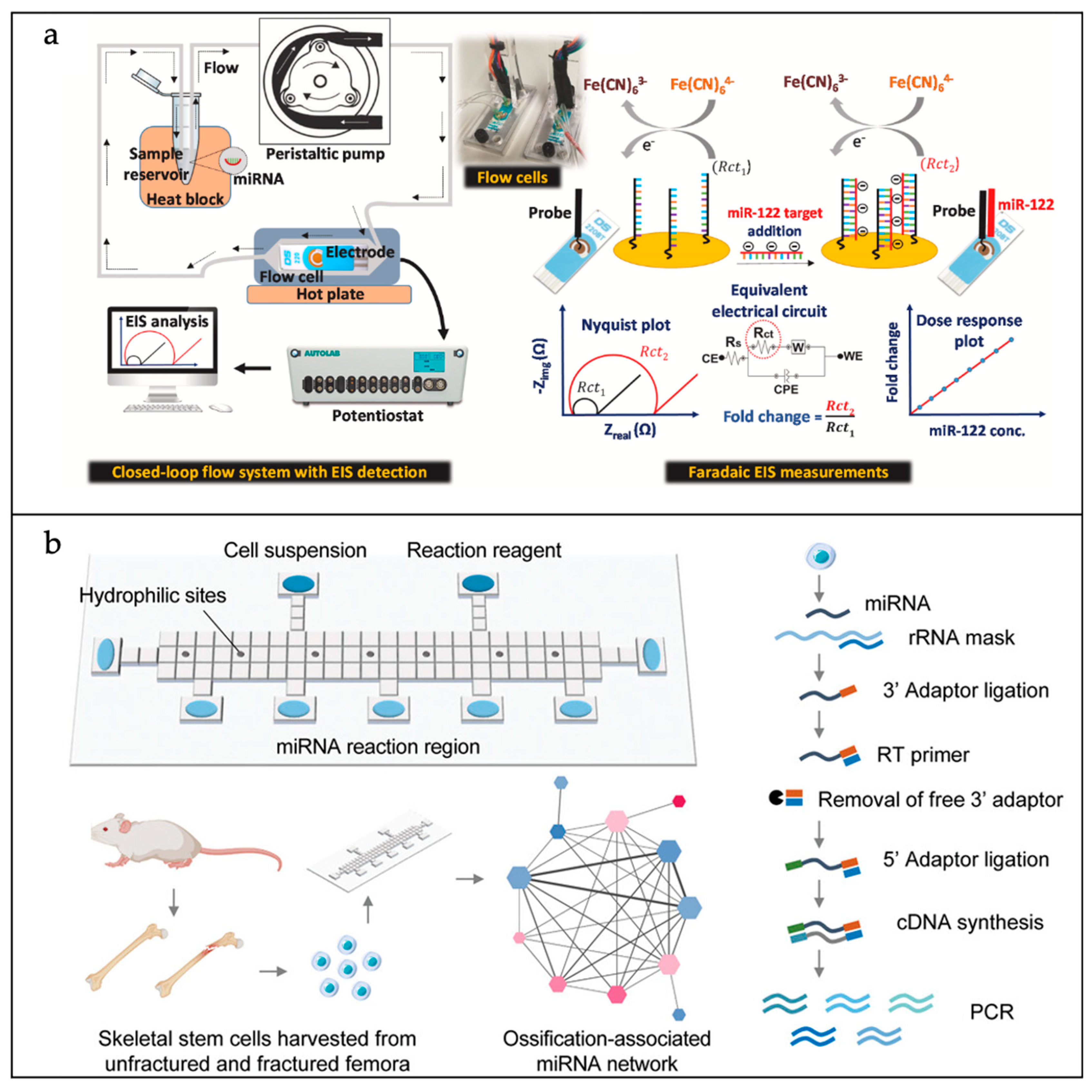
3.1.3. miRNA
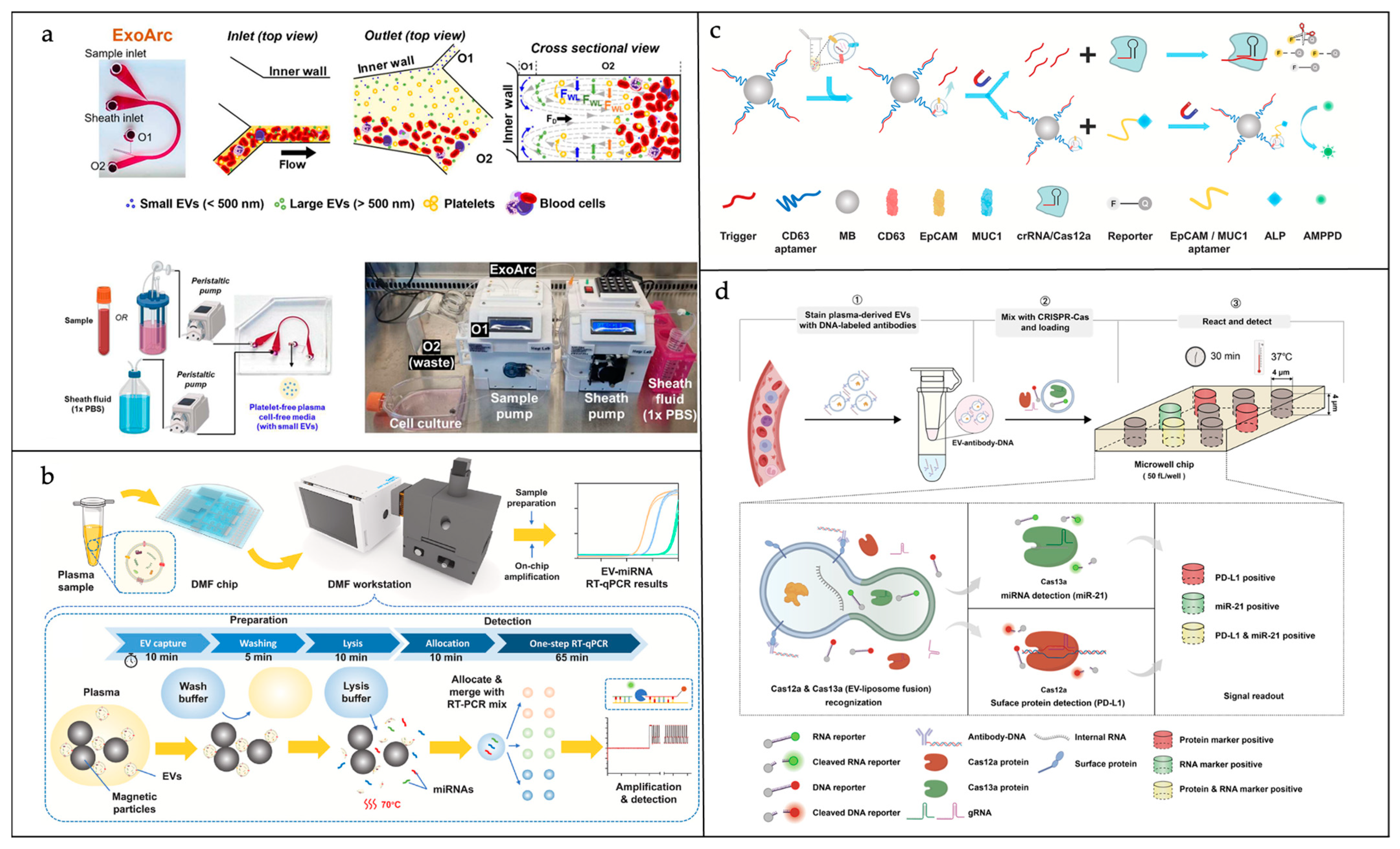
3.1.4. Exosome
3.1.5. Other Biomarkers
3.2. Pathogenic Bacteria Assessment
3.2.1. Pathogenic Bacteria Identification
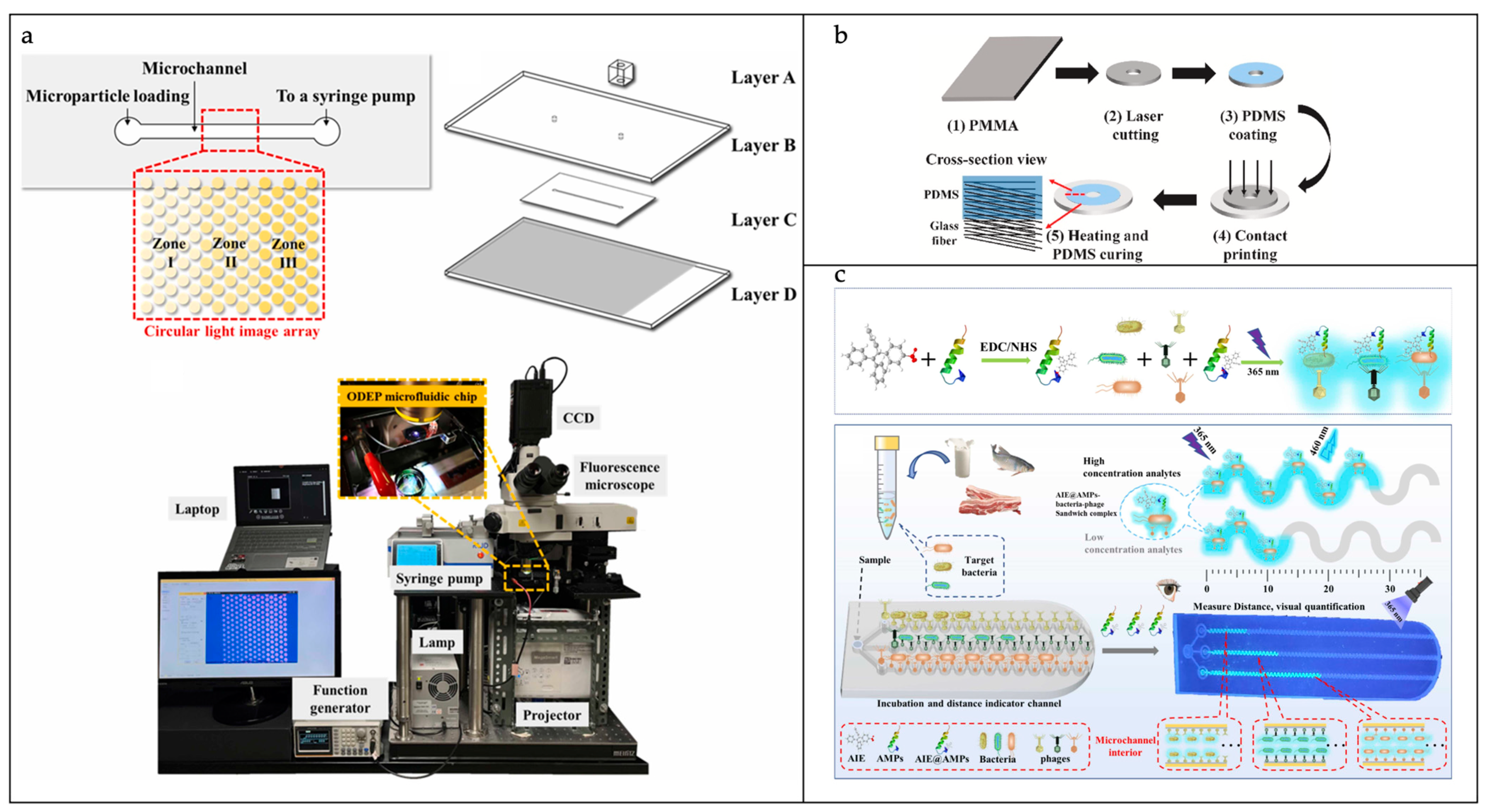
3.2.2. Pathogenic Bacteria Isolation
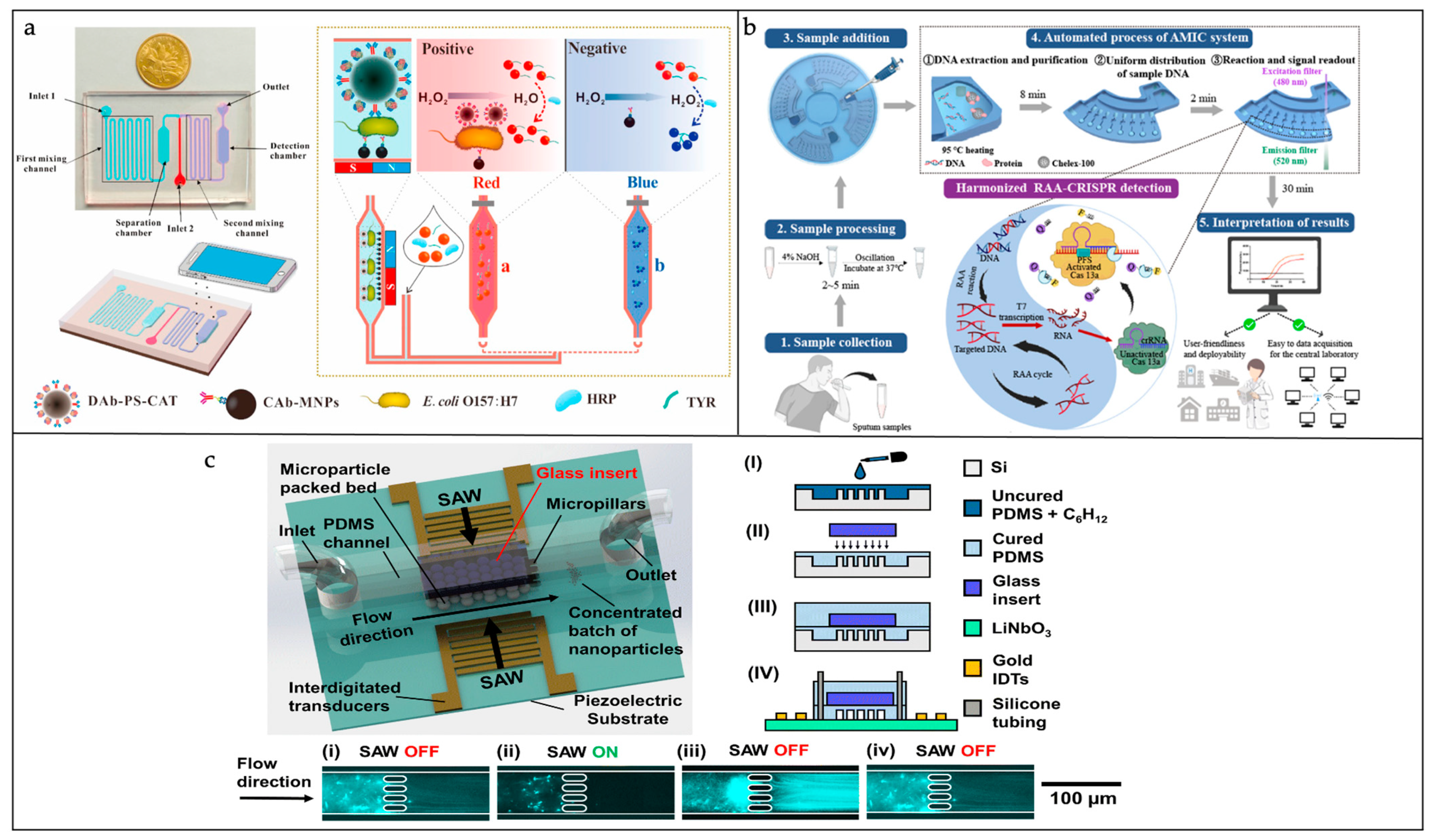
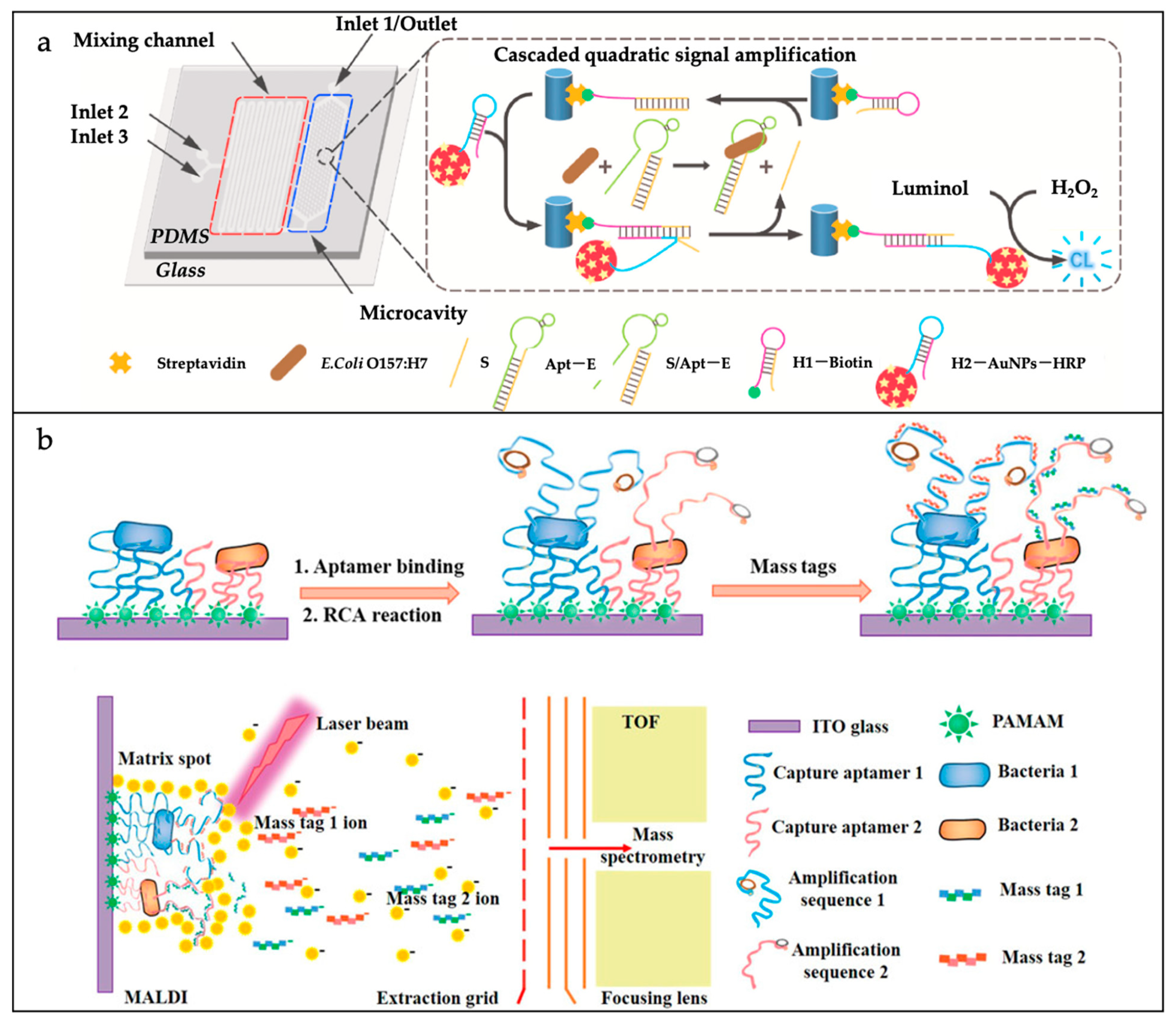
3.2.3. Pathogenic Bacteria Detection
3.3. Point-of-Care Testing (POCT)
3.3.1. Inflammation Biomarker Point-of-Care Testing
3.3.2. Infectious Disease Point-of-Care Testing
3.3.3. Chronic Disease Point-of-Care Testing
4. Summary: Challenges and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rackus, D.G.; Shamsi, M.H.; Wheeler, A.R. Electrochemistry, biosensors and microfluidics: A convergence of fields. Chem. Soc. Rev. 2015, 44, 5320–5340. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Shankar, S.; Marchetti, M.C.; Wu, Y. Viscoelastic control of spatiotemporal order in bacterial active matter. Nature 2021, 590, 80–84. [Google Scholar] [CrossRef]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- Squires, T.M.; Quake, S.R. Microfluidics: Fluid physics at the nanoliter scale. Rev. Mod. Phys. 2005, 77, 977–1026. [Google Scholar] [CrossRef]
- Naresh, V.; Lee, N. A review on biosensors and recent development of nanostructured materials-enabled biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef]
- Jayanthi, V.S.P.K.S.A.; Das, A.B.; Saxena, U. Recent advances in biosensor development for the detection of cancer biomarkers. Biosens. Bioelectron. 2017, 91, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.W.; Shen, Y.; Palomba, S.; Vigolo, D. Nanofluidic lab-on-a-chip systems for biosensing in healthcare. Small 2024, 21, 2407478. [Google Scholar] [CrossRef]
- Dendukuri, D.; Doyle, P.S. The synthesis and assembly of polymeric microparticles using microfluidics. Adv. Mater. 2009, 21, 4071–4086. [Google Scholar] [CrossRef]
- Terry, S.C.; Jerman, J.H.; Angell, J.B. A gas chromatographic air analyzer fabricated on a silicon wafer. IEEE Trans. Electron Devices 1979, 26, 1880–1886. [Google Scholar] [CrossRef]
- Mellors, J.; Gorbounov, V.; Ramsey, R.; Ramsey, J. Fully integrated glass microfluidic device for performing high-efficiency capillary electrophoresis and electrospray ionization mass spectrometry. Anal. Chem. 2008, 80, 6881–6887. [Google Scholar] [CrossRef]
- Powell, L.; Wiederkehr, R.S.; Damascus, P.; Fauvart, M.; Buja, F.; Stakenborg, T.; Ray, S.C.; Fiorini, P.; Osburn, W.O. Rapid and sensitive detection of viral nucleic acids using silicon microchips. Analyst 2018, 143, 2596–2603. [Google Scholar] [PubMed]
- Kim, B.J.; Meng, E. Review of polymer MEMS micromachining. J. Micromech. Microeng. 2015, 26, 013001. [Google Scholar]
- Unger, M.A.; Chou, H.-P.; Thorsen, T.; Scherer, A.; Quake, S.R. Monolithic microfabricated valves and pumps by multilayer soft lithography. Science 2000, 288, 113–116. [Google Scholar]
- Wu, H.; Wheeler, A.; Zare, R.N. Chemical cytometry on a picoliter-scale integrated microfluidic chip. Proc. Natl. Acad. Sci. USA 2004, 101, 12809–12813. [Google Scholar]
- Klank, H.; Kutter, J.P.; Geschke, O. CO2-laser micromachining and back-end processing for rapid production of PMMA-based microfluidic systems. Lab Chip 2002, 2, 242–246. [Google Scholar] [PubMed]
- Becker, H.; Gärtner, C. Polymer microfabrication technologies for microfluidic systems. Anal. Bioanal. Chem. 2008, 390, 89–111. [Google Scholar]
- Ren, K.; Dai, W.; Zhou, J.; Su, J.; Wu, H. Whole-Teflon microfluidic chips. Proc. Natl. Acad. Sci. USA 2011, 108, 8162–8166. [Google Scholar] [PubMed]
- Domachuk, P.; Tsioris, K.; Omenetto, F.G.; Kaplan, D.L. Bio-microfluidics: Biomaterials and biomimetic designs. Adv. Mater. 2010, 22, 249–260. [Google Scholar]
- Martinez, A.W.; Phillips, S.T.; Whitesides, G.M. Three-dimensional microfluidic devices fabricated in layered paper and tape. Proc. Natl. Acad. Sci. USA 2008, 105, 19606–19611. [Google Scholar]
- Gao, B.; Liu, H.; Gu, Z. Bottom-up fabrication of paper-based microchips by blade coating of cellulose microfibers on a patterned surface. Langmuir 2014, 30, 15041–15046. [Google Scholar]
- Bertassoni, L.E.; Cecconi, M.; Manoharan, V.; Nikkhah, M.; Hjortnaes, J.; Cristino, A.L.; Barabaschi, G.; Demarchi, D.; Dokmeci, M.R.; Yang, Y. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab Chip 2014, 14, 2202–2211. [Google Scholar] [PubMed]
- Utada, A.S.; Lorenceau, E.; Link, D.R.; Kaplan, P.D.; Stone, H.A.; Weitz, D. Monodisperse double emulsions generated from a microcapillary device. Science 2005, 308, 537–541. [Google Scholar] [PubMed]
- Lee, T.Y.; Lee, S.; Kim, Y.H.; Kim, D.J.; Amstad, E.; Lee, C.S.; Kim, S.H. Microfluidic fabrication of capsule sensor platform with double-shell structure. Adv. Funct. Mater. 2019, 29, 1902670. [Google Scholar]
- Chu, L.-Y.; Utada, A.S.; Shah, R.K.; Kim, J.-W.; Weitz, D.A. Controllable monodisperse multiple emulsions. Angew. Chem. Int. Ed. 2007, 46, 8970. [Google Scholar]
- Lin, L.; Mawatari, K.; Morikawa, K.; Pihosh, Y.; Yoshizaki, A.; Kitamori, T. Micro/extended-nano sampling interface from a living single cell. Analyst 2017, 142, 1689–1696. [Google Scholar]
- Andersson, J.; Järlebark, J.; Kk, S.; Schaefer, A.; Hailes, R.; Palasingh, C.; Santoso, B.; Vu, V.-T.; Huang, C.-J.; Westerlund, F. Polymer brushes on silica nanostructures prepared by aminopropylsilatrane click chemistry: Superior antifouling and biofunctionality. ACS Appl. Mater. Interfaces 2023, 15, 10228–10239. [Google Scholar]
- Frykholm, K.; Müller, V.; Sriram, K.; Dorfman, K.D.; Westerlund, F. DNA in nanochannels: Theory and applications. Q. Rev. Biophys. 2022, 55, e12. [Google Scholar]
- Roushan, M.; Kaur, P.; Karpusenko, A.; Countryman, P.J.; Ortiz, C.P.; Fang Lim, S.; Wang, H.; Riehn, R. Probing transient protein-mediated DNA linkages using nanoconfinement. Biomicrofluidics 2014, 8, 034113. [Google Scholar]
- Lee, S.; Vörös, J. An aqueous-based surface modification of poly (dimethylsiloxane) with poly (ethylene glycol) to prevent biofouling. Langmuir 2005, 21, 11957–11962. [Google Scholar]
- Fukuda, S.; Xu, Y. A biomimetic anti-biofouling coating in nanofluidic channels. J. Mater. Chem. B 2022, 10, 2481–2489. [Google Scholar]
- Persson, F.; Fritzsche, J.; Mir, K.U.; Modesti, M.; Westerlund, F.; Tegenfeldt, J.O. Lipid-based passivation in nanofluidics. Nano Lett. 2012, 12, 2260–2265. [Google Scholar]
- Shirai, K.; Mawatari, K.; Kitamori, T. Extended nanofluidic immunochemical reaction with femtoliter sample volumes. Small 2014, 10, 1514–1522. [Google Scholar]
- Shirai, K.; Mawatari, K.; Ohta, R.; Shimizu, H.; Kitamori, T. A single-molecule ELISA device utilizing nanofluidics. Analyst 2018, 143, 943–948. [Google Scholar] [PubMed]
- Víšová, I.; Houska, M.; Vaisocherová-Lísalová, H. Biorecognition antifouling coatings in complex biological fluids: A review of functionalization aspects. Analyst 2022, 147, 2597–2614. [Google Scholar] [PubMed]
- Zhang, T.; Cain, A.K.; Semenec, L.; Pereira, J.V.; Hosokawa, Y.; Yalikun, Y.; Li, M. Bacteria separation and enrichment using viscoelastic flows in a straight microchannel. Sens. Actuators B Chem. 2023, 390, 133918. [Google Scholar]
- Zhuang, J.; Zhao, Z.; Lian, K.; Yin, L.; Wang, J.; Man, S.; Liu, G.; Ma, L. SERS-based CRISPR/Cas assay on microfluidic paper analytical devices for supersensitive detection of pathogenic bacteria in foods. Biosens. Bioelectron. 2022, 207, 114167. [Google Scholar]
- Li, P.; Wang, C.; Qiu, J.; Song, F.; Huang, Y.; Zhang, Y.; Zhang, K.; Ji, H.; Sang, Y.; Blaker, J.J.; et al. Inhibitory effect of zinc oxide nanorod arrays on breast cancer cells profiled through real-time cytokines screening by a single-cell microfluidic platform. BMEMat 2023, 1, e12040. [Google Scholar]
- Oudeng, G.; Benz, M.; Popova, A.A.; Zhang, Y.; Yi, C.; Levkin, P.A.; Yang, M. Droplet microarray based on nanosensing probe patterns for simultaneous detection of multiple HIV retroviral nucleic acids. ACS Appl. Mater. Interfaces 2020, 12, 55614–55623. [Google Scholar]
- Wang, K.; Meng, X.; Yan, X.; Fan, K. Nanozyme-based point-of-care testing: Revolutionizing environmental pollutant detection with high efficiency and low cost. Nano Today 2024, 54, 102145. [Google Scholar]
- Miri, A.K.; Nieto, D.; Iglesias, L.; Goodarzi Hosseinabadi, H.; Maharjan, S.; Ruiz-Esparza, G.U.; Khoshakhlagh, P.; Manbachi, A.; Dokmeci, M.R.; Chen, S. Microfluidics-enabled multimaterial maskless stereolithographic bioprinting. Adv. Mater. 2018, 30, 1800242. [Google Scholar]
- Cho, S.K.; Moon, H.; Kim, C.-J. Creating, transporting, cutting, and merging liquid droplets by electrowetting-based actuation for digital microfluidic circuits. J. Microelectromech. Syst. 2003, 12, 70–80. [Google Scholar]
- Choi, K.; Ng, A.H.; Fobel, R.; Wheeler, A.R. Digital microfluidics. Annu. Rev. Anal. Chem. 2012, 5, 413–440. [Google Scholar]
- Mazur, F.; Tjandra, A.D.; Zhou, Y.; Gao, Y.; Chandrawati, R. Paper-based sensors for bacteria detection. Nat. Rev. Bioeng. 2023, 1, 180–192. [Google Scholar]
- Chen, J.L.; Njoku, D.I.; Tang, C.; Gao, Y.; Chen, J.; Peng, Y.K.; Sun, H.; Mao, G.; Pan, M.; Tam, N.F.Y. Advances in microfluidic paper-based analytical devices (µPADs): Design, fabrication, and applications. Small Methods 2024, 8, 2400155. [Google Scholar]
- Emmert-Buck, M.R.; Bonner, R.F.; Smith, P.D.; Chuaqui, R.F.; Zhuang, Z.; Goldstein, S.R.; Weiss, R.A.; Liotta, L.A. Laser capture microdissection. Science 1996, 274, 998–1001. [Google Scholar]
- Herzenberg, L.A.; Parks, D.; Sahaf, B.; Perez, O.; Roederer, M.; Herzenberg, L.A. The history and future of the fluorescence activated cell sorter and flow cytometry: A view from Stanford. Clin. Chem. 2002, 48, 1819–1827. [Google Scholar] [PubMed]
- Fröhlich, J.; König, H. New techniques for isolation of single prokaryotic cells. FEMS Microbiol. Rev. 2000, 24, 567–572. [Google Scholar]
- Seyfoori, A.; Seyyed Ebrahimi, S.A.; Samandari, M.; Samiei, E.; Stefanek, E.; Garnis, C.; Akbari, M. Microfluidic-assisted CTC isolation and in situ monitoring using smart magnetic microgels. Small 2023, 19, 2205320. [Google Scholar]
- Akbarnataj, K.; Maleki, S.; Rezaeian, M.; Haki, M.; Shamloo, A. Novel size-based design of spiral microfluidic devices with elliptic configurations and trapezoidal cross-section for ultra-fast isolation of circulating tumor cells. Talanta 2023, 254, 124125. [Google Scholar]
- Ma, J.; Chen, Y.; Ren, J.; Zhou, T.; Wang, Z.; Li, C.; Qiu, L.; Gao, T.; Ding, P.; Ding, Z. Purification of circulating tumor cells based on multiantibody-modified magnetic nanoparticles and molecular analysis toward epithelial ovarian cancer detection. ACS Sens. 2023, 8, 3744–3753. [Google Scholar]
- Liu, W.; Zhang, R.; Huang, S.; Li, X.; Liu, W.; Zhou, J.; Zhu, L.; Song, Y.; Yang, C. Quantification of intracellular proteins in single cells based on engineered picoliter droplets. Langmuir 2022, 38, 7929–7937. [Google Scholar] [CrossRef]
- Ruan, Q.; Ruan, W.; Lin, X.; Wang, Y.; Zou, F.; Zhou, L.; Zhu, Z.; Yang, C. Digital-WGS: Automated, highly efficient whole-genome sequencing of single cells by digital microfluidics. Sci. Adv. 2020, 6, eabd6454. [Google Scholar] [CrossRef]
- Ruan, Q.; Yang, J.; Zou, F.; Chen, X.; Zhang, Q.; Zhao, K.; Lin, X.; Zeng, X.; Yu, X.; Wu, L. Single-cell digital microfluidic mass spectrometry platform for efficient and multiplex genotyping of circulating tumor cells. Anal. Chem. 2021, 94, 1108–1117. [Google Scholar] [PubMed]
- Strati, A.; Nikolaou, M.; Georgoulias, V.; Lianidou, E. RNA-based CTC analysis provides prognostic information in metastatic breast cancer. Diagnostics 2021, 11, 513. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Q.; Song, J.; Ruan, Q.; Ruan, W.; Chen, Y.; Yang, J.; Zhang, X.; Song, Y.; Zhu, Z. A highly sensitive, accurate, and automated single-cell RNA sequencing platform with digital microfluidics. Anal. Chem. 2020, 92, 8599–8606. [Google Scholar] [CrossRef] [PubMed]
- Khajvand, T.; Huang, P.; Li, L.; Zhang, M.; Zhu, F.; Xu, X.; Huang, M.; Yang, C.; Lu, Y.; Zhu, Z. Interfacing droplet microfluidics with antibody barcodes for multiplexed single-cell protein secretion profiling. Lab Chip 2021, 21, 4823–4830. [Google Scholar] [CrossRef]
- Tewhey, R.; Warner, J.B.; Nakano, M.; Libby, B.; Medkova, M.; David, P.H.; Kotsopoulos, S.K.; Samuels, M.L.; Hutchison, J.B.; Larson, J.W.; et al. Microdroplet-based PCR enrichment for large-scale targeted sequencing. Nat. Biotechnol. 2009, 27, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Ou, C.-Y.; Vu, T.; Grunwald, J.T.; Toledano, M.; Zimak, J.; Toosky, M.; Shen, B.; Zell, J.A.; Gratton, E.; Abram, T.J. An ultrasensitive test for profiling circulating tumor DNA using integrated comprehensive droplet digital detection. Lab Chip 2019, 19, 993–1005. [Google Scholar] [CrossRef]
- Cao, X.; Ge, S.; Zhou, X.; Mao, Y.; Sun, Y.; Lu, W.; Ran, M. A dual-signal amplification strategy based on pump-free SERS microfluidic chip for rapid and ultrasensitive detection of non-small cell lung cancer-related circulating tumour DNA in mice serum. Biosens. Bioelectron. 2022, 205, 114110. [Google Scholar] [CrossRef]
- Cao, X.; Ge, S.; Hua, W.; Zhou, X.; Lu, W.; Gu, Y.; Li, Z.; Qian, Y. A pump-free and high-throughput microfluidic chip for highly sensitive SERS assay of gastric cancer-related circulating tumor DNA via a cascade signal amplification strategy. J. Nanobiotechnol. 2022, 20, 271. [Google Scholar] [CrossRef]
- Hsieh, S.-A.; Shamsaei, D.; Eitzmann, D.R.; Anderson, J.L. Digital droplet loop-mediated isothermal amplification featuring a molecular beacon assay, 3D printed droplet generation, and smartphone imaging for sequence-specific DNA detection. Anal. Chem. 2022, 94, 11949–11956. [Google Scholar] [PubMed]
- Reyes, J.C.B.; Solon, J.A.A.; Rivera, W.L. Development of a loop-mediated isothermal amplification assay for detection of Trichomonas vaginalis. Diagn. Microbiol. Infect. Dis. 2014, 79, 337–341. [Google Scholar] [PubMed]
- Hwang, H.; Mendell, J. MicroRNAs in cell proliferation, cell death, and tumorigenesis. Br. J. Cancer 2007, 96, R40–R44. [Google Scholar]
- Chen, Z.-P.; Yang, P.; Yang, Z.-Z.; Chai, Y.-Q.; Yuan, R.; Zhuo, Y.; Liang, W.-B. One-step digital droplet auto-catalytic nucleic acid amplification with high-throughput fluorescence imaging and droplet tracking computation. Anal. Chem. 2022, 94, 9166–9175. [Google Scholar]
- Roychoudhury, A.; Dear, J.W.; Kersaudy-Kerhoas, M.; Bachmann, T.T. Amplification-free electrochemical biosensor detection of circulating microRNA to identify drug-induced liver injury. Biosens. Bioelectron. 2023, 231, 115298. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Na, X.; Zhang, Y.; Li, Z.; Chen, X.; Cai, L.; Song, J.; Xu, R.; Yang, C. Highly multiplexed, efficient, and automated single-cell microRNA sequencing with digital microfluidics. Small Methods 2024, 8, 2301250. [Google Scholar]
- Chen, S.; Zhao, J.; Sakharov, I.Y.; Xu, J.; Xu, C.; Zhao, S. An ultrasensitive multivariate signal amplification strategy based on microchip platform tailored for simultaneous quantification of multiple microRNAs in single cell. Biosens. Bioelectron. 2022, 203, 114053. [Google Scholar]
- Shi, J.; Zhang, Y.; Fan, Y.; Liu, Y.; Yang, M. Recent advances in droplet-based microfluidics in liquid biopsy for cancer diagnosis. Droplet 2024, 3, e92. [Google Scholar]
- Leong, S.Y.; Lok, W.W.; Goh, K.Y.; Ong, H.B.; Tay, H.M.; Su, C.; Kong, F.; Upadya, M.; Wang, W.; Radnaa, E. High-throughput microfluidic extraction of platelet-free plasma for microRNA and extracellular vesicle analysis. ACS Nano 2024, 18, 6623–6637. [Google Scholar]
- Tong, Z.; Xu, X.; Shen, C.; Yang, D.; Li, Y.; Li, Q.; Yang, W.; Xu, F.; Wu, Z.; Zhou, L. All-in-one multiple extracellular vesicle miRNA detection on a miniaturized digital microfluidic workstation. Biosens. Bioelectron. 2025, 270, 116976. [Google Scholar]
- Guan, X.; Zhao, J.; Sha, Z.; Liang, Y.; Huang, J.; Zhang, J.; Sun, S. CRISPR/Cas12a and aptamer-chemiluminescence based analysis for the relative abundance determination of tumor-related protein positive exosomes for breast cancer diagnosis. Biosens. Bioelectron. 2024, 259, 116380. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Y.; Liu, J.; Wei, S.; Li, N.; Yao, X.; Wang, M.; Su, X.; Jing, G.; Xu, J.; et al. Concurrent detection of protein and miRNA at the single extracellular vesicle level using a digital dual CRISPR-Cas assay. ACS Nano 2024, 19, 1271–1285. [Google Scholar] [CrossRef]
- Cui, B.; Liu, C.; Yao, S. One-step RT-PCR for detection of micrornas in exosomes using droplet microfluidics. In Proceedings of the 2020 IEEE 33rd International Conference on Micro Electro Mechanical Systems (MEMS), Vancouver, BC, Canada, 18–22 January 2020; pp. 142–146. [Google Scholar]
- Zhang, L.; Li, W.; Parvin, R.; Wang, X.; Fan, Q.; Ye, F. Screening prostate cancer cell-derived exosomal microRNA expression with photothermal-driven digital PCR. Adv. Funct. Mater. 2022, 32, 2207879. [Google Scholar]
- Lin, B.; Tian, T.; Lu, Y.; Liu, D.; Huang, M.; Zhu, L.; Zhu, Z.; Song, Y.; Yang, C. Tracing tumor-derived exosomal PD-L1 by dual-aptamer activated proximity-induced droplet digital PCR. Angew. Chem. Int. Ed. 2021, 60, 7582–7586. [Google Scholar]
- Zhao, J.; Guan, X.; Zhang, S.; Sha, Z.; Sun, S. Weak value amplification-based biochip for highly sensitive detection and identification of breast cancer exosomes. Biosensors 2024, 14, 198. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Lin, L.; Lin, S.; Wang, X.; Chen, Y.; Zhu, H.; Zhu, Z.; Yang, L.; Xu, X.; Yang, C. Highly Multiplexing, Throughput and Efficient Single-Cell Protein Analysis with Digital Microfluidics. Small Methods 2024, 8, 2400375. [Google Scholar]
- Hou, Y.; Lin, J.; Yao, H.; Wu, Z.; Lin, Y.; Lin, J.-M. Linking Metastatic Behavior and Metabolic Heterogeneity of Circulating Tumor Cells at Single-Cell Level Using an Integrative Microfluidic System. Adv. Sci. 2025, 2413978. [Google Scholar] [CrossRef]
- Abafogi, A.T.; Kim, J.; Lee, J.; Mohammed, M.O.; van Noort, D.; Park, S. 3D-printed modular microfluidic device enabling preconcentrating bacteria and purifying bacterial DNA in blood for improving the sensitivity of molecular diagnostics. Sensors 2020, 20, 1202. [Google Scholar] [CrossRef]
- Kim, H.-J.; Choi, S.-J. Rapid single-cell detection of pathogenic bacteria for in situ determination of food safety. Anal. Methods 2020, 12, 5621–5627. [Google Scholar]
- Yang, C.-M.; Chu, P.-Y.; Wu, A.-Y.; Hsieh, P.-H.; Hsieh, C.-H.; Wu, M.-H. Development of an optically induced dielectrophoresis (ODEP) microfluidic system with a virtual gel filtration chromatography (GFC)-inspired mechanism for the high-performance sorting and separation of microparticles based on their size differences. Sens. Actuators B Chem. 2023, 395, 134443. [Google Scholar] [CrossRef]
- Xu, H.; Mao, X.; Zeng, Q.; Wang, S.; Kawde, A.-N.; Liu, G. Aptamer-functionalized gold nanoparticles as probes in a dry-reagent strip biosensor for protein analysis. Anal. Chem. 2009, 81, 669–675. [Google Scholar]
- Li, S.; Jiang, Y.; Yang, X.; Lin, M.; Dan, H.; Zou, S.; Cao, X. In situ rolling circle amplification surface modifications to improve E. coli O157: H7 capturing performances for rapid and sensitive microfluidic detection applications. Anal. Chim. Acta 2021, 1150, 338229. [Google Scholar]
- Zhu, Z.; Lv, Z.; Wang, L.; Tan, H.; Xu, Y.; Li, S.; Chen, L. A pump-free paper/PDMS hybrid microfluidic chip for bacteria enrichment and fast detection. Talanta 2024, 275, 126155. [Google Scholar]
- Liu, F.; Huang, Y.; Xu, J.; Wu, H.; Li, T.; Yu, Z.; Huang, S.; Gan, N. A multi-channel microfluidic chip based on fluorescent distance readout-mode for rapid, simultaneous and visual detection of multiplex pathogens using phage-AIEgen-antimicrobial peptide-encoded tags. Sens. Actuators B Chem. 2025, 424, 136867. [Google Scholar]
- Kwiatek, M.; Parasion, S.; Nakonieczna, A. Therapeutic bacteriophages as a rescue treatment for drug-resistant infections—An in vivo studies overview. J. Appl. Microbiol. 2020, 128, 985–1002. [Google Scholar]
- Kim, K.H.; Park, S.J.; Park, C.S.; Seo, S.E.; Lee, J.; Kim, J.; Lee, S.H.; Lee, S.; Kim, J.-S.; Ryu, C.-M. High-performance portable graphene field-effect transistor device for detecting Gram-positive and-negative bacteria. Biosens. Bioelectron. 2020, 167, 112514. [Google Scholar]
- Qiao, Z.; Fu, Y.; Lei, C.; Li, Y. Advances in antimicrobial peptides-based biosensing methods for detection of foodborne pathogens: A review. Food Control 2020, 112, 107116. [Google Scholar]
- Ma, X.; Ding, W.; Wang, C.; Wu, H.; Tian, X.; Lyu, M.; Wang, S. DNAzyme biosensors for the detection of pathogenic bacteria. Sens. Actuators B Chem. 2021, 331, 129422. [Google Scholar]
- Mi, F.; Guan, M.; Hu, C.; Peng, F.; Sun, S.; Wang, X. Application of lectin-based biosensor technology in the detection of foodborne pathogenic bacteria: A review. Analyst 2021, 146, 429–443. [Google Scholar]
- Cai, G.; Zheng, L.; Liao, M.; Li, Y.; Wang, M.; Liu, N.; Lin, J. A microfluidic immunosensor for visual detection of foodborne bacteria using immunomagnetic separation, enzymatic catalysis and distance indication. Microchim. Acta 2019, 186, 757. [Google Scholar]
- Kwon, K.; Gwak, H.; Hyun, K.-A.; Kwak, B.-S.; Jung, H.-I. High-throughput microfluidic chip for magnetic enrichment and photothermal DNA extraction of foodborne bacteria. Sens. Actuators B Chem. 2019, 294, 62–68. [Google Scholar]
- Hou, Y.; Cai, G.; Zheng, L.; Lin, J. A microfluidic signal-off biosensor for rapid and sensitive detection of Salmonella using magnetic separation and enzymatic catalysis. Food Control 2019, 103, 186–193. [Google Scholar]
- Zheng, L.; Cai, G.; Wang, S.; Liao, M.; Li, Y.; Lin, J. A microfluidic colorimetric biosensor for rapid detection of Escherichia coli O157: H7 using gold nanoparticle aggregation and smart phone imaging. Biosens. Bioelectron. 2019, 124, 143–149. [Google Scholar]
- Wang, L.; Qi, W.; Wang, M.; Jiang, F.; Ding, Y.; Xi, X.; Liao, M.; Li, Y.; Lin, J. A pipette-adapted biosensor for Salmonella detection. Biosens. Bioelectron. 2022, 218, 114765. [Google Scholar]
- Jin, N.; Xue, L.; Ding, Y.; Liu, Y.; Jiang, F.; Liao, M.; Li, Y.; Lin, J. A microfluidic biosensor based on finger-driven mixing and nuclear track membrane filtration for fast and sensitive detection of Salmonella. Biosens. Bioelectron. 2023, 220, 114844. [Google Scholar]
- Lu, X.; Chow, J.J.M.; Koo, S.H.; Tan, T.Y.; Jiang, B.; Ai, Y. Enhanced molecular diagnosis of bloodstream candida infection with size-based inertial sorting at submicron resolution. Anal. Chem. 2020, 92, 15579–15586. [Google Scholar]
- Xiang, X.; Ren, X.; Wen, Q.; Xing, G.; Liu, Y.; Xu, X.; Wei, Y.; Ji, Y.; Liu, T.; Song, H. Automatic microfluidic harmonized RAA-CRISPR diagnostic system for rapid and accurate identification of bacterial respiratory tract infections. Anal. Chem. 2024, 96, 6282–6291. [Google Scholar]
- Ang, B.; Sookram, A.; Devendran, C.; He, V.; Tuck, K.; Cadarso, V.; Neild, A. Glass-embedded PDMS microfluidic device for enhanced concentration of nanoparticles using an ultrasonic nanosieve. Lab Chip 2023, 23, 525–533. [Google Scholar]
- Wang, S.; Duan, H.; Lin, J. A centrifugal microfluidic platform for rapid detection of pathogens based on chitosan nucleic acid extraction and RAA-T7-CRISPR/Cas13a nucleic acid detection. Sens. Actuators B Chem. 2023, 393, 134223. [Google Scholar]
- Xiao, Y.; Zhou, M.; Liu, C.; Gao, S.; Wan, C.; Li, S.; Dai, C.; Du, W.; Feng, X.; Li, Y. Fully integrated and automated centrifugal microfluidic chip for point-of-care multiplexed molecular diagnostics. Biosens. Bioelectron. 2024, 255, 116240. [Google Scholar]
- Ahn, H.; Batule, B.S.; Seok, Y.; Kim, M.-G. Single-step recombinase polymerase amplification assay based on a paper chip for simultaneous detection of multiple foodborne pathogens. Anal. Chem. 2018, 90, 10211–10216. [Google Scholar] [CrossRef]
- Sun, D.; Fan, T.; Liu, F.; Wang, F.; Gao, D.; Lin, J.-M. A microfluidic chemiluminescence biosensor based on multiple signal amplification for rapid and sensitive detection of E. coli O157:H7. Biosens. Bioelectron. 2022, 212, 114390. [Google Scholar] [CrossRef]
- Li, N.; Zhang, W.; Lin, J.; Xing, G.; Li, H.; Lin, J.-M. A specific mass-tag approach for detection of foodborne pathogens using MALDI-TOF mass spectrometry. Anal. Chem. 2022, 94, 3963–3969. [Google Scholar] [CrossRef]
- Wang, C.-H.; Wu, J.-J.; Lee, G.-B. Screening of highly-specific aptamers and their applications in paper-based microfluidic chips for rapid diagnosis of multiple bacteria. Sens. Actuators B Chem. 2019, 284, 395–402. [Google Scholar] [CrossRef]
- Li, T.; Ou, G.; Chen, X.; Li, Z.; Hu, R.; Li, Y.; Yang, Y.; Liu, M. Naked-eye based point-of-care detection of E. coli O157: H7 by a signal-amplified microfluidic aptasensor. Anal. Chim. Acta 2020, 1130, 20–28. [Google Scholar] [CrossRef]
- Trinh, T.N.D.; La, H.C.; Lee, N.Y. Fully integrated and foldable microdevice encapsulated with agarose for long-term storage potential for point-of-care testing of multiplex foodborne pathogens. ACS Sens. 2019, 4, 2754–2762. [Google Scholar] [CrossRef]
- Witkowska, E.; Łasica, A.M.; Niciński, K.; Potempa, J.; Kamińska, A. In search of spectroscopic signatures of Periodontitis: A SERS-based magnetomicrofluidic sensor for detection of Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans. ACS Sens. 2021, 6, 1621–1635. [Google Scholar] [CrossRef]
- Jiang, J.; Wu, H.; Su, Y.; Liang, Y.; Shu, B.; Zhang, C. Electrochemical cloth-based DNA sensors (ECDSs): A new class of electrochemical gene sensors. Anal. Chem. 2020, 92, 7708–7716. [Google Scholar] [CrossRef]
- Hou, Y.; Tang, W.; Qi, W.; Guo, X.; Lin, J. An ultrasensitive biosensor for fast detection of Salmonella using 3D magnetic grid separation and urease catalysis. Biosens. Bioelectron. 2020, 157, 112160. [Google Scholar] [CrossRef]
- Li, Y.; Wang, T.; Wu, J. Capture and detection of urine bacteria using a microchannel silicon nanowire microfluidic chip coupled with MALDI-TOF MS. Analyst 2021, 146, 1151–1156. [Google Scholar] [CrossRef]
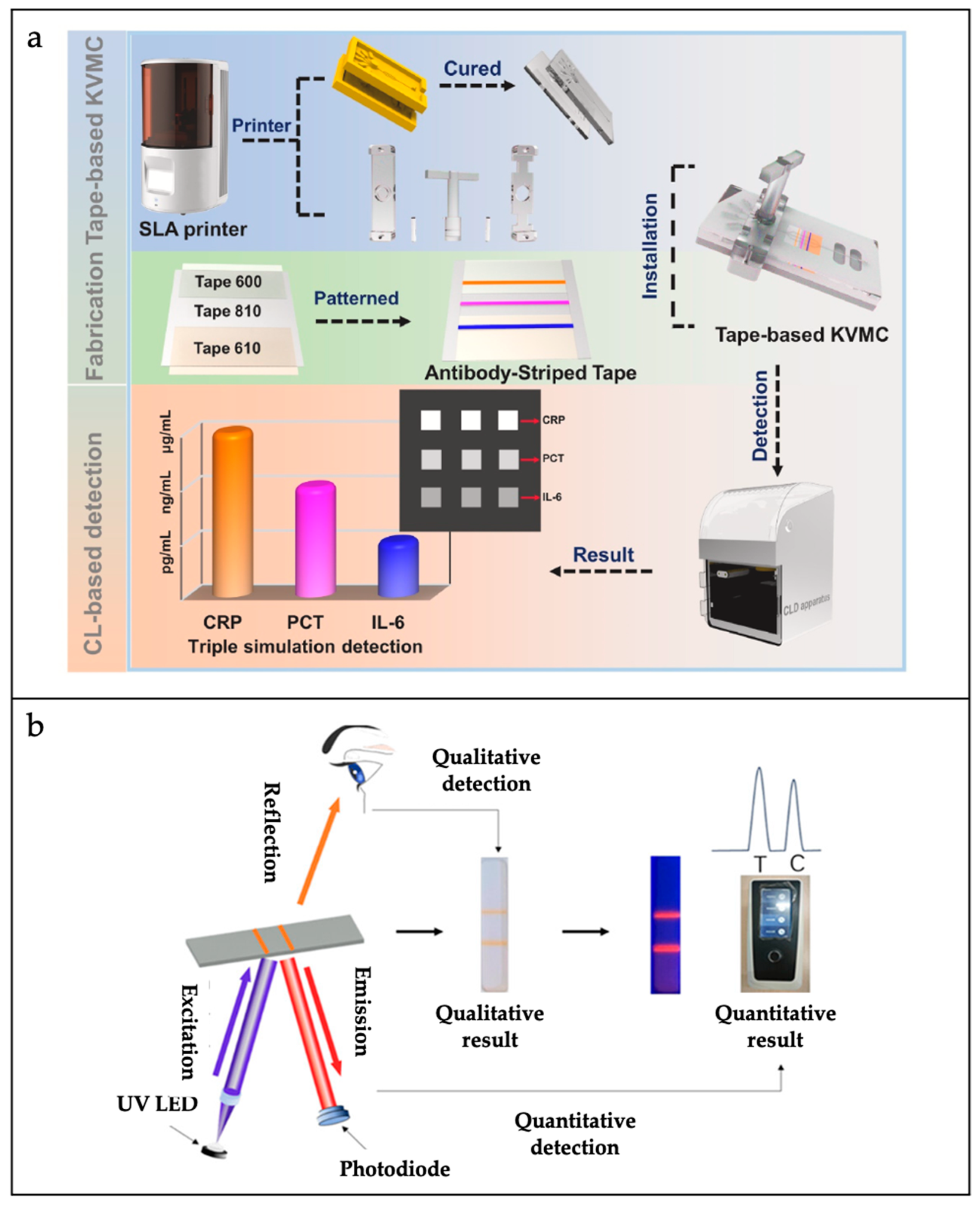
- Yin, B.; Qian, C.; Wan, X.; Muhtasim Fuad Sohan, A.S.M.; Lin, X. Tape integrated self-designed microfluidic chip for point-of-care immunoassays simultaneous detection of disease biomarkers with tunable detection range. Biosens. Bioelectron. 2022, 212, 114429. [Google Scholar]
- Fan, L.; Yan, W.; Chen, Q.; Tan, F.; Tang, Y.; Han, H.; Yu, R.; Xie, N.; Gao, S.; Chen, W.; et al. One-component dual-readout aggregation-induced emission nanobeads for qualitative and quantitative detection of C-reactive protein at the point of care. Anal. Chem. 2024, 96, 401–408. [Google Scholar]
- Boonkaew, S.; Szot-Karpińska, K.; Niedziółka-Jönsson, J.; de Marco, A.; Jönsson-Niedziółka, M. NFC smartphone-based electrochemical microfluidic device integrated with nanobody recognition for C-reactive protein. ACS Sens. 2024, 9, 3066–3074. [Google Scholar]
- Yang, N.; Peng, J.; Wu, L.; Han, X.; Shaheen, N.; Zou, X. Hand-held zoom micro-imaging system based on microfluidic chip for point-of-care testing (POCT) of vaginal inflammation. IEEE J. Transl. Eng. Health Med. 2021, 9, 2800109. [Google Scholar]
- Tu, J.; Min, J.; Song, Y.; Xu, C.; Li, J.; Moore, J.; Hanson, J.; Hu, E.; Parimon, T.; Wang, T.-Y.; et al. A wireless patch for the monitoring of C-reactive protein in sweat. Nat. Biomed. Eng. 2023, 7, 1293–1306. [Google Scholar]
- Yin, J.; Li, M.; Yan, H.; Zhou, S.; Xia, Z. Laboratory diagnosis for malaria in the elimination phase in China: Efforts and challenges. Front. Med. 2022, 16, 10–16. [Google Scholar] [CrossRef]
- Malpartida-Cardenas, K.; Miscourides, N.; Rodriguez-Manzano, J.; Yu, L.-S.; Moser, N.; Baum, J.; Georgiou, P. Quantitative and rapid Plasmodium falciparum malaria diagnosis and artemisinin-resistance detection using a CMOS Lab-on-Chip platform. Biosens. Bioelectron. 2019, 145, 111678. [Google Scholar]
- Malpartida-Cardenas, K.; Moser, N.; Ansah, F.; Pennisi, I.; Ahu Prah, D.; Amoah, L.E.; Awandare, G.; Hafalla, J.C.R.; Cunnington, A.; Baum, J. Sensitive detection of asymptomatic and symptomatic malaria with seven novel parasite-specific LAMP assays and translation for use at point-of-care. Microbiol. Spectr. 2023, 11, e05222. [Google Scholar]
- Shang, Y.; Xing, G.; Lin, H.; Sun, Y.; Chen, S.; Lin, J.-M. Development of nucleic acid extraction and real-time recombinase polymerase amplification (RPA) assay integrated microfluidic biosensor for multiplex detection of foodborne bacteria. Food Control 2024, 155, 110047. [Google Scholar] [CrossRef]
- Ye, X.; Fan, L.; Zhang, L.; Wang, D.; Ma, Y.; Kong, J.; Fang, W.; Hu, J.; Wang, X. Rapid and simultaneous detection of common childhood diarrhea viruses by microfluidic-FEN1-assisted isothermal amplification with ultra-high specificity and sensitivity. Biosens. Bioelectron. 2024, 264, 116677. [Google Scholar]
- Dai, C.; Xiong, H.; He, R.; Zhu, C.; Li, P.; Guo, M.; Gou, J.; Mei, M.; Kong, D.; Li, Q.; et al. Electro-optical multiclassification platform for minimizing occasional inaccuracy in point-of-care biomarker detection. Adv. Mater. 2024, 36, 2312540. [Google Scholar]
- Li, S.; Zhang, Y.; Liu, J.; Wang, X.; Qian, C.; Wang, J.; Wu, L.; Dai, C.; Yuan, H.; Wan, C.; et al. Fully integrated and high-throughput microfluidic system for multiplexed point-of-care testing. Small 2024, 20, 2401848. [Google Scholar]
- Liu, K.-Z.; Tian, G.; Ko, A.C.-T.; Geissler, M.; Brassard, D.; Veres, T. Detection of renal biomarkers in chronic kidney disease using microfluidics: Progress, challenges and opportunities. Biomed. Microdevices 2020, 22, 29. [Google Scholar]
- Jiao, D.; Zhang, R.; Zhang, H.; Ma, H.; Zhang, X.; Fan, X.; Chang, H. Rapid detection of glycosylated hemoglobin levels by a microchip liquid chromatography system in gradient elution mode. Anal. Chim. Acta 2024, 1288, 342186. [Google Scholar]
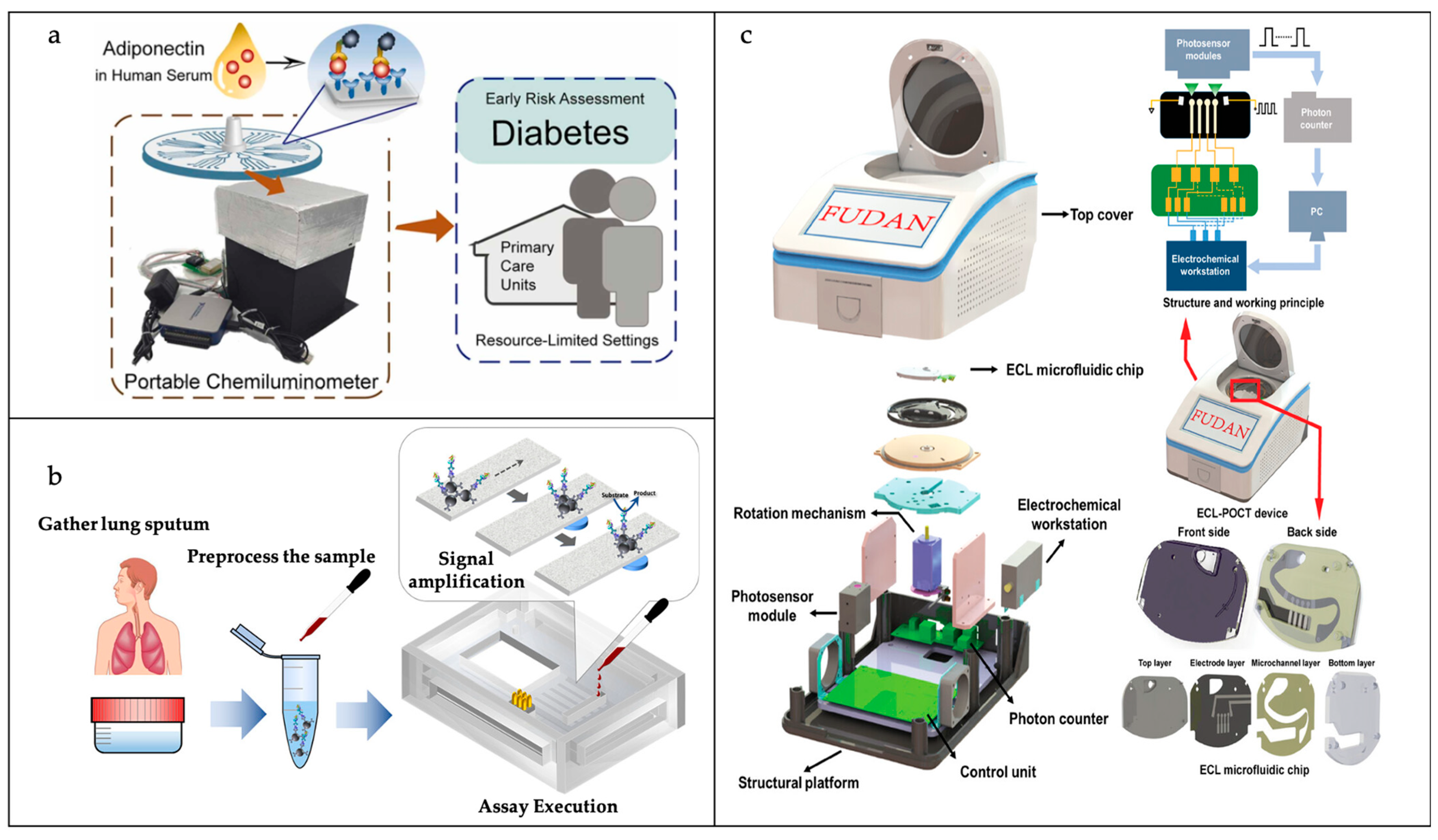
- Li, F.; Wang, W.; Li, H.; Yang, M.; Wu, J.; Zhang, L.; Gao, J.; Pu, Q. A low-cost portable microfluidic chemiluminometer for point-of-care testing of adiponectin for early risk assessment of diabetes. Sens. Actuators B Chem. 2023, 380, 133384. [Google Scholar]
- Gutiérrez-Capitán, M.; Sanchís, A.; Carvalho, E.O.; Baldi, A.; Vilaplana, L.; Cardoso, V.F.; Calleja, Á.; Wei, M.; de la Rica, R.; Hoyo, J.; et al. Engineering a point-of-care paper-microfluidic electrochemical device applied to the multiplexed quantitative detection of biomarkers in sputum. ACS Sens. 2023, 8, 3032–3042. [Google Scholar]
- Xiong, H.; Zhu, C.; Dai, C.; Ye, X.; Li, Y.; Li, P.; Yang, S.; Ashraf, G.; Wei, D.; Chen, H. An alternating current electroosmotic flow-based ultrasensitive electrochemiluminescence microfluidic system for ultrafast monitoring, detection of proteins/miRNAs in unprocessed samples. Adv. Sci. 2024, 11, 2307840. [Google Scholar]
- Pająk, A.; Jankowski, P.; Zdrojewski, T. The burden of cardiovascular disease risk factors: A current problem. Pol. Heart J. 2022, 80, 5–15. [Google Scholar]
- Yu, Z.; Gong, H.; Xue, F.; Zeng, Y.; Liu, X.; Tang, D. Flexible and high-throughput photothermal biosensors for rapid screening of acute myocardial infarction using thermochromic paper-based image analysis. Anal. Chem. 2022, 94, 13233–13242. [Google Scholar]
- Zhou, Y.; Cui, A.; Xiang, D.; Luan, Y.; Wang, Q.; Huang, J.; Liu, J.; Yang, X.; Wang, K. Point-of-care testing of four chronic disease biomarkers in blood based on a low cost and low system complexity microfluidic chip with integrated oxygen-sensitive membrane. Sens. Actuators B Chem. 2024, 398, 134734. [Google Scholar]
- Yang, S.; Zhu, J.; Zhao, L.; Yang, L.; Fa, H.; Wang, Y.; Huo, D.; Hou, C.; Zhong, D.; Yang, M. MBene nanosheets with DNA adsorbability for circulating tumor DNA assay via fluorescence biosensing and paper-based microfluidic POCT. Adv. Funct. Mater. 2024, 35, 2415074. [Google Scholar]


| Materials | Advantages | Disadvantages | |
|---|---|---|---|
| Silicon/Glass-based | High resistance, thermal conductivity, transparency, insulation. Good for electrophoresis, reactions, and cell culture. | High cost. Complex fabrication. Fragile. Limited to specific environments. | |
| Polymer-based | Thermoplastics | Ease of processing and prototyping. Recyclable. Wide range of mechanical properties. | Lower thermal stability. May deform under high temperatures. Potential for leaching of additives. |
| Thermosets | High thermal stability. Good chemical resistance. Excellent mechanical properties. | Cannot be remolded or recycled. Lengthy curing process. Potential for shrinkage during curing. | |
| Elastomers | Biocompatibility. Flexibility. Excellent for soft lithography. | Permeability to certain gasses and solvents. Lower mechanical strength. Potential for swelling in certain solvents. | |
| Hydrogel | Promotes cell adhesion. Suitable for cell culture applications. | Limited strength. Susceptible to degradation. | |
| Paper-based | Low cost. Ease of use. Suitable for point-of-care applications. | Limited sensitivity. Susceptible to environmental factors like humidity and evaporation. | |
| Capillary assembly | 3D flow paths. Minimal fluid–wall interaction. Simple assembly. | Manual assembly. Slower production speed. Less efficient for large-scale production. | |
| Elements | Content Description |
|---|---|
| Detection targets | Circulating tumor cells (CTCs). Circulating tumor DNA (ctDNA). MicroRNA (miRNA). Exosomes. Other biomarkers (e.g., proteins and metabolites). |
| Purpose | Early diagnosis. Accurate treatment. Prognostic assessment. |
| Microfluidics applications | The efficient processing and analysis of trace biological samples. Precise isolation, enrichment and detection of cancer-related molecules. |
| Technical advantages | Enhanced detection sensitivity and specificity. Automated and integrated processes. Reduced sample consumption. |
| Future directions | Developing novel tumor biomarker recognition elements and innovative signal transduction mechanisms. Enhancing precision in single-cell analysis to more thoroughly resolve tumor cell heterogeneity. Integrating microfluidic biosensors with a range of assays, including genomics, transcriptomics, proteomics, and metabolomics, for comprehensive multimodal analysis of liquid biopsies. Advancing the clinical utility of microfluidic biosensors in tumor liquid biopsy, which includes the creation of universally accessible liquid biopsy devices and assays, as well as the establishment of comprehensive clinical application guidelines. |
| Elements | Content Description |
|---|---|
| Key Technical Features | Rapid identification. High sensitivity. Elevated specificity. |
| Technology Used | Antibody–antigen interaction. Nucleic acid aptamer binding. Phage biorecognition. Antibiotic/antimicrobial peptide recognition strategies. |
| Technical Advantages | Higher selectivity and accuracy than traditional methods. Allow specific identification in complex samples. Efficient purification and detection of live pathogenic bacteria. |
| Future Directions | Exploring more sensitive and specific recognition elements, such as novel antibodies, nucleic acid aptamers, phages, etc., to enhance the accuracy and reliability of pathogenic bacteria detection. Optimizing microfluidic chip design to enhance pathogen isolation efficiency and purity, yielding high-quality samples for subsequent testing and analysis. Developing novel pathogen detection methods to increase the sensitivity and specificity of pathogen detection. Utilizing the deep learning and computational analysis of data generated by microfluidic biosensors with artificial intelligence algorithms to improve the accuracy and reliability of test results and support clinical decision-making. |
| Elements | Content Description |
|---|---|
| Technical advantages | Low consumption: reduce the amount of reagents used. High sensitivity: improve the sensitivity and accuracy of the test. Portability: easy to carry out in different settings. |
| Application areas | Inflammation. Infectious diseases. Chronic diseases. |
| Key challenges | Accuracy and consistency of results. Simplicity and maneuverability of equipment. Application in resource-constrained environments. |
| Future directions | Optimizing microfluidic chip design to streamline operational steps, enhance device simplicity and operability, and reduce the entry barrier for use. Developing POCT devices capable of detecting multiple biomarkers simultaneously to advance multiparameter testing for a more thorough disease assessment. Integrating POCT equipment with portable devices like smartphones for wireless data transmission and analysis, enabling patient self-monitoring and health management. Exploring new application domains, such as environmental monitoring, food safety, and drug discovery, leveraging the miniaturization, integration, and automation of POCT devices. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Guan, X.; Sun, S. Microfluidic Biosensors: Enabling Advanced Disease Detection. Sensors 2025, 25, 1936. https://doi.org/10.3390/s25061936
Wang S, Guan X, Sun S. Microfluidic Biosensors: Enabling Advanced Disease Detection. Sensors. 2025; 25(6):1936. https://doi.org/10.3390/s25061936
Chicago/Turabian StyleWang, Siyue, Xiaotian Guan, and Shuqing Sun. 2025. "Microfluidic Biosensors: Enabling Advanced Disease Detection" Sensors 25, no. 6: 1936. https://doi.org/10.3390/s25061936
APA StyleWang, S., Guan, X., & Sun, S. (2025). Microfluidic Biosensors: Enabling Advanced Disease Detection. Sensors, 25(6), 1936. https://doi.org/10.3390/s25061936






